Abstract
Background: Chronic kidney disease (CKD) is associated with morbidity and mortality in dogs. Plasma fibroblast growth factor‐23 (FGF‐23) concentration is an independent predictor of CKD progression and survival in cats and people with CKD.
Objectives: To investigate the relationship among FGF‐23, parathyroid hormone (PTH), vitamin D metabolites, and other clinical variables with survival time in dogs with CKD.
Animals: Twenty‐seven azotemic CKD dogs.
Methods: Dogs were recruited prospectively into the study and followed until death or study conclusion. Dogs were International Renal Interest Society (IRIS) staged into stage 2 (n = 9), stage 3 (n = 12), and stage 4 (n = 6) CKD. Survival times were calculated from the date of study inclusion. Univariable Cox regression was used to assess variables associated with survival including body condition score (BCS), muscle condition score, hematocrit, creatinine, CKD stage, serum phosphorus, urine protein:creatinine ratio (UPC), calcium phosphorus product (CaPP), PTH, 25‐hydroxyvitamin D, 1,25‐‐dihydroxyvitamin D, and FGF‐23 concentrations.
Results: Significant hazard ratios (hazard ratio; 95% confidence interval; P value) were as follows: BCS < 4/9 (1.579; 1.003‐2.282; P = .05), muscle atrophy (2.334; 1.352‐4.030; P = .01), increased creatinine (1.383; 1.16‐1.64; .01), hyperphosphatemia (3.20; 1.357‐7.548; P = .005), increased UPC (3.191; 1.310‐7.773; P = .01), increased CaPP (4.092; 1.771‐9.454; P = .003), and increased FGF‐23 (2.609; 1.090‐6.240; P = .05). Survival times for each IRIS CKD stage were significantly different (P = .01).
Conclusions and Clinical Importance: Multiple variables, including FGF‐23, were associated with duration of survival in CKD dogs. FGF‐23 could be a prognostic marker in dogs with CKD.
Keywords: dog, fibroblast growth factor‐23, mortality, prognostic, renal
Abbreviations
- 1,25(OH)2D
1,25‐dihydroxyvitamin D
- 25(OH)D
25‐hydroxyvitamin D
- BCS
body condition score
- CaPP
calcium × phosphorus product
- CKD
chronic kidney disease
- CKD‐MBD
chronic kidney disease‐mineral and bone disorders
- FGF‐23
fibroblast growth factor‐23
- iCa
ionized calcium
- IRIS
International Renal Interest Society
- MCS
muscle condition score
- PTH
parathyroid hormone
- RC
reference cut‐off
- RHPT
renal secondary hyperparathyroidism
- UPC
urine protein:creatinine ratio
1. INTRODUCTION
Chronic kidney disease (CKD) in dogs is characterized by progressive loss of renal function, with a prevalence of up to 25% of dogs in referral institutions.1, 2, 3 Major consequences of CKD include development of renal secondary hyperparathyroidism (RHPT) and CKD‐mineral and bone disorders (CKD‐MBD). The development of RHPT is influenced by complex interactions among ionized calcium (iCa), phosphorus, vitamin D metabolites, parathyroid hormone (PTH), fibroblast growth factor‐23 (FGF‐23), and klotho.
Dogs with CKD have higher plasma FGF‐23 and serum PTH, but lower serum 25‐hydroxyvitamin D (25[OH]D), 1,25‐dihydroxyvitamin D (1,25[OH]2D), and 24,25‐dihydroxyvitamin D (24,25[OH]2D) concentrations than healthy dogs.4, 5 In people and cats with CKD, increased FGF‐23 concentrations are an independent predictor of mortality.6, 7, 8 Additionally, in people, increased PTH and decreased 25(OH)D concentrations are associated with decreased duration of survival.6, 7 Other clinical variables correlated with survival in either people, cats, or dogs with CKD include hematocrit, body condition score (BCS), muscle condition score (MCS), albumin, creatinine, phosphorus, calcium × phosphorus product (CaPP), and urine protein:creatinine (UPC) ratio.9, 10, 11, 12, 13, 14, 15
The primary aim of this study was to determine which markers of CKD‐MBD (eg, FGF‐23, PTH, 25(OH)D, 1,25(OH)2D, calcium, and phosphorus) predicted survival time in dogs with International Renal Interest Society (IRIS) stages 2, 3, and 4 CKD. A secondary aim of this study was to evaluate the relationship between survival and other relevant clinical measures.
