Abstract
Background
Gastroesophageal reflux and microaspiration (MA) of gastric juice are associated with various human respiratory diseases but not in dogs.
Objective
To detect the presence of bile acids in bronchoalveolar lavage fluid (BALF) of dogs with various respiratory diseases.
Animals
Twenty‐seven West Highland White Terriers (WHWTs) with canine idiopathic pulmonary fibrosis (CIPF), 11 dogs with bacterial pneumonia (BP), 13 with chronic bronchitis (CB), 9 with eosinophilic bronchopneumopathy (EBP), 19 with laryngeal dysfunction (LD), 8 Irish Wolfhounds (IWHs) with previous BPs, 13 healthy WHWTs, all privately owned dogs, and 6 healthy research colony Beagles
Methods
Prospective cross‐sectional observational study with convenience sampling of dogs. Bile acids were measured by mass spectrometry in BALF samples. Total bile acid (TBA) concentration was calculated as a sum of 17 different bile acids.
Results
Concentrations of TBA were above the limit of quantification in 78% of CIPF, 45% of BP, 62% of CB, 44% of EBP, 68% of LD, and 13% of IWH dogs. In healthy dogs, bile acids were detected less commonly in Beagles (0/6) than in healthy WHWTs (10/13). Concentrations of TBA were significantly higher in CIPF (median 0.013 μM, range not quantifiable [n.q.]‐0.14 μM, P < .001), healthy WHWTs (0.0052 μM, n.q.‐1.2 μM, P = .003), LD (0.010 μM, n.q.‐2.3 μM, P = .015), and CB (0.0078 μM, n.q.‐0.073 μM, P = .018) groups compared to Beagles (0 μM, n.q.).
Conclusion and Clinical Importance
These results suggest that MA occurs in various respiratory diseases of dogs and also in healthy WHWTs.
Keywords: bile acid, bronchoalveolar lavage fluid, canine, microaspiration
Abbreviations
- BALF
bronchoalveolar lavage fluid
- BP
bacterial pneumonia
- CB
chronic bronchitis
- CIPF
canine idiopathic pulmonary fibrosis
- EBP
eosinophilic bronchopneumopathy
- GER
gastroesophageal reflux
- IWH
Irish Wolfhounds
- LD
laryngeal dysfunction
- MA
microaspiration
- n.q.
not quantifiable
- TBA
total bile acid
- WHWT
West Highland White Terriers
1. INTRODUCTION
Gastroesophageal reflux (GER), defined as a return of gastric contents into the esophagus, is a usual cause of esophagitis in humans but can also cause extraesophageal manifestations, such as cough and laryngitis.1 Normally, the respiratory tract is well protected from aspiration by several esophagopharyngolaryngeal reflexes as well as by the cough reflex and mucociliary barrier.2 However, recent evidence in people suggests that GER with microaspiration (MA) of small amounts of gastric juice plays an important role in the induction and exacerbation of respiratory diseases. Microaspiration occurs in several diseases including idiopathic pulmonary fibrosis (IPF),3, 4 asthma,5, 6, 7 cystic fibrosis,8 and chronic obstructive pulmonary disease (COPD).6, 9 It has been strongly suggested that IPF develops after repeated, chronic MA and that the use of medical or surgical treatment of GER delays its progression.10 Detection of MA includes assessment of gastrointestinal compounds such as bile acids and pepsin in bronchoalveolar lavage fluid (BALF).2, 11 Bile acids in BALF are used in several studies as a marker of reflux aspiration in humans.2 Gastric juice contents cause injury in lungs of human patients,12 and bile acids have been cytotoxic to the lung in studies with experimental rats,13 supporting the need to look for similar effects in dogs with respiratory disease.
Dogs develop a number of respiratory conditions similar to those that occur in humans, however the role of MA in these disorders has not been evaluated and the etiology of inflammatory conditions of the lung remains unknown. Of the respiratory diseases affecting dogs, canine idiopathic pulmonary fibrosis (CIPF) shares many clinical and pathological similarities with human IPF14, 15, 16 and has a similarly poor prognosis, with no specific treatment modalities available. Canine eosinophilic bronchopneumopathy (EBP) and chronic bronchitis (CB), the 2 most common inflammatory lung diseases, are typically steroid responsive yet the cause for disease exacerbations, which result in progressive lung dysfunction, has not been fully evaluated. Laryngeal dysfunction (LD) is a predisposing factor for aspiration pneumonia in dogs,17, 18 however the role of MA in these cases has not been examined. Specific treatment for GER is increasingly used in human patients with respiratory disease because of the recognized connections between the gastro‐intestinal and respiratory tracts, however the evidence is still quite limited.2 If such a relationship could be described in dogs, it would provide a basis for institution of similar treatment in affected individuals.
The aim of this study was to measure bile acid concentrations in BALF samples of dogs with respiratory diseases and determine existence of MA in different respiratory diseases. We assessed the prevalence of MA in BALF in dogs with typical respiratory diseases, including CIPF, bacterial pneumonia (BP), EBP, CB, LD, and Irish Wolfhounds (IWHs) with recurrent BPs and compared results to findings in BALF of healthy dogs. We hypothesized that many respiratory diseases can be associated with MA, as evidenced by detection of bile acids in BALF.
