Abstract
Background
Intestinal absorption of bile acids is mediated by the apical sodium‐dependent bile acid transporter (ASBT). Fecal bile acid dysmetabolism has been reported in dogs with chronic inflammatory enteropathy (CIE).
Objective
Characterization of ASBT distribution along the intestinal tract of control dogs and comparison to dogs with CIE.
Animals
Twenty‐four dogs with CIE and 11 control dogs.
Methods
The ASBT mRNA and protein expression were assessed using RNA in situ hybridization and immunohistochemistry, respectively. The concentrations of fecal bile acids were measured by gas chromatography‐mass spectrometry. The fecal microbiota dysbiosis index was assessed with a quantitative polymerase chain reaction panel.
Results
In control dogs, ASBT mRNA expression was observed in enterocytes in all analyzed intestinal segments, with highest expression in the ileum. The ASBT protein expression was restricted to enterocytes in the ileum, cecum, and colon. Dogs with CIE had significantly decreased expression of ASBT protein in the ileum (P = .001), which was negatively correlated with histopathological score (ρ = −0.40; P corr = .049). Additionally, dogs with CIE had a significantly increased percentage of primary bile acids in feces compared to controls (P = .04). The fecal dysbiosis index was significantly higher in dogs with CIE than in control dogs (P = .01).
Conclusions and Clinical Importance
These findings indicate that ileal protein expression of ASBT is downregulated in dogs with CIE. This change may be linked to the inflammatory process, intestinal dysbiosis, and fecal bile acid dysmetabolism observed in these patients.
Keywords: dysbiosis, ileal bile acid transporter, inflammatory bowel disease, SLC10A2
Abbreviations
- ASBT
apical sodium‐dependent bile acid transporter
- BA
bile acid
- C4
7a‐hydroxycholest‐4‐en‐3one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CIBDAI
canine inflammatory bowel disease activity index
- CIE
chronic inflammatory enteropathy
- DCA
deoxycholic acid
- DI
dysbiosis index
- ESAVI
Finnish National Animal Experiment Board
- FFPE
formalin‐fixed paraffin‐embedded
- GC/MS
gas chromatography‐mass spectrometry
- IBD
inflammatory bowel disease
- IACUC
Institutional Animal Care and Use Committee
- IgG
immunoglobulin G
- ISH
in situ hybridization
- LCA
lithocholic acid
- SLC10A2
solute carrier family 10, member 2
- UDCA
ursodeoxycholic acid
1. INTRODUCTION
Chronic inflammatory enteropathy (CIE) in dogs is characterized by gastrointestinal signs such as vomiting and diarrhea that persist for more than 3 weeks, histologic findings of intestinal inflammation, and exclusion of known specific causes (eg, infectious, neoplastic, and extra‐gastrointestinal diseases).1, 2 Based on response to treatment, CIE can be classified as food‐responsive, antibiotic‐responsive, or steroid‐responsive.2, 3 Bile acid (BA) dysmetabolism, characterized by an increased proportion of primary BAs in the feces, has been described in human patients with inflammatory bowel disease (IBD)4, 5 and in dogs with CIE.6, 7, 8 Bile acid malabsorption in people and rodents can cause diarrhea because of increased colonic secretion of water and electrolytes, increased intestinal permeability, and impaired lipid digestion.9
Primary BAs, namely cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized from cholesterol by hepatocytes, and subsequently conjugated with glycine or taurine before excretion in bile into the small intestinal lumen.10 Approximately 90% of conjugated BAs are actively reabsorbed in the ileum through the apical sodium‐dependent bile acid transporter (ASBT) and returned to the liver via the portal system.10 Bile acids can be deconjugated by the gut microbiota in the distal small intestine and large intestine.10 After deconjugation, the microbiota can 7α‐dehydroxylate CA and CDCA to form the secondary BAs deoxycholic acid (DCA) and lithocholic acid (LCA), respectively.10
Bile salt hydrolases responsible for BA deconjugation are produced primarily by Gram‐positive commensal bacteria.11 The generation of secondary BA by 7α‐dehydroxylation is restricted to a narrow phylogenetic group of commensal bacteria11 within Clostridium clusters XIVa and VI11 and Eubacterium species.12 In both humans and dogs, IBD and CIE are associated with intestinal dysbiosis,13, 14 which may cause impaired BA metabolism because of defective biotransformation.5
The ileum is considered the main site of BA uptake in many mammalian species15 and the ASBT, expressed primarily in this site, is considered the major pathway for intestinal uptake of conjugated primary BAs in rodents and humans.10 The ASBT can be found in lower abundance in other portions of the small and large intestine,16 as well as on cholangiocytes in the liver17 and on the epithelium of the proximal renal tubules.18 Physiologically, ASBT transcription is downregulated by activation of the nuclear BA receptor in enterocytes, known as farnesoid X receptor, by BAs in the intestinal lumen.8 Studies in people with IBD have identified decreased expression of ASBT19, 20, 21 because of repression of gene expression by inflammatory cytokine signaling.22
Our objectives were to characterize the distribution of ASBT expression along the canine gastrointestinal tract and to compare ASBT mRNA and protein expression between dogs with CIE and control dogs. Additionally, associations among ASBT expression with the canine inflammatory bowel disease activity index (CIBDAI), histopathological scores, fecal BAs, and fecal dysbiosis index (DI) were investigated.
