Abstract
Background: In humans, temporal lobe epilepsy (TLE), is a type of focal epilepsy occurring mainly in the mesial TLE (mTLE), commonly associated with hippocampal sclerosis (HS).
Objectives: According to recent studies, TLE might also occur in dogs and could be associated with hippocampal atrophy (HA)/HS. To date, hippocampal lesions have not been correlated with electroencephalographic (EEG) findings in epileptic dogs.
Animals: An EEG examination, brain magnetic resonance imaging, and volumetric assessment of the hippocampus were performed in 16 nonepileptic and 41 epileptic dogs.
Methods: In this retrospective study, the presence and localization of EEG‐defined epileptiform discharges (EDs) was blindly evaluated. The hippocampus was measured and assessed for unilateral atrophy. The results of EEG and volumetric findings were correlated to determine whether the functional epileptic focus is equivalent to structural changes.
Results: The median hippocampal asymmetric ratio (AR) in epileptic dogs was significantly greater than in the control group (P < .001). Using a cut‐off threshold AR of >6%, 56% (23/41) of the dogs were characterized with unilateral HA. Of those animals, 35% (8/23) had EDs in the temporal leads and 26% (6/23) had no EDs. In 88% (7/8) of dogs with EDs in the temporal leads that had unilateral HA, the EDs correlated with the side of the decreased hippocampal volume.
Conclusions and Clinical Importance: The results indicate an association between the presence of EDs detectable on EEG and a decrease in the unilateral hippocampal volume in some cases of canine idiopathic epilepsy that might reflect features of human mTLE.
Keywords: canine, electroencephalography, hippocampus, idiopathic epilepsy, magnetic resonance imaging
Abbreviations
- EDs
epileptiform discharges
- EEG
electroencephalography
- HA
hippocampal atrophy
- MRI
magnetic resonance imaging
- mTLE
mesial temporal lobe epilepsy
- HS
hippocampal sclerosis
1. INTRODUCTION
Epilepsy is one of the most common neurological diseases in both humans and dogs. It is estimated that more than 50 million people worldwide have epilepsy.1 In dogs, the prevalence of epilepsy is estimated to range from 0.5% to 5%. The International Veterinary Epilepsy Task Force (IVETF) reports that idiopathic epilepsy may be the most common form of epilepsy and occurs in up to 75% of epileptic dogs.2, 3 Temporal lobe epilepsy (TLE) was defined in 1989 by the International League Against Epilepsy as a condition distinguished by recurrent, unprovoked seizures originating from the mesial or lateral temporal lobe.4 Seizures associated with this condition consist of focal, focal aware, or focal impaired awareness.5 Hippocampal sclerosis (HS)/necrosis (HN)/atrophy (HA) is found to be very often associated with mesial TLE (mTLE). It is predicted that HS is related to approximately 80% of all TLE epilepsies in adult humans.6 According to recent studies, HS, HA, or both play an important role in canine epileptogenesis, and may reflect TLE or mTLE.7, 8, 9
Electroencephalographic (EEG) shows the function of the forebrain and can be useful in determining the diagnosis of epilepsy, defining the type of seizure disorder and the epileptogenic zone, including changes specific for TLE/mTLE.10, 11, 12, 13 The correlation of EEG and MRI studies is useful in determining whether the “functional” epileptic focus is the same as the structural one.14 TLE occurring in both species might be caused by functional and structural changes in the area of the temporal lobe of the cerebral cortex and associated structures, including the hippocampus.15 Volumetric MRI is particularly useful in the diagnosis of seizures associated with morphologically altered temporal lobes of the brain, which are reported to be difficult or impossible to detect on routine visual radiological analysis of brain MRI.14 Because of the fact that none of these methods alone suffices in the determination of the location of the epileptic focus, the interictal EEG, MR, and MR volumetry complement one other.16, 17, 18, 19 In canine epileptology, it is still unclear whether there is a direct correlation between changes observed in EEG recordings and volumetric brain analysis and whether lesions in the hippocampus (HA, HS, or both with or without MRI signal changes) can be the cause of seizures.20 Because of the fact that there are no histological studies of the hippocampus in dogs with epilepsy, combining structural and functional studies (MRI with volumetry and EEG) in dogs may enable in vivo diagnosis of HS as a cause of seizures and confirm the occurrence of canine mesial TLE.
To date, the EEG‐defined position of epileptiform discharges (EDs) has not been correlated with a detailed MR volumetric evaluation of the hippocampus in dogs with suspected mTLE. We hypothesized that dogs diagnosed with idiopathic epilepsy would have unilateral hippocampal atrophy. We also assumed that dogs with HA would show unilateral ED discharges from temporal leads corresponding to the side of the hippocampal volume loss. Hence, the aim of this study was to evaluate the correlation between MR volumetric and EEG and to evaluate whether the functional temporal lobe dysfunction correlates with the morphological damage in the hippocampus.