2. MATERIALS AND METHODS
2.1. Study subjects
Stored BALF samples from privately owned pet dogs and from healthy experimental Beagles (Helsinki University colony) were used. Dogs with respiratory tract disease were grouped accordingly: West Highland White Terriers (WHWTs) with CIPF (n = 27), dogs with acute BP (n = 11), CB (n = 13), EBP (n = 9), LD (n = 19), and IWHs with previous recurrent BPs (n = 8). Of the dogs in the LD group, 6/19 were examined at the Veterinary Teaching Hospital of the University of Helsinki, Finland and 13/19 at the William R. Pritchard Veterinary Medical Teaching Hospital of University of California, Davis. Healthy WHWTs (n = 13) and Beagles (n = 6) exhibited no signs, clinical examination findings or radiological findings suggestive of lung disease.
Diagnostic work‐up included hematology, serum biochemistry, arterial blood gas analyses, cervicothoracic radiographs, laryngoscopy, and bronchoscopy with BALF sampling, at the discretion of the attending clinician. High‐resolution computed tomography (HRCT) was performed in 24/27 of CIPF dogs, in all healthy WHWTs and in all IWHs. Detailed history was asked from all dog owners and none of the dogs had signs of acute or chronic gastric diseases at the sampling time.
Inclusion criteria were defined separately for each group. The diagnosis of CIPF was based on typical findings in clinical examinations of progressive respiratory difficulty, tachypnea, and crackles on auscultation along with either HRCT findings of ground glass opacities (24/27) or postmortem lung histopathology (14/27). Diagnoses of BP were based on typical signs (including fever, cough, lethargy, tachypnea), radiological findings (alveolar or interstitial consolidation), BALF bacterial growth or intracellular bacteria, and response to antimicrobial treatment.19 IWHs included here had experienced at least 2 previous episodes of BPs characterized by an acute onset of clinical signs and findings suggestive of BP in clinical examinations and thoracic radiographs but were asymptomatic during sampling for this study. Dogs with CB had a history of cough (duration >2 months during a year) and airway inflammation for which other respiratory and cardiac causes were excluded. EBP diagnosis was based on sterile eosinophilic inflammation in BALF (eosinophils >17%) and exclusion of other causes for eosinophilia (eg, parasites). LD was diagnosed by the presence of laryngeal paresis or paralysis before and after doxapram stimulation when indicated. In LD dogs, 13/19 had additional lower respiratory tract diseases including CB (4), BP (6), EBP (2), and brachycephalic obstructive airway syndrome (1) but were grouped as LD dogs for total bile acid (TBA) assessment.
2.2. Sample collection
Bronchoscopy was performed and BALF samples were collected from left and right caudal lobes with physiological saline (2 mL/kg/lobe, divided to 2 aliquots) except in IWHs, BP and part of the LD dogs. In IWHs, the right middle and left caudal lobe were lavaged. In BP dogs, samples were collected at the onset of clinical signs and the most affected lobe was selected based on radiographs and bronchoscopy findings and the amount of saline used was 0.5‐1 mL/kg once or twice except for 1 dog that underwent a transtracheal wash.19 In LD dogs examined at the University of California, BALF samples were collected with individual aliquots of 5‐20 mL per site depending on body size (mean and SD 1.93 ± 0.65 mL/kg, range 0.93‐3.29 mL/kg) and were collected from 2 to 3 sites considered most abnormal on visual inspection or on radiographic evaluation. The supernatant was separated by centrifugation (10 min, 100g) and stored at −112°F (−80°C).
2.3. Bile acid concentration analysis
Bile acids analyzed included 17 different bile acids. Total bile acid concentration was calculated as a sum of these. Bile acids were analyzed in the laboratory HUSLAB, Helsinki University Central Hospital, by high pressure liquid chromatography‐tandem mass spectrometry.20 All measurements were carried out by a Nexera X2 UPLC system (Shimazdu, Kyoto, Japan) coupled to a 5500 Qtrap mass spectrometer interfaced with an electrospray ion source (ABSciex, Toronto, Ontario, Canada). Before liquid chromatographic‐mass spectrometric quantification, the BALF samples were purified by solid phase extraction by a Strata‐X polymeric reversed‐phase 96‐well plate (10 mg/well, Phenomenex, Torrance, California). In brief, the samples (150 μL) were mixed with 50 μL of deuterium labeled internal standard solution and 0.005% formic acid to a final volume of 250 μL and drawn through the preconditioned extraction plate. The wells were washed with 100 μL 0.005% formic acid and 100 μL 5% methanol, and the analytes were then eluted with 100 μL methanol followed by 100 μL acetonitrile. Finally, the sample extracts were dried with a centrifugal evaporator (GeneVac, Thermo Fisher Scientific) and reconstituted in 100 μL of 60% methanol. The BA reference samples (quality controls and calibration standards) were prepared in water. The extraction recoveries were over 65% for all compounds except for Lithocholic acid (55%) and 7‐OH‐4‐cholesten‐3‐one (40%). The relative poor extraction recoveries for lithocholic acid and 7‐alpha‐hydroxy‐4‐cholesten‐3‐one were corrected using corresponding deuterium labeled internal standards. All bile acids showed a good linearity (r > 0.999) over the concentration levels from limit of quantification to 5 μM, and the day‐to‐day (n = 6) coefficient of variation was below 10% at 0.01 and 1.0 μM for all bile acids. The chromatographic separation was achieved on Atlantis T3 (2.1 x 100 mm, 3 μm particle size; Waters. Corp., Milford, Massachusetts), and the mass spectrometer was operated in negative multi reaction monitoring mode, except for 7‐OH‐4‐cholesten‐3‐one that was analyzed in positive mode (ion transition m/z 401 ➔ 177). The chromatographic conditions and the mass spectrometric parameters for individual compounds are previously described in detail elsewhere.20
The limit of quantification was 0.001 μM for tauroursodeoxycholic acid, glycocholic acid, glycolithocholic acid, chenodeoxycholic acid, glycoursodeoxycholic acid, deoxycholic acid, glycodeoxycholic acid, taurolithocholic acid, taurochenodeoxycholic acid, and cholic acid. For the remaining bile acids (ursodeoxycholic acid, glycochenodeoxycholic acid, taurocholic acid, hyodeoxycholic acid, taurodeoxycholic acid, lithocholic acid, and 7‐OH‐4‐cholesten‐3‐one), the limit of quantification was 0.005 μM.
