Abstract
Background
Current recommendation for performing the ACTH stimulation test (ACTHST) for diagnosis of hyperadrenocorticism (HAC) advocates the collection of baseline serum cortisol concentration (BC), but no references for interpretation of its results exist.
Objective
Evaluate the contribution of BC of the ACTHST to the diagnosis of HAC.
Animals
Fifty‐four dogs were evaluated for suspected HAC at a referral hospital.
Methods
Records of dogs that had been evaluated by ACTHST for suspected HAC were reviewed. Receiver operator characteristics (ROC) analyses were used to assess the performance of BC, post‐stimulation serum cortisol concentrations (PC), post‐to‐baseline cortisol concentration difference (DeltaC) and quotient (RatioC) for the diagnosis of HAC by comparing the area under the ROC curve (AUC) of PC to each of the other tests.
Results
The AUC of PC (95% confidence interval [CI]: 0.92; 95% CI, 0.81‐0.98) was significantly higher than AUCs of BC (0.70; 95% CI, 0.56‐0.82; P = .01) and RatioC (0.55; 95% CI, 0.41‐0.69; P < .001), and was not significantly different from AUC of DeltaC (0.86; 95% CI, 0.74‐0.94; P = .09). An optimal cutoff value of 683 nmol/L (24.8 μg/dL) for PC yielded a sensitivity of 86% and a specificity of 94%, respectively, and a cutoff value of 718 nmol/L (26.0 μg/dL) yielded a specificity of 100% with of 81% sensitivity for the diagnosis of pituitary‐dependent HAC.
Conclusion and Clinical Importance
The PC had good discriminatory ability for the diagnosis of HAC. It was comparable to DeltaC, whereas BC and RatioC were ineffective. Current recommendations to collect samples for BC appear redundant.
Keywords: added‐value, basal, Cushing's, post‐stimulation
Abbreviations
- BC
serum cortisol concentrations
- PC
post‐stimulation cortisol concentration
- DeltaC
post‐to‐baseline cortisol concentrations difference
- RatioC
post‐to‐baseline cortisol concentrations quotient
- ACTHST
adrenocorticotropic hormone stimulation test
- ACVIM
American College of Veterinary Internal Medicine
- PDHAC
pituitary‐dependent hyperadrenocorticism
- AT
adrenal tumor
- AUC
area under the ROC curve
- CI
confidence interval
- HAC
hyperadrenocorticism
- LDDST
low‐dose dexamethasone suppression test
- NAI
non‐adrenal illness
- ROC
receiver operator characteristics
1. INTRODUCTION
Naturally occurring hyperadrenocorticism (HAC) is a frequently diagnosed endocrine disorder in dogs.1 The result of excessive ACTH secretion or ACTH‐independent cortisol overproduction, HAC is characterized by a constellation of clinical signs and clinicopathological laboratory abnormalities.1, 2 It is a common cause of dermatological lesions as well as polyuria, polydipsia, and polyphagia in dogs.1, 2, 3, 4 Rarely, it may lead to serious potentially life‐threatening complications, including thromboembolism, infections, and urolithiasis.1, 2, 3
Treatment of HAC entails considerable financial expenses, and potentially serious adverse effects. On the other hand, withholding treatment may lead to exacerbation of clinical signs and increased risk of complications.1, 2, 3 Both false positive and false negative test results are therefore a concern. Diagnosis of HAC is hindered by inadequate sensitivities and specificities of existing diagnostic tests.2, 3, 5, 6, 7, 8, 9, 10 Their performance, however, is greatly improved when performed on a population of dogs with compatible clinical signs and laboratory abnormalities.8, 10
The ACTH stimulation test (ACTHST) often is advocated as the initial screening tool for dogs suspected of HAC because of its simplicity, short duration, and higher specificity and positive predictive value.7, 10 However, in the latest American College of Veterinary Internal Medicine (ACVIM) consensus statement on the diagnosis of spontaneous HAC in dogs, the panel recommended the low‐dose dexamethasone suppression test (LDDST) as the screening test of choice because of its higher sensitivity as compared to the ACTHST.3, 11 In practice, both tests are commonly used.