2. MATERIALS AND METHODS
2.1. Study population
The retrospective study with prospective elements involved neurological cases presented to the Department of Internal Medicine and Clinic for Horses, Dogs and Cats between 2014 and 2017, MRI studies were performed at the Centre for Experimental Diagnostics and Biomedical Innovations of the Faculty of Veterinary Medicine, Wrocław University of Environmental and Life Sciences, Poland. The dogs' owners provided informed consent for their pets to participate in the EEG and the MRI studies. Dogs were included in the control group from our clinical database based on a normal clinical, neurological, EEG, and MRI examination, where the owners gave informed consent to the use of their dogs' data for future research. This group was gathered in a fully legal and clear circumstances. In accordance with the Polish law, this study did not require the approval of the Ethical Committee (Experiments on Animals Act from January 15, 2015, Journal of Laws of the Republic of Poland from 2015, item. 266). All dogs underwent the same diagnostic procedures and were included in the study if they met the following inclusion criteria: (1) a clinical and a neurological examination, (2) extensive blood analysis (complete blood count, differential leukocyte count, and the concentrations of sodium, chloride, potassium, calcium, total protein, albumin, globulin, urea nitrogen, creatinine phosphokinase, glucose, cholesterol, lipase, alanine aminotransferase, aspartate transaminase, alkaline phosphatase, ammonia, preprandial bile acids, and total bilirubin), (3) a readable EEG recording, (4) MRI of the brain according to the IVETF epileptic patient recommendations,21 and (5) cerebrospinal fluid analysis in dogs with seizures.
Dogs were divided into the following 2 groups based on the results of the above listed examinations: the IE group, dogs with idiopathic epilepsy; and the N group, control, nonepileptic dogs. The IE group included dogs with a history of at least 2 epileptic seizures, a normal blood workup, normal CSF analysis, and no apparent intracranial lesions on MRI (consistent with IVETF Tier II22). Dogs were included in the N group if they had no history of seizures and if the examinations mentioned above were within the reference range. Dogs were excluded from the study if they did not meet the above mentioned criteria, or were of a brachycephalic breed (attributable to the frequent presence of ventriculomegaly). In the N group, dogs were excluded if they had a history of forebrain signs.
2.2. EEG recording
The dogs were subjected to a 30‐minute EEG study using a protocol described previously.23 All the EEG recordings were performed using medetomidine sedation (Narcostart, Livisto, Gdynia, Poland) at a dose of 20 mcg/kg, administered IM into the right triceps muscle. Subdermal wire electrodes (Ives EEG Solutions, Newburyport, MA) were used. The recordings were performed before general anesthesia necessary for MRI and CSF examinations. The examined dogs were placed in sternal recumbency in order to facilitate videometry, which recorded possible movements of the dogs during EEG. The recordings were carried out with the use of an EEG unit (Nikon Kohden, Rosbach, Germany) using the following settings: sensitivity 70 μV/cm: band pass filter 0.5‐0.30 Hz; a 0.3 seconds time constant and an inserted 50 Hz notch filter. Each recording was carried out using a 10‐channel referential montage (F3, F4, C3, C4, T3, T4, O1, O2‐Ref., the reference electrode was placed on the frontal bone, the ground electrode was inserted in the neck and in a standard bipolar montage [F3‐C3, C3‐T3, T3‐O1, F4‐C4, C4‐T4, T4‐O2]). The ECG‐Ref. electrode was placed SC at the level of the left 5th intercostal space near the chondrocostal junction. A light stimulation using a stroboscope lamp was conducted in the 10th minute of the EEG recording. The initial stimulation frequency was 0.5 Hz. It was gradually increased to 60 Hz, then steadily decreased to the base point over a 5‐minute period (as described in another veterinary protocol, a similar protocol is used in human medicine).23, 24 All EEGs were subjected to visual analysis. The recordings were blindly studied by a veterinary neurologist using monopolar and simultaneously bipolar montage with videometric control. The visual analysis took into account the physiologic superimposed transients (SITs) and pathologic EDs. Currently accepted25 nomenclature was used to identify EDs, as described elsewhere.23
Special attention was paid to the ED detection and localization. The ED localization was defined based on the highest amplitude of the discharge in a reference montage and reversed polarity in a bipolar montage recording.26 The EDs were differentiated from physiologic SITs (including sleep spindles, K‐complexes, occipital intermittent rhythmic delta activity or frontal intermittent rhythmic delta activity, and others) and artifacts such as muscle activity, movements, and ocular movements or blinks, together defined as “muscle artifacts.” EEG analyses were reviewed independently by 2 observers (AC and MW).
2.3. MRI examination
Following the EEG recording, the dogs were subjected to MRI using a 1.5 Tesla scanner (Philips, Ingenia device). The IVETF protocol21 was used to scan the brains of the dogs using 3D T1 (TR 25 ms/TE 4 ms, slice thickness 1 mm, FOV 200 × 200 mm, matrix size 268 × 266, voxel 0.75 × 0.75 mm, slice thickness 1 mm, slice gap—0.38 mm), T2 (TR 8042 ms/TE 100 ms, FOV 120 × 100 mm, matrix size 268 × 171 mm, voxel 0.45 × 0.569 mm, slice thickness 2 mm, slice gap 1 mm) weighted sequence, FLAIR (TR 9000 ms/TE 140 ms/TI 2450 ms), T2* sequence and 3D T1 sequence with a contrast agent. Transverse plane T2‐W images were acquired in all dogs.
