Abstract
Background
Hypertension is common in older cats. There is limited evidence for predictors of survival after diagnosis.
Hypothesis/Objectives
Investigate blood pressure assessment (BPA) and hypertension diagnosis in cats attending UK primary care practices (PCPs) and factors that influence survival.
Animals
Cats (347 889) attending 244 UK PCPs enrolled in the VetCompass program between January 1, 2012, and December 31, 2013. Cats identified as hypertensive (282) were included in descriptive and survival analyses.
Methods
All electronic patient records (EPRs) were searched to identify cats that potentially had received BPA. EPRs were read in detail to identify those that had BPA. The proportion that received BPA was evaluated using a stratified analysis and the incidence of hypertension estimated. A retrospective cohort study was used to investigate survival after diagnosis (Cox proportional hazard model).
Results
Estimated incidence risk was 19.5% (95% confidence interval [CI], 17.5‐21.6) from the estimated 1.34% (1.30%‐1.38%) of cats that received BPA. Few cats had BPA more than once after diagnosis (median, 1; interquartile range [IQR], 0‐3), with only 9.9% of diagnosed hypertensive cats having urine protein:creatinine ratio determined. Cats diagnosed as a result of monitoring of pre‐existing disease had improved survival (hazard ratio [HR], 0.58; 95% CI, 0.37‐0.89; P = .01) compared to cats diagnosed after clinical signs were recognized. Cats that had an amlodipine dose change had improved survival (HR, 0.56; 95% CI, 0.36‐0.87; P = .01) compared to those with no dose change.
Conclusions and clinical importance
These data suggest improved blood pressure monitoring in clinical practice may decrease the morbidity associated with hypertension.
Keywords: blood pressure, prognosis, feline, VetCompass
Abbreviations
- ACVIM
American College of Veterinary Internal Medicine
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
- CI
confidence interval
- CKD
chronic kidney disease
- EPR
electronic patient record
- HR
hazard ratio
- IQR
interquartile range
- IRIS
International Renal Interest Society
- LRT
likelihood ratio test
- QOL
quality of life
- TOD
target organ damage
- UK
United Kingdom
- UPC
urine protein:creatinine ratio
1. INTRODUCTION
Hypertension is a common disease in older cats, with up to 13% of healthy cats ≥9 years of age being diagnosed with hypertension.1, 2, 3 This frequency increases to 87% in studies in which cats have concurrent disease.2, 4, 5 Previous prevalence estimates are difficult to compare because of variation in case definition of hypertension. The American College of Veterinary Internal Medicine (ACVIM) guidelines and the International Renal Interest Society (IRIS) staging system have attempted to standardize the definition of hypertension by categorizing blood pressure based on estimated risk of target organ damage (TOD). A systolic blood pressure (SBP) <150 mm Hg is considered normotensive (minimal risk), a SBP of 150‐159 mm Hg is considered borderline hypertensive (low risk), a SBP of 160‐179 mm Hg is considered hypertensive (moderate risk) and a SPB ≥ 180 mm Hg is considered severely hypertensive (severe risk).6, 7 Hypertension in cats frequently is associated with an underlying disease, with chronic kidney disease (CKD) and hyperthyroidism most commonly reported.2, 4, 8, 9 Idiopathic hypertension is estimated to occur in up to 20% of cases.10, 11 The aim of identifying hypertension early is to decrease the risk of TOD.6 Target organ damage can occur in the brain,12 eyes,13 heart,12 or kidneys6 with ocular TOD most easily recognized in association with hypertension in general practice. Target organ damage is associated with considerable morbidity, including blindness, ventricular hypertrophy, proteinuria, and hypertensive encephalopathy.5, 13, 14 Amlodipine, a calcium channel blocker, is recommended for the treatment of hypertension6, 7 and has been found to be effective at decreasing blood pressure in hypertensive cats.8, 15, 16
Limited research has been done into survival after diagnosis of hypertension in cats that are presented to primary care practice (PCP) in the United Kingdom (UK). Median survival time previously has been estimated to be up to 490 days in cats that were not proteinuric at diagnosis, whereas median survival time has been estimated to be 162 days in cats that were proteinuric at diagnosis.8 Urine protein:creatinine ratio (UPC) has been found to be correlated with decreased survival when adjusted for IRIS stage, both when assessed at initial diagnosis and as the time‐averaged UPC while on treatment.8 Much of the published data however derive from a small number of practices and referral centers where specific screening as well as diagnostic and treatment protocols tend to be followed. Thus, there is a deficiency of information on hypertension that is diagnosed in cats presented to PCP in the UK and how these cats are managed by general practitioners. The use of data from electronic patient records (EPRs) allows epidemiological studies on a large dataset that can be generalized to the cat population presented to PCP in the UK. The aims of our study were to estimate the proportion of cats in the UK receiving blood pressure assessment (BPA), describe cats diagnosed with hypertension, and investigate survival after diagnosis of hypertension in cats presented to PCP in the UK.
2. MATERIALS AND METHODS
The Veterinary Companion Animal Surveillance System (VetCompass)17 project collects and collates anonymized EPR data from primary care veterinary practices that have enrolled in the project. Patient demographic data (species, breed, date of birth, sex, and body weight) and clinical data (free clinical text, VeNom diagnosis terms,18 and treatment fields) are uploaded in real time to the secure database where the EPR can be accessed for epidemiological studies. Ethical approval for the VetCompass project was provided by the Royal Veterinary College Ethics and Welfare Committee and is supported by the Royal College of Veterinary Surgeons.17 This study was approved by the Royal Veterinary College Clinical Research Ethical Review Board (URN M2015 0051).