Performing the ACTHST involves determining baseline (BC) and post‐stimulation serum cortisol concentrations (PC).1, 3 Baseline serum cortisol concentration alone is unsuitable for the diagnosis of HAC, owing to marked daily fluctuations in cortisol secretion, and the effects of systemic disease.2, 3, 5, 9, 12, 13, 14 Interpretation of ACTHST results is based solely on the PC in the majority of veterinary medical textbooks and scientific articles.1, 3 The ratio or percentage change between BC and PC occasionally is taken into consideration by veterinary practitioners. In a preliminary survey among general practitioners, conducted by the authors, both BC and PC were considered in result interpretation in 77% of 73 cases, whereas 22% and 1% considered only PC or only BC results, respectively. When both measurements were considered, either post‐to‐baseline serum cortisol concentration difference (DeltaC) or post‐to‐baseline serum cortisol concentrations quotient (RatioC) was assessed in 57% and 4% of cases, respectively. Neither the clinical relevance nor the diagnostic utility of such calculations have been studied, to the best of our knowledge. Notwithstanding the above, the latest ACVIM consensus statement still advocates the collection of baseline samples when conducting an ACTHST.3 Therefore, we sought to evaluate the contribution of the prestimulation BC of the ACTHST in the diagnosis of HAC in dogs. We hypothesized that PC would prove superior to BC, DeltaC, or RatioC in the final diagnosis of HAC.
2. MATERIALS AND METHODS
2.1. Study groups and case selection
The database of the Hebrew University Veterinary Teaching Hospital was screened for all cases for which an ACTHST had been performed in suspicion of HAC between the years 2014 and 2018. Medical records were retrospectively reviewed by 2 board‐certified internists (R. Nivy and M. Mazaki‐Tovi). Eligible cases had an ACTHST result, compatible history and physical examination findings, and/or ultrasonographic evidence of an adrenal mass or bilateral adrenomegaly.3 Left and right adrenal gland enlargement was determined by ultrasonographic evaluation of adrenal thickness (>0.62 and >0.60 cm, respectively, for dogs that weighed ≤12 kg, and >0.70 and >0.72 cm, respectively, for dogs that weighed >12 kg).15 Hyperadrenocorticism was diagnosed based on compatible clinical, physical examination, and imaging findings, together with a positive screening test (ACTHST, LDDST, or both)3 and a positive response to treatment. The ACTHST was performed by injecting tetracosactide (125 μg for dogs <5 kg or 250 μg for dogs >5 kg) IM, and obtaining baseline and 1‐hour post‐injection blood samples for serum cortisol concentration measurement. The LDDST was performed by injecting 0.01 mg/kg dexamethasone IV, and obtaining baseline, 4‐ and 8‐hour post‐injection blood samples for serum cortisol concentration measurement. Serum cortisol concentration was measured using a chemiluminescent immunoassay (Elecsys/Cobas Cortisol, Roche Diagnostics GmbH, Mannheim, Germany).16 A positive ACTHST was defined as a PC > 550 nmol/L (19.9 μg/dL), and a positive LDDST was defined as an 8‐hour serum cortisol concentration > 30 nmol/L (1.1 μg/dL), based on our laboratory's reference interval for these tests and previous reports.7, 10, 11 Hyperadrenocorticism was determined as pituitary‐dependent (PDHAC) if some suppression was present on LDDST (4‐hour post‐dexamethasone serum cortisol concentration < 1.1 μg/dL or 4‐ or 8‐hour post‐dexamethasone serum cortisol concentration < 50% of BC) or bilaterally normal or enlarged adrenal glands were identified by ultrasonography. Hyperadrenocorticism was deemed caused by cortisol‐secreting adrenal tumor (AT) when no suppression was demonstrated on LDDST, when performed, together with corroborative ultrasonographic findings, including moderate asymmetry of the adrenal glands with contralateral adrenal gland width < 5 mm.3 A non‐adrenal illness (NAI) was diagnosed regardless of the ACTHST results when an alternative diagnosis had been reached, or when clinical signs had resolved without specific treatment for HAC (eg, resolution of polyuria). When cases could not be classified as HAC or NAI by the above‐mentioned criteria, they were independently re‐evaluated by each of the reviewers and subsequently included only if the 2 reviewers concurred.