2. MATERIALS AND METHODS
Client‐owned dogs diagnosed with CKD at The Ohio State University Veterinary Medical Center between January 2014 and July 2015. A diagnosis of CKD was made based on the presence of at least 2 episodes, over at least 3 months, of minimally concentrated urine (urine specific gravity < 1.030) with stable azotemia in the absence of other diseases likely to cause polyuria or polydipsia. Additional factors used to determine eligibility included the presence of renal proteinuria or ultrasonographic changes consistent with CKD (eg, loss of corticomedullary distinction, contour irregularities, and decreased size).
Based on IRIS staging guidelines, dogs were assigned to either IRIS stage 2 (creatinine 1.4‐2.0 mg/dL), stage 3 (creatinine 2.1‐5.0 mg/dL), or stage 4 (creatinine >5.0 mg/dL). These cases had been included in 2 previous studies.4, 5 They were substaged based on UPC and blood pressure. Dogs were considered proteinuric with a UPC > 0.5. Dogs were considered hypertensive when systolic blood pressure exceeded 150 mm Hg. Dogs were further divided into normophosphatemic or hyperphosphatemic based on cut‐offs from an expert panel suggesting that maintenance of serum phosphorus concentration within the following ranges is optimal management for dogs with CKD: 2.5‐4.5 mg/dL for dogs with stage 2 CKD, 2.5‐5.0 mg/dL for stage 3, and 2.5‐6.0 mg/dL for stage 4.16
Dogs <1 year of age, those diagnosed with concurrent diseases, or those receiving medications known to affect PTH concentrations were excluded. Dogs diagnosed with acute kidney injury or suspected of acute exacerbation of CKD were excluded.
At the time of enrollment, each CKD dog had a complete physical examination performed, including body weight, BCS, and MCS. Doppler systolic blood pressure was measured in all dogs. Blood was collected by jugular venipuncture and urine was collected by cystocentesis for hematology, serum biochemistry, serum iCa concentration, urinalysis, urine culture, and UPC ratio. CBCs were performed with a hematology analyzer (Siemens Advia 2120i), serum chemistry with an automated chemistry system (Roche Cobas C501), and iCa with a chemistry analyzer (Nova pHOX analyzer). All dogs had FGF‐23, PTH, and vitamin D metabolites measured and reported as described previously.4, 5
After inclusion into the study, all dogs were followed serially by phone contact, with either the owner, the primary care veterinarian, or both, until reaching study endpoint (all‐cause death) or conclusion of the observational study period in March of 2017. Treatments, monitoring, and reason for euthanasia were not controlled during the study period and left to the discretion of the attending clinician and owners based on results of individual canine assessments and clinicopathologic results.
3. STATISTICAL ANALYSIS
Data were tested for normality using the Shapiro Wilk test and presented as either mean with SD or median and range. Survival times using all‐cause death were calculated from the date of study enrollment to the date of death or euthanasia. Enrollment was recorded as the date on which the second documentation of necessary inclusion criteria was obtained. Dogs alive at conclusion of the study were censored. Left censoring was not applicable in this study population. Statistical analysis was performed using commercial statistical software (SPSS for Macintosh [SPSS, Chicago, IL]; GraphPad Prism). Statistical significance was set at ≤ .05.