2.3.1. MR volumetric analysis
A volumetric analysis of the hippocampus was performed using a semiautomatic method with the use of Osirix 8 software in a T2‐W sequence in the transverse plane. The hippocampal volume was measured using described anatomic landmarks and borderlines27, 28, 29, 30 based on a previously described protocol of volumetric analysis.8 The hippocampus was measured at the border of the lateral cerebral ventricle, where it was easily identifiable (Figures 1, 2, 3). The hippocampal volume was calculated as the sum of the volumes obtained by multiplying the surface of each slice (cm2) with the slice interval (cm). The volume of the left and right hippocampus and their total volume was calculated. The asymmetric ratio (AR) was used to assess the unilateral hippocampal atrophy, which was obtained by dividing the absolute volume difference between the left and right hippocampi by the volume of the larger hippocampus.8 The cut‐off value of 6% (equal to 0.3 cm3 for 0.5 cm3 of hippocampal volume) was applied for AR in order to define pathologic hippocampal atrophy.8 MR volumetric images were blindly reviewed independently by 2 observers (AC, MW, and PP).
Figure 1.
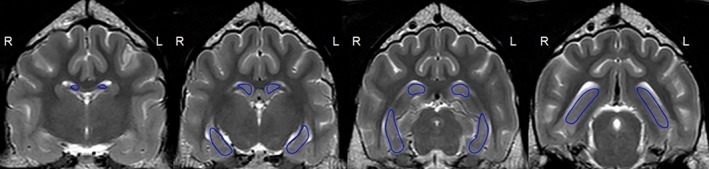
T2‐weighted volumetry in the transverse plane with a 2D manually marked region of interest in the hippocampus in a nonepileptic 3‐year‐old Golden Retriever (group N)
Figure 2.
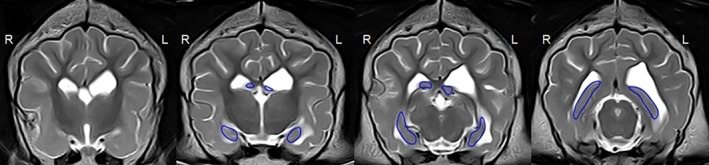
T2‐weighted volumetry in the transverse plane with a 2D manually marked region of interest in the hippocampus in an epileptic 2‐year‐old Rottweiler (idiopathic epilepsy group). The left (L) ventricle is enlarged, the left (L) hippocampus is visibly atrophic with no change in the signal intensity
Figure 3.
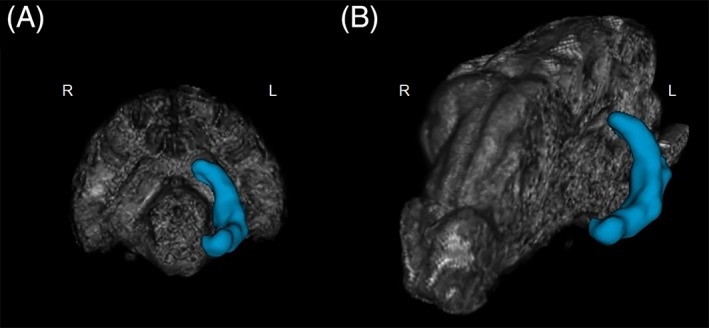
Three‐dimensional volumetric analysis of the hippocampus (marked in blue): (A) in transverse view and (B) in sagittal view
2.4. Statistical methods
The volumes of the left and right hippocampus were recorded and the AR was calculated manually. The normality of data distribution was analyzed using the Shapiro‐Wilk test. All the analyzed data were nonparametric; hence, the nonparametric Mann‐Whitney U test was used to compare hippocampal volumes (StatSoft Inc, 2011 STATISTICA [data analysis software system, version 13.1]). Statistical significance was determined at P < .05. The correlation between the groups was analyzed using Spearman's correlation coefficient. Data were presented as a box plots.
3. RESULTS
We included 41 dogs with IE and 16 dogs in the N group in the study. The IE group consisted of 22 males and 19 females, aged from 9 and 108 months (median 49.2 months) and weighting between 10 and 48 kg (median 24. 2 kg). The IE group included the following breeds: 10 mixed breeds, 4 Border Collies, 4 Beagles, 2 Siberian Huskies, 2 German Shepherds, 2 Giant Schnauzers, 2 Cocker Spaniels, 2 Cane Corso, 2 Weimaraners, and 1 each of the following: Polish hunting dog, Polish Hound, Greater Swiss Mountain dog, Alaskan Malamute, Polish Lowland Sheepdog, Golden Retriever, Labrador Retriever, West Highland White Terrier, Cotton de Tulear, Bloodhound, and Akita Inu. The neurological ictal signs included generalized seizures (n = 30), focal seizures evolving into generalized (epileptic) seizures (n = 8) or focal seizures (n = 3).
The N group contained 9 males and 7 females, aged from 48 and 108 months (median 78.7 months), weighing between 18 and 50 kg (median 31.9 kg). It included the following breeds: 4 mixed breeds, 2 Golden Retrievers, 2 Siberian Huskies, and 1 each of the following: Australian Shepherd, Akita Inu, German Shepherd, Black Russian Terrier, Rottweiler, Doberman, Labrador Retriever, and Miniature Schnauzer. The N group consisted of dogs diagnosed with disc herniation, the cauda equina syndrome, cervical spondylomyelopathy, and no history with seizures.
3.1. EEG findings
In the IE group, the EEG analysis revealed EDs in the form of spikes, polyspikes, and sharp waves in 27 dogs and no EDs in 14 dogs. Ten dogs had EDs from temporal leads, 11 dogs had EDs from central leads, and 6 dogs had EDs from frontal leads. In the N group, 3 dogs had single EDs in the form of spikes from the central and occipital leads.
