Abstract
Six essential oils (EOs) from the Alliaceae family, namely garlic (Allium sativum), onion (Allium cepa), leek (Allium porrum), Chinese chive (Allium tuberosum), shallot (Allium ascalonicum) and chive (Allium schoenoprasum) were characterized by GC and GC-MS and evaluated for their functional food properties. Antibacterial properties were tested on five food-borne pathogens: Two Gram-positive Staphylococcus aureus (ATCC 25923), Listeria monocytogenes (ATCC 19115) and three Gram-negative Salmonella Typhimurium (ATCC 14028), Escherichia coli (ATCC 8739) and Campylobacter jejuni (ATCC 33291) bacteria. Antioxidant and radical-scavenging properties were tested by means of Folin-Ciocalteu and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays. Garlic, Chinese chive and onion EOs had the highest antibacterial activity whereas shallot and leek EOs were the strongest antioxidants. Heating caused a decrease in the antioxidant activity of these Eos, as shown in the Total Polar Materials (TPM) test. Suggestions on relationships between chemical composition and biological activities are presented. Results show that the EOs could be of value in the food industry as alternatives to synthetic antioxidants.
Keywords: essential oils, Allium species, antioxidant activity, antibacterial activity
1. Introduction
Allium species, the most important genus of the Alliaceae family, are among the oldest cultivated vegetables. They have been used as ornamentals, spices, vegetables, or as medicines for curing various diseases. The Allium genus includes more than 700 species widely distributed all over the world [1] and appreciated due to their flavor, easy growth and long storage time. The species may differ in form and taste, but they are close in biochemical and phytochemical contents. Allium species are characterized by their rich content in sulfur compounds that are responsible for the organoleptic parameters [2,3] and contribute to the antioxidant and antimicrobial activities of these vegetables [4,5,6,7,8]. These volatile components also constitute the major part of the essential oils of these plants [9].
Garlic (Allium sativum) and onion (Allium cepa) are the most important Allium species consumed all over the world. The allicin derivative products (diallyl disulfide, diallyl trisulfide) found in garlic essential oils have shown good antimicrobial [10,11] and antioxidant activities [12]. The major compounds presented in onion EO are dipropyl disulfide and dipropyl trisulfide [10] and they have been reported to have antimicrobial and antioxidant activities [13]. Leek (Allium porrum), Chinese chive (Allium tuberosum), shallot (Allium ascalonicum) and chive (Allium schoenoprasum) are widely cultivated in Asian countries. They constitute important ingredients in many European and Asian diets [14] and they have been known for their medicinal properties [15,16,17,18,19,20]. The essential oils of these plants are believed to have antimicrobial and antioxidant activities, which are mostly associated with their sulfur compounds [21]. However, little attention has been paid to the compositions and properties of the essential oils of these Allium species.
Lipid oxidation and microbial alteration are the main reasons for the deterioration of quality, safety and shelf life of food. In fact, the presence of pathogens in food might be responsible for serious diseases leading to death. Gram-positive Staphylococcus aureus, Listeria monocytogenes and Gram-negative Salmonella Typhimurium, Escherichia coli and Campylobacter jejuni are among the most common bacteria present in food. Staphylococcus aureus is a pathogen of great importance in food processing. Some strains produce a poisonous toxin to humans which is resistant to high temperatures, gastric acidity and proteases; it can grow at high salt concentrations, low water activities and a relatively low pH. Listeria monocytogenes has the ability to grow at 4 °C; it can be present at high concentrations in cases of long storage. Its transmission might be maternal-fetal and through contaminated food. Salmonella Typhimurium is the major cause of food-borne diseases; it is a severe pathogen for humans associated with outbreaks caused mainly by contaminated meat and eggs. Escherichia coli is often used as an indicator of fecal contamination in food products; it constitutes about 80% of human intestinal flora. Finally, Campylobacter jejuni is the second most common cause of food poisoning after Salmonella; it has a high mobility which is important in the phenomenon of colonization in the intestinal tract.
Due to the increasing demand for natural ingredients, the use of essential oils for food preservation appears as a viable and healthy alternative to unpopular synthetic antioxidants. Therefore, this work was carried out to evaluate the chemical composition of six essential oils of garlic, onion, leek, Chinese chive, shallot and chive as well as their antioxidant and antimicrobial activities. Foremost, the extractions of EO were optimized using turbo hydrodistillation, then they were analyzed for their possible antioxidant activities by two methods, namely the Folin-Ciocalteu assay for the determination of the total phenol contents and the DPPH assay for their radical scavenging activity. Antibacterial tests using the disk diffusion method have been conducted against the five important food-borne pathogens mentioned above. In addition to that, the possible use of EOs in food processing under thermal conditions has been studied. The objective of this study was to determine the possibility of using the EOs as food additives for the food industry in order to satisfy the consumer demands by reducing the use of synthetic antioxidants.
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
Table 1 shows the chemical constituents, the relative percentage of the total chromatogram area according to the total compounds and the retention indexes of garlic, onion, leek, Chinese chive, shallot and chive essential oils.
Table 1.
Chemical composition of the essential oils of garlic, onion, leek, Chinese chive, shallot and chive.