Clinical variables correlated with survival in people, cats or dogs with CKD, including hematocrit, 25(OH)D, BCS, MCS, albumin, creatinine, phosphorus, CaPP, PTH, FGF‐23, and UPC were included in analyses. Kaplan‐Meier time‐to‐event curves were generated for all dogs with each variable of interest. In the analysis, variables were categorized based on concentrations above and below cut‐off points. For BCS, dogs were allotted to one of the 3 groups: underweight (BCS 1‐3), moderate weight (BCS 4‐6), or overweight. (BCS 7‐9). Decreased MCS included all cases of mild, moderate, and severe atrophy. Cut‐off points were based on biologically relevant cut points or results generated from healthy dogs for this study.4, 5 Log‐rank test was used to determine if the survival curves were significantly different. Adjusted risk estimates for all‐cause mortality were calculated using univariable Cox regression.
4. RESULTS
Twenty‐seven dogs were included in the survival analysis. Breeds included mixed breed dogs (n = 9), Labrador Retrievers (n = 2), Cocker Spaniels (n = 2), Shetland Sheepdogs (n = 2), and one of each of the following: Boxer, Fox Terrier, German Shepherd Dog, Greyhound, Jack Russell Terrier, Miniature Schnauzer, Pekingese, Pomeranian, Shih Tzu, Viszla, and Whippet. Thirteen dogs were females (all spayed) and 14 dogs were males (1 entire, 13 neutered).
Complete descriptive data have been previously published on this study population.4, 5 Variables of interest that were used in the survival analysis are reported in Table 1. The median age at diagnosis was 9.5 [3.2, 15.7] years. Twenty‐three dogs (85%) had died or were euthanized at or before the end of the follow‐up period in March 2017. Total population median survival time was 11.2 [0.32, 38.35] months from time of enrollment. Median survival time of IRIS CKD stages 2, 3, and 4 were: 14.78 [9.14, 37.39], 11.14 [0.46, 38.35], and 1.98 [0.32, 12.78] months, respectively, from time of enrollment. The 4 dogs right censored in the study included 3 dogs with stage 2 and 1 dog with stage 3 CKD, which were nonproteinuric, nonhypertensive, and normophosphatemic at the time of study enrollment. The FGF‐23 concentrations of the 4 right censored dogs were 152, 257, 273, and 340 pg/mL. No dogs were lost to follow‐up.
Table 1.
Laboratory results from variables included in survival analyses
| All dogs | CKD stage 2 | CKD stage 3 | CKD stage 4 | |
|---|---|---|---|---|
| Number of dogs | 27 | 9 | 12 | 6 |
| Hematocrit (%)a | 39 (23, 61) | 45 (35, 61) | 36.5 (24, 47) | 36.5 (23, 42) |
| UPC (mg2/dL2)a | 0.7 (0.1, 9.6) | 0.2 (0.1, 1.7) | 1.05 (0.1, 9.2) | 1.5 (0.7, 4.7) |
| Creatinine (mg/dL)a | 2.6 (1.4, 12.9) | 1.7 (1.4, 2.0) | 3.25 (2.4, 4.8) | 8.2 (6.3, 12.9) |
| Phosporus (mg/dL)a | 4.8 (1.6, 14.4) | 3.7 (1.6, 7.3) | 4.9 (2.7, 6.6) | 10.6 (8.1, 11.8) |
| 25(OH)D (ng/mL)a | 44.5 (3.5, 95.8) | 55.7 (34.5, 93.5) | 42.7 (3.5, 95.8) | 25.0 (5.7, 52.5) |
| 1,25(OH)D (pg/mL)a | 106.1 (19, 286) | 143.2 (96.4, 286.0) | 104.8 (29.2, 228.7) | 64.7 (19.0, 91.1) |