2.4. Statistical analysis
All statistical analyses were performed by SAS System for Windows, version 9.3 (SAS Institute, Inc, Cary, North Carolina). The transformed data satisfied normality assumptions based on Shapiro‐Wilk tests and Normal QQ‐plots.
For WHWTs, differences in TBA between CIPF and healthy WHWTs were analyzed by analysis of variance (ANOVA). When the TBA concentration was below the limit of quantification, the value was replaced with half of the limit of quantification to minimize the error in estimating mean values. Imputed data for all variables were logarithmically transformed to meet the normality‐assumption of the ANOVA‐analyses. The ANOVA‐models included health status (CIPF/healthy WHWTs), age, sex, and the storage time of BALF samples as fixed effects.
In all dog groups when compared to the control group (Beagles), bile acid data were dichotomized (zero‐concentrations versus others), because of the large number of concentrations below the lower limit of quantification in the control group. Pair wise association of the group (CIPF/healthy WHWTs/BP/IWHs/CB/ EBP/LD versus Beagles) and the dichotomized bile acid variable were analyzed by Fisher's exact test. Correlation analysis was made by Spearman's rank correlation coefficient (r s).
The effect of possible confounding factors (sex, age, and storage time of BALF samples) was analyzed by logistic regression within each dog group. The model included sex, age, and storage time as fixed effects and the dichotomized bile acid variable as the response. P‐values <.05 were considered statistically significant in all analyses.
3. RESULTS
A total of 106 BALF samples were analyzed from dogs clinically diagnosed with CIPF (27), LD (19), CB (13), BP (11), EBP (9), and from IWHs (8), healthy WHWTs (13), and Beagles (6). Dogs with BP, CB, EBP, and LD were comprised of different breeds (listed in Supporting Information Table S1). In all dog groups, age, sex, or storage time did not affect detection of TBA in BALF (Supporting Information Table S2).
Concentrations of TBA are presented in Figure 1. These concentrations were above the limit of quantification in 78% of WHWTs with CIPF (21/27, binomial confidence interval [CI] 58%‐91%), 68% of dogs with LD (13/19, CI 43%‐87%), 62% of dogs with CB (8/13, CI 32%‐86%), 45% of dogs with BP (5/11, CI 17%‐77%), 44% of dogs with EBP (4/9, CI 14%‐79%), and 13% of IWHs (1/8, CI 0.3%‐53%) as well as in 77% of healthy WHWTs (10/13, CI 46%‐95%) and in 0% of healthy Beagles (0/6, CI 0%‐46%). Concentrations of TBA were significantly higher in CIPF (median 0.013 μM, range not quantifiable [n.q.]‐0.14 μM, P < .001), healthy WHWT (0.0052 μM, n.q.‐1.2 μM, P = .003), LD (0.010 μM, n.q.‐2.3 μM, P = .015), and CB (0.0078 μM, n.q.‐0.073 μM, P = .018) groups compared to Beagles (0 μM, n.q.). In WHWTs, TBA concentrations did not differ significantly between CIPF and healthy dogs (P = .373).
Figure 1.