2.2. Statistical analyses
Descriptive statistics are presented as medians and range. Frequencies of clinical signs, imaging findings, and clinicopathological data were compared between dogs with HAC or NAI using Fisher's exact test.
Receiver operator characteristics (ROC) analyses were used to assess the predictive accuracy of BC alone, PC alone, DeltaC, and RatioC in the final diagnosis of HAC. The area under the ROC curve (AUC) of the PC was compared to each of the AUCs’ of BC, DeltaC, and RatioC. The ROC analysis also was used to assess the performance of PC in the diagnosis of HAC. The Youden index (J) was used to locate the optimal cutoff point with the least misclassifications. Fisher's exact test was used to compare the frequencies of normal and increased (>110 nmol/L or >4.0 μg/dL) BC, normal and increased (>550 nmol/L or >19.9 μg/dL) post‐ACTH serum cortisol concentrations, and the frequencies of cortisol ratio ≤2.0 and >2.0 between dogs with or without a final diagnosis of HAC in each group. Analyses were made using a statistical software package (MedCalc software bvba version 14.8 and IBM SPSS statistics version 25). P < .05 was considered significant.
3. RESULTS
Fifty‐seven dogs initially were enrolled. Adrenal tumor was diagnosed in only 3 dogs that were therefore excluded from the study because of the small sample size, which may be nonrepresentative. A diagnosis of PDHAC or NAI was determined in 36 and 18 of the remaining 54 dogs, respectively. Thirty dogs were diagnosed with HAC based solely on the ACTHST. In the remaining 6 dogs, HAC was diagnosed based on the LDDST alone (3 dogs) or both tests (3 dogs).
Median (range) age and body weights in PDHAC and NAI dogs were 10 years (range, 5.0‐16.0 years) versus 12.0 years (range, 5.0‐14.0 years) and 14.0 kg (range, 3.9‐53.0 kg) versus 15.0 kg (range, 6.0‐42.0 kg), respectively. Polydipsia, polyuria, and polyphagia were observed commonly in both PDHAC and NAI dogs, whereas pendulous abdomen, panting, exercise intolerance, and dermatological signs (including alopecia, lichenification, hyperpigmentation, erythema, bacterial pyoderma, calcinosis cutis, bruising, and nonhealing skin lesions) were overrepresented in dogs with PDHAC (Table 1). Similarly, many clinicopathological derangements were observed equally in both dogs with PDHAC and NAI, except for hypercholesterolemia that was more frequently observed in dogs with PDHAC (Table 2). Adrenal size was not significantly different between dogs with PDHAC and NAI (P = .56 and .29 for right and left adrenal glands, respectively). Bilateral and unilateral adrenomegaly was observed in 17/36 (47%) and 3/36 (8%) dogs with PDHAC and 4/18 (22%) and 3/18 (17%) dogs with NAI.
Table 1.
Clinical signs in 54 dogs evaluated for suspected hyperadrenocorticism at a university teaching hospital
| Clinical sign n (%) | HAC (n = 36) | NAI (n = 18) | P valuea |
|---|---|---|---|
| Polyuria/polydipsia | 32 (89) | 13 (72) | .14 |
| Polyphagia | 16 (44) | 8 (44) | 1 |
| Decreased appetite | 1 (3) | 2 (11) | .25 |
| Pendulous abdomen | 14 (39) | 1 (6) | .01 |
| Panting | 20 (56) | 3 (17) | .009 |
| Dermatological signsb | 20 (56) | 4 (22) | .02 |
| Exercise intolerance | 21 (58) | 3 (17) | .004 |
| Vomiting | 1 (3) | 2 (11) | .25 |
| Diarrhea | 1 (3) | 2 (11) | .25 |
HAC, hyperadrenocorticism; NAI, non‐adrenal disease.