3.2. Volumetric findings
In the IE group, the median unilateral hippocampus volume was 0.55 cm3 (0.3653‐0.6779 cm3) for left side, 0.55 cm3 (0.4616‐0.7284 cm3) for right side and the median total hippocampus volume was 1.075 cm3 (0.952‐1.3899 cm3). The median AR was 6.9%. In the N group, the median unilateral hippocampus volume was 0.66 cm3 (0.5728‐0.7826 cm3) for left side, 0.63cm3 (0.5385‐0.7789 cm3) for right side and the median total hippocampus volume was 1.3119 cm3 (1.319‐1.569 cm3). The median AR in the N group was 1.8%. The AR of the IE group was significantly (P < .001) greater in comparison to N group (Figure 4). In the N group, no significant difference was observed between the volume of the left and the right hippocampus. There was a significant difference in the overall hippocampal volumes between the 2 groups (P < .001).
Figure 4.
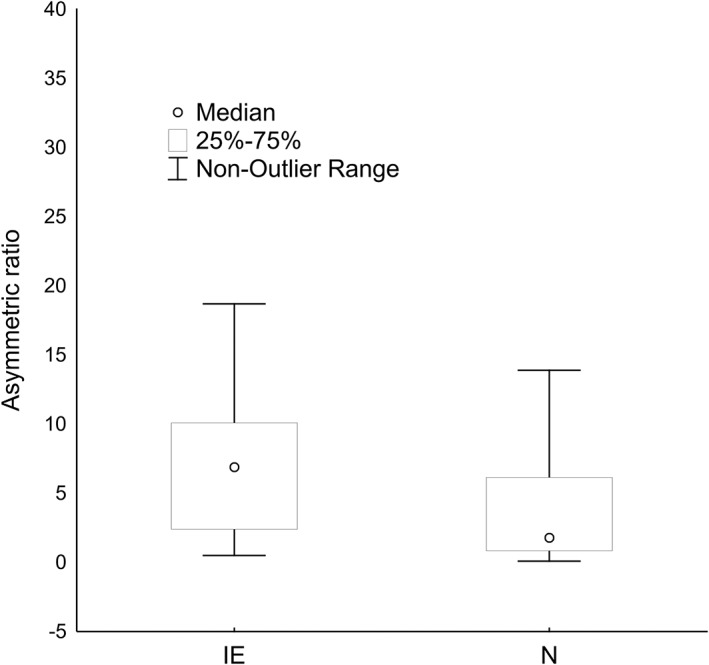
A significantly greater median asymmetric ratio in epileptic dogs than the control group measured using Mann‐Whitney U test (P < .001). IE, idiopathic epilepsy group; N, control group
3.3. Volumetry and EEG correlation
Using a cut‐off threshold AR of >6%, 56% (23/41) of dogs in IE group were characterized with unilateral hippocampus atrophy (HA) (Figures 1, 2, and 5). Of those 23 dogs, 35% (8/23) had EDs in the temporal leads (Figures 6 and 7), reflecting pathological temporal lobe activity, and EDs were not detected in 26% of the dogs (6/23). In 30% (7/23) of the dogs with HA, and 88% (7/8) with HA and ED in the temporal leads, the EDs correlated with a decreased hippocampal volume on the same side (Figure 8).
Figure 5.
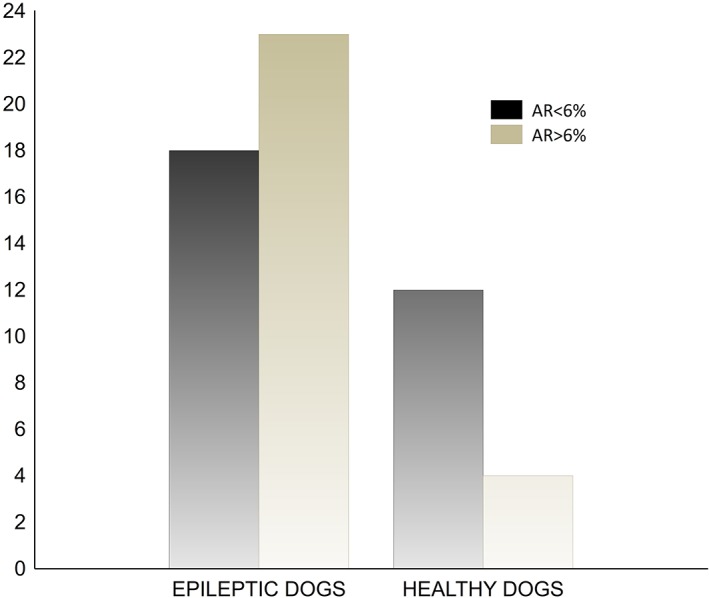
The differences of an asymmetric ratio (AR) in both groups (light gray; AR > 6%, dark gray; AR < 6%)
Figure 6.