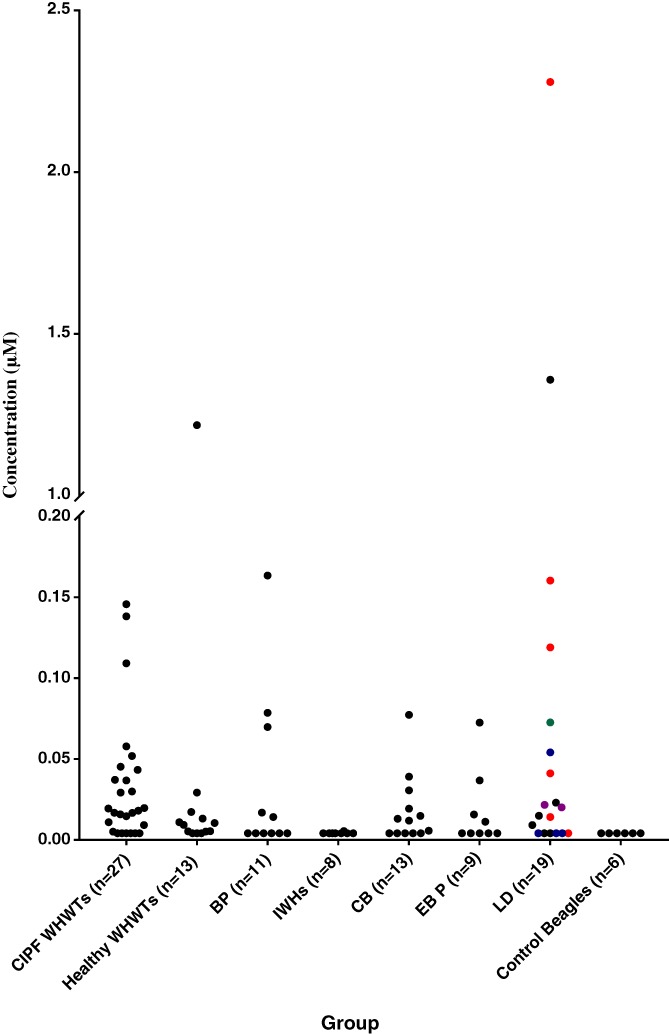
Total bile acid (TBA) concentrations in bronchoalveolar lavage fluid (BALF) samples of different dog groups. CIPF, canine idiopathic pulmonary fibrosis; WHWTs, West Highland White Terriers; BP, bacterial pneumonia; IWHs, Irish Wolfhounds; CB, chronic bronchitis; EBP, eosinophilic bronchopneumopathy; LD, laryngeal dysfunction. In LD group colors are used to express the additional respiratory disease diagnosis of each dog. Red, BP; Blue, CB; Green, Brachycephalic obstructive airway syndrome (BOAS); Purple, EBP; Black, no additional respiratory disease diagnosis
Correlations between TBA concentrations and BALF cytologic values are shown in Table 1. In BP group, strong positive correlations were found between TBA concentrations and total cell counts, neutrophils, eosinophils, and lymphocytes. There were no significant correlations between TBA concentrations and arterial blood gas values (Supporting Information Table S3). In dogs with BP there were strong positive correlation between TBA concentrations and hospitalization days (median 1 day, range 0‐5 days; P = .017, r s = 0.72).
Table 1.
Correlations between total bile acid (TBA) concentrations and total cell count and differential cell counts of bronchoalveolar lavage fluid (BALF)
| Variable | CIPF WHWTs (n = 27) |
Healthy WHWTs (n = 13) |
CB (n = 13) |
BP (n = 11) |
IWHs (n = 8) |
EBP (n = 9) |
LD (n = 19) |
|---|---|---|---|---|---|---|---|
| RV (%) Median Range |
35 14‐69 |
54 33‐74 |
26 13‐48 |
54 33‐74 |
22 7.8‐45 |
27 12‐52 |
49a
26‐64 |
| TCC, n/μL Median |
860 | 360 | 350 | 220** | 175 | 620 | 1046 |
| Range | 220‐2600 | 270‐630 | 20‐1380 | 70‐3670 | 30‐470 | 100‐1920 | 60‐33 600 |
| r s | 0.30 | 0.12 | 0.17 | 0.82 | 0.41 | 0.64 | −0.02 |
| Mac, n/μL Median |
650* | 280 | 250 | 87 | 125 | 180 | 570 |
| Range | 156‐2340 | 190‐470 | 9.0‐560 | 31‐6130 | 7.0‐270 | 77‐880 | 45‐4900 |
| r s | 0.41 | 0.01 | 0.09 | 0.57 | 0.41 | 0.24 | −0.02 |
| Mac % Median |
77 | 79 | 71 | 48* | 65 | 46 | 55 |
| Range | 26‐96 | 69‐86 | 31‐95 | 3.0‐64 | 24‐79 | 18‐77 | 12‐91 |
| r s | 0.05 | −0.28 | 0.31 | −0.66 | −0.41 | −0.30 | −0.21 |
| Neu, n/μL Median |
52 | 16 | 24 | 30** | 6,2 | 48 | 106 |
| Range | 2.6‐370 | 3.0‐110 | 0.4‐78 | 5.0‐30 400 | 1.9‐20 | 1.4‐680 | 1.6‐26 900 |
| r s | 0.24 | 0.17 | −0.11 | 0.87 | 0.58 | 0.38 | −0.27 |
| Neu (%) Median |
6.4 | 4.1 | 4.2 | 12** | 4.6 | 5.7 | 9.5 |
| Range | 0.7‐38 | 1.0‐10 | 0.4‐52 | 3.4‐98 | 0.4‐16 | 1.0‐14 | 0.7‐87 |
| r s | 0.17 | 0.43 | 0.003 | 0.90 | 0.25 | −0.11 | −0.33 |
| Eos, n/μL Median |
1.2 | 1.8 | 9.0 | 9.1 | 1.4 | 310 | 37 |
| Range | 0.5‐22 | 0–12 | 0–110 | 0–260 | 0–17 | 17‐940 | 0‐1600 |
| r s | −0.038 | 0.08 | 0.31 | −0.21 | 0.43 | 0.62 | −0.006 |
| Eos (% Median) |
0.2 | 0.5 | 1.4 | 3.6** | 0.9 | 34 | 4.5 |
| Range | 0‐2.4 | 0‐6.0 | 0‐22 | 0–21 | 0–11 | 17‐69 | 0–62 |
| r s | −0.13 | −0.11 | 0.28 | −0.82 | 0.09 | 0.57 | −0.11 |
| Mast, n/μL Median |
4.8 | 0.8 | 3.0 | 0.0* | 1.3 | 9.7 | 2.3 |
| Range | 0–64 | 0‐3.0 | 0‐9.0 | 0‐7.3 | 0‐4.3 | 0‐36 | 0–38 |
| r s | 0.33 | 0.42 | 0.42 | −0.73 | 0.42 | 0.25 | 0.22 |
| Mast (%) Median |
0.70 | 0.20 | 1.0 | 0* | 0.4 | 0.70 | 0.50 |
| Range | 0‐2.5 | 0‐0.9 | 0‐2.6 | 0‐4.4 | 0‐2.4 | 0‐7.7 | 0‐3.0 |
| r s | 0.27 | 0.12 | 0.38 | −0.73 | 0.26 | 0 | 0.22 |
| Lymp n/μL Median |
60 | 56 | 34 | 25 | 41 | 74 | 112 |
| Range | 12‐340 | 40‐93 | 3.0‐710 | 0‐150 | 3.0‐190 | 4.0‐150 | 7.0‐2030 |
| r s | 0.14 | 0.064 | 0.31 | −0.19 | 0.41 | 0.35 | −0.06 |
| Lymp % Median |
7.4 | 15.7 | 9.7 | 20** | 21 | 11 | 8.3 |