Frequencies were compared between groups by the Fisher's exact test.
dermatological signs included alopecia, lichenification, hyperpigmentation, erythema, bacterial pyoderma, calcinosis cutis, bruising, and nonhealing skin lesions.
Table 2.
Selected laboratory measures from 54 dogs evaluated for suspected hyperadrenocorticism in a university teaching hospital
| Analytea | HAC | NAI | RI | ||||
|---|---|---|---|---|---|---|---|
| Median (range) | Below RI n (%) | Above RI n (%) | Median (range) | Below RI n (%) | Above RI n (%) | ||
| Leukocytes (×103/mL) | 10.7 (5.0‐23.4) | 1/34 (3) | 6/34 (18) | 9.5 (4.4‐15.8) | 1/17 (6) | 2/17 (12) | 5.2‐13.9 |
| Red blood cells (×106/mL) | 7.0 (4.3‐8.7) | 3/34 (9) | 0/34 (0) | 6.9 (5.3‐8.0) | 3/17 (18) | 0/17 (0) | 5.7‐8.8 |
| Platelets (103/μL) | 402 (122‐646) | 1/34 (3) | 17/34 (50) | 336 (61‐523) | 2/17 (12) | 5/17 (29) | 143‐400 |
| Albumin (g/dL) | 3.6 (2.5‐4.6) | 5/34 (15) | 2/34 (6) | 3.4 (2.6‐4.4) | 3/18 (17) | 0/18 (0) | 3.0‐4.4 |
| Alkaline phosphatase (U/L) | 402 (47‐1954) | 0/36 (0) | 30/36 (83) | 223 (48‐1093) | 0/18 (0) | 11/18 (61) | 21‐170 |
| γ glutamyl transferase (U/L) | 7.7 (0.0‐140.8) | 0/28 (0) | 16/28 (57) | 3.5 (0.0‐53.8) | 0/14 (0) | 4/14 (29) | 0‐6 |
| Alanine transaminase (U/L) | 102 (36‐620) | 0/36 (0) | 26/36 (72) | 125 (4‐370) | 1/18 (6) | 12/18 (67) | 19‐67 |
| Aspartate transaminase (U/L) | 26 (6‐173) | 6/25 (24) | 7/25 (28) | 31 (20‐150) | 0/11 (0) | 5/11 (45) | 19‐42 |
| Cholesterol (mg/dL)b | 347 (128‐759) | 1/30 (3) | 14/30 (47) | 300 (192‐1250) | 0/15 (0) | 2/15 (13) | 135‐361 |
| Triglycerides (mg/dL) | 205 (58‐1186) | 0/26 (0) | 17/26 (65) | 209 (59‐754) | 0/11 (0) | 7/11 (64) | 19‐133 |
| Creatinine (mg/dL) | 0.6 (0.2‐2.5) | 1/36 (3) | 1/36 (3) | 1.0 (0.5‐2.0) | 0/18 (0) | 3/18 (17) | 0.3‐1.2 |
| Total bilirubin (mg/dL) | 0.1 (0.0‐0.4) | 0/33 (0) | 2/33 (6) | 0.2 (0.0‐0.5) | 0/18 (0) | 2/18 (11) | 0.0‐0.2 |
| Glucose (mg/dL) | 98 (58‐420) | 2/33 (6) | 2/33 (6) | 99 (46‐131) | 2/18 (11) | 1/18 (6) | 64‐123 |
RI, reference interval; HAC, hyperadrenocorticism; NAI, non‐adrenal illness.
Frequencies were compared between groups by the Fisher's exact test.
P = .04.
The frequencies of an increased BC or a RatioC exceeding 2 were not significantly different between dogs with PDHAC or NAI (69% versus 56%; P = .50 and 97% versus 89%; P = .40, respectively). The frequency of an increased PC was significantly higher in dogs with a final diagnosis of PDHAC compared to dogs with NAI (92% versus 39%; P = .0001).