| PTH (pmol/L)a | 2.85 (1.2, 155.5) | 1.7 (1.2, 3.4) | 3.6 (1.6, 14.2) | 23.2 (5.1, 155.5) |
| FGF‐23 (pg/mL)a | 2112 (142, 41 265) | 336 (142, 704) | 2301 (455, 24 409) | 7732.5 (2520, 41 265) |
| tCa (mg/dL)a | 11 (8.1, 13) | 10.8 (10.3, 13) | 11.1 (9.4, 12.2) | 10.6 (8.1, 11.8) |
| iCa (mg/dL)a | 5.22 (4.03, 6.02) | 5.17 (5.0, 5.36) | 5.17 (4.88, 5.58) | 5.3 (4.03, 6.02) |
| CaPP (mg2/dL2)a | 54.5 (17.3, 133.9) | 39.1 (17.3, 94.9) | 54.5 (29.7, 75.6) | 95 (44.6, 133.9) |
| BCSa | 5 (2, 8) | 5 (3, 8) | 5 (3, 8) | 5 (2, 7) |
| MCS | Normal (normal, severe) | Normal (normal, mild) | Normal (normal, severe) | Mild (normal, severe) |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; 1,25(OH)D, 1,25‐hydroxyvitamin D; BCS, body condition score; CaPP, calcium × phosphorus product; CKD, chronic kidney disease; FGF‐23, fibroblast growth factor‐23; iCa, ionized calcium; MCS, muscle condition score; PTH, parathyroid hormone; tCa, total calcium; UPC, urine protein:creatinine ratio.
All data reported as median (range).
For Kaplan‐Meier and Cox proportional hazards regression analysis, reference cut‐off points were determined by IRIS guidelines (creatinine, phosphorus, and UPC), institutional reference ranges (hematocrit, total calcium [tCa], and iCa), as well as biologically relevant values from a control population of dogs for the remaining variables.4, 5 Reference cut‐off (RC) values are presented in Table 2.
Table 2.
Hazard ratios for hematocrit, UPC, phosphorus, 25(OH)D, 1,25(OH)D, PTH, FGF‐23, CaPP, iCa, tCa, albumin, BCS, and MCS
| Reference cut‐off | Units | Median survival below RC (months) | Median survival above RC (months) | Hazard ratio | 95% CI of hazard ratio | P value | |
|---|---|---|---|---|---|---|---|
| Hematocrit | 36 | % | 10.71 | 12.35 | 0.96771 | 0.414 to 2.259 | .9 |
| UPC | 0.5 | mg2/dL2 | 19.2 | 8.769 | 3.191 | 1.310 to 7.773 | .01 |
| Phosphorus | 4.5/5.0/6.0 | mg/dL | 19.2 | 6 | 3.2 | 1.357 to 7.548 | .005 |
| 25(OH)D | 50.4 | ng/mL | 10.02 | 13.64 | 0.7343 | 0.3179 to 1.697 | .15 |
| 1,25(OH)D | 169 | pg/mL | 10.36 | 13.7 | 0.7565 | 0.2574 to 2.224 | .25 |
| PTH | 1.8 | pmol/L | 10.92 | 11.17 | 0.9773 | 0.3307 to 2.888 | .45 |
| FGF‐23 | 450 | pg/mL | 26.08 | 10.29 | 2.609 | 1.09 to 6.24 | .05 |
| CaPP | 70 | mg2/dL2 | 12.84 | 3.13 | 4.092 | 1.771 to 9.454 | .003 |
| iCa | 4.9 | mg/dL | 1.546 | 11.04 | 1.045 | 0.2477 to 4.404 | .95 |
| tCa | 9.3 | mg/dL | 1.108 | 11.17 | 10.08 | 2.364 to 43 | .001 |
| Alb | 2.9 | mg/dL | 3.138 | 11.48 | 28.38 | 2.621 to 307.3 | .005 |
| BCS | 4 | NA | 3.393 | 11.964 | 1.579 | 1.003 to 2.282 | .05 |
| MCS | Abnormal | NA | 7.615 | 12.78 | 2.334 | 1.352 to 4.030 | .01 |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; 1,25(OH)D, 1,25‐hydroxyvitamin D; Alb, albumin; BCS, body condition score; CaPP, calcium × phosphorus product; CKD, chronic kidney disease; FGF‐23, fibroblast growth factor‐23; iCa, ionized calcium; MCS, muscle condition score; PTH, parathyroid hormone; RC, reference cut‐off; tCa, total calcium; UPC, urine protein:creatinine ratio.