| Range | 0.9‐30 | 10‐21 | 3.7‐52 | 0–30 | 10‐42 | 4.0‐16 | 1.0‐35 |
| r s | −0.14 | −0.11 | 0.051 | −0.85 | 0.58 | −0.18 | 0.013 |
Statistically significant difference * <.05 and ** <.01.
Abbreviations: BP, bacterial pneumonia; CB, chronic bronchitis; CIPF, canine idiopathic pulmonary fibrosis; EBP, eosinophilic bronchopneumopathy; Eos, Eosinophil; IWHs, Irish Wolfhounds; LD, laryngeal dysfunction; Lymp, Lymphocyte; Mac, Macrophage; Mast, Mast cell; Neu, Neutrophil; r s, Spearman's rank correlation coefficient; TCC, total cell count; RV, Recovered volume; WHWTs, West Highland White Terriers.
Data available only from 6 dogs.
4. DISCUSSION
This study was designed to detect the presence of bile acids in BALF of dogs with various respiratory diseases with the goal of investigating the potential role of MA in these diseases. We were able to detect presence of bile acids in BALF in dogs from all disease groups but not in any of 6 control Beagles. Bile acids were found in 60% of diseased dogs and were most prevalent in group of WHWTs with CIPF (detectable TBA concentrations in 78% of dogs). Although not detected in BALF of healthy Beagle dogs, bile acids were present in the BALF in an equal proportion of healthy WHWTs (77%). Bile acids, gastrointestinal compounds that are produced in the canine liver and secreted in bile into the gut, should not be present in the pulmonary epithelial lining fluid, thus suggesting that GER followed by MA is common in respiratory conditions of dogs.
Gastroesophageal disease (ie, GER with signs such as heartburn and regurgitation) is common in humans21 and MA of GER has been detected in various respiratory diseases in humans, including IPF, cystic fibrosis, COPD, and asthma.3, 4, 6, 7, 8, 9 GER occurs commonly in dogs under anesthesia and with various disease processes,22, 23, 24 and a recent study suggests that GER disease might be more common in dogs than previously suspected.25 In healthy dogs GER without MA has been shown to occur (Grobman et al. Abstract, 27th ECVIM‐CA Congress, 2017).
Bile acids and gastric pepsin are commonly used for detection of MA from different type of samples including BALF, tracheal aspirates, exhaled breath condensate, and sputum.2 We chose the most commonly used sample type, BALF, to investigate bile acid detection as evidence for possible MA. Samples were collected by techniques commonly used clinically and with small variations in the lavage volumes. Bile acid concentrations were measured by mass spectrometry,20 because other techniques, such as enzymatic methods, are less sensitive for low concentrations.26, 27
It is likely that GER and MA occur as a consequence of impairment in protective barriers (including esophageal sphincters, normal esophageal motility, and upper airway reflexes that exclude material from the airways, such as laryngospasm and cough).2 It has been speculated that the detection of MA can be affected by variation in the duration of MA as well as by the frequency, volume, and gastric juice contents in each episode of GER and MA.10 Also, clearance of gastric juice contents from the lung can vary among individuals10, 13 and can decrease because of primary respiratory disease resulting in variable resistance to disease related to MA. Finally, underlying respiratory disease can result in additional challenges to protective barriers and can augment the predisposition to MA.2 Therefore, it remains unclear whether GER and MA are primary causes of respiratory disease or represent factors that aggravate or potentiate lung diseases.