The cohort of cats presented to VetCompass practices during the study period was used to identify cats that received BPA and those diagnosed with hypertension. A retrospective cohort study was used to explore survival after hypertension diagnosis in cats. All cats attending 244 primary care clinics enrolled in the VetCompass program from January 1, 2012, to December 31, 2013, were included in the study. The EPR was searched using VeNom diagnosis codes (hypertensive disorder, hypertension, retinal separation/detachment, blindness, retinal haemorrhage, and high blood pressure), clinical free text terms (hyperten*, BP, blood pressure, blind*, retin* detach*, hyphaema~1, retin* haem*~1, and amlod*), and treatments (amlod*, istin, and blood pressure) associated with blood pressure measurement and hypertension diagnosis. The results from the searches were merged and duplicates removed. A random sample of 30.5% of these potential cases was reviewed in detail to confirm BPA and hypertension diagnosis. A cat was considered to have had its blood pressure assessed if a blood pressure measurement was recorded in the EPR or the veterinarian considered the cat normotensive or hypertensive after ocular examination. A cat was considered hypertensive if a diagnosis of hypertension was made in the EPR or a blood pressure measurement was recorded in the EPR and antihypertensive medication was started afterward. Demographic data were extracted automatically and additional data (date of diagnosis, reason for presentation, date of death, method of death, reason for death, blood pressure at all measurements, number of blood pressure measurements, ocular exam, clinical signs, goitre palpation, thoracic auscultation, urine protein measurement, treatments, and co‐morbidities) were collected manually from the EPR of hypertensive cats. Data were exported to commercially available software (Microsoft Excel 13) for checking and cleaning and then to statistical software (Stata 11, StataCorp LP, College Station, Texas) for statistical analysis.
Sample size calculations indicated that 212 hypertensive cats would be required to detect an all‐cause mortality hazard ratio (HR) of 0.5 for a variable to which 75% of cats were exposed (eg, amlodipine use) with a power of 80% and 95% confidence, assuming, on average, 365 days of follow‐up.19
2.1. Statistical Analysis
For the calculations of proportion of cats that received BPA and the incidence risk of hypertension, age was categorized as <9 years and ≥9 years or as <4.5 years, 4.5 to <9 years, 9 to <13.5 years, 13.5 to <18 years, 18 to <22.5 years and ≥22.5 years. Reason for presentation at time of BPA was categorized as for: owner‐reported clinical signs (any clinical signs), anesthetic monitoring, geriatric health check, monitoring of pre‐existing disease (typically CKD or hyperthyroidism), monitoring of pre‐existing hypertension, and others. Cats that had a blood pressure measurement to monitor pre‐existing hypertension only were included in the calculations for the proportion of cats that received a BPA.
For the survival analysis, median and interquartile range (IQR) were calculated for all continuous variables. Age was categorized as <9 and ≥9 years. Breed was categorized into crossbred and purebred, where purebred cats had a breed name recognized by International Cat Care.20 Blood pressure was categorized into quartiles and by ACVIM guidelines risk ranges (for SBP: minimum risk <150 mm Hg, mild risk 150‐159 mm Hg, moderate risk 160‐179 mm Hg, and severe risk ≥180 mm Hg). Number of blood pressure measurements after diagnosis was categorized as 0, 1, and ≥2. The UPC was categorized as ≤0.2, 0.21‐0.39, and ≥0.4. Treatments prescribed were categorized as no amlodipine or benazepril, amlodipine only, benazepril only, and amlodipine and benazepril combined. Comparison of continuous variables among groups was performed using the Mann‐Whitney test for non‐normally distributed variables and Student's t test for normally distributed variables.
A weighted stratified analysis was performed, using Stata survey commands, to account for the sampling strategy when estimating the proportion of cats that received BPA. Strata 1 consisted of cats that had had their EPR read in detail (a random sample of 30.5% of cats identified by the key word clinical free text and VeNom diagnosis searches) and were ascribed a sampling weight of 1/30.5. Strata 2 consisted of all the cats that were not identified when their EPR were searched for terms associated with hypertension and blood pressure measurement and were ascribed a sampling weight of 1/100 (Figure 1). The sampling weights corrected for the fact that not all cats had their EPRs read in detail, allowing an estimate of the proportion of cats that had their blood pressure assessed to be calculated.21
Figure 1.
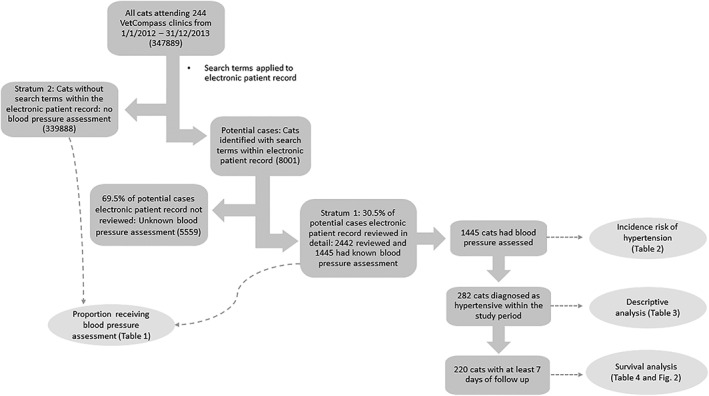
Flowchart describing the electronic patient record (EPR) search, stratification process, and of the number of cases used for the analyses
Only cats that were newly diagnosed during the study period were included in the incidence calculations. Incidence of hypertension was calculated as the proportion of all cats that had their blood pressure assessed during the study period, and that were diagnosed as hypertensive. Confidence intervals (CI) were calculated by Stata using exact methods.22
Clinical notes were followed until December 31, 2015. All cats with ≥7 days follow‐up were included in the survival analysis. A univariable Cox proportional hazard model was used to investigate associations between variables and survival. Any variable broadly associated (P < .2) with survival was taken forward to the multivariable analysis. A manual forward stepwise model construction approach was used to build the multivariable model. Confounders were assessed by examining changes to the HR > 10% when included in the model. Biologically plausible interactions were assessed using the likelihood ratio test. Collinearity of continuous predictors was evaluated for by examining Pearson's correlation. The proportional hazard assumption was tested by examining the log cumulative hazard plot and assessment of Schoenfeld residuals. Model fit was assessed by examining Cox‐Snell residuals, and competing models were assessed using the Akaike information criterion and the Bayesian information criterion. Predictive ability of the model was assessed using Harrell's C statistic, and outliers were evaluated using deviance residuals.23 Statistical significance was set at the 5% level.