| Compounds | Essential Oils (% ± SD) | Identification Methods | ||||||
|---|---|---|---|---|---|---|---|---|
| LRIHP5 | Garlic | Onion | Leek | Chinese Chive | Shallot | Chive | ||
| Isoamyl alcohol | 762 | - | 0.13 ± 0.03 | - | - | - | - | SM, LRI, Std |
| Methyl 1-propenyl sulfide a | 764 | - | - | - | - | tr | - | SM, LRI |
| Dimethyl disulfide | 767 | 1.12 ± 0.18 | tr | 0.28 ± 0.02 | 19.58 ± 2.62 | tr | tr | SM, LRI, Std |
| (Z)-3-hexenal | 769 | 0.15 ± 0.01 | - | tr | tr | 0.16 ± 0.03 | tr | SM, LRI, Std |
| 2-methylpentenal a | 776 | 0.15 ± 0.02 | - | - | - | 0.51 ± 0.07 | - | SM, LRI |
| Hexanal | 802 | - | - | tr | tr | 0.17 ± 0.02 | - | SM, LRI, Std |
| Propanal diethyl acetal t | 813 | - | - | tr | - | - | 0.16 ± 0.03 | SM |
| 2-Ethylpyridine | 836 | 0.10 ± 0.01 | - | - | - | - | - | SM, LRI |
| (E)-hexenol | 848 | - | - | tr | - | - | - | SM, LRI, Std |
| 1,3-Propanedithiol t | 851 | - | - | tr | - | - | 0.17 ± 0.01 | SM |
| Diallyl sulfide | 854 | 6.59 ± 0.55 | tr | tr | 1.47 ± 0.10 | tr | tr | SM, LRI, Std |
| n-Hexanol | 866 | - | - | 0.25 ± 0.02 | - | - | - | SM, LRI, Std |
| Allyl propyl sulfide | 867 | 0.09 ± 0.01 | - | 0.15 ± 0.01 | - | 0.16 ± 0.03 | - | SM, LRI |
| Bis-(1-propenyl)-sulfide a | 884 | 0.08 ± 0.01 | - | - | 0.21 ± 0.01 | - | - | SM, LRI |
| Dimethyl thiophene a | 871 | - | - | - | - | - | 0.15 ± 0.01 | SM, LRI |
| Nonane | 900 | - | - | - | - | - | tr | SM, LRI, Std |
| Dimethyl thiophene a | 902 | 0.08 ± 0.01 | 0.18 ± 0.01 | 0.34 ± 0.03 | - | 0.21 ± 0.01 | 0.26 ± 0.01 | SM, LRI |
| Allyl methyl disulfide | 915 | 3.69 ± 0.02 | - | - | 14.37 ± 0.36 | - | - | SM, LRI, Std |
| Methyl propyl disulfide | 926 | 0.25 ± 0.01 | 2.11 ± 0.16 | 4.48 ± 0.33 | 1.21 | 3.26 ± 0.27 | 2.55 ± 0.13 | SM, LRI |
| Methyl 1-propenyl disulfide a | 934 | 0.46 ± 0.02 | 0.51 ± 0.04 | 0.27 ± 0.03 | 6.07 ± 0.13 | 1.33 ± 0.13 | 0.62 ± 0.03 | SM, LRI |
| Dimethyl trisulfide | 962 | 0.33 ± 0.01 | 0.30 ± 0.01 | 0.12 ± 0.01 | 14.34 ± 0.05 | 1.22 ± 0.06 | 0.65 ± 0.02 | SM, LRI, Std |
| 2-Pentylfuran | 990 | - | - | - | - | - | 0.14 ± 0.01 | SM, LRI |
| Diallyl disulfide | 1084 | 37.90 ± 0.07 | - | - | 5.14 ± 0.21 | 0.13 | - | SM, LRI, Std |
| Allyl propyl disulfide | 1088 | - | 0.42 ± 0.08 | 0.73 ± 0.04 | - | 0.55 ± 0.06 | 0.44 ± 0.02 | SM, LRI |
| Linalool | 1101 | - | - | - | 1.75 ± 0.14 | - | - | SM, LRI, Std |
| Dipropyl disulfide | 1105 | 0.25 ± 0.06 | 30.92 ± 0.03 | 47.70 ± 0.03 | 1.24 ± 0.05 | 15.17 ± 0.18 | 19.49 ± 0.08 | SM, LRI |
| Ethyl-3-(methylthio)propionate | 1113 | 0.09 ± 0.01 | - | - | - | - | - | SM, LRI |
| 1-Propenyl propyl disulfide a | 1116 | - | 7.26 ± 0.06 | 3.75 ± 0.02 | - | 4.57 ± 0.05 | 5.84 ± 0.05 | SM, LRI |
| 2,4,5-tTrithiahexane t | 1118 | - | - | - | 0.15 ± 0.01 | - | - | SM |
| 3,5-Dimethyl-1,2,4-trithiolane | 1126 | - | 0.12 ± 0.01 | 0.21 ± 0.01 | - | - | - | SM, LRI |
| Allyl methyl trisulfide | 1131 | 7.26 ± 0.05 | - | - | 7.24 ± 0.38 | - | 0.2 | SM, LRI |
| Menthone | 1145 | - | - | - | 1.91 ± 0.12 | - | - | SM, LRI, Std |
| Methyl propyl trisulfide | 1148 | - | 5.20 ± 0.02 | 3.19 ± 0.02 | - | 9.20 ± 0.10 | 8.47 ± 0.10 | SM, LRI |
| Methyl 1-propenyl trisulfide | 1153 | - | 0.34 ± 0.01 | 0.13 ± 0.01 | - | 0.50 ± 0.08 | 0.36 ± 0.01 | SM, LRI |
| Methyl-1-(methylthio)ethyl-disulfide t | 1159 | 0.47 ± 0.05 | 0.15 ± 0.01 | - | 0.68 ± 0.05 | 0.51 ± 0.02 | SM | |
| n-Nonanol | 1172 | - | 0.47 ± 0.01 | - | - | 0.15 ± 0.01 | SM, LRI, Std | |
| 3,4-Dihydro-3-vinyl-1,2-dithiin t | 1177 | 0.13 ± 0.02 | - | - | - | - | - | SM |
| Borneol | 1183 | - | - | - | - | 0.33 ± 0.01 | 0.85 ± 0.01 | SM, LRI, Std |
| α-Terpineol | 1187 | - | - | - | 0.32 ± 0.01 | - | - | SM, LRI, Std |
| Methyl salicylate | 1189 | - | - | - | 0.46 ± 0.01 | - | - | SM, LRI, Std |
| Methyl chavicol | 1196 | - | - | - | - | 0.11 ± 0.01 | - | SM, LRI, Std |
| Dimethyl tetrasulfide | 1206 | 0.56 ± 0.01 | 0.15 ± 0.01 | - | 2.82 ± 0.19 | 0.46 ± 0.01 | 0.39 ± 0.01 | SM, LRI, Std |
| 3-Ethyl-5-methyl-1,2,4-trithiolane a, t | 1216 | - | 0.13 ± 0.01 | - | - | 0.11 ± 0.01 | - | SM |
| 3-Ethyl-5-methyl-1,2,4-trithiolane a, t | 1219 | - | 0.16 ± 0.01 | 0.54 ± 0.01 | - | 0.17 ± 0.01 | - | SM |
| Butyl thiocyante | 1239 | - | - | - | - | 0.21 ± 0.01 | - | SM, LRI |
| Methyl 1-(methylthiopropyl) disulfide | 1249 | - | 0.36 ± 0.04 | 0.12 ± 0.01 | 0.59 ± 0.04 | 0.52 ± 0.06 | 0.20 ± 0.01 | SM, LRI |
| 2-Undecanone | 1292 | - | 0.53 ± 0.01 | - | 0.62 ± 0.04 | 0.82 ± 0.01 | 0.10 ± 0.01 | SM, LRI |
| Tridecane | 1301 | - | 0.49 ± 0.05 | - | - | - | - | SM, LRI, Std |
| Diallyl trisulfide | 1305 | 28.06 ± 0.63 | - | - | - | - | - | SM, LRI |
| 3-Methoxyoctane t | 1311 | 1.10 ± 0.03 | - | - | - | 0.88 ± 0.01 | 1.24 ± 0.02 | SM |
| Dipropyl trisulfide | 1328 | tr | 17.10 ± 0.28 | 15.01 ± 0.27 | tr | 11.14 ± 0.14 | 15.21 ± 0.18 | SM, LRI |
| 1-Propenyl propyl trisulfide a | 1332 | - | - | 0.43 ± 0.02 | - | 1.36 ± 0.02 | - | SM, LRI |
| Allyl propyl trisulfide | 1334 | - | 1.84 ± 0.01 | 1.58 ± 0.03 | - | 1.97 ± 0.01 | 1.92 ± 0.02 | SM, LRI |
| Di-1-propenyl trisulfide | 1347 | 0.23 ± 0.05 | 3.07 ± 0.01 | - | - | 0.26 ± 0.02 | 0.14 ± 0.01 | SM, LRI |
| Eugenol | 1356 | 0.21 ± 0.01 | - | - | 0.26 0.01 | - | - | SM, LRI |