Kaplan‐Meier survival curves were significantly different based on increased plasma FGF‐23 concentration (P < .05), total hypocalcemia (P = .001), hypoalbuminemia (P < .01), increasing CKD stage (P = .01), hyperphosphatemia (P = .05), increased CaPP >70 (P = .003), and proteinuria (P = .01; Figures 1 and 3). Decreased BCS (P = .05) and MCS (P = .01) were both significantly associated with reduced survival (Figure 2). Dogs had significantly increased case fatality rate (P = .01) with each increase in IRIS stage.
Figure 1.
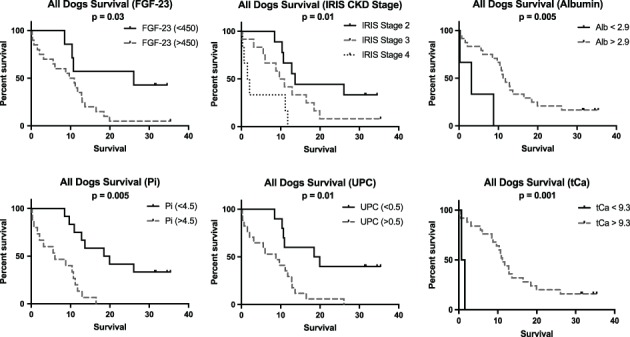
Kaplan‐Meier survival curves based on increased fibroblast growth factor concentration, advancing International Renal Interest Society chronic kidney disease stage, hypoalbuminemia, increased phosphorus, proteinuria, and increased total calcium
Figure 3.

Kaplan‐Meier survival curves based on decreased hematocrit, ionized hypocalcemia, decreased 25‐hydroxyvitamin D concentration, decreased 1,25‐dihydroxyvitamin D concentration, increased parathyroid hormone concentration, and increased calcium × phosphorus product above 70
Figure 2.
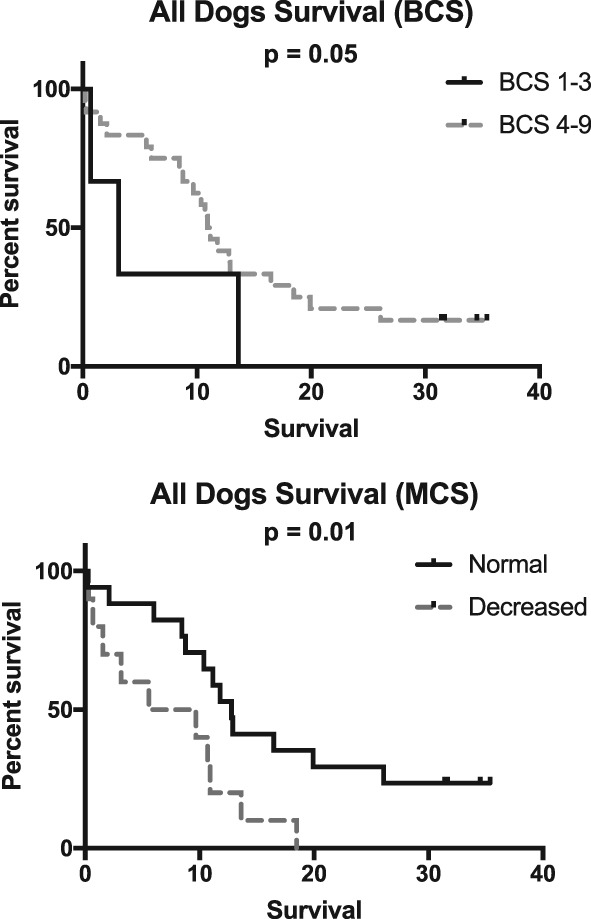
Kaplan‐Meier survival curves based on body condition score and muscle condition score
Decreased hematocrit (P = .94), ionized hypocalcemia (P = .72), decreased 25(OH)D concentration (P = .73), decreased 1,25(OH)2D concentration (P = .44), or increased PTH concentration (P = .21) were not associated with survival (Figure 3).