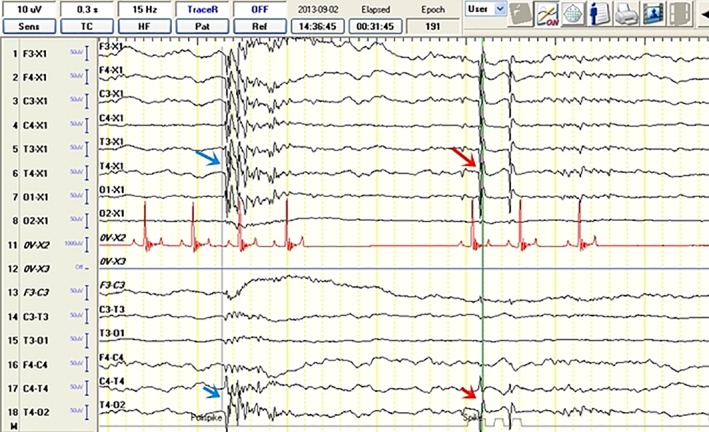
Electroencephalographic recordings from a 2‐year‐old female Siberian husky (idiopathic epilepsy group) with unilateral hippocampal atrophy on the right showing spike activity (read arrow) and polyspike activity (blue arrow) from the T4 lead. The discharge localization is defined based on the highest amplitude in the reference montage (arrow) and reversed polarity in the bipolar montage recording (arrow)
Figure 7.
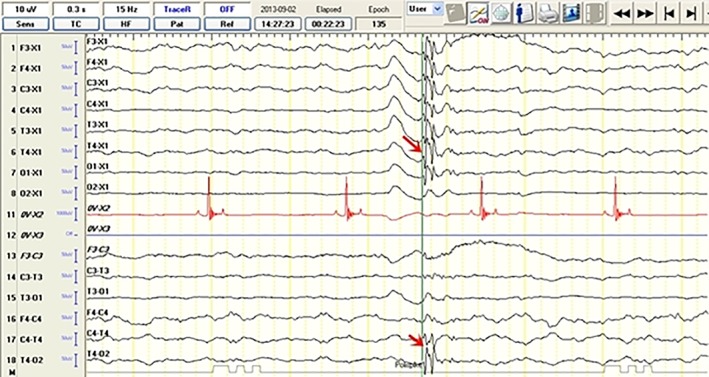
Electroencephalographic recordings from a 2‐year‐old female Siberian husky (idiopathic epilepsy group) with unilateral hippocampal atrophy on the right showing polyspike activity (read arrow) from the T4 lead. The discharge localization is defined based on the highest amplitude in the reference montage (arrow) and reversed polarity in the bipolar montage recording (arrow)
Figure 8.
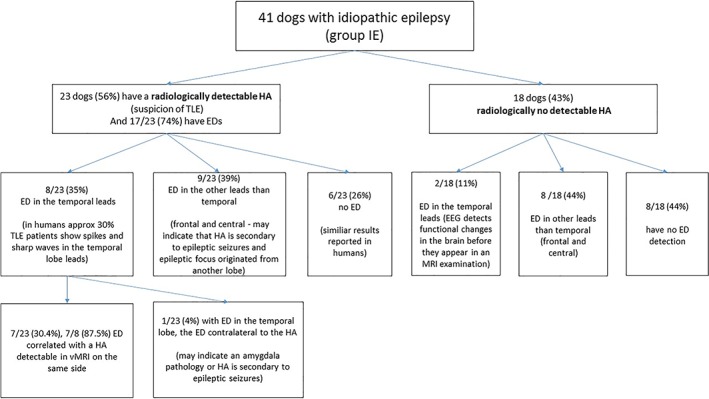
Schematic representation of the electroencephalographic findings with particular emphasis on the location of epileptic activity in the idiopathic epilepsy group of dogs with radiologically detectable and no detectable hippocampal atrophy
4. DISCUSSION
In this study, 56% (23/41) of the 41 idiopathic epileptic dogs had radiologically detectable HA, which might be indicative of HS resulting in TLE. In those dogs, the EEG detectability for EDs was 74% (17/23) (Figure 8). Thirty‐five percent of the dogs (8/23) had EDs from temporal leads and 39% (9/23) of the dogs had EDs from the frontal and central leads (Figure 8). In human medicine, inter‐ictal EEG or single routine EEG is normal or with nonspecific abnormalities in half of the patients with mTLE.31 Only a third of the dogs with mTLE show classic spikes or sharp waves and slow‐wave foci in the cranial temporal lobe electrode. In humans, standard EEG consists of a 10‐20 recording montage with 3 electrodes placed on each side that collect activity from the temporal lobes: F7/F8 (the so called sylvian‐frontal or cranial temporal‐inferior frontal electrode), T3/T4 (mesial temporal electrode), and T5/T6 (caudal temporal electrode).32 This montage provides limited coverage of the temporal regions, because the T5/T6 electrodes collect activity from the caudal part of the temporal lobe (which is indicative of caudal temporal epilepsy) and the F7/F8 electrodes are temporal frontal electrodes that are located above the latero‐caudal frontal area. They record activity both from the frontal cortex and the cranial part of the temporal lobe. The T3/T4 electrodes help to localize the region of interest, that is, if the activity is recorded by the F4 (frontal electrodes) and F8 electrodes, then the epileptic field is located in the frontal lobe, if it is recorded by the F8 and T4 electrodes, then this activity is likely to be located in the cranial part of the temporal lobe.32, 33 Therefore, additional T1 and T2 (the so‐called Silverman/Sphenoidal electrodes) electrodes are placed over the zygomatic arch to record activities strictly from the medial temporal region.32, 33 The typical epileptiform abnormalities in mTLE include spike or sharp waves over the temporal basal electrodes F7/ F8, especially from T1/T2.32 In this study, discharges from the T3,T4 electrodes were considered to originate from the canine temporal lobe,34 resembling human F7, F8, T1, T2 leads, as they were located above the zygomatic arch (corresponding to the T1/T2 electrodes in human medicine) and recorded activity from the Sylvian gyrus and the temporal lobe area.35, 36 Recently, the use of the F7/F8 electrodes, located on the zygomatic arch37, was described. In our opinion, the use of these electrodes increases the value of the EEG examination and should definitely be considered for use in an optional preoperative diagnosis of HS.37 In 26% of the dogs (from group IE, 6/23) with unilateral HA, no EDs were observed. Dogs in this study were subjected to a single EEG study lasting 30 minutes. In humans, this type of examination gives a 50% seizure detection rate. Prolonged EEG monitoring increases the yield to 70%‐80%, and a repeated second EEG examination further enhances the yield to 90% abnormalities.31, 32, 33 Approximately 10% of the patients with mTLE will have normal EEGs between seizures.32, 33
In those dogs that had radiologically detectable HA with ED from frontal and central leads (35%), HA could have developed secondary to epileptic seizures, with an epileptic focus originating from the frontal lobe and parietal lobe.9, 20