| Benzyl thiocyanate | 1359 | - | - | 0.20 ± 0.02 | 0.27 ± 0.02 | - | - | SM, LRI, Std |
| α-Copaene | 1370 | - | - | - | - | - | 0.14 ± 0.01 | SM, LRI, Std |
| Allyl methyl tetrasulfide | 1371 | 1.07 ± 0.03 | - | - | 1.21 ± 0.09 | - | - | SM, LRI |
| Benzyl methyl disulfide t | 1373 | - | - | - | 0.37 ± 0.04 | - | - | SM |
| Geranyl acetate | 1386 | - | - | 0.83 ± 0.01 | - | - | - | SM, LRI |
| Methyl eugenol | 1405 | - | - | - | - | 0.14 ± 0.03 | - | SM, LRI, Std |
| 6,10-Dimethyl 2-undecanone | 1406 | - | - | - | - | - | 0.32 ± 0.01 | SM, LRI |
| β-Caryophyllene | 1410 | - | - | - | - | - | 0.17 ± 0.01 | SM, LRI, Std |
| 3,6-Dimethyl-2,4,5,7 tetrathioctane t | 1423 | - | - | 0.39 ± 0.04 | - | - | 0.11 ± 0.01 | SM |
| 2-Hexyl-5-methyl 3(2H)-furanone | 1440 | - | 1.26 ± 0.01 | 0.13 ± 0.01 | - | 5.40 ± 0.15 | 0.15 ± 0.01 | SM, LRI |
| β-Selinene | 1445 | - | - | - | - | - | 0.25 ± 0.03 | SM, LRI |
| (E)-β-Farnesene | 1457 | - | - | - | - | - | 1.50 ± 0.06 | SM, LRI |
| β-Ionone | 1481 | - | - | - | 0.13 ± 0.01 | - | 0.30 ± 0.04 | SM, LRI, Std |
| Methyl-1-propylthioethyl tetrasulfide | 1487 | - | - | 0.43 ± 0.01 | - | - | - | SM, LRI |
| 2-Tridecanone | 1496 | - | 0.32 ± 0.03 | - | - | 0.63 ± 0.01 | - | SM, LRI |
| γ-Cadinene | 1506 | 0.10 ± 0.01 | - | - | - | - | - | SM, LRI |
| α-Farnesene | 1509 | - | - | - | - | - | 2.56 ± 0.08 | SM, LRI |
| β-Sesquiphellandrene | 1521 | - | - | - | - | - | 0.15 ± 0.01 | SM, LRI |
| Diallyl tetrasulfide | 1538 | 4.14 ± 0.11 | - | - | 0.22 ± 0.02 | - | - | SM, LRI, Std |
| 2-Methyl-3,4-dithiaheptane | 1558 | - | 6.48 ± 0.08 | 2.03 ± 0.04 | - | 4.42 ± 0.05 | 3.70 ± 0.05 | SM, LRI |
| Dipropyl tetrasulfide | 1573 | - | 0.55 ± 0.04 | - | - | - | 0.35 ± 0.01 | SM, LRI |
| Tetradecanal | 1607 | - | - | 0.15 ± 0.02 | - | - | - | SM, LRI |
| Dimethyl pentasulfide | 1686 | - | - | - | 0.23 ± 0.02 | - | - | SM, LRI |
| Propyl-1-(propylthio)ethyl trisulfide t | 1700 | - | - | 0.28 ± 0.01 | - | 0.44 ± 0.03 | 1.12 ±0.02 | SM |
| 6,10,14-Trimethyl-2-pentadecanone | 1845 | - | - | 0.52 ± 0.01 | 0.16 ± 0.01 | 0.23 ± 0.01 | 1.35 ± 0.01 | SM, LRI |
| Benzyl salicylate | 1857 | - | - | - | - | 0.27 ± 0.01 | - | SM, LRI |
| 2,4-Diméthyl-5,6-dithia-2,7-nonadienal | 1885 | 0.44 ± 0.02 | - | - | - | - | - | SM, LRI |
| Methyl palmitate | 1928 | - | 0.81 ± 0.04 | 0.17 ± 0.01 | 0.15 ± 0.01 | 1.39 ± 0.02 | 0.30 ± 0.01 | SM, LRI, Std |
| Palmitic acid | 1970 | - | - | 0.76 ± 0.01 | 1.58 ± 0.10 | - | 2,17 ± 0.06 | SM, LRI, Std |
| Ethyl palmitate | 1996 | - | 0.42 ± 0.02 | 0.48 ± 0.01 | 0.41 ± 0.03 | 0.43 ± 0.01 | 0.56 ± 0.01 | SM, LRI, Std |
| Methyl linoleate | 2093 | - | 0.55 ± 0.01 | - | 0.14 ± 0.01 | - | 0.25 ± 0.01 | SM, LRI, Std |
| Methyl linolenate | 2099 | - | - | - | 0.16 ± 0.01 | - | - | SM, LRI, Std |
| Phytol | 2113 | - | - | 0.16 ± 0.01 | 0.61 ± 0.03 | - | - | SM, LRI |
| Ethyl linoleate | 2161 | - | - | 0.26 ± 0.01 | 0.21 ± 0.01 | 0.42 ± 0.04 | 0.35 ± 0.01 | SM, LRI, Std |
| Ethyl oleate | 2167 | - | 0.18 ± 0.01 | - | - | 0.36 ± 0.01 | - | SM, LRI, Std |
| Ethyl linolenate | 2168 | - | - | 0.21 ± 0.01 | - | - | 0.35 ± 0.02 | SM, LRI |
| Ethyl α-linolénate | 2244 | - | - | - | 0.19 ± 0.02 | - | - | SM, LRI |
SD = Standard Deviation. Compositional values less than 0.1% are denoted as traces (tr). Presence of a compound is indicated by its GC-FID percentage with SD, absence is indicated by “-”. a: Correct isomer not identified. t: Tentative.
GC-MS analysis of garlic EO identified 27 constituents representing more than 94.63% of the total EO. The major components were diallyl disulfide (37.90%), diallyl trisulfide (28.06%), allyl methyl trisulfide (7.26%), diallyl sulfide (6.59%), diallyl tetrasulfide (4.14%) and allyl methyl disulfide (3.69%). The profile obtained in this study was similar to that presented by Banerjee et al. [22] and Kim et al. [10]. Different studies on the composition of garlic essential oil show that diallyl disulfide and diallyl trisulfide are the two major compounds [4,23].
Thirty one constituents which represent more than 82.36% of the total EO were identified in the onion EO. The main components were dipropyl disulfide (30.92%), dipropyl trisulfide (17.10%), 1-propenyl propyl disulfide (7.26%) and methyl propyl trisulfide (5.20%). Dipropyl disulfide was reported by Corzomartinez et al. [4] to be the major compound present, which is in accordance with our results.
When leek EO was analyzed, 41 compounds which constitute more than 86.90% of the total EO were identified. Dipropyl disulfide was the major component, representing 47.70% followed by dipropyl trisulfide (15.01%), methyl propyl disulfide (4.48%), 1-propenyl propyl disulfide (3.75%) and methyl propyl trisulfide (3.19%). These results are in agreement with Casella et al. [23] who reported that dipropyl disulfide and dipropyl trisulfide were also the major compounds.
In the Chinese chive EO, 37 compounds were identified, representing 85.79% of the total oil. The EO consisted mainly of dimethyl disulfide (19.58%), allyl methyl disulfide (14.37%), dimethyl trisulfide (14.34%), allyl methyl trisulfide (7.24%), methyl 1-propenyl disulfide (6.07%) and diallyl disulfide (5.14%).