In this study, TBA concentrations were significantly higher in both CIPF and healthy WHWTs compared to Beagles, and no significant differences in TBA concentrations existed between healthy and CIPF WHWT groups. Canine IPF shares many similarities with human IPF, both histopathologically and in clinical features.14, 15, 16 Proximal GER is common in people with IPF,4, 28, 29 and it has been suggested that MA is an etiological factor in IPF leading to direct injury of lung alveolar epithelium, dysregulated wound healing, and finally to lung fibrosis.10, 30 The severity of GER and presence and concentration of gastric content in BALF are positively correlated with the degree of pulmonary fibrosis.4 Bile acids are cytotoxic and induce inflammation and fibrosis, noted both in cell cultures and in experimental aspiration studies in rats.13, 31 The finding that progression of IPF in some human cases can be delayed with anti‐reflux drugs and anti‐reflux surgery that targets reduction of GER,32, 33, 34, 35 supports the hypothesis that MA can be a contributing factor in IPF. The presence of bile acids in BALF in 77% of healthy WHWTs could be an indication that MA is a predisposing factor for development of fibrosis in CIPF. Peribronchiolar accentuation of fibrosis has been described in CIPF WHWTs, which could suggest involvement of inhaled etiological factors16 such as bile acids. Usually CIPF is found in WHWTs at middle to old age,14, 15 but the disease development might have started several years earlier. This could explain why apparently healthy WHWTs, which median age was 2 years lower, also had increased concentrations of TBA in BALF. Two WHWTs in this study that were healthy at the time of BALF sampling and had TBA concentrations in the lowest third of the group, were subsequently diagnosed with CIPF 4 and 6 years after sampling, both at the age of 15 years. Unfortunately, BALF was not available for analysis after the diagnosis had been made to allow further investigation of the role for MA in CIPF.
In the LD group, TBA concentrations were significantly higher compared to Beagles, with values above the detectable level in 68% of the dogs. Idiopathic polyneuropathy, the most common cause of laryngeal paralysis, is related to the presence of both laryngeal and esophageal dysfunction, which can both predispose to aspiration.22, 36 Most of the LD dogs had other simultaneous respiratory tract diseases, including BP and CB. It is interesting to note that 3 of the 4 highest TBA values in LD dogs were from dogs with BP. LD is often found in dogs with cough lacking other obvious neurological deficits and the most typical signs of LD, such as stress related inspiratory effort or stridor.37 Unfortunately, the effect of LD on MA cannot be evaluated further in other groups in which laryngeal function was not examined.
Bile acids were detected in BALF of 62% of CB dogs, and TBA concentrations were significantly higher compared to Beagles. The etiology of CB in dogs is unknown. Disease is characterized by cough and inflammation, similar to its closest corresponding disease in humans, COPD.38 The specific cause and effect relationship between GER and COPD has not been clarified, but it has been noted that GER can increase severity of disease and cause acute exacerbation,39, 40, 41 and this could also occur in canine CB. Further study is needed to evaluate the potential role of MA as a contributor to chronic respiratory disease or even as an etiological factor in development and progression of CB. Interestingly, bile acids were also detected in the other inflammatory disease process diagnosed here (EBP) although there was no specific difference in this proportion compared to healthy Beagle control dogs. Also, there was no correlation between TBA concentrations and BALF neutrophils or eosinophils in these inflammatory conditions.
Almost half of the dogs with BP demonstrated bile acids in BALF although there was no statistical difference compared to control Beagles. The limited number of dogs might have impacted these results, however, detection of bile acids in some cases of BP could support aspiration as 1 possible etiology of pneumonia.
IWHs were all healthy at the time of sampling, and a low concentration of bile acids was detected in BALF of only 1 dog. In a study by Greenwell and Brain,42 IWHs had a high incidence of BP and were suspected to be predisposed to aspiration. Possible predisposing factors for aspiration including abnormal laryngeal and esophageal function were not examined thoroughly in their study.42 Analysis of BALF TBA during times of BP in IWHs could assist in determining the role of aspiration injury in this disease.
We evaluated correlations between TBA and BALF cytology in different dog groups and found strong positive correlations between TBA concentrations and BALF total cell, neutrophil, eosinophil, and lymphocyte counts only in BP group. Airway neutrophilia has been detected in the acute phase of lung injury after gastric fluid aspiration in experimental animal models.12 However, because neutrophilia is the key change in BP dogs' BALF, the noted correlation can be only incidental and refer to the severity of BP. The finding of strong positive correlation between TBA concentrations and hospitalization days indicate that bile acid aspiration increases the severity of disease, either because of aspiration associated with respiratory distress or because bile acids increase the severity of inflammation in BP. In humans, there are limited data available about correlations between MA biomarkers and BALF cytology in corresponding diseases.
Limitations of the study include the small number of dogs in some groups (especially in the healthy Beagles group), the heterogeneity in the LD group, and that laryngeal function was specifically evaluated only in the LD group. Storage time could affect bile acid concentrations, although, we did not find this effect in statistical analysis. Finally, bile acids were commonly present in BALF of healthy WHWTs and whereas we propose that this could reflect a role for MA in the pathogenesis or exacerbation of disease, further studies are required to investigate this hypothesis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the Ethics Committee for Animal Experimentation at Helsinki University, Finland (statements 5B/2008, April 30, 2013, January 2, 2014/2014, and 4/2014) and by the Committee for Experimental Animals of Southern Finland (ESAVI/9116/04.10.07, ESAVI/7383/04.October 7, 2013, ESLH‐2008‐05403/Ym‐23, HY 132‐05). Dog owners provided a written consent for the use of leftover BALF samples (University of California, April 21, 2015 to January12, 2016).
Supporting information
Table S1 The dog breeds in dog groups consisting several breeds.
Table S2 Age, sex and storage time of bronchoalveolar lavage fluid (BALF) samples and their effect on total bile acid concentrations (TBA) in different groups.
