Abstract
Background
Dietary interventions are thought to modify gut microbial communities in healthy individuals. In dogs with chronic enteropathies, resolution of dysbiosis, along with remission of clinical signs, is expected with treatment.
Hypothesis/Objective
To evaluate changes in the fecal microbiota in dogs with food‐responsive chronic enteropathy (FRE) and in healthy control (HC) dogs before and after an elimination dietary trial with an animal protein‐free diet (APFD).
Animals
Dogs with FRE (n = 10) and HC (n = 14).
Methods
Dogs were fed the APFD for 60 days. Fecal microbiota was analyzed by Illumina 16S rRNA sequencing and quantitative polymerase chain reaction (PCR).
Results
A significantly lower bacterial alpha‐diversity was observed in dogs with FRE compared with HC dogs at baseline, and compared with FRE dogs after the trial. Distinct microbial communities were observed in dogs with FRE at baseline compared with HC dogs at baseline and compared with dogs with FRE after the trial. Microbial communities still were different in FRE dogs after the trial compared with HC dogs at baseline. In HC dogs, the fecal microbiota did not show a significant modification after administration of the APFD.
Conclusion and Clinical Importance
Our results suggest that, in FRE dogs, treatment with the APFD led to a partial recovery of the fecal microbiota by significantly increasing microbiota richness, which was significantly closer to a healthy microbiota after the treatment. In contrast, no changes were detected in the fecal microbiota of HC dogs fed the same APFD.
Keywords: canine chronic enteropathies, dysbiosis, food‐responsive chronic enteropathy, microbiota
Abbreviations
- ANOSIM
analysis of similarity
- BCS
body condition score
- CE
chronic enteropathy
- CIBDAI
canine inflammatory bowel disease activity index
- FRE
food‐responsive chronic enteropathy
- FS
fecal score
- GI
gastrointestinal
- HC
healthy control
- IBD
inflammatory bowel disease
- LDA
linear discriminant analysis
- LEfSe
linear discriminant analysis effect size
- qPCR
quantitative PCR
1. INTRODUCTION
Food‐responsive enteropathy (FRE) is a phenotype of inflammatory chronic enteropathy (CE) that typically responds to an elimination dietary trial.1, 2 Typically, FRE dogs are young and frequently show signs of large bowel disease.2, 3 Treatment of FRE is based on a feeding trial with an elimination diet containing a “novel protein” or a hydrolyzed diet.4, 5, 6
In most cases, many different types of food have been provided to dogs over the years. Therefore, it is not easy to identify all of the protein sources with which the dog has come into contact.4, 7 Moreover, some commercial petfood diets, even those specified for an elimination dietary trial, contain animal proteins other than those declared in the ingredient list.8, 9 Vegetable proteins can be found in traces in pet food, but are less likely to cause allergic reactions.7 Therefore, a balanced diet, containing exclusively proteins of vegetable origin, could be an alternative elimination diet for dogs with FRE.
In recent years, the gastrointestinal (GI) microbiota has been subject of considerable interest because of its potential etiopathological role in host health and disease.10, 11, 12, 13, 14 Studies in veterinary species have associated intestinal dysbiosis with various GI disorders, such as acute diarrhea, CE (eg, inflammatory bowel disease [IBD]), granulomatous colitis and colorectal polyps.12, 15, 16, 17, 18 Although a relationship exists between the gut microbiota and these diseases, it is unclear, in most cases, if alterations in the microbiota are a cause or an effect of the disease and whether manipulation of the gut microbiota could help to prevent or even treat the disease.13, 19
Many factors can alter GI microbial communities, such as infection, inflammation, diet and medication.13, 20 Studies have shown that the amount and type of food, feeding frequency and diet composition have impact on GI function and GI microbiota.21, 22, 23, 24, 25, 26, 27 However, most of these studies used only healthy research dogs and markedly different diets (with respect to macronutrients such as protein, fat or fiber). Limited information is available about the effects of normal balanced animal protein‐free diets (APFD) on the GI microbiota of healthy dogs or dogs with FRE.
Consequently, the aim of our study was to evaluate changes in fecal microbiota in dogs with FRE before and after an elimination dietary trial with an APFD. The same APFD trial was carried out in healthy control (HC) dogs to evaluate changes in fecal microbiota before and after the trial and to compare them to FRE dogs.
2. MATERIALS AND METHODS
2.1. Diet
Dogs were fed a specifically formulated dry food (provided by Effeffe Pet Food S.p.A., Italy) based on the following ingredients: corn, corn gluten meal, potato protein, animal purified fat, mineral salts, linen seed, fish oil, sunflower oil, beet pulp, dried yeasts, chicory pulp, fructo‐oligosaccharides, and Yucca schidigera. The complete ingredient list is available as Supporting Information material (see 1).