Concerning the shallot EO, 42 compounds which represent more than 70.29% of the total EO were identified. The major components were dipropyl disulfide (15.17%), dipropyl trisulfide (11.14%), methyl propyl trisulfide (9.20%), 1-propenyl propyl disulfide (4.57%) and methyl propyl disulfide (3.26%).
GC-MS analysis of chive EO identified 48 components, representing more than 76.36% of the total EO. The main compounds found in chive EO were dipropyl disulfide (19.49%), dipropyl trisulfide (15.21%), methyl propyl trisulfide (8.47%) and 1-propenyl propyl disulfide (5.84%).
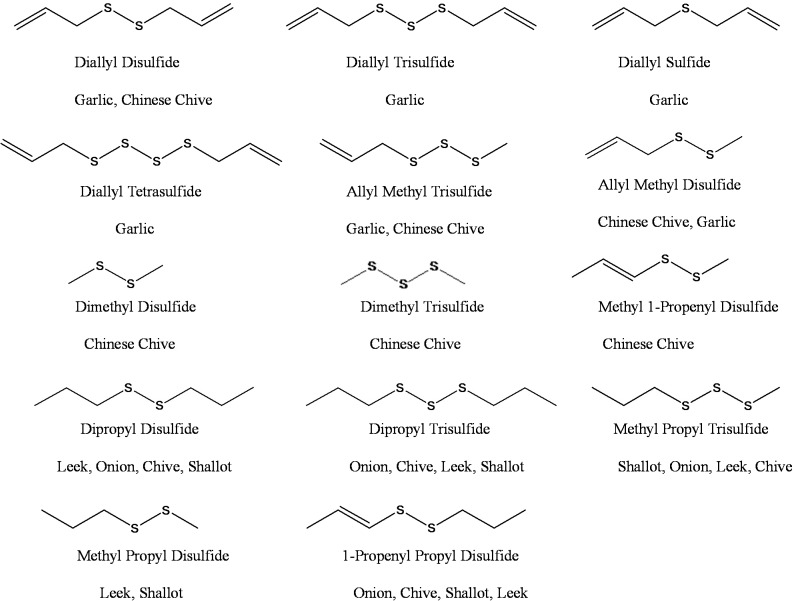
According to these results, diallyl disulfide was mainly present in garlic and Chinese chive EO whereas dipropyl disulfide and dipropyl trisulfide were the most representative compounds found in the essential oils of onion, leek, shallot and chive. The structures of the major bioactive compounds are presented in Figure 1.
Figure 1.
Structure of major compounds of the essential oils of garlic, onion, leek, Chinese chive, shallot and chive.
2.2. In Vitro Antimicrobial Activity
The in vitro antibacterial activity of the six essential oils against the tested microorganisms (Gram-positive and Gram-negative bacteria) was assessed by the disc diffusion method by measuring the inhibition zones. According to the results presented in Table 2, the essential oils with the highest antibacterial effects produced inhibition zones larger than 20 mm diameter.
Table 2.
Antibacterial activity of garlic, onion, leek, Chinese chive, shallot, chive EO and positive control after 48 h 1.
| Pathogens | Inhibition Diameter (mm) 2 Including Disk Diameter of 6.0 mm | |||
|---|---|---|---|---|
| Garlic | Onion | Leek | ||
| Staphylococus aureus | 20.0 ± 0.0 a | 15.5 ± 2.1 a | 10.0 ± 0.0 a | |
| Salmonella Typhimurium | 9.0 ± 1.0 c | 12.0 ± 1.8 a b | 6 mm | |
| Listeria monocytogenes | 23.0 ± 1.4 a | 15.0 ± 1.4 a | 6 mm | |
| Escherichia coli | 9.3 ± 0.9 c | 6 mm | 6 mm | |
| Campylobacter jejuni | 12.6 ± 2.1 b | 9.0 ± 1.2 b | 9.3 ± 1.9 a | |
| Pathogens | Inhibition Diameter (mm) 2 Including Disk Diameter of 6.0 mm | |||
| Chinese Chive | Shallot | Chive | Positive Control | |
| Staphylococus aureus | 18.5 ± 0.7 a | 20.0 ± 0.1 a | 11.5 ± 0.7 a | 30.6 ± 0.6 a |
| Salmonella Typhimurium | 9.3 ± 1.2 b | 11.3 ± 2.3 b | 6 mm | 25.6 ± 1.3 b |
| Listeria monocytogenes | 6 mm | 6 mm | 6 mm | 28.0 ± 0.5 a b |
| Escherichia coli | 9.0 ± 1.4 b | 6 mm | 6 mm | 25.5 ± 1.1 b |
| Campylobacter jejuni | 21.0 ± 1.7 a | 11.6 ±1.5 b | ±2.1 a | 25.3 ± 1.2 b |
1 Results are analyzed according to Ponce et al., [24]; 2 Results are mean ± SD values of three replications. For the same essential oil, values followed by different letters within the same column are significantly different (p < 0.05) according to Tukey’s HSD test.
The positive control amoxicillin/clavulanic acid was extremely effective on all tested bacteria, with inhibition zones ranging from 25.3 to 30.6 mm. Staphylococcus aureus was highly sensitive to the control (30.6 mm, p < 0.05), followed by Listeria monocytogenes (28.0 mm). The three Gram-negative bacteria were also highly sensitive with no statistical difference between them (25.6 mm for Salmonella Typhimurium, 25.5 mm for Escherichia coli and 25.3 mm for Campylobacter jejuni).
Among the essential oils, the most effective in this respect was garlic oil inhibiting all five bacteria tested with different sensitivities. Garlic EO was highly effective (p < 0.05) on Staphylococcus aureus and Listeria monocytogenes, showing inhibitory zones of 20.0 and 23.0 mm, respectively. Garlic oil exhibited lower inhibition activity against Campylobacter jejuni, Escherichia coli and Salmonella Typhimurium with diameter inhibition halos of 12.6, 9.3 and 9.0 mm, respectively.
The two second most effective EOs were Chinese chive and onion oils. They inhibited four bacteria. Campylobacter jejuni and Staphylococcus aureus were highly sensitive (p < 0.05) to Chinese chive EO with inhibition zones of 21.0 and 18.5 mm, respectively. Salmonella Typhimurium and Escherichia coli were sensitive to Chinese chive EO (9.3 and 9.0 mm, respectively) whereas Listeria monocytogenes was resistant to the same EO. Staphylococcus aureus and Listeria monocytogenes were highly sensitive (p < 0.05) to onion oil with diameters of 15.5 and 15.0 mm, respectively. Salmonella Typhimurium and Campylobacter jejuni were also sensitive with inhibition zones of 12.0 and 9.0 mm, respectively. Escherichia coli was the only resistant bacteria to this essential oil.
Shallot essential oil inhibited three of the five bacteria tested; it was extremely effective (p < 0.05) on Staphylococcus aureus (20.0 mm) and active on Campylobacter jejuni (11.6 mm) and Salmonella Typhimurium (11.3 mm).