Forty‐three percentage (18/41) of the dogs had no radiologically detectable HA. Eleven percent (2/18) of those dogs had EDs in the temporal leads during the EEG study. This might point to functional changes in the brain detectable on EEG before structural changes detectable in MRI.25 Forty‐four percent (8/18) of the dogs had EDs in the frontal and central leads, indicating an epileptic focus originating in the frontal and parietal lobe. Forty‐four percent (8/18) of the dogs had no EDs.
The medial temporal lobe contains the hippocampus and the amygdala. Some studies in human medicine showed that 5% of patient with mTLE had a seizure onset in the amygdala36 and it has been suggested that the pathophysiology of mTLE can sometimes relate to abnormalities in the amygdala. Some findings support the hypothesis that there might be a subgroup of patients without MRI signs of HS or any other abnormality based on a visual analysis of MRI, known as “MRI‐negative” patients, who have no changes in the hippocampal volume.38 The volumetric study of the amygdala in some of those patients suggests that an enlarged amygdala can be responsible for the epileptogenic process.39 Most of those patients have unilateral EDs (interictal spikes localized primarily in the sphenoidal electrodes [T1/T2] or in the cranial temporal‐inferior frontal region [F7/F8]) ipsilateral to the enlarged amygdala. These findings indicate that if there is no apparent HA in MRI or if HA is contralateral to a functional epileptic focus on EEG, an enlarged amygdala should be considered as a cause of mTLE.36 In our study, the measurement of the amygdala was not carried out because of its small size and difficulty in differentiating it from other structures in the T2 sequences using 1.5 Tesla imaging. In one of the dogs with unilateral HA, EDs were detected in the contralateral temporal lead that could reflect amygdala involvement36, 38 and that HA is secondary to epileptic seizures. The lack of the amygdala measurements is one of our study limitations.
Although seizures in mTLE usually initiate with focal seizures evolving into generalized (epileptic) seizures, our study also includes dogs with apparent generalized seizures, as it is often impossible to determine the seizure onset in this species.
In a previous study of the hippocampal volume,8 28 out of 58 (48%) dogs had unilateral hippocampal atrophy. In our study, 23 out of 41 (56%) dogs had HA. Those results are similar to our findings and indicate that applying an AR > 6% of the hippocampus enables an accurate assessment of HA in dogs.8 The second possible and described protocol for the assessment of unilateral atrophy of the hippocampus is the use of an AR > 12.6%.7 It has been suggested that this protocol is more specific for identifying canine epileptic hippocampal asymmetry, although the protocol applied in this study was also used by other authors.8 It is based on a human study affirming that a difference in hippocampal volume of 0.3 cm3 was associated with a satisfactory sensitivity and specificity. Therefore, it is reasonable to consider that an AR 6% or more indicates pathologic hippocampal atrophy.40 In some dogs in this study, the difference in the unilateral volume of the hippocampus was visible during a visual radiological evaluation of the MRI scans, which is also described in human patients with mTLE.41 A volumetric analysis of chosen intracranial structures is used to support the radiological evaluation of subtle changes in brain tissue in MRI images.15 In veterinary medicine, there is no information regarding the correlation between structural changes observed in the hippocampus and EEG findings. However, in humans, interictal EEG abnormalities correlated very well with the ictal onset zone (spikes 90%) and interictal spikes correlated with the structural abnormalities detected by MRI in the majority of patients with mTLE.42 To validate the in vivo occurrence of mTLE in dogs with IE and to expand the research into canine epilepsy, there is a needed to perform repeat EEG examinations (minimum 2) or 24 hour video‐EEG monitoring and refine the volumetric method taking into account the amygdala according to the methodology carried out in human studies. This may allow future application of resection treatment in dogs with refractory epilepsy.43, 44TLE is often resistant to medical treatment and it is estimated that 30% of IE dogs are resistant to medical treatment.43, 44 Thus, surgical management of drug‐resistant epilepsy is considered as an alternative. Hence, there is a necessity for further development of advanced diagnostic methods to detect epileptogenic foci in dogs. The results of this study form the basis of further research into mTLE in dogs and future application of surgical management of this type of epilepsy.