2.2. Inclusion and exclusion criteria
Diseased dogs were included if they had a history of chronic GI signs with a duration >3 weeks. Compatible GI signs were diarrhea, vomiting, weight loss, decreased appetite, or some combination of these signs. Only dogs >1 year of age were included, because the APFD was formulated to meet the nutritional requirements of adult dogs. The presence of CE was suspected based on previously established criteria1, 6: no parasite infection based on negative fecal parasitology examination (performed by direct smear and zinc sulfate flotation and centrifugation) with or without treatment with fenbendazole (50 mg/kg q24h for 5 days) and no clinically relevant abnormalities on routine hematology or serum biochemistry. Further examinations were performed at discretion of the attending clinicians to exclude other causes of chronic GI signs. Dogs with CE included in the study were fed exclusively with the APFD. Dogs that responded to the dietary trial without relapse were defined as having FRE and were enrolled in the study. A positive response to the treatment was defined as clinical improvement of GI signs (Canine Inflammatory Bowel Disease Activity Index [CIBDAI]28 score, 0–3 point; clinically not relevant) with a normal Fecal Score (FS; 7‐point Nestlè Purina Fecal Scoring System; FS < 6)29 after the dietary trial.
Exclusion criteria for CE dogs were previous administration of an elimination diet, and treatment with antimicrobials, corticosteroids, nonsteroid anti‐inflammatory drugs or some combination of these up to 3 weeks before presentation, presence of concurrent diseases or both. Lastly, dogs that failed to respond to the dietary trial and required other therapies (eg, tylosin, corticosteroids), dogs that refused to eat, and dogs that were not fed the diet exclusively were excluded.
Healthy control dogs from volunteer owners, were included if they had normal physical examination findings, and no history of acute, chronic or episodic GI signs in the last 3 weeks. The HC dogs were exclusively fed the APFD and were excluded if they refused the diet or if the owners did not feed the diet exclusively.
2.3. Study design and fecal sample collection
The HC and FRE dogs were clinically evaluated at the first visit (baseline), at which time clinical score indices were calculated (CIBDAI, FS and 9‐point body condition score chart [BCS]).30 Both FRE and HC dogs started receiving the elimination APFD from baseline (day 0). Dogs were re‐evaluated at day 30 or 60 or both, and CIBDAI, FS, and BCS were calculated at each visit.
Owners were asked to collect naturally voided fecal samples for microbiota analysis. The samples were frozen (–20°C) ≤20 minutes after evacuation and brought frozen to the laboratory where they were stored at −20°C until analyzed. Fecal samples in FRE and HC dogs were collected at baseline and after 30 or 60 days or both of the diet trial; the most recent fecal sample was used for analysis.
2.4. Analysis
2.4.1. DNA extraction
An aliquot from each fecal sample (∼100 mg of feces) was used for DNA extraction, according to the manufacturer's protocol (Power Soil, Mo Bio, Carlsbad, California).
2.4.2. Illumina sequencing
Amplification and sequencing of the V4 variable region 16S rRNA gene was performed as previously reported.16 Raw sequence data were screened, trimmed, denoised, chimera‐depleted and filtered using QIIME pipeline version 1.8.0.31 Sequence data were uploaded into the Sequence Read Archive of the National Centre for Biotechnology Information (NCBI), GenBank database, under submission number SRP 101454.
The sequencing data was used to calculate a microbiota index, using a previously described mathematical model, and compared between the different animal groups.32
2.4.3. Quantitative polymerase chain reaction (qPCR)
Selected bacterial groups (Universal, Faecalibacterium spp., Turicibacter spp., Streptococcus spp., E. coli, Blautia spp., Fusobacterium spp., Clostridium Hiranonis)33 in the fecal microbiota were analyzed by qPCR assays, as described previously for canine fecal samples.18, 34
The qPCR assays were performed to confirm the pyrosequencing results or quantitate bacterial groups or both that typically are present at very low abundance or under‐represented in 16S rRNA gene sequencing data, based on our experience from previous studies.16, 35, 36, 37
2.5. Statistical analysis
All statistical analyses were performed using commercially available software (Prism version 7.0, Graph Pad Software). Assessment of data for normality was calculated by applying the D'Agostino‐Pearson test. Data were expressed as frequency or median and range (minimum and maximum); significance was set at P < .05.
To evaluate if any differences in signalment were present between FRE and HC dogs, with age, sex, breed, and body weight being compared using Mann Whitney's U test.
In FRE dogs, clinical improvement was evaluated by comparing clinical scores (CIBDAI, FS, and BCS) before and after the dietary trial using the Wilcoxon matched‐pairs signed rank test.