Leek and chive essential oils were both active on two bacteria, with inhibition zones for Staphylococcus aureus of 10.0 and 11.5 mm, respectively, and for Campylobacter jejuni of 9.3 and 10.3 mm respectively. The results were in a accordance with the ones already published showing that garlic, onion, leek, Chinese chive, shallot and chive EO had antibacterial activity against Gram-negative and Gram-positive bacteria [10,14,21,23,25,26,27,28].
Studies show that diallyl sulfide exerts good antimicrobial activity [4,7,29,30]. In fact, in sulfide compounds, a greater number of sulfur atoms is found to result in stronger antimicrobial activity [10,11,14]. This explains the good antimicrobial effect of garlic and Chinese chive EO which have diallyl disulfide in their compositions (37.90% and 5.14% respectively). Moreover, garlic EO also contains diallyl trisulfide (28.06%) and diallyl tetrasulfide (4.14%). Thus, the richness in sulfur atoms may have contributed in the effectiveness of the EO activity. The high antimicrobial activity of Chinese chive EO may also be attributed to other sulfide compounds such as dimethyl disulfide (19.58%), allyl methyl disulfide (14.37%), dimethyl trisulfide (14.34%) and allyl methyl trisulfide (7.24%).
Studies on the antimicrobial activity of dipropyl disulfide and dipropyl trisulfide, which are the main components in onion, leek, shallot and chive EO, are scarce. However, it was noted that dipropyl trisulfide demonstrated antimicrobial activity against Staphylococcus aureus [10]. The antibacterial activity of these EO may be related to the propyl derivatives.
Several studies report that EO act more on Gram-positive than Gram-negative bacteria [31]; this is due to the difference in cell wall composition [32]. However, there is no general rule with respect to the Gram sensitivity because many controversies exist in the various published works. Thus the Gram-negative bacteria Campylobacter jejuni has been described as particularly sensitive to the action of EO [33]. This is in agreement with our results since Gram-negative Campylobacter jejuni showed sensitivity to the six essential oils. Among the Gram-positive bacteria, Staphylococcus aureus was sensitive to all EOs.
Due to the complexity of the chemical composition of the essential oils, their mechanisms of action have not been clarified yet. However, assumptions on their activity for targeting different bacterial structures might be proposed. Their hydrophobicity allows them to attack the phospholipid membrane of the cell and to increase its permeability. Therefore, the contents of the cells are lost leading to bacterial death [34]. More specifically, the relation between chemical structure and antibacterial activities of sulfides is not fully understood. However, Kyung [35] reported that the sulfides can damage the microbial cells by reacting with SH groups of cellular proteins to generate mixed disulfides.
2.3. Total Phenolic Content
The Total Phenolic Contents (TPCs) of the garlic, onion, leek, chive, shallot and Chinese chive essential oils are presented in Table 3. The positive control BHT had the highest phenol content (p < 0.05, 46.77 mg GAE/g). Among the six essential oils, shallot and leek oils showed the highest TPCs (p < 0.05, 1.14 and 10.79 mg GAE/g, respectively). Chive and garlic EO had lower TPCs (6.76 and 5.61 mg GAE/g, respectively) whereas Chinese chive and onion EO showed the lowest amount of TPCs (4.24 and 3.29 mg GAE/g respectively) with no statistical difference between them. Data on the TPCs of these essential oils is not available. However, Lu et al., and Yang et al. [36,37] showed that shallot extracts had the highest phenol content (17.18 mg GAE/g fresh weight and 114.70 mg GAE/100 g sample, respectively) among different onion varieties. Leek extracts were also reported to be rich in phenols [16,19]. Phenolic contents are good indicators of antioxidant activity, their high redox potential enables them to act as hydrogen donors or radical scavengers [38,39]. They are also reported to play an important role in the antimicrobial potential of the EO [40,41,42].
Table 3.
Total Phenol contents (TPCs) of garlic, onion, leek, Chinese chive, shallot, chive EO and BHT.
| Essential Oils | Total Phenol Contents GAE * (mg/g) |
|---|---|
| Garlic | 5.61 ± 0.69 c |
| Onion | 3.29 ± 0.12 d |
| Leek | 10.79 ± 0.53 b |
| Chinese chive | 4.24 ± 0.11 d |
| Shallot | 11.14 ± 0.43 b |
| Chive | 6.76 ± 0.37 c |
| BHT | 46.77 ± 0.81 a |
*: GAE: Gallic acid equivalent (mg/g). Values followed by the same letter within the same column are not significantly different (p > 0.05) according to Tukey’s HSD test.
2.4. DPPH Radical Scavenging Activity
The stable free radical DPPH was used to test the ability of the essential oils and BHT standard to donate the hydrogen atom. Table 4 shows the percentage of DPPH inhibition by the different concentrations of each EO and BHT. A concentration-dependent scavenging activity was found for the studied EO. All essential oils were able to reduce the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) to the yellow diphenylpicrylhydrazine with varying degrees of scavenging capacities. Great bleaching action (from purple to yellow) reflected a higher antioxidant activity and thus a lower IC50 (Table 4). The values of IC50 were in the following order: BHT ˂ shallot ˂ leek ˂ chive ˂ garlic ˂ Chinese chive ˂ onion. Positive control BHT was the strongest antioxidant with IC50 value of 0.03 mg/mL. Among essential oils, shallot and leek EO showed the strongest radical scavenging effect with IC50 values of 2.70 and 4.49 mg/mL, respectively. This activity was followed by chive (5.59 mg/mL) and garlic EO (7.67 mg/mL). Chinese chive and onion EO showed the lowest scavenging activity with IC50 values of 12.16 and 20.19 mg/mL, respectively.
Table 4.
Antioxidant activity of garlic, onion, leek, Chinese chive, shallot, chive EO and BHT at different concentrations measured by DPPH method.
| Essential Oils | DPPH Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|
| 2 mg/mL | 4 mg/mL | 8 mg/mL | 12 mg/mL | 16 mg/mL | 20 mg/mL | IC50 * | |
| Garlic | 31.35 ± 1.91 | 37.93 ± 1.59 | 51.07 ± 0.67 | 64.22 ± 0.78 | 77.37 ± 0.79 | 90.52 ± 0.59 | 7.67 |
| Onion | 22.30 ± 0.97 | 25.34 ± 0.19 | 31.43 ± 1.60 | 37.52 ± 0.32 | 43.61 ± 0.42 | 49.70 ± 1.49 | 20.19 |
| Chinese chive | 17.93 ± 0.89 | 24.24 ± 1.39 | 36.85 ± 1.38 | 49.46 ± 0.30 | 62.08 ± 0.68 | 74.69 ± 0.31 | 12.16 |
| Chive | 39.05 ± 0.33 | 45.15 ± 0.11 | 57.34 ± 1.14 | 69.54 ± 1.45 | 81.73 ± 0.11 | 93.93 ± 1.70 | 5.59 |
| 1 mg/mL | 2 mg/mL | 3 mg/mL | 4 mg/mL | 5 mg/mL | |||
| Leek | 9.90 ± 1.32 | 21.37 ± 1.70 | 32.84 ± 1.46 | 44.31 ± 0.64 | 55.78 ± 1.49 | 4.49 | |
| Shallot | 29.95 ± 1.18 | 42.59 ± 1.11 | 51.99 ± 1.44 | 61.38 ± 1.58 | 70.77 ± 1.64 | 2.70 | |
| 0.01 mg/mL | 0.02 mg/mL | 0.04 mg/mL | 0.06 mg/mL | 0.08 mg/mL | |||
| BHT | 16.01 ± 1.71 | 35.3 ± 1.27 | 68.93 ± 2.01 | 85.50 ± 0.13 | 91.21 ± 0.41 | 0.03 | |
* IC50: concentration (mg/mL) for a 50% inhibition.