3. RESULTS
3.1. Blood pressure assessment
Of the 347 889 cats that were presented to 244 PCPs from January 1, 2012, to December 31, 2013, 8001 were identified as potentially having had their blood pressure assessed in the searches and 2442 (30.5%) of these were reviewed in detail. Of cats for which clinical notes were reviewed, 1445 (59.2%) had their blood pressure assessed during the study period (Figure 1). This resulted in an estimated 1.34% (95% CI, 1.30‐1.38) of cats that received BPA during the study period. This percentage increased to 4.4% (95% CI, 4.3‐4.6) in cats ≥9 years. Most cats (94.8%; 1370) were assessed using blood pressure measurement. Seventy (4.8%) cats were predicted to be normotensive or hypertensive by ocular examination alone and 5 (0.04%) on clinical signs alone. The proportion of cats having their blood pressure assessed increased with age. Presentation with clinical signs was the most common reason for a cat to have its blood pressure assessed (Table 1). Owners of further 0.50% (95% CI, 0.46‐0.53) or 535 cats were offered a blood pressure measurement during the study period but declined.
Table 1.
Proportion of all cats receiving blood pressure assessment
| Variable | N | Blood pressure assessed | Proportion blood pressure assesseda (%) | 95% CIa | |
|---|---|---|---|---|---|
| Overall | 347 889 | 1445 | 1.34 | 1.30‐1.38 | |
| Age (years) | <9 | 254 698 | 300 | 0.38 | 0.34‐0.28 |
| ≥9 | 80 025 | 1139 | 4.4 | 4.3‐4.6 | |
| 0 to <4.5 | 187 460 | 142 | 0.25 | 0.20‐0.28 | |
| 4.5 to <9 | 67 238 | 158 | 0.76 | 0.65‐0.88 | |
| 9 to <13.5 | 44 185 | 413 | 3.0 | 2.7‐3.2 | |
| 13.5 to <18 | 28 401 | 566 | 6.1 | 5.7‐6.5 | |
| 18 to <22.5 | 7054 | 155 | 6.6 | 5.7‐7.6 | |
| ≥22.5 | 385 | 5 | 4.0 | 0.6‐7.4 | |
| Sex | Male | 165 360 | 702 | 13.6 | 13.6‐14.1 |
| Female | 177 749 | 739 | 13.4 | 12.8‐14.5 | |
| Neuter | Entire | 58 105 | 78 | 0.4 | 0.3‐0.5 |
| Neutered | 249 002 | 1119 | 1.5 | 1.4‐1.51 | |
| Breed | Crossbred | 309 233 | 1237 | 1.29 | 1.24‐1.34 |
| Purebred | 35 059 | 201 | 1.84 | 1.60‐2.09 | |
| Reason for presentation at blood pressure assessmentb | Clinical signs | 1445 | 471 | 32.6 | 30.2‐35.0 |
| Anaesthetic monitoring | 1445 | 425 | 29.4 | 27.1‐31.8 | |
| Geriatric health check | 1445 | 40 | 2.8 | 1.9‐3.6 | |
| Monitoring of pre‐existing disease | 1445 | 419 | 29.0 | 26.7‐31.3 | |
| Other | 1445 | 5 | 0.4 | 0.04‐0.7 | |
| Monitoring of pre‐existing hypertension | 1445 | 85 | 5.9 | 4.7‐7.1 | |
Abbreviation: CI, confidence interval.
Calculated using stratified analysis and Stata survey commands.
Proportion of cats receiving blood pressure assessment presented because of each category. This group only includes the 1445 cats that had their blood pressure assessed.
3.2. Incidence of hypertension
Of the 1445 cats identified that had their blood pressure assessed during the study period (2 years), 282 cats were diagnosed as hypertensive during the study period. This resulted in an estimated incidence risk of 19.5% (95% CI, 17.5‐21.6) over the study period. Incidence increased with age and was higher in crossbred cats than in purebred cats. Hypertension was most frequently diagnosed in cats presented for evaluation of clinical signs (Table 2).
Table 2.
Incidence risk of hypertension diagnosis during study period
| Variable | N | Hypertension diagnosed | Incidence risk of hypertension (%) | 95% CIa | |
|---|---|---|---|---|---|
| Overall | 1445 | 282 | 19.5 | 17.5‐21.7 | |
| Age (years) | <9 | 300 | 9 | 3.0 | 1.4‐5.6 |
| ≥9 | 1139 | 270 | 23.7 | 21.3‐26.3 | |
| 0 to <4.5 | 142 | 1 | 0.7 | 0.02‐3.9 | |
| 4.5 to <9 | 158 | 8 | 5.1 | 2.2‐9.7 | |
| 9 to < 13.5 | 413 | 44 | 10.7 | 7.8‐14.0 | |
| 13.5 to <18 | 566 | 167 | 29.5 | 25.8‐33.5 | |
| 18 to <22.5 | 155 | 56 | 36.1 | 28.6‐44.2 | |
| 22.5+ | 5 | 3 | 60.0 | 14.7‐94.7 | |
| Sex | Male | 702 | 145 | 20.7 | 17.7‐23.8 |
| Female | 793 | 136 | 18.4 | 15.7‐21.4 | |
| Neuter | Entire | 78 | 22 | 28.2 | 18.6‐39.5 |
| Neutered | 1119 | 228 | 20.4 | 18.1‐22.9 | |
| Breed | Crossbred | 1237 | 260 | 21.0 | 18.8‐23.3 |
| Purebred | 201 | 19 | 9.5 | 5.4‐13.5 | |
| Reason for presentation | Clinical signs | 471 | 178 | 37.8 | 33.4‐42.3 |
| Anaesthetic monitoring | 425 | 3 | 0.7 | 0.01‐2.1 | |
| Geriatric health check | 40 | 12 | 30.0 | 16.6‐46.5 | |
| Monitoring of concurrent disease | 419 | 88 | 21.0 | 1.7‐25.2 | |
| Other | 5 | 1 | 20.0 | 0.5‐71.6 | |
Abbreviation: CI, confidence interval.