2. MATERIAL AND METHODS
2.1. Ethics approval
The protocols for sample collection from CIE dogs were reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee (IACUC, Animal use protocols 2012‐083 and 2015‐0069) or by the Finnish National Animal Experiment Board (ESAVI/6973/04.10.03/2011 and ESAVI/10384/04.10.07/2014), for dogs enrolled at Texas A&M University or the University of Helsinki, respectively. Written consent was obtained from each owner. Dogs in the control group were euthanized for reasons unrelated to this study and the bodies were donated for teaching and research purposes; the post‐euthanasia collection of samples was exempted by the Texas A&M University IACUC.
2.2. Animal population and samples
The control group consisted of 11 privately owned dogs without clinical signs of gastrointestinal disease or histological intestinal lesions. None had been treated with ursodeoxycholic acid (UDCA), antibiotics, or immunomodulatory drugs in the preceding 6 months. All of the control dogs were presented to the Texas A&M Veterinary Teaching Hospital with traumatic injuries and were euthanized at the owners' request. Full‐thickness intestinal samples were collected within 20 minutes of euthanasia, fixed in formalin, and processed for histology. Fecal samples were collected and stored in a −80°C freezer until processed. Formalin‐fixed paraffin‐embedded (FFPE) samples of duodenum, jejunum, ileum, cecum, and colon from 6 control dogs were used to characterize the distribution of ASBT mRNA (in situ hybridization [ISH]) and protein expression (immunohistochemistry) along the gastrointestinal tract. Immunohistochemistry and ISH also were performed in samples of ileum and colon from all 11 control dogs for comparison to CIE dogs.
Twenty‐four client‐owned dogs with CIE were included in this study. Affected dogs had gastrointestinal signs (eg, vomiting, diarrhea, tenesmus, hematochezia, or weight loss) for >3 weeks, with intestinal inflammation and exclusion of other possible causes of these signs (eg, infectious, neoplastic, or extra‐gastrointestinal diseases) by standard examinations (hematology, serum biochemistry profile, fecal analysis, abdominal ultrasonography, gastrointestinal endoscopy or laparoscopy, and histology). The severity of clinical signs at presentation was evaluated by the attending veterinarian using the CIBDAI scoring system (determined by the dog's attitude, activity, appetite, vomiting, fecal consistency, defecation frequency, and weight loss; cumulative score ranges from 0 to 18).23 Serum albumin concentration was determined in all dogs with CIE and serum cobalamin concentration was measured in 11/24. None of the CIE dogs had received immunomodulatory drugs or UDCA in the month before sample collection. Most of the CIE dogs (21/24) had not received antibiotics in the month before sample collection. Three dogs had received antibiotics within 7 days of sample collection; these individuals were not included in the portion of the study that evaluated the fecal microbiota and concentrations of fecal BAs. Endoscopic (20/24) or full‐thickness (4/24) biopsy samples were obtained from the ileum and colon of all dogs with CIE. Histological changes were classified according to the World Small Animal Veterinary Association Gastrointestinal Standardization Group histopathologic criteria using a grading system (0 = normal, 1 = mild lesions, 2 = moderate lesions, and 3 = severe lesions).24 A cumulative histopathological score calculated as the sum of individual lesions scores was assigned to the ileum (0‐30) and colon (0‐24) for each CIE dog. Fecal samples stored at −80°C were available for 11/24 CIE dogs and were used to measure fecal BA concentrations and to determine the DI.