The antioxidant activity of these plants is attributed partly to its sulfur compounds, which represent the main constituents of these essential oils [4,7,43]. Amagase et al. [12] reported that diallyl polysulphides contributed to the antioxidant properties of the essential oils.
The different antioxidant activities between these essential oils may be due to the variability of the composition and concentration of sulfides. It may also be attributed to the presence and synergy of different minor compounds. It is interesting to note that a good correlation between DPPH and Folin-Ciocalteu test was found, which is in the same order for all essentials oils. Both tests confirm that shallot exerts the strongest antioxidant activity and onion the lowest. This result indicates that the phenolic compounds of Allium species contribute to their antioxidant properties.
2.5. Heating Test
All samples (sunflower oil used as control and its mixture with each EO) display similar TPM results since the total heating times required to reach the maximal TPM value of 25% were quite close (ranging from 12 to 15 h). This might be attributed to the thermal degradation of sulfur and phenolic compounds present in the EO. These findings are in agreement with Wangcharoen and Morasuk [44] who reported that heat treatment caused the degradation of these constituents.
3. Experimental Section
3.1. Plant Materials, Chemicals and Standards
The bulbs of garlic, onion, leek, Chinese chive, shallots and chive were purchased from a local supermarket in Avignon province (France). Glycerol and Butylated Hydroxytoluene (BHT) were purchased from Sigma Aldrich (St. Louis, MO, USA), Trypticase Soy Agar was obtained from Biomerieux (Marcy-l’Étoile, France). Nutrient Broth and Mueller Hinton Agar were bought from VWR (London, UK), Nutrient Agar and Amoxicillin/Clavulanic acid were purchased from Himedia (Mumbai, India), Peptone water was obtained from SRL (Mumbai, India). Methanol was supplied by Fisher Scientific (Loughborough, UK). Folin-Ciocalteu reagent and Gallic acid were obtained from Isitec Lab-Seppal (Montauban, France) and DPPH was supplied by Sigma Aldrich (Munich, Germany).
3.2. Essential Oils: Extraction and Yield
The EO were extracted by turbo hydrodistillation (Figure 2). This technique is similar to that of the conventional one. The difference lies in the installation of a stainless steel stirrer in the apparatus. It is equipped with blades to cut the bulbs into small pieces during distillation to increase and speed up the extraction process. Five kilograms of the different fresh Allium vegetables were soaked in distilled water (8000 mL) and submitted to turbo hydrodistillation with a Clevenger-type apparatus. The studied EOs, which have a higher density than water, sank to the bottom while water floated to the top. An amount of the EOs was lost due to their backflow into the apparatus. The EO extractions were optimized by adding petroleum ether to the system. This solvent trapped the essential oils at the top avoiding their decantation and consequently their loss. Then, the EOs were separated and filtered over sodium sulfate to eliminate all traces of water. The petroleum ether was evaporated in a rotary evaporator at 40 °C. The EOs were collected and stored at 4 °C away from light until use. The yields of the essential oils per 1 ton (g/t) of raw material were: 1300 to 2000 g/t for garlic, 60 to 130 g/t for onion, 80 to 110 g/t for leek, 200 to 300 g/t for Chinese chive, 80 to 120 g/t for shallot and 30 to 40 g/t for chive.
Figure 2.
Extraction of essential oils by turbo hydrodistillation.
3.3. GC-FID and GC-MS Identification
The essential oils were analyzed using gas chromatography-mass spectrometry (GC-MS) to identify their chemical constituents. Essential oils were analyzed first by gas chromatography coupled to flame ionization detector (FID). These analyses were performed by using a 7890A Gas Chromatograph (Agilent, Massy, France) with a non-polar HP-5MS column (5% phenyl 95% methylsiloxane, 30 m × 0.25 mm × 0.25 µm) (Agilent). Injection of 0.4 µL samples was carried out with a split ratio 1:100, the carrier gas was H2, and injection temperature was 250 °C. The oven temperature range progressed from 40 to 250 °C at 2 °C·min−1 (for 60 min). Then, gas chromatography coupled with mass-spectra were performed on a 5975C mass spectrometry detector (Agilent), using the same column as GC-FID. GC-MS spectra were obtained using the following conditions: the ratio split was 1:100, injection volume was 0.4 µL and the carrier gas was helium. Injection temperature was 250 °C and the oven temperature range progressed from 40 to 250 °C at 2 °C·min−1 (for 60 min). The ionization mode used was electronic impact at 70 eV and the mass range between 35 and 400 was scanned. Essential oils were identified by comparison of their GC linear retention indices (RI), determined with reference to a homologous series of alkanes. Identification was confirmed by comparison of their spectral mass with authentic samples, with those stored in the MS database (home-made and commercial libraries: Wiley 6N, NIST 98) and with index retention literature data [45] using the Automatic Mass Spectral Deconvolution and Identification System (AMDIS) software. Three repeated injections were performed for the quantitative analysis of the constituents.
3.4. Bacterial Strains
Two Gram-positive bacteria Staphylococcus aureus (ATCC 25923), Listeria monocytogenes (ATCC 19115) and two Gram-negative bacteria Salmonella Typhimurium (ATCC 14028), Escherichia coli (ATCC 8739) were supplied by the Lebanese Agricultural Research Institute (LARI, Fanar, Lebanon). Gram-negative bacteria Campylobacter jejuni (ATCC 33291) was supplied by the Lebanese University, Faculty of Agricultural Engineering and Veterinary Medicine.
Bacterial cultures were frozen at −20 °C in Nutrient Broth containing 20% glycerol (v/v). Throughout the experiments, the strains were subcultured every month on Trypticase Soy Agar and kept at 4 °C. Before use, bacteria were activated in Nutrient Broth and incubated for 24 h at 42 °C for Campylobacter jejuni under microaerophilic conditions and 24 h at 37 °C for the other strains. The bacterial suspension was then diluted in Peptone water to provide initial cell counts of about 106 CFU/mL.
3.5. Screening for Antibacterial Activity
The paper disc diffusion method was applied to determine the antibacterial activity of the essential oils. One mL of the suspension of the tested microorganism (106 CFU/mL) was spread on plates containing 20 mL Muller Hinton Agar. Filter paper discs of 6.0 mm diameter (Whatman n° 40) were individually impregnated with 15 µL of essential oil, then laid on to the surface of the inoculated plates. A disc containing 30 μg amoxicillin/clavulanic acid was placed in the plate as a positive control. At the end of incubation time (48 h at 42 °C for Campylobacter jejuni under microaerophilic conditions and 48 h at 37 °C for the other bacteria), positive antibacterial activities were established by the presence of measurable inhibition zones. The antimicrobial activity was recorded as the width (in millimetres, diameter of the disc included) of the inhibition zones after incubation using a ruler. This sensitivity was classified according to Ponce et al. [24] as follows: not sensitive for diameter less than 8 mm; sensitive for diameter of 9–14 mm; very sensitive for diameter of 15–19 mm and extremely sensitive for diameter larger than 20 mm. Each test was performed in three replicates.