Univariable Cox regressions analysis revealed dogs with increased FGF‐23, increased creatinine, hypoalbuminemia, decreased total calcium, hyperphosphatemia, and proteinuria had an increased risk of death at any given time during study time period. As compared to dogs with stages 3 and 4 CKD, dogs with stage 2 CKD had a hazard ratio of 0.346 (95% CI: 0.119 to 0.999, P = .05) and 0.16 (95% CI: 0.046 to 0.552, P < .01), respectively. Using creatinine as a continuous variable, increased creatinine was associated with a hazard ratio of 1.383 (95% CI: 1.166 to 1.640, P = .001). Other variables of interest, including hematocrit, iCa, albumin, 25(OH)D, 1,25(OH)D, and PTH concentrations did not result in significant hazard ratios in this study. Hazard ratios for these variables are reported in Table 2.
5. DISCUSSION
This study examined variables, including plasma FGF‐23, that were associated with survival time in dogs with azotemic CKD. Increased plasma FGF‐23 concentration, proteinuria, hyperphosphatemia, total hypocalcemia, hypoalbuminemia, decreased BCS, decreased MCS, increased CaPP, and advanced CKD stage were all associated with being a risk factor for all‐cause death in a population of dogs with azotemic CKD. These results are similar to findings in a study of CKD in cats.8
None of the other variables examined, including hematocrit, iCa, serum PTH, 25(OH)D, and 1,25(OH)2D concentrations were found to be associated with survival time using all‐cause death. This is in contrast to other reports where these factors have been associated with a worse prognosis in people with CKD.6, 7, 10, 13
The univariable analysis is consistent with previous reports in cats and people.17, 18, 19, 20, 21 Importantly, this is the first report of an association between FGF‐23 and all‐cause death in dogs. It has been proposed that FGF‐23 is a good marker of CKD‐MBD; however, meta‐analyses in human medicine have shown poor correlation between other markers of CKD‐MBD and mortality.22 Furthermore, multiple studies have also demonstrated a stronger relationship with mortality between FGF‐23 than other markers of CKD‐MBD.23, 24, 25 This combined data implies there might be an association between FGF‐23 and death that extends beyond dysregulation in CKD‐MBD. In people, there is a strong correlation between FGF‐23 and cardiovascular death, but this has not been extensively studied in veterinary medicine.23 Another explanation is that FGF‐23 is a marker of reduced GFR and advanced CKD. Thereby, the increases in FGF‐23 levels are an indicator of advancement and progression of CKD.
Lastly, there is limited evidence that FGF‐23 might have a directly toxic effect on the body that might affect survival.24, 26 However, this is a preliminary finding and has yet to be substantiated with additional studies and thus requires further evaluation. Further studies should be conducted to determine the pathophysiologic relationship among FGF‐23, direct toxicosis, and case outcomes. As more information is ascertained from the relationship between FGF‐23 and survival, this will eventually allow for better application of the survival data described here in a clinical setting and in other studies, as well as determine the feasibility of FGF‐23 as a disease marker.