Table S3 Correlations between total bile acid (TBA) concentrations of bronchoalveolar lavage fluid (BALF) and arterial blood gas values in dog groups where arterial blood gas analysis were available.
ACKNOWLEDGMENTS
We thank Jouni Junnila from 4Pharma for his contribution to statistical analysis. We also thank laboratory staff for their contribution to laboratory analytics and Laura Parikka for technical assistance. The study was done in the department of Equine and Small Animal Medicine, Faculty of Veterinary Medicine, University of Helsinki. Presented as a poster at the European College of Veterinary Internal medicine—Companion animal, St Julians, Malta, September 2017. Study was supported in part by grants from Finnish Veterinary Foundation and Finnish Foundation of Veterinary Research.
Määttä OLM, Laurila HP, Holopainen S, et al. Reflux aspiration in lungs of dogs with respiratory disease and in healthy West Highland White Terriers. J Vet Intern Med. 2018;32:2074–2081. 10.1111/jvim.15321
Funding information Finnish Foundation of Veterinary Research; Finnish Veterinary Foundation
REFERENCES
- 1. Tsoukalia E, Sifrim D. Investigation of extraesophageal gastroesophageal reflux disease. Ann Gastroenterol. 2013;26:290‐295. [PMC free article] [PubMed] [Google Scholar]
- 2. Houghton LA, Lee AS, Badri H, DeVault KR, Smith JA. Respiratory disease and the oesophagus: reflux, reflexes and microaspiration. Nat Rev Gastroenterol Hepatol. 2016;13:445‐460. [DOI] [PubMed] [Google Scholar]
- 3. Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savarino E, Carbone R, Marabotto E, et al. Gastro‐oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur Respir J. 2013;42:1322‐1331. [DOI] [PubMed] [Google Scholar]
- 5. Perng D‐W, Chang K‐T, Su K‐C, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor‐β1 production and fibroblast proliferation. Chest. 2007;132:1548‐1556. [DOI] [PubMed] [Google Scholar]
- 6. Timms C, Thomas PS, Yates DH. Detection of gastro‐oesophageal reflux disease (GORD) in patients with obstructive lung disease using exhaled breath profiling. J Breath Res. 2012;6:016003. [DOI] [PubMed] [Google Scholar]
- 7. Hunt EB, Ward C, Pover S, et al. The potential role of aspiration in the asthmatic airway. Chest. 2017;151:1272‐1278. [DOI] [PubMed] [Google Scholar]
- 8. Brodlie M, Aseeri A, Lordan JL, et al. Bile acid aspiration in people with cystic fibrosis before and after lung transplantation. Eur Resp J. 2015;46:1820‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee AL, Button BM, Denehy L, et al. Exhaled breath condensate pepsin: potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Resp Care. 2015;60:244‐250. [DOI] [PubMed] [Google Scholar]
- 10. Raghu G, Mayer KC. Silent gastro‐oesophageal reflux and microaspiration in IPF: mounting evidence for anti‐reflux therapy? Eur Respir J. 2012;39:242‐245. [DOI] [PubMed] [Google Scholar]
- 11. Trinick R, Johnston N, Dellzell AM, et al. Reflux aspiration in children with neurodisability—a significant problem, but can we measure it? J Ped Surg. 2012;47:291‐298. [DOI] [PubMed] [Google Scholar]
- 12. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration‐induced lung injury. Crit Care Med. 2011;39:818‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen B, You WJ, Liu XQ, Xue S, Qin H, Jiang HD. Chronic microaspiration of bile acids induces lung fibrosis through multiple mechanisms in rats. Clin Sci. 2017;131:951‐963. [DOI] [PubMed] [Google Scholar]
- 14. Corcoran BM, Cobb M, Martin MW, et al. Chronic pulmonary disease in West Highland White Terriers. Vet Rec. 1999;144:611‐616. [DOI] [PubMed] [Google Scholar]
- 15. Heikkilä HP, Lappalainen AK, Day MJ, Clercx C, Rajamäki MM. Clinical, bronchoscopic, histopathologic, diagnostic imaging, and arterial oxygenation findings in West Highland White Terriers with idiopathic pulmonary fibrosis. J Vet Int Med. 2011;25:433‐439. [DOI] [PubMed] [Google Scholar]
- 16. Syrjä P, Heikkilä HP, Lilja‐Maula L, et al. The histopathology of idiopathic pulmonary fibrosis in west highland white terriers shares features of both non‐specific interstitial pneumonia and usual interstitial pneumonia in man. J Comp Pathol. 2013;149:303‐313. [DOI] [PubMed] [Google Scholar]
- 17. Kogan DA, Johnson LR, Sturges BK, Jandrey KE, Pollard RE. Etiology and clinical outcome in dogs with aspiration pneumonia: 88 cases (2004‐2006). J Am Vet Med Assoc. 2008;233:1748‐1755. [DOI] [PubMed] [Google Scholar]
- 18. Tart KM, Babski DM, Lee JA. Potential risks, prognostic indicators, and treatment modalities affecting survival in dogs with presumptive aspiration pneumonia: 122 cases (2005‐2008). J Vet Emerg Crit Care. 2010;20:319‐329. [DOI] [PubMed] [Google Scholar]
- 19. Viitanen SJ, Laurila HP, Lilja‐Maula LI, Melamies MA, Rantala M, Rajamäki MM. Serum C‐reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J Vet Int Med. 2014;28:84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ, Niemi M. High performance liquid chromatography‐tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Cromatogr. 2010;878:51‐60. [DOI] [PubMed] [Google Scholar]