3.6. Folin-Ciocalteu Assay
The Total Phenol Contents (TPCs) was determined using Folin–Ciocalteu reagent following the procedure described by the supplier of the kit [46]. A methanolic solution of essential oil (EO; 20 mg/mL, 100 µL) was introduced into test tubes followed by Folin-Ciocalteu reagent (2 mL) and alkaline buffer (1 mL). The tubes were vortexed and allowed to stand for 1 h in the dark. Absorption at 760 nm was measured with a 8453 diode-array spectrophotometer (Hewlett-Packard, Waldbronn, Germany) and compared to a gallic acid solution (3 g/L) used as standard. TPCs were calculated using the following formula: TPCs = 3 × (sample absorbance-blank absorbance)/(standard absorbance-blank absorbance). BHT was used as positive control. The results were expressed as mg gallic acid equivalents (GAE)/g sample. Each assay was carried out in triplicates.
3.7. DPPH Free-Radical-Scavenging Assay
The antioxidant activity of garlic, onion, leek, Chinese chive, shallot and chive essential oils was measured in terms of hydrogen-donating or radical scavenging ability by bleaching of purple colored methanolic solution of the stable radical DPPH [47]. The diluted solutions were prepared in methanol to obtain final concentrations ranging from 20 to 1 mg/mL of the stock essential oil solutions (20 mg/mL) and were introduced into test tubes.
Two milliliters of fresh methanolic solution of DPPH at a concentration of 6 × 10−5 M were added. BHT (0.1 mg/mL) prepared in diluted methanolic concentrations ranging from 0.01 to 0.08 mg/mL was used as positive control. The samples were shaken in the dark for four hours.
The decrease in absorbance at 517 nm was determined using a Hewlett-Packard 8453 diode-array spectrophotometer for all samples. Methanol was used to zero the spectrophotometer; Methanol and DPPH were used as negative control. All the samples were tested in triplicates.
The concentration of the sample required to decrease the absorbance of DPPH by 50% (IC50) was calculated graphically. The inhibition percentage of the DPPH radical was calculated according to the formula of Yen and Duh [48]:
| I = (A0 − Ai)/A0 × 100 | (1) |
where I = DPPH inhibition (%), A0 = absorbance of control sample (No antioxidant) t = 0, and Ai = absorbance of the tested sample at the end of the reaction (t = 4 h).
3.8. Frying Oil Test
The antioxidant effect of essential oils under heating conditions was studied by the determination of Total Polar Materials (TPMs). The objective of this test consisted of studying whether the addition of any of the EOs would increase the shelflife of fried sunflower oil. Each of the six essential oils (0.25 g) was added separately to sunflower oils (250 g) and the samples were heated under domestic frying conditions, i.e., 180 ± 5 °C during several hours [49]. The temperature was monitored by a thermocouple (ATC-300) inserted directly into the domestic deep-fat electric fryers. All samples were evaluated before the first heating sessions and every 1 h of heating until oil discard using a cooking oil tester (Testo 270, Testo Sàrl, Forbach, France). The end of the heating assays was determined by the value of TPM, where a maximum value of 25% is tolerated in accordance with the French law (Article 3-3 of decree No 86-857 of 18/07/86). This maximal legal content of TPM in frying oils, including hydrolysis products (diglycerides, monoglycerides and free fatty acids) and a complex distribution of oxidation products encompassing polymers, is formed at temperatures below 180 °C (French law No 86-857)The TPM value, usually assessed in restaurants and the agrofood industry by fast commercial tests (mostly based on colorimetric readings), has proven to correlate well with values obtained by official standards [49].
3.9. Statistical Analysis
Means and standard deviations of the assays were calculated using conventional statistical methods. Each treatment was performed in three replicates. Statistical analysis (ANOVA) was applied to the data to determine differences (p < 0.05). Means differences were made by using Tukey’s HSD test. The statistical analysis was carried out using Statgraphics XV.I for windows.
4. Conclusions
There are many ongoing studies on the biological properties of essential oils for their possible use as alternatives to synthetic antioxidant such as BHT. This study evaluates the composition of the essential oils of the six Allium plants, their antimicrobial, antioxidant activities and their possible use in food processing against thermal effects.
All the essential oils showed antioxidant properties with different degrees of scavenging activity. Shallot and leek oils were the strongest antioxidants. In the antimicrobial activity tests, all the essential oils inhibited a good range of Gram-positive and Gram-negative bacteria, while garlic, onion and Chinese chive were amongst the strongest. These activities are mainly attributed to the presence of the sulfur compounds in their compositions. Moreover, the variability in the composition, structure and concentration of the different sulfides present in the essential oils, play an important role in the determination of their antimicrobial and antioxidant activities.
Some components such as allyl sulfide group were reported for their biological properties, but other active components have not been fully investigated. Thus, further studies are needed on the sulfur compounds to link the chemical content with particular functional properties. Based on the results of the antimicrobial and antioxidant tests, essential oils can be applied as natural alternatives to food synthetic preservatives. However, heating should be carefully considered when the EO are used in cooking for antioxidant protection.
Acknowledgments
We would like to thank the Lebanese Agricultural Research Institute for providing the microbial strains.