Differences in bacterial abundances (detected α‐rarefaction data and qPCR) between the HC and FRE groups at baseline and after the dietary trial were evaluated using Mann‐Whitney's U test. Fecal samples collected before and after the dietary trial within each group (FRE and HC) were compared using a Wilcoxon matched‐pairs signed rank test.
To evaluate differences in overall microbiota composition (β‐diversity) between the groups, analysis of similarities (ANOSIM) was performed on the weighted and unweighted UniFrac distance matrices.
An unweighted UniFrac calculation assigns equal importance to rare and common bacteria within a sample and is similar to a presence/absence type of analysis. UniFrac can be weighted based on the percent abundance of bacteria within a sample. A weighted UniFrac calculation gives a higher importance to the most abundant bacteria in the sample.38, 39
Linear discriminant analysis (LDA) effect size (LEfSe)40 was used to evaluate differentially abundant bacterial taxa between the animal groups.
For the evaluation of bacterial abundances, all qPCR data were adjusted for multiple comparisons using a Bonferroni correction; an adjusted P < .05 was considered significant.
3. RESULTS
3.1. Animals included and excluded
A total of 58 possible cases were identified and recruited. Of those, 10 fulfilled the inclusion criteria and had a positive response to the treatment; they therefore were enrolled in the study. Twenty‐two healthy dogs met the inclusion criteria. Of these, 14 were enrolled in the study. The drop‐in/drop‐off flowchart is available as Supporting Information material (2).
3.2. Clinical and clinicopathological data
Of the 10 FRE dogs included in the study, 6 were intact males, 3 were females and 1 was a spayed female. One FRE dog was mixed breed and the other 9 dogs were pure breeds (1 each of the following: Chihuahua, German Shepherd Dog, Golden Retriever, Labrador Retriever, Cane Corso, Broholmer, Greater Swiss Mountain Dog, Boxer, and Dogue de Bordeaux).
Fecal parasitological examinations were performed in 9 dogs with negative results; 1 dog only was treated with fenbendazole, 4/10 dogs had both parasitological examination and treatment. All FRE dogs had hematology and serum biochemistry tests performed. Other specific serum analyses were required for 8 dogs, and abdominal ultrasonography was requested for 6 dogs. Major clinicopathological and abdominal ultrasound findings are provided as Supporting Information material (see 3 and 4).
Clinical score indices improved significantly from baseline to after the APFD trial in dogs with FRE, as reported in the Supporting Information material (5).
The HC dogs included in the study were as follows: 1 intact male, 2 castrated males, 4 females and 7 spayed females. Four dogs were mixed breed, 4 were Golden Retrievers and the remaining dogs were 1 each of Newfoundland, Bernese Mountain Dog, Border Collie, Beagle, Australian Shepherd Dog, and English Setter. Median clinical scoring indices were normal in all dogs at baseline and remained unchanged after the APFD trial (Supporting Information 5).
At baseline, no significant differences were found between HC and FRE dogs in terms of age, breed, body weight, and BCS.
3.3. Sequence analysis
Illumina analysis was performed on 48 fecal samples (20 from FRE dogs and 28 from HC dogs); total count yielded 5 802 628 quality sequences, with an average of 119 140 sequences per sample (range, 55,888‐243,612). To account for unequal sequencing depth across samples, subsequent analysis was performed on a randomly selected subset of 55,880 sequences per sample. A total of 14 phyla and 285 genera were identified.
3.4. Diversity metrics
The PCoA plots showed significant differences in β‐diversity between the fecal samples of FRE dogs at baseline and after the dietary trial, between FRE and HC dogs at baseline and between FRE after the dietary trial and HC at baseline (weighted UniFrac distances, ANOSIM; r = 0.14 and P = .032; r = 0.389 and P = .001; r = 0.288 and P = .001, respectively; Figure 1A and Table 1). The PCoA plots demonstrated similarity in β‐diversity between the fecal samples of HC before and after the trial (weighted UniFrac distances, ANOSIM; r = 0.094 and P = .023; Figure 1A and Table 1). The results of the ANOSIM of unweighted UniFrac distances are presented in Table 1 and Figure 1B.
Figure 1.