5. CONCLUSION
The results indicate an association between the presence of EDs detectable on EEG and a decrease in the unilateral hippocampal volume in some cases of canine idiopathic epilepsy that might reflect features of human mTLE.
The combination of EEG and volumetric MR could be an appropriate protocol for the diagnosis of TLE in dogs. However, 2 EEG examinations at different time points or, preferably, over a 24‐hour period are likely to significantly improve the precision of this diagnosis. Both functional and structural diagnostic techniques are useful in the diagnosis of epilepsy and provide the highest diagnostic value when performed together.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
ACKNOWLEDGMENTS
The support of the Faculty of Veterinary Medicine of the Wrocław University of Environmental and Life Science, Poland, is gratefully acknowledged. The work was funded from the Faculty funds and a KNOW donation. The study was performed at the Department of Internal Medicine and Clinic for Horses, Dogs and Cats and at the Centre for Experimental Diagnostics and Biomedical Innovations of the Faculty of Veterinary Medicine, Wrocław University of Environmental and Life Sciences, Poland. Presented at the 30st Annual Symposium of the ESVN‐ECVN in Helsinki, Finland at September 21–23, 2017.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The dog's owners provided informed consent for their pets to participate in the EEG and the MRI studies. In accordance with the Polish law, this study did not require the approval of the Ethical Committee (Experiments on Animals Act from January 15, 2015, Journal of Laws of the Republic of Poland from 2015, item. 266).
Czerwik A, Płonek M, Podgórski P, Wrzosek M. Comparison of electroencephalographic findings with hippocampal magnetic resonance imaging volumetry in dogs with idiopathic epilepsy. J Vet Intern Med. 2018;32:2037–2044. 10.1111/jvim.15323
REFERENCES
- 1. Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy—a review. Epilepsy Res. 2009;32:2037‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hülsmeyer VI, Fischer A, Mandigers JJP, et al. International veterinary epilepsy task force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. 2015;11:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berendt M, Farquhar GR, Mandigers JJP, et al. International Veterinary Epilepsy Task Force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tèllez‐Zenteno JF, Hernȃndez‐Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer RS, Cross JH, D'Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizures types. Epilepsia. 2017;58:531‐542. [DOI] [PubMed] [Google Scholar]
- 6. Blair RDG. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012;2012:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estey CM, Dewey CW, Rishniw M, et al. A subset of dogs with presumptive idiopathic epilepsy show hippocampal asymmetry: a volumetric comparison with non‐epileptic dog using MRI. Front Vet Sci. 2017;4:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuwabara T, Hasegawa D, Kobayashi M, Fujita M, Orima H. Clinical magnetic resonance volumetry of the hippocampus in 58 epileptic dogs. Vet Radiol Ultrasound. 2010;51:485‐490. [DOI] [PubMed] [Google Scholar]
- 9. Lőrincz BA, Anson A, CsЀbi P, et al. Novel approach to magnetic resonance imaging of epileptic dogs—T2 relaxometry of the brain with emphasised hippocampus. Acta Vet Hung. 2017;65:185‐197. [DOI] [PubMed] [Google Scholar]
- 10. Wrzosek M, Nicpoń J, Bergamasco L, et al. Visual and quantitative electroencephalographic analysis of healthy young and cats under medetomidine sedation. Vet J. 2008;180:221‐230. [DOI] [PubMed] [Google Scholar]
- 11. Brauer C, Kästner SBR, Rohn K, et al. Electroencephalographic recordings in dogs suffering from idiopathic and symptomatic epilepsy: diagnostic value of interictal short time EEG protocol supplemented by two activation techniques. Vet J. 2012;193:185‐192. [DOI] [PubMed] [Google Scholar]
- 12. James F. Introduction to electroencephalography In: De Risio L, Platt S, eds. Canine and Feline Epilepsy: Diagnosis and Treatment. Oxfordshire, UK: CABI; 2014:325‐346. [Google Scholar]
- 13. Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47:14‐22. [DOI] [PubMed] [Google Scholar]
- 14. Placantonakis DG, Schwartz TH. Localization of epilepsy. Neurol Clin. 2009;27:1015‐1030. [DOI] [PubMed] [Google Scholar]
- 15. Giorgio A, Stefano De N. Clinical use of brain volumetry. J Magn Reson Imaging. 2013;37:1‐14. [DOI] [PubMed] [Google Scholar]
- 16. Connor SEJ, Jarosz JM. Magnetic resonance imaging of patients with epilepsy. Clin Radiol. 2001;56:787‐801. [DOI] [PubMed] [Google Scholar]
- 17. Cendes F, Li LM, Watson C, et al. Is ictal recording mandatory in temporal lobe epilepsy? Not when the interictal electroencephalogram and hippocampal atrophy coincide. Arch Neurol. 2000;57:497‐500. [DOI] [PubMed] [Google Scholar]
- 18. Cendes F, Caramanos Z, Andermann F. Proton magnetic resonance spectroscopic images and MRI volumetric studies for lateralization of temporal lobe epilepsy. Ann Neurol. 1997;42:737‐746. [DOI] [PubMed] [Google Scholar]
- 19. Woeremann FG, Functional MR. MR spectroscopy. Paper presented at: European epilepsy academy (EUREPA) seminar: update on neuroimaging, 5th European congress on Epileptology; October 6‐10, 2002; Madrid, Spain.