The AUC of PC for a final diagnosis of PDHAC was significantly higher than all other AUCs excluding DeltaC (Figure 1, Table 3). Sample size was determined sufficient to identify a potential difference between PC and DeltaC AUCs, because the calculated post hoc sample size required for identifying a statistically significant difference, with a power of 100%, between an ideal DeltaC AUC of 1.00 and the actual PC AUC of .92 was 35 dogs, whereas 54 dogs actually were recruited.
Figure 1.
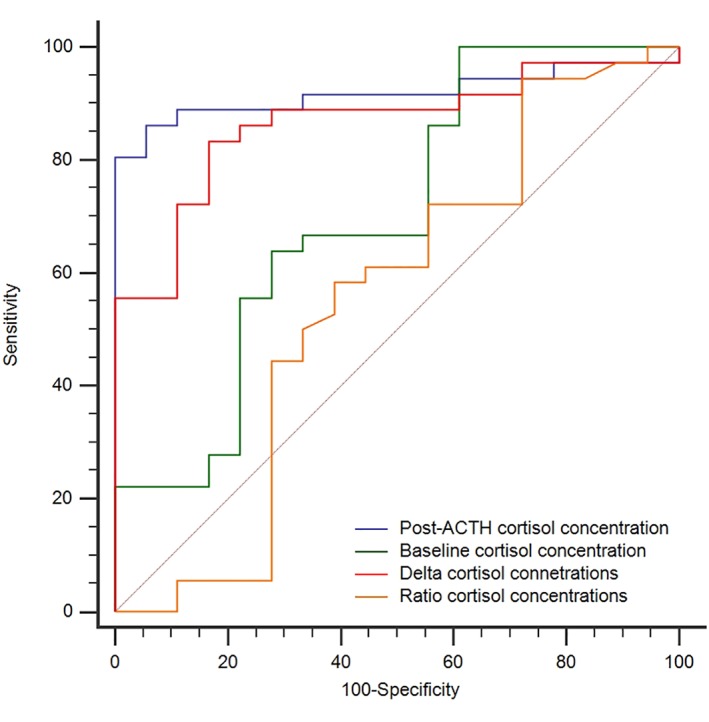
Receiver operating characteristic (ROC) curves of baseline cortisol, post‐ACTH stimulation cortisol, delta and ratio (post to baseline) serum cortisol concentrations for predicting a final diagnosis pituitary‐dependent hyperadrenocorticism in 54 dogs
Table 3.
Median (range) and areas under the ROC curve (AUCs, 95% confidence interval) of baseline cortisol, post‐ACTH stimulation cortisol, ratio and delta (post‐to‐baseline) serum cortisol concentrations in the diagnosis of pituitary‐dependent hyperadrenocorticism
| Median (range) | ROC curve | ||||
|---|---|---|---|---|---|
| HAC | NAI | AUC | 95% CI | P a | |
| Post‐ACTH stimulation cortisol | 0.92 | 0.81‐0.98 | ‐ | ||
| (nmol/L) | 878 (208‐1753) | 529 (244‐718) | |||
| (μg/dL) | 31.8 (7.5‐63.5) | 19.2 (8.8‐26.0) | |||
| Baseline cortisol | 0.70 | 0.56‐0.82 | .01 | ||
| (nmol/L) | 147 (61‐600) | 113 (19‐263) | |||
| (μg/dL) | 5.3 (2.2‐21.7) | 4.1 (0.7‐9.5) | |||
| Delta cortisol | 0.86 | 0.74‐0.94 | .09 | ||
| (nmol/L) | 730 (134‐1601) | 396 (218‐658) | |||
| (μg/dL) | 26.4 (4.8‐58.0) | 14.3 (7.9‐23.8) | |||
| Ratio cortisol | 0.55 | 0.41‐0.69 | <.001 | ||
| 6.9 (1.6‐15.0) | 5.4 (0.4‐30.4) | ||||
P value for comparison of each AUC with post‐stimulation cortisol AUC.