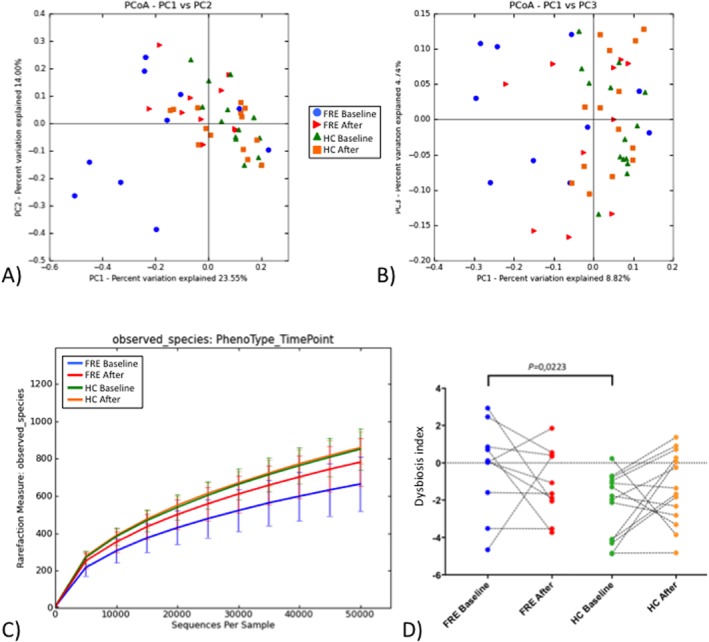
Bacterial diversity measures and microbiota index. Principal finding of sequencing analysis PCoA plot representing beta diversity of microbial communities, based on weighted (A) and unweighted (B) UniFrac distance matrices. Analysis of similarity between groups showed significant differences between FRE dogs at baseline compared to FRE dogs after the dietary trial and HC dogs (more detailed data available in Table 1). C, α‐rarefaction curves of different groups as determined by observed species. Differences were found between FRE and HC dogs at baseline and between FRE dogs before and after the treatment (see Table 2 for more details). D, 16S rRNA sequence‐based microbiota index calculated based on abundance of specific bacterial taxa. Paired samples are connected with lines. Significant differences were found between FRE and HC dogs at baseline. FRE, food‐responsive enteropathy: HC, healthy control; Baseline, time of first visit; After, after 30 or 60 days of APFD
Table 1.
Beta diversity metrics
| Unweighted analysis | Weighted analysis | |||
|---|---|---|---|---|
| Group | R‐Statistic | P value | R‐Statistic | P value |
| FRE Baseline versus HC Baseline | 0.275 | 0.002 | 0.389 | .001 |
| FRE Baseline versus FRE After | 0.118 | 0.066 | 0.14 | .032 |
| HC Baseline versus HC After | 0.013 | 0.632 | 0.094 | .023 |
| FRE After versus HC Baseline | 0.151 | 0.03 | 0.288 | .001 |
Results of beta diversity metrics are reported, significant differences are in bold.
Abbreviations: Baseline, T0, before dietary trial with APFD; FRE, food‐responsive enteropathy dog; HC, healthy control; After, after 30 or 60 days of dietary trial with APFD.
The α‐diversity was used to determine the taxonomic diversity with regard to species richness and evenness in the animal groups (Figure 1C). Samples from FRE dogs at baseline showed significant differences compared with HC dogs at baseline for Chao1 metric, number of observed species, Shannon index, and goods coverage (P = .0015, P = .0016, P = .0005, P = .0011, respectively; Table 2). No differences were detected between FRE after the dietary trial and HC at baseline. Comparing samples from FRE at baseline and after the dietary trial, differences were only found in Chao 1 metric and goods coverage (P = .0273 and P = .0488, respectively; Table 2).
Table 2.
Alpha diversity metrics
| FRE baseline | FRE after | HC baseline | HC after | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Metric | Median | (Min‐max) | Median | (Min‐max) | Median | (Min‐max) | Median | (Min‐max) | Sign. P value |
| Chao1 | 1283*,§ | (670–1547) | 1556 | (1122–1768) | 1528*,§ | (1019–2455) | 1593 | (1227–1914) | *.0015; §.0273 |
| Observed OTUs | 689* | (432–886) | 809 | (575–956) | 837* | (586–1120) | 858 | (723–984) | *.0016 |
| Shannon | 4.17* | (2.38‐5.04) | 467 | (3.29‐5.91) | 5.15* | (4.27‐5.78) | 5.27 | (4.09‐5.82) | *.0005 |
| Goods coverage | 0.99*,§ | (0.99‐1) | 0.99 | (0.99‐0.99) | 0.99*,§ | (0.99‐0.99) | 0.99 | (0.99‐0.99) | *.0011; §.0488 |
Results of alpha diversity metrics are reported, significant differences are indicated with (*) or (§).
Abbreviations: After, after 30 or 60 days of dietary trial with APFD; Baseline, T0, before dietary trial with APFD; FRE, food‐responsive enteropathy dog; HC, healthy control; Min., minimum; Max., maximum.
No differences were detected in the samples from HC before and after the dietary trial.
3.5. Taxonomic summary
The LEfSe was used to determine differentially abundant bacterial taxa between the animal groups. A total of 15 bacterial groups were differentially expressed (α = 0.01, LDA score > 2.0) between the FRE and HC dogs (Figure 2). Other comparisons are available as Supporting Information materials (6).
Figure 2.