In this study, a thin BCS and muscle atrophy at the time of diagnosis were significantly associated with shorter survival. This is similar to results from studies of people and dogs with CKD.9, 27, 28, 29 Hypoalbuminemia also was significantly associated with survival, similar to people with CKD and previous reports of dogs.9, 29, 30, 31 Similarly, proteinuria, CaPP, and hyperphosphatemia were associated with greater hazard ratios and decreased survival, related to mechanisms described elsewhere.12, 13, 14, 32, 33, 34, 35, 36 PTH, iCa and vitamin D metabolites were not associated with survival in this study, contrary to previous reports for some of these variables in dogs and humans.6, 7, 13 Importantly, when analyzing the data on non‐significant variables in this study, it is useful to look at the frequency at which dogs were outside the established reference ranges. In each of these categories, only a few dogs were outside the cut‐off points for reference ranges. As a result, the statistical analysis was not robust enough to detect potential differences in the groups based on these variables. A larger study should be conducted with more dogs with each of these abnormalities to assess their association with survival.
There were a number of limitations in this study. Dogs were enrolled in various stages of the disease as they were presented to the teaching hospital. All‐cause mortality was used in this survival analysis, and as the majority of dogs in this study were euthanized, there may have been reasons for euthanasia unrelated to CKD, which can bias the analysis. Moreover, treatment decisions were left to the discretion of the owner, attending clinician, as well as dog receptiveness in each individual case. Both treatment decisions and euthanasia are personal decisions and can be biased by individual dog factors, owner factors, and clinician preferences. This study was not appropriately powered for a multiple variable analysis.
In conclusion, increased FGF‐23 concentration was associated with an increased risk of premature death in dogs with azotemic CKD as well as previously identified prognostic markers of proteinuria, hyperphosphatemia, advanced CKD stage and body composition. Future studies are necessary to determine the role of FGF‐23 as a marker of disease severity versus mediator of disease progression in dogs with CKD and how it relates to prognosis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the IACUC at The Ohio State University. The study was also approved through the College of Veterinary Medicine CRC committee.
ACKNOWLEDGMENT
Presented in oral abstract form at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
Rudinsky AJ, Harjes LM, Byron J, et al. Factors associated with survival in dogs with chronic kidney disease. J Vet Intern Med. 2018;32:1864–1873. 10.1111/jvim.15322
REFERENCES
- 1. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336‐1341. [PubMed] [Google Scholar]
- 2. Bartlett PC, Van Buren JW, Neterer M, et al. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med. 2010;94:264‐271. [DOI] [PubMed] [Google Scholar]
- 3. Pelander L, Ljungvall I, Egenvall Aet al. Incidence of and mortality from kidney disease in over 600,000 insured Swedish dogs. Vet Rec. 2015; 176:656. [DOI] [PubMed] [Google Scholar]
- 4. Harjes LM, Parker VJ, Dembek K, et al. Fibroblast growth Factor‐23 concentration in dogs with chronic kidney disease. J Vet Intern Med. 2017;31:784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parker VJ, Harjes LM, Dembek K, Young GS, Chew DJ, Toribio RE. Association of Vitamin D Metabolites with parathyroid hormone, fibroblast growth Factor‐23, calcium, and phosphorus in dogs with various stages of chronic kidney disease. J Vet Intern Med. 2017;31:791‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pilz S, Tomaschitz A, Friedl C, et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603‐3609. [DOI] [PubMed] [Google Scholar]
- 7. Shardlow A, McIntyre NJ, Fluck RJ, et al. Associations of fibroblast growth factor 23, vitamin D and parathyroid hormone with 5‐year outcomes in a prospective primary care cohort of people with chronic kidney disease stage 3. BMJ Open. 2017;7:e016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geddes RF, Elliott J, Syme HM. Relationship between plasma fibroblast growth Factor‐23 concentration and survival time in cats with chronic kidney disease. J Vet Intern Med. 2015;29:1494‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med. 2011;25:1306‐1311. [DOI] [PubMed] [Google Scholar]