Sensitivity and specificity for the optimal cutoff value of PC (>683 nmol/L or >24.8 μg/dL) were 86% and 94%, respectively. The lower traditional cutoff value (>550 nmol/L or >19.9 μg/dL) yielded a sensitivity of 92% and a specificity of 61%, whereas a higher cutoff value of (>718 nmol/L or 26.0 μg/dL) yielded a sensitivity of 81% with a specificity of 100%.
4. DISCUSSION
In our study, BC provided no added value in the interpretation of the ACTHST for a diagnosis of PDHAC, as neither the difference nor the ratio between PC and BC improved the diagnostic accuracy of the ACTHST in predicting a final diagnosis of PDHAC compared to PC alone. As expected, BC alone was ineffective in the diagnosis of PDHAC.
The ACTHST and the LDDST are commonly used as screening tests for HAC in dogs.1, 3 The former test is based on identifying excessive cortisol secretion in response to exogenous ACTH. Its sensitivity varies among studies, is higher in PDHAC compared to functional ATs, and ranges between 57 and 95%.1, 2, 3, 7, 9, 10 The LDDST test is based on identifying decreased sensitivity of the hypothalamic–pituitary–adrenal axis to negative feedback by exogenous glucocorticoids. Despite better sensitivity (85%‐100%), the test lacks specificity (44%‐73%).1, 2, 3, 7, 10, 11 The ACTHST is considered superior to LDDST in terms of simplicity and specificity.7, 10 Therefore, it frequently is used as a screening test in the diagnosis of HAC. Furthermore, it is commonly used in monitoring response to treatment,1 in addition to newly introduced approaches employing BC for monitoring trilostane treatment.17 Current guidelines regarding the technical aspects of performing the ACTHST, including the latest ACVIM consensus statement on the diagnosis of spontaneous HAC, recommend collection of both baseline and post‐stimulation samples.1, 3 Notwithstanding the above, current guidelines regarding the interpretation of the ACTHST results are solely concerned with PC, when PC exceeding 550‐600 nmol/L (19.9‐21.7 μg/dL) often are considered positive.1, 7, 10, 11 To the best of our knowledge, only a single veterinary textbook refers to the futility of BC in the diagnosis of spontaneous HAC, regarding the practice as a “habit” and “not informative.”18
Owing to its pivotal role in maintaining various physiological functions, as well as the body's response to stressful events, serum cortisol concentrations commonly fluctuate during the day in response to a plethora of physical, inflammatory, neurogenic, and emotional stimuli.4, 12, 13, 14 Unlike humans, a circadian rhythm in cortisol secretion has not been consistently identified in dogs, but pulsatile cortisol secretion is common, as a result of episodic secretion of ACTH.12, 13, 14 Furthermore, dogs with HAC also have episodic cortisol secretion and consequently may have BC within the reference range.2, 7 Therefore, BC is neither sensitive nor specific in the diagnosis of spontaneous HAC,2, 4, 7 and previous studies as well as ours failed to find statistically significant differences in BC between dogs with spontaneous HAC and dogs with NAI.2, 5, 8, 9 Although the absolute result of BC was uncommonly addressed by general practitioners in a preliminary survey conducted by us, the majority used both measurements in result interpretation including DeltaC and RatioC. To the best of our knowledge, no references for such calculations and their interpretation exist in the veterinary literature, except for a reference in which a post‐to‐baseline cortisol ratio of >2 in urinary 17‐ketogenic steroids after the ACTHST was regarded as confirmatory for adrenocortical hyperplasia.19 In our study, no statistically significant differences in the proportion of increased RatioC (ie, >2) in the blood were found between dogs diagnosed with spontaneous PDHAC and those in which the disease had been ruled out.
Two medical conditions require BC measurement when performing the ACTHST. The first is iatrogenic HAC, where both baseline and post‐stimulation measurements are used to confirm adrenal suppression.3 The second is relative adrenal insufficiency of critical illness, where DeltaC constitutes a criterion in diagnosis.4, 20, 21 With those 2 exceptions, neither the difference nor the ratio between baseline and post‐ACTH stimulation measurements is addressed in the interpretation of the ACTHST in the variety of medical conditions in which the test is employed.