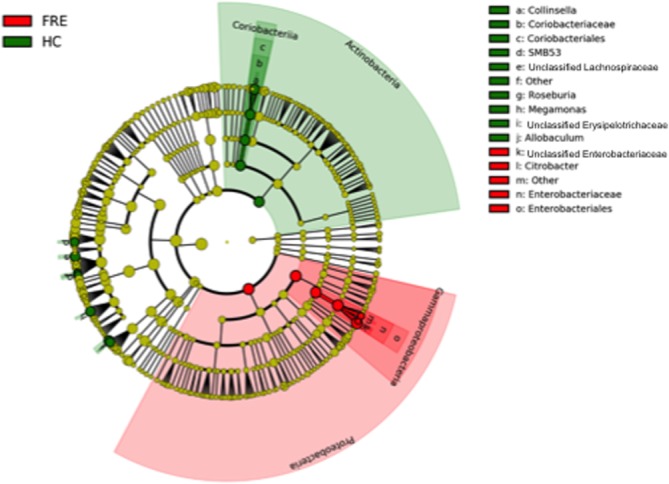
LDA effect size (LEfSe) of Illumina data sets based on 16S rRNA gene sequences. Taxonomic distribution of bacterial groups significant in FRE and HC dogs is available as Supporting Information material (5)
3.6. Microbiota index
The microbiota index, based on the sequencing data, was calculated for all fecal samples. A significantly higher index was detected in samples from FRE compared with HC dogs at baseline, but no significant difference was detected between FRE dogs after the dietary trial and HC dogs at baseline. No significant differences were found between baseline samples and samples after the dietary trial, neither in HC nor in FRE dogs (Figure 1D).
3.7. qPCR analysis of fecal microbial communities
The abundance of Blautia spp. was significantly decreased in FRE compared with HC dogs at baseline (adjusted P = .0304; Figure 3). Abundances of other bacterial groups (Universal, Faecalibacterium spp., Turicibacter spp., Streptococcus spp., E. coli, Fusobacterium spp., Clostridium hiranonis spp.) were not significantly different in FRE and HC dogs in any comparison. All of the results described above are provided in the Supporting Information material (Figure 3; Supporting Information 7).
Figure 3.
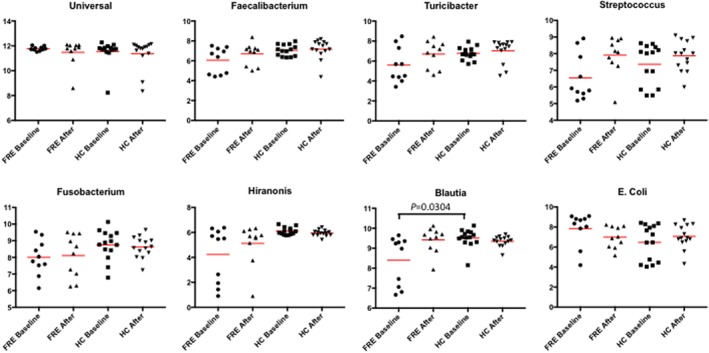
Results of quantitative PCR assays for selected bacterial groups. Abundance of Blautia spp. was significantly decreased in FRE in respect to HC dogs (adjusted P = .0304). Complete details are available as Supporting Information material (7). FRE, food‐responsive enteropathy: HC, healthy control; Baseline, time of first visit; After, after 30 or 60 days of APFD; Red line, median
4. DISCUSSION
Our results of suggest that, in FRE dogs, treatment with the APFD led to a significant changes of the fecal microbiota after the treatment. In contrast, no changes were detected in the fecal microbiota of HC dogs fed the same APFD.
Microbiota analyses identified significantly decreased α‐diversity and distinct microbial communities in dogs with FRE compared with HC dogs at baseline. These findings are similar to those of previous studies, where lower bacterial diversity and richness and different microbial communities in dogs with acute and chronic GI disease compared to those in HC dogs were reported.16, 18, 41, 42, 43, 44 At the phylum level, an increased abundance of Proteobacteria was detected, based on the univariate analyses. The potentially pathogenic bacterium E. coli belongs to this group, and our data from the qPCR analysis showed increased abundance of E. coli in FRE compared with HC dogs at baseline, but this difference was not significant after being adjusted for multiple comparisons. On the other hand, the abundance of the phyla Firmicutes and Actinobacteria was lower in FRE dogs at baseline. This finding was mainly because of the decreased presence of taxa within the family Lachnospiraceae (Clostridia clusters XIVa and XIVb). Increased or decreased abundances of those bacteria have been found to play an important role in the GI health of humans, rodents, and dogs, with regard to microbiota‐host interaction and host immunity. In the qPCR assays, we detected significant changes in the abundance of Blautia spp. between FRE and HC dogs at baseline.