Calculated using exact method
3.3. Descriptive statistics
All further analysis was undertaken on the 282 incident cases of hypertension. The median age at diagnosis of hypertension was 16 years (IQR, 14.6‐17.5). Body weight within 1 month of diagnosis was recorded in 27.7% (79) of cats. Median body weight within 1 month of diagnosis was 3.4 kg (IQR, 3.1‐4.1). Sex was recorded in 99.7% (281) of cats and 51.6% (145) were female. Neuter status was recorded in 63.1% (178) of cats and 88.2% (157) were neutered. Breed was recorded in 98.9% (279) of cats and most (92.2%; 260) were crossbred. Clinical signs accounted for 63.1% (178) of presentations when hypertension was diagnosed, with a further 31.2% (88) being presented for monitoring of concurrent disease, 4.3% (13) for geriatric health evaluations, and 1.1% (3) for anesthetic monitoring. Blood pressure was measured in 78.4% (221) of cats at diagnosis and was recorded in 75.2% (212) of EPRs. Median blood pressure at diagnosis was 206 mm Hg (IQR, 190‐230). Most cats (92.9%; 197) were within the ACVIM severe risk category at diagnosis, with 6.1% (14) within the moderate risk category and 0.9% (2) in the low risk category at diagnosis of hypertension. Of the 61 (21.6%) cats that did not have a blood pressure measurement as the basis for diagnosis of hypertension, most (56; 91.8%) were diagnosed after an ocular examination that disclosed signs compatible with hypertensive ocular damage. The remaining 5 cats were diagnosed based on the clinical signs for which they presented (eg, sudden onset blindness, neurological signs, and hyperthyroidism). The median number of blood pressure measurements after hypertension diagnosis was 1 (IQR, 0‐3). Ocular examination was performed in 61.4% (173) of cats. Clinical assessment of hypertensive cats and clinical signs of hypertension reported at diagnosis are presented in Table 3. Amlodipine was the most common treatment prescribed (68.4%; 193). The most common initial dose was 0.625 mg daily (50.6%; 90) with an additional 37.1% (66) of cats started on 1.25 mg daily. A total of 43 (22.3%) cats receiving amlodipine had a dose increase during their follow‐up, with a median time to dose change of 38 days from starting amlodipine (IQR, 14‐156). Cats requiring a dose increase had higher blood pressure at diagnosis (median, 235.7 mm Hg; IQR, 201.0‐270.0) in comparison to cats that did not (median, 209.3; IQR, 190.0‐220.0; Mann‐Whitney P < .001). Median number of blood pressure measurements was 2 (IQR, 1‐4) in cats not receiving a dose change and 4 (IQR, 3‐6) in cats receiving a dose change (Mann‐Whitney P < .0001). Benazepril was prescribed to 39.4% (111) of cats. The most frequent reason for benazepril prescription in these cats was for blood pressure control (46.0%; 51), other reasons being concurrent CKD (39.6%; 44), practitioner‐diagnosed cardiac disease (12.6%; 14), and protein‐losing nephropathy (1.8%; 2). Just over a quarter (28%; 79) of cats received amlodipine and benazepril combined, with 11.4% (32) receiving benazepril alone. Other treatments prescribed were propranolol (0.3%; 1), atenolol (4.3%; 12), and enalapril (0.3%; 1). Just under a fifth (19.5%; 55) did not receive any treatment for their hypertension. Most of these cats (83.6%; 46) were euthanized within 7 days of diagnosis. Chronic kidney disease was the most common co‐morbidity diagnosed before or at the same time as hypertension (46.1%). Hyperthyroidism was diagnosed in 24.5% of cats before or at the time of hypertension diagnosis. Diabetes mellitus was diagnosed in 2.13% of hypertensive cats, all diagnosed before hypertension diagnosis. Just under a third (30.5%; 86) of hypertensive cats did not have a concurrent disease diagnosed (idiopathic hypertensive cats). Only 50% (43) of these “idiopathic hypertensive” cats had investigations performed at or after hypertension diagnosis.
Table 3.
Clinical investigations performed in hypertensive cats and clinical signs of hypertension recorded at diagnosis (based on total 282 incident cases)
| N | % | ||
|---|---|---|---|
| Blood pressure measurements | None | 61 | 21.6 |
| At diagnosis only | 73 | 25.9 | |
| After diagnosis | 148 | 52.5 | |
| Urine protein assessment | Urine protein measured | 100 | 35.5 |
| • Urine protein:creatinine ratio | 28 | 9.9 | |
| • Urine dipstick | 72 | 25.5 | |
| Proteinuria classification | Non‐proteinuric | 68 | 68.0 |
| Proteinuric | 23 | 23.0 | |
| No classification made | 9 | 9.0 | |
| Clinical Signs | Blind | 78 | 27.7 |
| Retinopathy | 132 | 46.8 | |
| • Retinal detachment | 80 | 28.4 | |
| • Tortuous vessels | 17 | 6.0 | |
| • Retinal hemorrhage | 55 | 19.5 | |
| • Hyphema | 31 | 11.0 | |
| Neurological signs | 44 | 15.6 | |
| • Seizures | 8 | 2.8 | |
| • Ataxia | 18 | 6.4 | |
| • Behavior change | 13 | 4.6 | |
| • Circling | 5 | 1.8 | |
| No clinical signs reported | 106 | 37.6 |
Bold indicates group level with individual investigations or clinical signs listed below. Cats may have had more than one clinical sign within each group.