- 10. Sato Y, Fujimoto S, Konta T, et al. Anemia as a risk factor for all‐cause mortality: obscure synergic effect of chronic kidney disease. Clin Exp Nephrol. 2018;22:388‐394. [DOI] [PubMed] [Google Scholar]
- 11. Lin TY, Peng CH, Hung SC, et al. Body composition is associated with clinical outcomes in patients with non‐dialysis‐dependent chronic kidney disease. Kidney Int. 2018;93:733‐740. [DOI] [PubMed] [Google Scholar]
- 12. Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000‐2002). J Vet Intern Med. 2008;22:1111‐1117. [DOI] [PubMed] [Google Scholar]
- 13. Lippi I, Guidi G, Marchetti V, Tognetti R, Meucci V. Prognostic role of the product of serum calcium and phosphorus concentrations in dogs with chronic kidney disease: 31 cases (2008‐2010). J Am Vet Med Assoc. 2014;245:1135‐1140. [DOI] [PubMed] [Google Scholar]
- 14. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. 2005;226:393‐400. [DOI] [PubMed] [Google Scholar]
- 15. Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med. 2006;20:528‐535. [DOI] [PubMed] [Google Scholar]
- 16. Vetoquinol . Phosphatemia Management in the Treatment of Chronic Kidney Disease, a Roundtable Discussion. Vetoquinol Academia; 2006. [Google Scholar]
- 17. Jialal I, Camacho F, Nathoo B, Tam P, Pahwa R, Wu GG. Fibroblast growth factor 23 predicts mortality and end‐stage renal disease in a Canadian Asian population with chronic kidney disease. Nephron. 2017;137:190‐196. [DOI] [PubMed] [Google Scholar]
- 18. Finch NC, Geddes RF, Syme HM, Elliott J. Fibroblast growth factor 23 (FGF‐23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med. 2013;27:227‐233. [DOI] [PubMed] [Google Scholar]
- 19. Geddes RF, Finch NC, Elliott J, Syme HM. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med. 2013;27:234‐241. [DOI] [PubMed] [Google Scholar]
- 20. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end‐stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432‐2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kendrick J, Cheung AK, Kaufman JS, et al. FGF‐23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta‐analysis. JAMA. 2011;305:1119‐1127. [DOI] [PubMed] [Google Scholar]
- 23. Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)‐23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792‐2796. [DOI] [PubMed] [Google Scholar]
- 25. Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22:956‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juppner H, Wolf M, Salusky IB. FGF‐23: more than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25:2091‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13:136‐143. [DOI] [PubMed] [Google Scholar]
- 28. Kovesdy CP, Anderson JE, Kalantar‐Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49:581‐591. [DOI] [PubMed] [Google Scholar]
- 29. Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight‐for‐height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136‐1148. [DOI] [PubMed] [Google Scholar]
- 30. Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458‐482. [DOI] [PubMed] [Google Scholar]
- 31. Kovesdy CP, George SM, Anderson JE, Kalantar‐Zadeh K. Outcome predictability of biomarkers of protein‐energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:108‐116. [DOI] [PubMed] [Google Scholar]
- 33. Elliott J, Barber PJ. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract. 1998;39:78‐85. [DOI] [PubMed] [Google Scholar]
- 34. Kuwahara Y, Ohba Y, Kitoh K, Kuwahara N, Kitagawa H. Association of laboratory data and death within one month in cats with chronic renal failure. J Small Anim Pract. 2006;47:446‐450. [DOI] [PubMed] [Google Scholar]
- 35. Finco DR, Brown SA, Crowell WA, Duncan RJ, Barsanti JA, Bennett SE. Effects of dietary phosphorus and protein in dogs with chronic renal failure. Am J Vet Res. 1992;53:2264‐2271. [PubMed] [Google Scholar]
- 36. Brown SA, Crowell WA, Barsanti JA, White JV, Finco DR. Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J Am Soc Nephrol. 1991;1:1169‐1179. [DOI] [PubMed] [Google Scholar]