Comparing fecal microbiota in dogs with FRE before and after 30 or 60 days of the APFD trial, both diversity and richness were significantly changed. Bacterial species richness was lower in FRE at baseline compared with HC at baseline. Interestingly, the fecal microbiota of FRE after the dietary trial was not different compared with that of HC at baseline, and the richness of fecal microbiota of FRE increased after the dietary trial. This change in fecal microbiota may be interpreted as partial recovery from dysbiosis. Several studies have evaluated how to modulate the intestinal microbiota in dogs with GI diseases. In dogs with IBD, previous reports showed no changes in fecal microbiota after treatment with immunosuppressive drugs.16, 18, 43 In dogs with acute diarrhea, after perturbation the microbiota seemed to return to the starting condition.45 Administration of antibiotics is a strong driver to shift GI microbial composition.16, 46, 47 In 1 study, treatment with diet, with or without a probiotic strain (Enterococcus faecium), lead to a significantly increased richness of the fecal bacterial microbiome in dogs with FRE, similar to our results.48
An explanation for why FRE appear to have partially recovered from dysbiosis, something not observed in IBD dogs, may be found in disease pathogenesis or in the length of the follow‐up sample collection. In FRE dogs, the exclusion of specific antigens from the diet potentially can drive major changes in intestinal homeostasis, leading to re‐established health of the microbial community, similar to what happens with acute diarrhea. In IBD dogs, instead, an on‐going chronic pathological process is suspected to remain after improvement of the clinical signs. Secondly, the follow‐up samples were collected after a shorter16 or equal18, 43 period in previous studies of IBD dogs. Therefore, the length of the time of follow‐up sampling may be sufficient to recover from FRE, but not from IBD.
Another aim of our study was to evaluate the effect of the APFD on fecal microbiota in HC, and no significant modification of the microbiota was detected. It is commonly believed that dietary changes lead to modifications of the microbiota in animals and humans, which is supported by several studies.21, 22, 23, 25, 26, 27, 49 However, these studies compared markedly different diets (or prebiotics) in terms of macronutrient components such as protein, fat, starch, or soluble and insoluble fiber. However, the macronutrient composition of the diet used in our study is similar to commercially available dry diets used for adult dogs. The microbial community (abundance and diversity) in HC dogs included in our study did not change after feeding the APFD.
We therefore conclude that the fecal microbiota of FRE dogs changed after the dietary trial, not directly because of the composition of the APFD, but because the diet promoted recovery from the disease. Recovery from disease was a strong driver to change the microbiota composition. This hypothesis is supported by the fact that the APFD did not drive any changes in the fecal microbiota of HC dogs.
Our study had several limitations. First, the number of dogs included was small. This was mainly because of the fact that only dogs fed exclusively with the APFD were included. The second limitation was that fecal samples taken after the APFD trial were collected either after 30 or 60 days or both. The collection of all fecal samples after 60 days could have led to the different results.
The third limitation was that trypsin‐like immunoreactivity was not assessed in all dogs, which could underestimate the prevalence of exocrine pancreatic insufficiency (EPI) in this population. However, because the dogs had a complete and prolonged response to the APFD without the need for additional medication or pancreatic enzyme supplementation, they were still considered food‐responsive and it is very unlikely that they suffered from undetected EPI.
The final limitation was that not all dogs had abdominal ultrasonography performed and none of dogs included had histological examinations performed to confirm intestinal inflammation and exclude other caused (eg, neoplastic disease).
In FRE dogs, improvement of clinical signs typically is expected to be seen quickly (ie, in few days) and usually no additional treatments or further examinations are necessary. These dogs are those included in our study. This quick response after an elimination dietary trial usually is not seen in antibiotic‐responsive enteropathy, immunosuppressant‐responsive enteropathy or in neoplastic processes. For this reason, endoscopy, although minimally invasive, is best reserved for animals that have failed diet and antibiotic trials.
In conclusion, the APFD did not drive substantial changes in the fecal microbiota in healthy individuals. Instead, in FRE dogs, the microbiota was different from that of HC dogs at first evaluation and was changed after the dietary trial, appearing more similar to the fecal microbiota of healthy individuals.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) or OTHER APPROVAL DECLARATION
The study was approved by the Scientific Ethics Committee for Experimentation on Animals of the Alma Mater Studiorum, University of Bologna (ID 639), and was conducted between October 2014 and May 2016.
Supporting information
Supporting Information 1: Ingredients of the animal protein‐free diet used in this study.
Supporting Information 2: Drop‐in/drop‐off flowchart
Supporting Information 3: Results of haematology and serum biochemistry assays in dogs with food‐responsive enteropathy (FRE).
Supporting Information 4: Main findings of the abdominal ultrasound.
Supporting Information 5: Clinical scores in FRE and HC dogs
Supporting Information 6: Abundance and statistical differences of different bacterial groups in faecal samples analysed with Illumina sequencing.