- 20. Hasegawa D. Diagnostic techniques to detect the epileptogenic zone: pathophysiological and presurgical analysis of epilepsy in dogs and cats. Vet J. 2016;215:64‐75. [DOI] [PubMed] [Google Scholar]
- 21. Rusbridge C, Long S, Jovanovik J, et al. International Veterinary Epilepsy Task Force recommendations for a veterinary epilepsy‐specific MRI protocol. BMC Vet Res. 2015;11:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Risio De L, Bhatti S, Muñana K, et al. International Veterinary Epilepsy Task Force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wrzosek M, Ives JR, Karczewski M, Dziadkowiak E, Gruszka E. The relationship between epileptiform discharges and background activity in the visual analysis of electroencephalographic examinations in dogs with seizures of different etiologies. Vet J. 2017;222:41‐51. [DOI] [PubMed] [Google Scholar]
- 24. Trenité K‐ND, Rubboli G, Hirsch E, et al. Methodology of photic stimulation revisited: updated European algorithm for visual stimulation in the EEG laboratory. Epilepsia. 2012;53:16‐24. [DOI] [PubMed] [Google Scholar]
- 25. Nordli DR, Riviello J, Niedermeyer E. Seizures and epilepsy in infants to adolescents In: Schomer DL, Da Silva FL, eds. Niedermeyer's Electroencephalography. Basic Principles, Clinical Applications and Related Fields. Philadelphia PA: Lippincott Williams & Williams; 2011:479‐540. [Google Scholar]
- 26. Tatum WO, Husain AM, Benbadis SR, Kaplan P.W. Handbook of EEG interpretation. Demos; 2008. [Google Scholar]
- 27. Leigh EJ, Mackillop E, Robertson ID, Hudson LC. Clinical anatomy of the canine brain using magnetic resonance imaging. Vet Radiol Ultrasound. 2008;49:113‐121. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt MJ, Kramer M. MRT atlas ZNS‐Befunde bei Hund und Katze. Enke. 2015;33‐55:219‐225. [Google Scholar]
- 29. De Lahunta A, Glass E, Kent M. Veterinary Neuroanatomy and Clinical Neurology. 4th ed. St Louis, MO: Elsevier; 2015:6‐42. [Google Scholar]
- 30. Evans HE, de Lahunta A. Miller's Anatomy of the Dog. 4th ed. St Louis, MO: Elsevier; 2013:681‐684. [Google Scholar]
- 31. Ladino LD, Moien F, Tellez‐Zenteno JF. A comprehensive review of temporal lobe epilepsy Neurological Disorders. Clinical Methods. 1st ed. ISBN 978‐1‐922227‐74‐4: iConcept Press; 2016:1‐35. [Google Scholar]
- 32. Abou–Khalil B, Misulis KE. Atlas of EEG and Seizure Semiology. Philadelphia, PA: Elsevier; 2006. [Google Scholar]
- 33. Javidan M. Electroencephalography in mesial temporal lobe epilepsy: a review. Epilepsy Res Treat. 2012;2012:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pellegrino FC, Sica RE. Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol. 2004;115:477‐487. [DOI] [PubMed] [Google Scholar]
- 35. Chassoux F, Semah F, Bouilleret V. Metabolic changes and electro‐clinical patterns in mesio‐temporal lobe epilepsy: a correlative study. Brain. 2004;127:164‐174. [DOI] [PubMed] [Google Scholar]
- 36. Lv R‐J, Sun Z‐R, Cui T, et al. Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy. BMC Neurol. 2014;14:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. James FMK, Cortez MA, Monteith G, et al. Diagnostic utility of wireless video—electroencephalography in unsedated dogs. J Vet Intern Med. 2017;31:1469‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh P, Kaur R, Saggar K, Singh G, Aggarwal S. Amygdala volumetry in patients with temporal lobe epilepsy and normal magnetic resonance imaging. Pol J Radiol. 2016;81:212‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coan AC, Morita ME, De Campos BM, Yasuda CL, Cendes F. Amygdala enlargement in patients with mesial temporal lobe epilepsy without hippocampal sclerosis. Front Neurol. 2013;4:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheon JE, Chang KH, Kim HD, et al. MR of hippocampal sclerosis: comparison of qualitative and quantitative assessments. AJNR Am J Neuroradiol. 1998;19:465‐468. [PMC free article] [PubMed] [Google Scholar]
- 41. Mitsueda‐Ono T, Ikeda A, Sawamoto N, et al. Internal structural changes in the hippocampus observed on 3‐tesla MRI in patients with mesial temporal lobe epilepsy. Intern Med. 2013;52:877‐885. [DOI] [PubMed] [Google Scholar]
- 42. Cascino GD, Trenerry MD, So EL, et al. Routine EEG and temporal lobe epilepsy: relation to long‐term EEG monitoring, quantitative MRI, and operative outcome. Epilepsia. 1996;37:651‐656. [DOI] [PubMed] [Google Scholar]
- 43. Martlè V, Van Ham L, Raedt R, et al. Non‐pharmacological treatment options for refractory epilepsy: an overview of human treatment modalities and their potential utility in dogs. Vet J. 2014;199:332‐339. [DOI] [PubMed] [Google Scholar]
- 44. Wieser HG. Presurgical diagnosis of epilepsies—concepts and diagnostic tools. J Epileptol. 2016;24:115‐140. [Google Scholar]