3.4. Survival Analysis
Thirty‐five cats diagnosed with hypertension had no follow‐up. Twenty‐seven cats had <7 days follow‐up and 220 cats had ≥7 days follow‐up.. Of the 35 cats with no follow‐up, 82.9% (29) were euthanized at the time of diagnosis of hypertension, and the remaining 6 were not presented to the practice again after diagnosis. Of the 27 cats that had <7 days follow‐up, 17 (63.0%) were euthanized, 1 (3.7%) died naturally and 9 (33.3%) were censored. The most common reasons for euthanasia were quality of life (QOL; 35.3%; n = 6), CKD (23.5%; n = 4) and blindness (11.8%; n = 2). All further analysis only includes those cats with ≥7 days follow‐up. Just over a third (38.6%; n = 85) of the cats with ≥7 days follow‐up were subsequently lost to follow‐up and 130 (59.1%) died during the follow‐up period (until December 31, 2015). Those cats (n = 5) that were alive at the end of the study period and those lost to follow‐up (n = 85) were censored in the survival analysis. The median follow‐up time for those cats lost to follow‐up was 375 days (IQR, 146‐772 days). The most common reasons reported for euthanasia were QOL (16.8%; 37), CKD (13.6%; 30), and neurological signs (5.9%; 13). For 16 of the 35 (55.2%) cats euthanized at diagnosis, the reason for euthanasia was recorded as clinical signs related to hypertension. Estimated median survival time of cats with ≥7 days follow‐up was 400 days (IQR, 147‐797; Figure 2). All‐cause mortality rate was estimated at 6.57 deaths per 10 hypertensive cat years at risk (95% CI, 5.54‐7.81).
Figure 2.
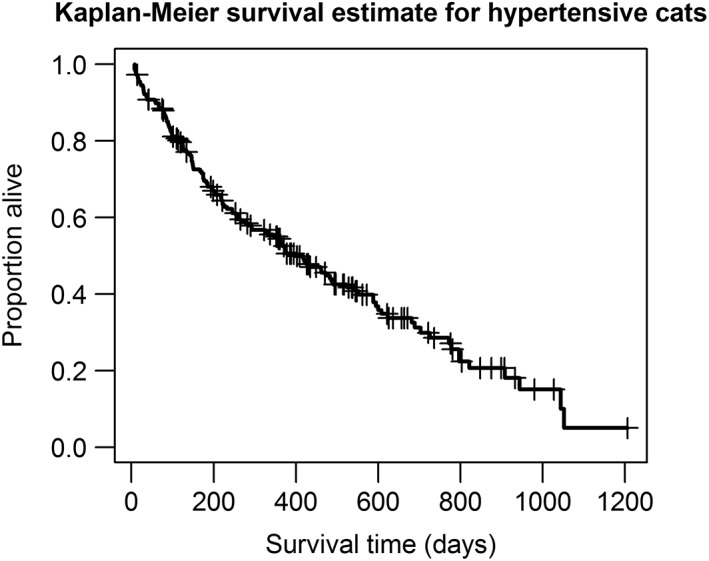
Kaplan‐Meier curve of all‐cause mortality of 220 cats diagnosed with hypertension. Dash indicates censoring of cat. Estimated median survival time was 400 (interquartile range [IQR], 147‐797) days
The univariable Cox proportional hazard model identified the following variables to be broadly associated with death after hypertension diagnosis: neuter status, reason for presentation, body condition score, body weight, number of times blood pressure was measured after diagnosis, ocular examination, blindness, retinopathy, tortuous vessels, seizures, behavioral change, proteinuria, UPC, amlodipine, atenolol, diabetes mellitus, and investigations performed. No clustering was identified at the veterinary group level.
The final multivariable model included retinal detachment, tortuous vessels, reason for presentation, investigations after diagnosis, amlodipine use, CKD diagnosis, and diabetes mellitus diagnosis (Table 4). Cats not receiving amlodipine treatment were at increased hazard of death (HR, 1.59; 95% CI, 0.98‐2.55; P = .06) compared to those that did receive it but had no dose change, although the difference was not significant at the 5% level in the multivariable analysis. Cats that received amlodipine but required a dose change were at decreased hazard of death (HR, 0.56; 95% CI, 0.36‐0.87; P = .01). Cats that had retinal detachment or tortuous vessels at diagnosis were at increased hazard of death, as were cats with a diagnosis of CKD or diabetes mellitus. Cats that were presented for monitoring of concurrent disease and cats that had investigations for underlying disease after diagnosis of hypertension were at decreased hazard of death. There was no evidence of interaction in the model and the proportional hazard assumption was met. Predictive ability of the model and model fit was adequate (Harrell's C, 0.68).
Table 4.
Mulitvariable Cox proportional hazard analysis of association with survival (including only cats that had >7 days survival, n = 220)
| Variable | N | Deaths (%) | HR | 95% CI | P‐value | ||
|---|---|---|---|---|---|---|---|
| Wald's test | LRT | ||||||
| Retinal detachment | No | 166 | 90 (54.2) | Reference | .01 | ||
| Yes | 54 | 40 (74.1) | 1.71 | 1.12‐2.62 | .01 | ||
| Tortuous vessels | No | 209 | 119 (56.9) | Reference | .008 | ||
| Yes | 11 | 11 (100.0) | 2.67 | 1.41‐5.07 | .003 | ||
| Reason for presentation | Clinical signs | 123 | 77 (62.6) | Reference | .03 | ||
| Anesthetic monitoring | 3 | 3 (100) | 2.61 | 0.76‐9.03 | .22 | ||
| Geriatric health screen | 12 | 6 (50) | 0.95 | 0.40‐2.26 | .91 | ||
| Monitoring of concurrent disease | 82 | 44 (53.7) | 0.58 | 0.37‐0.89 | .01 | ||
| Investigation after diagnosis | No | 41 | 31 (75.6) | Reference | .0006 | ||
| Yes | 179 | 99 (55.3) | 0.42 | 0.26‐0.68 | <.001 | ||
| Amlodipine | No | 44 | 30 (68.2) | 1.59 | 0.98‐2.55 | .06 | |
| Yes ‐ no dose change | 116 | 67 (57.8) | Reference | .0006 | |||
| Yes ‐ dose change | 60 | 33 (55) | 0.56 | 0.36‐0.87 | .01 | ||
| Concurrent CKD | No | 101 | 61 (60.4) | Reference | .002 | ||
| Yes | 119 | 69 (58.0) | 2.05 | 1.40‐3.22 | .002 | ||
| Concurrent Diabetes Mellitus | No | 214 | 124 (57.9) | Reference | .02 | ||
| Yes | 6 | 6 (100) | 3.31 | 1.40‐7.82 | .006 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; LRT, likelihood ratio test.