Supporting Information 7: Abundance and statistical differences of different bacterial groups in faecal samples analysed with qPCR.
ACKNOWLEDGMENTS
The clinical trial and sample collection was performed at the Department of Veterinary Medical Sciences, University of Bologna, Italy. The analysis of fecal sample were performed at Gastrointestinal Laboratory, Texas A&M University, College Station, TX, USA. Amplification and sequencing of the V4 variable region 16S rRNA gene was performed at MR DNA, Shallowater, TX, USA. This study was supported, in part, by Effeffe Pet Food S.p.A, which provided and formulated the vegetable‐diet for this study. Preliminary results of this study were presented as a poster at the 2017 ACVIM Forum, National Harbor, MD.
Bresciani F, Minamoto Y, Suchodolski JS, et al. Effect of an extruded animal protein‐free diet on fecal microbiota of dogs with food‐responsive enteropathy. J Vet Intern Med. 2018;32:1903–1910. 10.1111/jvim.15227
REFERENCES
- 1. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. JVet Intern Med. 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 2. Dandrieux JRS. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? JSmall Anim Pract. 2016;1–11. [DOI] [PubMed] [Google Scholar]
- 3. Kathrani A, Werling D, Allenspach K. Canine breeds at high risk of developing inflammatory bowel disease in the south‐eastern UK. Vet Rec. 2011;169:635. [DOI] [PubMed] [Google Scholar]
- 4. Verlinden A, Hesta M, Millet S, Janssens GPJ. Food allergy in dogs and cats: A review. Crit Rev Food Sci Nutr. 2006;46:259–273. [DOI] [PubMed] [Google Scholar]
- 5. Mandigers PJ, Biourge V, van den Ingh TS, Ankringa N, German AJ. A Randomized, open‐label, positively‐controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. JVet Intern Med. 2010;24:1350–1357. [DOI] [PubMed] [Google Scholar]
- 6. Simpson KW, Jergens AE. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet Clin North Am Small Anim Pract. 2011;41:381–398. [DOI] [PubMed] [Google Scholar]
- 7. Diez M. Nutritional Treatment of Food Allergy in Dogs and Cats: An Update. 25th ECVIM‐CA Congress. Lisboa, 2015.
- 8. Ricci R, Granato A, Vascellari M, et al. Identification of undeclared sources of animal origin in canine dry foods used in dietary elimination trials. JAnim Physiol Anim Nutr (Berl). 2013;97:32–38. [DOI] [PubMed] [Google Scholar]
- 9. Maine IR, Atterbury R, Chang K‐C. Investigation into the animal species contents of popular wet pet foods. Acta Vet Scand. 2015;57:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hooda S, Minamoto Y, Suchodolski JS, Swanson KS. Current state of knowledge: The canine gastrointestinal microbiome. Anim Health Res Rev. 2012;13:78–88. [DOI] [PubMed] [Google Scholar]
- 11. Kamada N, Seo S‐U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. [DOI] [PubMed] [Google Scholar]
- 12. Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J Gastroenterol. 2014;20:16489–16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suchodolski JS. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet J. 2016;215:30–37. [DOI] [PubMed] [Google Scholar]
- 14. Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation‐driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suchodolski JS, Dowd SE, Wilke V, Steiner JM, Jergens AE. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the Duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 2012;7:e39333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minamoto Y, Otoni CC, Steelman SM, et al. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 2015;6:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Igarashi H, Ohno K, Horigome A, et al. Fecal dysbiosis in miniature dachshunds with inflammatory colorectal polyps. Res Vet Sci. 2016;105:41–46. [DOI] [PubMed] [Google Scholar]
- 18. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott KP, Antoine J‐M, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. 2015;26:25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suchodolski J, Jergens A. Recent advances and understanding of using probiotic‐based interventions to restore homeostasis of the microbiome for the prevention/therapy of bacterial diseases. Microbiol Spectr. 2016;4:1–14. [DOI] [PubMed] [Google Scholar]
- 21. Zentek J. Influence of diet composition on the microbial activity in the gastro‐intestinal tract of dogs. II. Effects on the microflora in the ileum chyme. JAnim Physiol Anim Nutr. 1995;74:53–61. [Google Scholar]
- 22. Biagi G, Cipollini I, Grandi M, Zaghini G. Influence of some potential prebiotics and fibre‐rich foodstuffs on composition and activity of canine intestinal microbiota. Anim Feed Sci Technol. 2010;159:50–58. [Google Scholar]