Author Contributions
FC designed research; DM, A-SF-T, EP, TH, NN, CF, XF performed research and analyzed the data; DM wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Tepe B., Sokmen M., Akpulat H., Sokmen A. In vitro antioxidant activities of the methanol extracts of five species from Turkey. Food Chem. 2005;92:89–92. doi: 10.1016/j.foodchem.2004.07.016. [DOI] [Google Scholar]
- 2.Benkeblia N., Lanzotti V. Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Food. 2007;1:193–201. [Google Scholar]
- 3.Lanzotti V. The analysis of onion and garlic. J. Chromatogr. A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Corzomartinez M., Corzo N., Villamiel M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007;18:609–625. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- 5.Leuschner R.G.K., Ielsch V. Antimicrobial effects of garlic, clove and red hot chilli on Listeria monocytogenes in broth model systems and soft cheese. Int. J. Food Sci. Nutr. 2003;54:127–133. doi: 10.1080/0963748031000084070. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa M., Mima T., Ohnishi S.T., Mori K. S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice. Planta Med. 2000;66:148–151. doi: 10.1055/s-2000-11124. [DOI] [PubMed] [Google Scholar]
- 7.Yin M., Cheng W. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003;63:23–28. doi: 10.1016/S0309-1740(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 8.Yin M., Cheng W. Antioxidant activity of several Allium members. J. Agric. Food Chem. 1998;46:4097–4101. doi: 10.1021/jf980344x. [DOI] [Google Scholar]
- 9.Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012;24:393–434. doi: 10.1080/10412905.2012.692918. [DOI] [Google Scholar]
- 10.Kim J.W., Huh J.E., Kyung S.H., Kyung K.H. Antimicrobial activity of alk(en)yl sulfides found in essential oils of garlic and onion. Food Sci. Biotechnol. 2004;13:235–239. [Google Scholar]
- 11.Tsao S., Yin M. In vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J. Med. Microbiol. 2001;50:646–649. doi: 10.1099/0022-1317-50-7-646. [DOI] [PubMed] [Google Scholar]
- 12.Amagase H., Petesch B., Matsuura H., Kasuga S., Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 13.Ye C.L., Dai D.H., Hu W.L. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.) Food Control. 2013;30:48–53. doi: 10.1016/j.foodcont.2012.07.033. [DOI] [Google Scholar]
- 14.Rattanachaikunsopon P., Phumkhachorn P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenis bacteria of chives (Allium schoenoprasum) Bionsci. Biotechnol. Biochem. 2008;72:2987–2991. doi: 10.1271/bbb.80482. [DOI] [PubMed] [Google Scholar]
- 15.Amin M., Kapadnis B.P. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Ind. J. Exp. Biol. 2005;43:751–754. [PubMed] [Google Scholar]
- 16.Bernaert N., de Paepe D., Bouten C., de Clercq H., Stewart D., van Bockstaele E., de Loose M., van Droogenbroeck B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum) Food Chem. 2012;134:669–677. doi: 10.1016/j.foodchem.2012.02.159. [DOI] [PubMed] [Google Scholar]
- 17.Fista G., Bloukas J., Siomos A. Effect of leek and onion on processing and quality characteristics of Greek traditional sausages. Meat Sci. 2004;68:163–172. doi: 10.1016/j.meatsci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Leelarungrayub N., Rattanapanone V., Chanarat N., Gebicki J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition. 2006;22:266–274. doi: 10.1016/j.nut.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Stajner D., Milic-DeMarino M., Canadanovic-Brunet J. Screening for antioxidant properties of leeks, Allium sphaerocephalon L. J. Herbs Spices Med. Plants. 2003;10:75–82. doi: 10.1300/J044v10n03_08. [DOI] [Google Scholar]
- 20.Yabuki Y., Mukaida Y., Saito Y., Oshima K., Takahashi T., Muroi E., Hashimoto K., Uda Y. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler) Food Chem. 2010;120:343–348. doi: 10.1016/j.foodchem.2009.11.028. [DOI] [Google Scholar]
- 21.Rattanachaikunsopon P., Phumkhachorn P. Shallot (Allium ascalonicum L.) oil: Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria. Afr. J. Microbiol. Res. 2009;3:747–750. doi: 10.1271/bbb.80482. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S., Mukherjee K., Maulik S. Garlic as an Antioxidant: The Good, The Bad and The Ugly. Phytother. Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 23.Casella S., Leonardi M., Melai B., Fratini F., Pistelli L. The Role of Diallyl Sulfides and Dipropyl Sulfides in the In Vitro Antimicrobial Activity of the Essential Oil of Garlic, Allium sativum L., and Leek, Allium porrum L. Phytother. Res. 2012;27:380–383. doi: 10.1002/ptr.4725. [DOI] [PubMed] [Google Scholar]
- 24.Ponce A.G., Fritz R., del Valle C.E., Roura S.I. Antimicrobial activity of essential oils on native microbial population of organic Swiss chard. Lebensm. Wiss. Technol. 2003;36:679–684. doi: 10.1016/S0023-6438(03)00088-4. [DOI] [Google Scholar]
- 25.Babu A.J., Sundari A.R., Indumathi J., Srujan R.V.N., Sravanthi M. Study on the Antimicrobial activity and Minimum Inhibitory Concentration of Essential Oils of Spices. Vet. World. 2011;4:311–316. doi: 10.5455/vetworld.4.311. [DOI] [Google Scholar]
- 26.Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT—Food Sci. Technol. 2004;37:263–268. doi: 10.1016/j.lwt.2003.09.001. [DOI] [Google Scholar]
- 27.Razavi Rohani S.M., Moradi M., Mehdizadeh T., Saei-Dehkordi S.S., Griffiths M.W. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. LWT—Food Sci. Technol. 2011;44:2260–2265. doi: 10.1016/j.lwt.2011.07.020. [DOI] [Google Scholar]
- 28.Zohri A.N., Abdel-Gawad K., Saber S. Antibacterial, antidermatophytic and antitoxigenic activities of onion (Allium cepa L.) oil. Microbiol. Res. 1995;150:167–172. doi: 10.1016/S0944-5013(11)80052-2. [DOI] [PubMed] [Google Scholar]
- 29.Kim J., Kim Y., Kyung K. Inhibitory activity of essential oils of garlic and onion against bacteria and yeasts. J. Food Prot. 2004;67:499–504. doi: 10.4315/0362-028x-67.3.499. [DOI] [PubMed] [Google Scholar]
- 30.O’Gara E., Hill D., Maslin D. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000;66:2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert R.J., Skandamis P., Coote P., Nychas G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oils, thymol and carvacrol. J. Appl. Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 32.Ratledge C., Wilkinson S. An overview of microbial lipids. In: Ratledge C., Wilkinson S.G., editors. Microbial Lipids. Volume 1. Academic Press; London, UK: 1988. p. 3. [Google Scholar]
- 33.Wannissorn B., Jarikasem S., Siriwangchai T., Thubthimthed S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia. 2005;76:233–236. doi: 10.1016/j.fitote.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Kyung K.H. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol. 2012;23:142–147. doi: 10.1016/j.copbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Lu X., Wang J., Al-Qadiri H.M., Ross C.F., Powers J.R., Tang J., Rasco B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011;129:637–644. doi: 10.1016/j.foodchem.2011.04.105. [DOI] [PubMed] [Google Scholar]
- 37.Yang J., Meyers K.J., van der Heide J., Liu R.H. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004;52:6787–6793. doi: 10.1021/jf0307144. [DOI] [PubMed] [Google Scholar]
- 38.Miguel M. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010;25:291–312. doi: 10.1002/ffj.1961. [DOI] [Google Scholar]
- 39.Viuda-Martos M., Mohamady M.A., Fernández-López J., Abd ElRazik K.A., Omer E.A., Pérez-Alvarez J.A., Sendra E. In vitro antioxidant and antibacterial activities of essentials oils obtained from Egyptian aromatic plants. Food Control. 2011;22:1715–1722. doi: 10.1016/j.foodcont.2011.04.003. [DOI] [Google Scholar]
- 40.Cosentino S., Tuberoso C.I., Pisano B., Satta M., Mascia V., Arzedi E., Palmas F. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999;29:130–135. doi: 10.1046/j.1472-765X.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 41.Dorman H., Deans S. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 42.Holley R., Patel D. Improvement of shelflife and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. doi: 10.1016/j.fm.2004.08.006. [DOI] [Google Scholar]
- 43.Lampe J. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999;70:475S–490S. doi: 10.1093/ajcn/70.3.475s. [DOI] [PubMed] [Google Scholar]
- 44.Wangcharoen W., Morasuk W. Effect of heat treatment on the antioxidant capacity of Garlic. Maejo Int. J. Sci. Technol. 2009;3:60–70. [Google Scholar]
- 45.Adams R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 46.Isitec Lab-Seppal, Indice de Folin/Polyphenols totaux. ISITEC; Montauban, France: 2013. [Google Scholar]
- 47.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 48.Yen G.C., Duh P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994;42:629–632. doi: 10.1021/jf00039a005. [DOI] [Google Scholar]
- 49.Casal S., Malheiro R., Sendas A., Oliveira B.P.P., Pereira J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010;48:2972–2979. doi: 10.1016/j.fct.2010.07.036. [DOI] [PubMed] [Google Scholar]