3.5. Blood pressure and treatment
Cats that received amlodipine treatment alone had significantly higher blood pressure at diagnosis (median, 210 mm Hg; IQR, 195‐232) in comparison to cats that received benazepril alone (median, 195 mm Hg; IQR, 185‐220; Mann‐Whitney P = .03). No difference was found in blood pressure at diagnosis between cats that received amlodipine or benazepril alone and amlodipine and benazepril combined.
4. DISCUSSION
Ours is the first study to determine the frequency with which blood pressure is measured in general veterinary practice in the UK, showing that 1 in 75 cats had their blood pressure assessed during the study period, increasing to just under 1 in 23 of cats ≥9 years. The findings suggest that blood pressure measurement is not commonly used to screen cats known to be at risk of developing hypertension (eg, the aging cat, those with CKD, and those with hyperthyroidism). The most common reason for measuring blood pressure was because of the presence of clinical signs compatible with hypertension. Furthermore, the results of our study suggest a benefit for cats that are screened for hypertension because they survived longer than those that had developed clinical signs of hypertension leading to their diagnosis. Whether early treatment of hypertension in cats that are screened decreases morbidity and mortality related to hypertension cannot be determined by this retrospective study. However, prospective experimental studies suggest that by lowering blood pressure, amlodipine does protect against hypertensive ocular damage.25
Cats with a diagnosis of CKD or hyperthyroidism or apparently healthy cats ≥9 years also are recommended to have regular blood pressure measurements because of increased risk of hypertension.2, 6, 26 In our study population, just under 24% of cats were ≥9 years of age. Previous research has estimated that 3.6% of cats have a diagnosis of CKD and 3% of cats have a diagnosis of hyperthyroidism in the PCP‐attending population.27 This suggests that blood pressure measurement is not being utilized routinely as a screening measure in higher risk cats, based on the lower proportion of cats receiving BPA and having it recommended. This could be because of the lack of experience, confidence, or training in measuring blood pressure in cats, and availability of the appropriate equipment in some practices or reluctance on the part of owners to pay for routine screening. The awareness of white coat hypertension also may mean veterinarians are reluctant to perform blood pressure measurements in a busy clinic because of the lack of confidence in the accuracy of the result.10 Very few cats <9 years had their blood pressure assessed. Without a baseline blood pressure measurement, as discussed in the ACVIM guidelines,6 it may be more difficult for veterinarians to assess if there has been an increase in blood pressure, potentially delaying the diagnosis of hypertension in some cats.
Calculated incidence risk was similar to that previously estimated from healthy cat populations, but previous studies were conducted on cats ≥9 years of age.1, 2, 3 In our study, most cats that received BPA were ≥9 years, which may explain the similarities with previous studies. Blood pressure assessment appears to have been targeted at high risk cats in our study population (ie, older cats, cats with pre‐existing disease, and cats with clinical signs). Subclinical disease may have been missed because few cats were diagnosed with hypertension before clinical signs were present. This may result in the incidence estimate calculated being an underestimate of the true incidence of hypertension.
Median blood pressure at diagnosis was within the severe risk ACVIM category, with most cats having blood pressure of ≥180 mm Hg at diagnosis. This finding is consistent with most cats showing clinical signs of TOD at diagnosis. Earlier diagnosis of hypertension may decrease the number of cats presenting with evidence of TOD, as has been seen in a study that enrolled cats with lower blood pressure at diagnosis of hypertension,15 which may decrease the morbidity associated with the condition. In another study that performed regular blood pressure measurements longitudinally in initially normotensive cats, 52% were found to have evidence of TOD at the point at which they were diagnosed with hypertension,2 lower than the proportion of cats with TOD at time of diagnosis in our study. Hypertension is considered to cause harm by resulting in TOD, and decreasing QOL in these cats.6 Quality of life was found to be decreased in hypertensive cats before starting treatment in 1 study,15 and QOL was the most frequently reported reason for euthanasia in our study. Some clinical signs associated with CKD have been found to negatively impact QOL of cats with CKD.28 Neurological signs have been reported to be the 6th most common reason for death in cats attending PCP.29 This observation would all suggest that clinical disease associated with TOD may decrease QOL in cats. Because all cats diagnosed with diabetes mellitus were euthanized, it is possible that the QOL impact on the cat and owner of this disease influenced the HR calculated in our study, and that the impact of hypertension on death in these cats is lower than calculated. Additionally, blood pressure at diagnosis was not associated with survival. However, an association was identified between TOD and survival, which suggests that severity of hypertension (as reflected by evidence of TOD rather than a single blood pressure measurement made in the clinic) is associated with survival after diagnosis.
Blood pressure monitoring of cats after diagnosis was limited, which may mean that control of blood pressure was inadequate in some cats and did not result in a decrease in blood pressure that would decrease the cat's risk of TOD. The UPC was measured in a minority of cats, despite the association between both UPC at diagnosis and the time averaged UPC after treatment and survival after hypertension diagnosis.8 Primary care veterinarians may not be aware of this association, or owners may decline to have UPC measured. The use of urine dipstick tests to assess cat urine for the presence of protein lacks sensitivity and specificity.24 Cats requiring an increase in amlodipine dose were found to have significantly higher blood pressure at diagnosis in comparison to those that did not. This finding is in agreement with a recent study,16 even though not all cats had a follow‐up blood pressure measurement to ensure adequate blood pressure control in our study. Because of inadequate monitoring, it was not possible to investigate the association between blood pressure control and survival after hypertension diagnosis.