- 23. Middelbos IS, Boler BMV, Qu A, White BA, Swanson KS, Fahey GC Jr. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One. 2010;5:e9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hang I, Heilmann RM, Grützner N, et al. Impact of diets with a high content of greaves‐meal protein or carbohydrates on faecal characteristics, volatile fatty acids and faecal calprotectin concentrations in healthy dogs. BMC Vet Res. 2013;9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hang I, Rinttila T, Zentek J, et al. Effect of high contents of dietary animal‐derived protein or carbohydrates on canine faecal microbiota. BMC Vet Res. 2012;8:90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beloshapka AN, Dowd SE, Suchodolski JS, Steiner JM, Duclos L, Swanson KS. Fecal microbial communities of healthy adult dogs fed raw meat‐based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol Ecol. 2013;84:532–541. [DOI] [PubMed] [Google Scholar]
- 27. Panasevich MR, Kerr KR, Dilger RN, et al. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br J Nutr. 2015;113:125–133. [DOI] [PubMed] [Google Scholar]
- 28. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. JVet Intern Med 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 29. Burton EN, O'Connor E, Ericsson AC, Franklin CL. Evaluation of Fecal Microbiota Transfer as Treatment for Postweaning Diarrhea in Research‐Colony Puppies. JAm Assoc Lab Anim Sci. 2016;55:582–587. [PMC free article] [PubMed] [Google Scholar]
- 30. Baldwin K, Bartges J, Buffington T, et al. AAHA Nutritional Assessment Guidelines for dogs and cats. JAm Anim Hosp Assoc. 2010;46:285–296. [DOI] [PubMed] [Google Scholar]
- 31. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vázquez‐Baeza Y, Hyde ER, Suchodolski JS, Knight R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. 2016;1:16177. [DOI] [PubMed] [Google Scholar]
- 33. Kitahara M, Sakamoto M, Benno Y. PCR detection method of Clostridium scindens and C. hiranonis in human fecal samples. Microbiol Immunol. 2001;45:263–266. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Mazcorro JF, Lanerie DJ, Dowd SE, et al. Effect of a multi‐species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol Ecol. 2011;78:542–554. [DOI] [PubMed] [Google Scholar]
- 35. Ritchie LE, Burke KF, Garcia‐Mazcorro JF, et al. Characterization of fecal microbiota in cats using universal 16S rRNA gene and group‐specific primers for Lactobacillus and Bifidobacterium spp. Vet Microbiol. 2010;144:140–146. [DOI] [PubMed] [Google Scholar]
- 36. Ritchie LE, Steiner JM, Suchodolski JS. Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66:590–598. [DOI] [PubMed] [Google Scholar]
- 37. Handl S, Dowd SE, Garcia‐Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol. 2011;76:301–310. [DOI] [PubMed] [Google Scholar]
- 38. Shobar RM, Velineni S, Keshavarzian A, et al. The effects of bowel preparation on microbiota‐related metrics differ in health and in Inflammatory Bowel Disease and for the mucosal and luminal microbiota compartments. Clin Trans Gastroenterol. 2016;7:e143 10.1038/ctg2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8:e1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004;53:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xenoulis PG, Palculict B, Allenspach K, et al. Molecular‐phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579–589. [DOI] [PubMed] [Google Scholar]
- 43. Rossi G, Pengo G, Caldin M, Piccionello AP, Steiner JM, Cohen ND, et al. Comparison of Microbiological, Histological, and Immunomodulatory Parameters in Response to Treatment with Either Combination Therapy with Prednisone and Metronidazole or Probiotic VSL#3 Strains in Dogs with Idiopathic Inflammatory Bowel Diseas. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guard BC, Barr JW, Reddivari L, et al. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS One. 2015;10:e0127259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bell J. a, Kopper JJ, Turnbull JA, Barbu NI, Murphy AJ, Mansfield LS. Ecological characterization of the colonic microbiota of normal and diarrheic dogs. Interdiscip Perspect Infect Dis. 2008;2008:149694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huse SM, Dethlefsen L, Huber JA, Mark Welch D, Relman DA, Sogin ML. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS Genet. 2008;4:e1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suchodolski JS, Dowd SE, Westermarck E, et al. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmitz S, Suchodolski JS, Guard BC, Steiner JM, Werling D, Allenspach K. Treatment with the probiotic Enterococcus faecium in dogs with inflammatory bowel disease: Effect on microbiome composition J Vet Intern Med. 2015;29:433–434. [Google Scholar]
- 49. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1: Ingredients of the animal protein‐free diet used in this study.
Supporting Information 2: Drop‐in/drop‐off flowchart
Supporting Information 3: Results of haematology and serum biochemistry assays in dogs with food‐responsive enteropathy (FRE).
Supporting Information 4: Main findings of the abdominal ultrasound.
Supporting Information 5: Clinical scores in FRE and HC dogs
Supporting Information 6: Abundance and statistical differences of different bacterial groups in faecal samples analysed with Illumina sequencing.
Supporting Information 7: Abundance and statistical differences of different bacterial groups in faecal samples analysed with qPCR.