- 21. Pandit S, Boktor M, Alexander JS, et al. Gastroesophageal reflux disease: a clinical overview for primary care physicians. Pathophysiolgy. 2017. [DOI] [PubMed] [Google Scholar]
- 22. Tarvin KM, Twedt DC, Monnet E. Prospective controlled study of gastroesophageal reflux in dogs with naturally occurring laryngeal paralysis. Vet Sur. 2016;45:916‐921. [DOI] [PubMed] [Google Scholar]
- 23. Shaver SL, Barbur LA, Jimenez DA, et al. Evaluation of Gastroesophageal reflux in anesthetized dogs with brachycephalic syndrome. J Am Anim Hosp Assoc. 2017;53(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 24. Mayhew PD, Marks SL, Pollard R, Culp WTN, Kass PH. Prospective evaluation of surgical management of sliding hiatal hernia and gastroesophageal reflux in dogs. Vet Surg. 2017;46(8):1098‐1109. [DOI] [PubMed] [Google Scholar]
- 25. Munster M, Hoerauf A, Vieth M. Gastro‐oesophageal reflux disease in 20 dogs (2012 to 2014). J Small Anim Pract. 2017;58:276‐283. [DOI] [PubMed] [Google Scholar]
- 26. Knight J, Lively MO, Johnston N, Dettmar PW, Koufman JA. Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Laryngoscope. 2005;115:1473‐1478. [DOI] [PubMed] [Google Scholar]
- 27. Parikh S, Brownlee IA, Robertson AG, et al. Are the enzymatic methods currently being used to measure bronchoalveolar lavage bile salt levels fit for purpose? J Heart Lung Transplant. 2013;32:418‐423. [DOI] [PubMed] [Google Scholar]
- 28. Raghu G, Freudenberger TD, Yang S, et al. High prevalence of gastro‐oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136‐142. [DOI] [PubMed] [Google Scholar]
- 29. Hoppo T, Komatsu Y, Jobe BA. Gastroesophageal reflux disease and patterns of reflux in patients with idiopathic pulmonary fibrosis using hypopharyngeal multichannel intraluminal impedance. Dis Esophagus. 2014;27:530‐537. [DOI] [PubMed] [Google Scholar]
- 30. Lee JS. The role of gastroesophageal reflux and microaspiration in idiopathic pulmonary fibrosis. Clin Pulm Med. 2014;21:81‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aldhahrani A, Verdon B, Ward C, et al. Effects of bile acids on human airway epithelial cells: implications for aerodigestive diseases. Eur Respir J Open Res. 2017;3:00107‐02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end‐stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438‐446. [DOI] [PubMed] [Google Scholar]
- 33. Lee JS, Collard HR, Anstrom KJ, et al. Anti‐acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomized controlled trials. Lancet Respir Med. 2013;1:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghebremariam YT, Cooke JP, Gerhart VV, et al. Pleiotropic effect of the proton pump inhibitor esomepratzole leading to suppression of lung inflammation and fibrosis. J Transl Med. 2015;13:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raghu G, Morrow E, Collins BF, et al. Laparoscopic anti‐reflux surgery for idiopathic pulmonary fibrosis at a single centre. Eur Respir J. 2016;48:826‐832. [DOI] [PubMed] [Google Scholar]
- 36. Stanley BJ, Hauptman JG, Fritz MC, et al. Esophageal dysfunction in dogs with idiopathic laryngeal paralysis: a controlled cohort study. Vet Surg. 2010;39:139‐149. [DOI] [PubMed] [Google Scholar]
- 37. Johnson LR. Laryngeal structure and function in dogs with cough. J Am Vet Med Assoc. 2016;249:195‐201. [DOI] [PubMed] [Google Scholar]
- 38. Williams K, Roman J. Studying human respiratory disease in animals—role of induced and naturally occurring models. J Path. 2016;238:220‐232. [DOI] [PubMed] [Google Scholar]
- 39. Casanova C, Baudet JS, del Valle VM, et al. Increased gastro‐oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23:841‐845. [DOI] [PubMed] [Google Scholar]
- 40. Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastroint Liver Dis. 2010;19:253‐256. [PubMed] [Google Scholar]
- 41. Lin YH, Tsai CL, Chien LN, Chiou HY, Jeng C. Newly diagnosed gastroesophageal reflux disease increased the risk of acute exacerbation of chronic obstructive pulmonary disease during the first year following diagnosis—a nationwide population‐based cohort study. Int J Clin Pract. 2015;69:350‐357. [DOI] [PubMed] [Google Scholar]
- 42. Greenwell CM, Brain PH. Aspiration pneumonia in the Irish wolfhound: a possible breed predisposition. J Small Anim Pract. 2014;55:515‐520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The dog breeds in dog groups consisting several breeds.
Table S2 Age, sex and storage time of bronchoalveolar lavage fluid (BALF) samples and their effect on total bile acid concentrations (TBA) in different groups.
Table S3 Correlations between total bile acid (TBA) concentrations of bronchoalveolar lavage fluid (BALF) and arterial blood gas values in dog groups where arterial blood gas analysis were available.