Retinal detachment and tortuous vessels identified at diagnosis of hypertension both were associated with increased hazard of death. These cats also may have had TOD in other organs, such as the heart, that may have predisposed them to more life‐limiting clinical signs. The owners of these cats also may have chosen to euthanize them sooner than cats without these clinical signs because of their perceived decreased QOL. Hypertensive cats that were diagnosed while being monitored for hypertension associated with a known predisposing disease were at lower hazard of death in comparison to cats that were diagnosed with hypertension after presentation for clinical signs. This finding was most likely because of hypertension being diagnosed earlier in cats being monitored for it, and therefore decreasing the risk of TOD in these cats. It is also possible that this association with survival is caused by lead time bias, because cats diagnosed while being monitored for a pre‐existing disease are diagnosed earlier and they are considered to have had hypertension longer than they would have had if they were not diagnosed until clinical signs of hypertension were present. Cats that received investigations for an underlying cause of their hypertension also had a lower hazard of death in comparison to those cats that did not. This is likely associated with the owners being more proactive in treatment of hypertension in their cats, and these cats may have had increased monitoring after diagnosis of hypertension and therefore better treatment. A dose change in amlodipine was associated with decreased hazard of death. Cats that had a dose change of amlodipine had significantly more blood pressure measurements after diagnosis of hypertension in comparison to cats that did not receive a dose change. It seems most likely that the association between dose change and survival was related to improved monitoring after diagnosis and better treatment, because these cats had blood pressure measurements after diagnosis to identify the lack of response to amlodipine at the initial dose. It is also possible that owners who are more committed to monitoring and treatment of hypertension in their cats are more likely to have uncontrolled hypertension identified and that improved survival associated with dose change is an indirect effect of this vigilance. Proteinuria was not found to be associated with survival, unlike findings in previous studies.8 This difference is likely because of the small numbers of cats that had UPC measured, leading to the study being underpowered to detect this association.
A subgroup analysis was undertaken to see if there were any associations between blood pressure at diagnosis and treatments received and between treatments received and average blood pressure after diagnosis. These analyses were performed primarily for hypothesis‐generating purposes. Cats receiving amlodipine treatment had higher blood pressure at diagnosis than did cats receiving benazepril. This observation may be caused by veterinarians being unwilling to prescribe amlodipine to cats they perceived to have mild hypertension “on the cascade” (a UK system for deciding what medicine should be used for a condition in a particular species30) because, at the time this study was conducted, amlodipine did not have a product authorization for cats.30 The preparations of amlodipine available for human in 2012‐2015 required tablets to be divided into much smaller doses for cats. It also may be that veterinarians were concerned about potential adverse effects of amlodipine, although it has been shown recently that amlodipine has no more adverse effects than a placebo.15 These considerations may explain why some veterinarians opted to give benazepril to cats with less severe hypertension. No controlling for confounding was performed in this subgroup analysis, and unrecognized confounding may be present.
Our study had a number of limitations. It is possible that potential cases were not identified from the searches because of veterinarians using different terms in the EPR. This limitation was shown to be of low significance by a pilot study carried out to informally assess the sensitivity and specificity of the search terms. The case definition relied on veterinarians performing a BPA and correctly identifying hypertension, no minimum blood pressure was required. Also, it was not possible to validate the techniques used by clinicians, the interpretation of the blood pressure measurement or differentiate between measurements from Doppler or oscillometric BP machines. This may mean that some cats were misclassified as hypertensive when they had white coat hypertension or normotensive when they actually were hypertensive. It also relied on veterinarians measuring blood pressure, and so cases may have been missed. The data were not recorded for research purposes, so there is the possibility of missing data resulting in misclassification of variables. Not all cats received follow‐up blood pressure measurements, so any analysis of control of hypertension may be biased or underpowered. There is also the possibility that cats that received follow‐up blood pressure measurements were different in some way from those cats that did not, resulting in bias. It is possible that some cats lost to follow‐up had misclassification of variables because of the lack of follow‐up in their available clinical notes. Insurance data were not available for our study. Insurance status may have been a confounder in the survival analysis. A lack of definitive diagnosis of practitioner‐diagnosed cardiac disease meant that adaptive hypertrophy secondary to hypertension could not be considered separately in the survival analysis.
Our study highlighted that inadequate blood pressure measurement is performed in cats attending PCP in the UK. Hypertension is associated with considerable morbidity in this population and limited monitoring after diagnosis of hypertension occurs. Cats with hypertension diagnosed before associated clinical signs occur have improved survival, as do cats that have regular blood pressure monitoring after diagnosis and institution of treatment. Encouraging more routine blood pressure monitoring in older cats (≥10 years of age) and in cats with CKD and hyperthyroidism, before signs of TOD become apparent, should improve survival and decrease morbidity associated with hypertension. Encouraging owners and veterinarians to implement routine blood pressure measurement as part of routine health screening in healthy cats may allow earlier diagnosis of hypertension and earlier implementation of treatment. Further research into why veterinarians do not carry out blood pressure monitoring more routinely and reasons for limited monitoring after diagnosis of hypertension would aid in the design of educational programs to improve owner and veterinarian use of blood pressure measurement in daily practice. By identifying hypertension early, we may be able to decrease associated morbidity and improve survival, thus improving the health and welfare of cats diagnosed with hypertension in the UK.
CONFLICT OF INTEREST DECLARATION
J.E. has acted as a consultant for Bayer ltd, Boehringer Ingelheim Ltd, Ceva Santé Animale, Novartis Animal Health, Elanco Ltd, Orion Ltd, Vetoquinol Ltd, Waltham Centre for Pet Nutrition, Idexx Ltd, Royal Canin, and Nextvet Ltd. He has received grants for research from Royal Canin, Zoetis Ltd, Orion Ltd, and Ceva Santé Animale.
ACKNOWLEDGMENTS
This study was supported by a grant from Ceva Santé Animale. The authors thank Noel Kennedy (Royal Veterinary College) and Peter Dron (Royal Veterinary College) for VetCompass database, software, and program development. The authors are grateful to the Medivet Veterinary partnership, Vets4Pets/ Companion Care, Blythwood Vets, Vets Now, and the other UK veterinary practices that contribute to VetCompass. The authors thank Dan O'Neill and members of the cat group (Royal Veterinary College) for feedback on database search terms and data to extract. This study was performed at the Royal Veterinary College, Hatfield, UK.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Royal Veterinary College Clinical Research Ethical Review Board (URN M2015 0051).