The ROC analysis was used to assess the diagnostic performance of either BC or PC, as well as DeltaC and RatioC for the diagnosis of PDHAC. The AUCs, as a measure of the overall accuracy of each variable, subsequently were compared to determine the clinical benefit of measuring BC.22, 23 When comparing the diagnostic performance of each variable, by comparison of AUCs, PC was superior to all other variables for the diagnosis of PDHAC. A singular exception was the DeltaC which performed similarly to PC, thus rendering the measurement of BC superfluous.
Employing a conventional cutoff point of 550 nmol/L (19.9 μg/dL)7, 10 yielded a comparably low specificity (61%), in contrast to the perception of the ACTHST as a relatively specific test.1 The optimal higher cutoff value (683 nmol/L or 24.8 μg/dL) yielded a much higher specificity (94%), while moderately compromising the sensitivity of the test (86%). A specificity of 100% was reached with an even higher cutoff value (718 nmol/L or 26.0 μg/dL), which resulted in a lower sensitivity of (81%). These cutoff values may be helpful for interpretation of the ACTHST.
The stringent selection criteria for performing the ACTHST probably improved its performance in this study. These criteria were based on inclusion of dogs with compatible clinical findings according to the ACVIM consensus statement.3 In addition, the exclusion of dogs with AT most likely also contributed to the higher sensitivity of the ACTHST in our study, because lower sensitivity of the test is reported in such cases.1
In conclusion, PC had good‐to‐excellent discriminatory power for a final diagnosis of PDHAC, which was comparable to DeltaC whereas BC and RatioC failed to discriminate between PDHAC and NAI. Therefore, there is no apparent added value in obtaining prestimulation BC for interpretation of the ACTHST in the diagnosis of spontaneous PDHAC. Additional studies are required to investigate the performance of the ACTHST in cases of HAC caused by functional AT.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
ACKNOWLEDGMENT
The study was carried out at the Hebrew University Veterinary Teaching Hospital, Koret School of Veterinary Medicine, The Hebrew University of Jerusalem.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Nivy R, Refsal KR, Ariel E, Kuzi S, Yas‐Natan E, Mazaki‐Tovi M. The interpretive contribution of the baseline serum cortisol concentration of the ACTH stimulation test in the diagnosis of pituitary dependent hyperadrenocorticism in dogs. J Vet Intern Med. 2018;32:1897–1902. 10.1111/jvim.15330
REFERENCES
- 1. Melian C, Perez‐Alenza MC, Peterson ME. Hyperadrenocorticism in dogs In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, MO: Saunders; 2010:1816‐1840. [Google Scholar]
- 2. Ling GV, Stabenfeldt GH, Comer KM, Gribble DH, Schechter RD. Canine hyperadrenocorticism: pretreatment clinical and laboratory evaluation of 117 cases. J Am Vet Med Assoc. 1979;32:1897‐1902. [PubMed] [Google Scholar]
- 3. Behrend EN, Kooistra HS, Nelson R, Reusch CE, Scott‐Moncrieff JC. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J Vet Intern Med. 2013;27:1292‐1304. [DOI] [PubMed] [Google Scholar]
- 4. Behrend EN. Canine hyperadrenocorticism In: Feldman EC, Nelson R, Reusch CE, Scott‐Moncrieff JC, eds. Canine and Feline Endocrinology and Reproduction. 4th ed. St. Louis, MO: Elsevier Saunders; 2015:377‐451. [Google Scholar]
- 5. Chastain CB, Franklin RT, Ganjam VK, Madsen RW. Evaluation of the hypothalamic pituitary‐adrenal axis in clinically stressed dogs. J Am Anim Hosp Assoc. 1986;22:435‐442. [Google Scholar]