Conroy M, Chang Y‐M, Brodbelt D, Elliott J. Survival after diagnosis of hypertension in cats attending primary care practice in the United Kingdom. J Vet Intern Med. 2018;32:1846–1855. 10.1111/jvim.15307
References
- 1. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med. 2014;32(1):1846‐1855. 10.1111/jvim.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bijsmans ES, Jepson RE, Chang YM, Syme HM, Elliott J. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J Vet Intern Med. 2015;29(3):855‐861. 10.1111/jvim.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jepson RE, Brodbelt D, Vallance C, Syme HM, Elliott J. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med. 2009;23(4):806‐813. 10.1111/j.1939-1676.2009.0339.x. [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi DL, Peterson ME, Graves TK, Nichols CE, Lesser M. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med. 1990;4(2):58‐62. 10.1111/j.1939-1676.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 5. Chetboul V, Lefebvre HP, Pinhas C, Clerc B, Boussouf M, Pouchelon J‐L. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med. 2003;17(1):89‐95. 10.1111/j.1939-1676.2003.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 6. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21(3):542‐558. 10.1111/j.1939-1676.2007.tb03005.x. [DOI] [PubMed] [Google Scholar]
- 7. IRIS . International Renal Interest Society. http://www.iris-kidney.com/. Published 2015. Accessed October 26, 2015.
- 8. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med. 2007;21(3):402‐409. 10.1892/0891-6640(2007)21%5B402:EOCOSB%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9. Williams TL, Elliott J, Syme HM. Renin‐angiotensin‐aldosterone system activity in hyperthyroid cats with and without concurrent hypertension. J Vet Intern Med. 2013;27(3):522‐529. 10.1111/jvim.12062. [DOI] [PubMed] [Google Scholar]
- 10. Stepien RL. Feline systemic hypertension diagnosis and management. J Feline Med Surg. 2011;13(1):35‐43. 10.1016/j.jfms.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott J, Fletcher M, Syme HM. Idiopathic feline hypertension: epidemiological study. J Vet Intern Med. 2003;17:754. [Google Scholar]
- 12. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med. 1994;8(2):79‐86. [DOI] [PubMed] [Google Scholar]
- 13. Elliott J, Barber PJ, Syme HM, Rawlings JM, Markwell PJ. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract. 2001;42(3):122‐129. 10.1111/j.1748-5827.2001.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 14. Kyles AE, Gregory CR, Wooldridge JD, et al. Management of hypertension controls postoperative neurologic disorders after renal transplantation in cats. Vet Surg. 1999;28(6):436‐441. 10.1111/j.1532-950X.1999.00436.x. [DOI] [PubMed] [Google Scholar]
- 15. Huhtinen M, Derré G, Renoldi HJ, et al. Randomized placebo‐controlled clinical trial of a chewable formulation of amlodipine for the treatment of hypertension in client‐owned cats. J Vet Intern Med. 2015;29(3):786‐793. 10.1111/jvim.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bijsmans ES, Doig M, Jepson RE, Syme HM, Elliott J, Pelligand L. Factors influencing the relationship between the dose of amlodipine required for blood pressure control and change in blood pressure in hypertensive cats. J Vet Intern Med. 2016;30(5):1630‐1636. 10.1111/jvim.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. College RV. VetCompass. http://www.rvc.ac.uk/vetcompass. Published 2016.
- 18. Venom Coding Group . Veterinary Nomenclature. http://www.venomcoding.org/VeNom/Welcome.html. Published 2016. Accessed October 31, 2016.
- 19. Sample Size Calculators , UCSF Clinical & Translational Science Institute 2016. URL: http://www.sample-size.net/sample-size-survival-analysis/ Accessed August 30, 2016.
- 20. International Cat Care . Cat Breeds. http://icatcare.org/advice/cat-breeds. Published 2015. Accessed August 1, 2016.
- 21. Birnbaum ZW, Sirken MG. Design of Sample Surveys to Estimate the Prevalence of Rare Diseases: Three Unbiased Estimates. Washington, D.C.: National Center for Health Statistics; 1965. [PubMed] [Google Scholar]
- 22. Kirkwood B, Sterne J. Essenial Medical Statistics. 2nd ed. Blackwell Science Ltd: Singapore; 2003. [Google Scholar]
- 23. Dohoo IR. Veterinary Epidemiologic Research. 2nd ed. VER Inc: Charlottetown, Canada; 2010. [Google Scholar]
- 24. Lyon SD, Sanderson MW, Vaden SL, Lappin MR, Jensen WA, Grauer GF. Comparison of urine dipstick, sulfosalicylic acid, urine protein‐to‐creatinine ratio, and species‐specific ELISA methods for detection of albumin in urine samples of cats and dogs. J Am Vet Med Assoc. 2010;236(8):874‐879. 10.2460/javma.236.8.874. [DOI] [PubMed] [Google Scholar]
- 25. Mathur S, Syme H, Brown CA, et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res. 2002;63(6):833‐839. 10.2460/ajvr.2002.63.833. [DOI] [PubMed] [Google Scholar]
- 26. Morrow L, Adams V, Elliott J, Syme H. Hypertension in hyperthyroid cats: prevalence, incidence, and predictors of its development. J Vet Intern Med. 2009;23:699. [Google Scholar]
- 27. O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in cats attending primary‐care veterinary practices in England. Vet J. 2014;202(2):286‐291. 10.1016/j.tvjl.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 28. Bijsmans ES, Jepson RE, Syme HM, Elliott J, Niessen SJM. Psychometric validation of a general health quality of life tool for cats used to compare healthy cats and cats with chronic kidney disease. J Vet Intern Med. 2016;30(1):183‐191. 10.1111/jvim.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg. 2015;17(2):125‐133. 10.1177/1098612X14536176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veterinary Medicine Directorate . The Cascade: Prescribing unauthorised medications. https://www.gov.uk/guidance/the-cascade-prescribing-unauthorised-medicines. Published 2015. Accessed June 24, 2017.