- 6. Eiler H, Oliver JW, Legendre AM. Stages of hyperadrenocorticism: response of hyperadrenocorticoid dogs to the combined dexamethasone suppression/ACTH stimulation test. J Am Vet Med Assoc. 1984;185:289‐294. [PubMed] [Google Scholar]
- 7. Feldman EC. Comparison of ACTH response and dexamethasone suppression as screening tests in canine hyperadrenocorticism. J Am Vet Med Assoc. 1983;182:506‐510. [PubMed] [Google Scholar]
- 8. Kaplan AJ, Peterson ME, Kemppainen RJ. Effects of disease on the results of diagnostic tests for use in detecting hyperadrenocorticism in dogs. J Am Vet Med Assoc. 1995;207:445‐451. [PubMed] [Google Scholar]
- 9. Peterson ME, Gilbertson SR, Drucker WD. Plasma cortisol response to exogenous ACTH in 22 dogs with hyperadrenocorticism caused by adrenocortical neoplasia. J Am Vet Med Assoc. 1982;180:542‐544. [PubMed] [Google Scholar]
- 10. Van Liew CH, Greco DS, Salman MD. Comparison of results of adrenocorticotropic hormone stimulation and low‐dose dexamethasone suppression tests with necropsy findings in dogs: 81 cases (1985‐1995). J Am Vet Med Assoc. 1997;211:322‐325. [PubMed] [Google Scholar]
- 11. Bennaim M, Shiel RE, Forde C, Mooney CT. Evaluation of individual low‐dose dexamethasone suppression test patterns in naturally occurring hyperadrenocorticism in dogs. J Vet Intern Med. 2018;32:967‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston SD, Mather EC. Canine plasma cortisol (hydrocortisone) measured by radioimmunoassay: clinical absence of diurnal variation and results of ACTH stimulation and dexamethasone suppression tests. Am J Vet Res. 1978;39:1766‐1770. [PubMed] [Google Scholar]
- 13. Kemppainen RJ, Sartin JL. Evidence for episodic but not circadian activity in plasma concentrations of adrenocorticotrophin, cortisol and thyroxine in dogs. J Endocrinol. 1984;103:219‐226. [DOI] [PubMed] [Google Scholar]
- 14. Kolevska J, B V, Svoboda M. Circadian rhythm of cortisol secretion in dogs of different daily activities. Acta Vet Brno. 2003;72:599‐605. [Google Scholar]
- 15. Bento PL, Center SA, Randolph JF, Yeager AE, Bicalho RC. Associations between sex, body weight, age, and ultrasonographically determined adrenal gland thickness in dogs with non‐adrenal gland illness. J Am Vet Med Assoc. 2016;248:652‐660. [DOI] [PubMed] [Google Scholar]
- 16. Wenger‐Riggenbach B, Boretti FS, Quante S, Schellenberg S, Reusch CE, Sieber‐Ruckstuhl NS. Salivary cortisol concentrations in healthy dogs and dogs with hypercortisolism. J Vet Intern Med. 2010;24:551‐556. [DOI] [PubMed] [Google Scholar]
- 17. Macfarlane L, Parkin T, Ramsey I. Pre‐trilostane and three‐hour post‐trilostane cortisol to monitor trilostane therapy in dogs. Vet Rec. 2016;179:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feldman EC, Nelson R. Caine hyperadrenocorticism In: Feldman EC, Nelson R, eds. Caniine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis, MO: Saunders; 2004:252‐357. [Google Scholar]
- 19. Siegel ET. Hyperadrenocorticism In: Kirk RW, ed. Current Veterinary Therapy IV. Philadelphia, PA: Elsevier Saunders; 1971:592‐593. [Google Scholar]
- 20. Burkitt JM, Haskins SC, Nelson RW, Kass PH. Relative adrenal insufficiency in dogs with sepsis. J Vet Intern Med. 2007;21:226‐231. [DOI] [PubMed] [Google Scholar]
- 21. Martin LG, Groman RP, Fletcher DJ, et al. Pituitary‐adrenal function in dogs with acute critical illness. J Am Vet Med Assoc. 2008;233:87‐95. [DOI] [PubMed] [Google Scholar]
- 22. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver‐operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23‐41. [DOI] [PubMed] [Google Scholar]
- 23. Moons KG, de Groot JA, Linnet K, et al. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012;58:1408‐1417. [DOI] [PubMed] [Google Scholar]