2.3. Immunohistochemistry and image analysis
Immunohistochemistry was performed on samples of ileum and colon from 24 dogs with CIE and 11 control dogs. The FFPE samples were cut at 3 μm, adhered to charged slides, and deparaffinized. Heat‐induced antigen retrieval was performed, followed by blocking of endogenous peroxidases. Sections were incubated with a goat anti‐mouse solute carrier family 10, member 2 (SLC10A2) polyclonal antibody (#PA5‐18990; Thermo Fisher Scientific, Rockford, Illinois) diluted 1:300 for 1 hour. Sections were incubated with goat immunoglobulin G (IgG) in lieu of the primary antibody as negative controls. After incubation, slides were washed and then incubated with a mouse monoclonal anti‐goat IgG‐biotin secondary antibody (#sc‐2023; Santa Cruz Biotechnology, Dallas, Texas). Slides then were incubated with avidin and biotinylated horseradish peroxidase. The target antigen was revealed by incubation in peroxidase substrate and 3,3′‐diaminobenzidine chromogen. Slides were counterstained with Mayer's hematoxylin and were mounted using a xylene‐based medium. Ten random fields in regions of well‐oriented villi were captured at ×400 magnification per slide with a digital camera for bright field microscopy (DP73; Olympus, Tokyo, Japan) by using cellSens standard software (Olympus, Tokyo, Japan). Images were analyzed by an immunohistochemistry image analysis toolbox for ImageJ software.25 The area corresponding to the immunolabeling was automatically evaluated by the plug‐in and the number of pixels corresponding to the labeled area was recorded for each image. The mean number of pixels corresponding to the immunolabeled area was calculated for the ileum and colon of each case.
2.4. In situ hybridization and scoring
For specific detection of ASBT mRNA, RNA ISH using 20 probes targeting region 89‐1032 of canine SLC10A2 mRNA (NM_001002968.1) was used. The ISH was performed on samples of ileum and colon from 24 dogs with CIE and 11 control dogs. The FFPE samples were cut at 3 μm, mounted on charged slides, and the RNAscope 2.5 HD red assay was performed according to the manufacturer's protocol (RNAscope; Advanced Cell Diagnostics, Hayward, California). Consecutive sections were incubated with a positive control probe targeting canine RNA polymerase II subunit A to verify RNA quality and a nonspecific bacterial RNA (dapB gene) probe was used as a negative control probe. Chromogenic detection with fast red was performed using alkaline phosphatase‐based detection. The final deposits were red dots or clusters, with each dot corresponding to an mRNA copy.26 Ten random fields in areas of villi were captured at ×400 magnification for each slide with a digital camera (DP73; Olympus) for bright field microscopy by using cellSens standard software (Olympus). The number of dots/cell and clusters was manually counted. The labeling was categorized into 6 scores: (0) negative, no staining or < 1 dot per 10 cells; (1) minimal, 1‐3 dots/cell; (2) mild, 4‐10 dots/cell with up to 10% of the dots forming clusters, (3) moderate, 10‐15 dots/cell with 10%‐20% of the dots forming clusters, (4) marked, 15‐20 dots/cells with 20%‐30% of the dots forming clusters, and (5) diffuse, with >20 dots/cell and >30% of the dots forming clusters. The mean score was calculated for the ileum and colon of each dog.
2.5. Fecal bile acids
The concentrations of fecal BAs were measured in 11 dogs with CIE with available feces and 11 control dogs, utilizing methods established by Guard.8 Fecal samples were kept frozen at −80°C until lyophilization (Labconco FreeZone 2.5 Plus, Kansas City, Missouri). Approximately 10‐15 mg of lyophilized feces were used for downstream extraction. A total volume of 200 μL of butanol containing the internal standards CA‐d4 and LCA‐d4 was added to each fecal sample. Twenty microliters of 37% HCl then was added for a final volume of 220 μL and vortexed for 30 seconds. Samples then were capped and incubated at 65°C for 4 hours. Next, samples were evaporated under nitrogen gas until dryness at 65°C for approximately 25 minutes. Two‐hundred microliters of derivatization agent (HMDS+TMCS+Pyridine, 3:1:9, Sigma‐Aldrich, St. Louis, Missouri) then were added to each sample and incubated at 65°C for 30 minutes. After incubation, samples again were evaporated under nitrogen gas until dryness at 65°C (approximately 25 minutes). Samples then were resuspended in 200 μL of hexane, vortexed briefly, and centrifuged at 4°C for 10 minutes at 3000 relative centrifugal force. An 80 μL aliquot then was transferred to a gas chromatography‐mass spectrometry (GC/MS) vial and the vial was capped for further downstream analysis. A GC/MS system (6890N and 5975 inert Mass Selective Detector, Agilent, Santa Clara, California) was used as described previously.8, 27 Deconjugated fecal BAs CA, CDCA, LCA, DCA, and UDCA were measured. The concentrations of BAs were calculated according to the original weight of the aliquot to normalize for variable starting fecal weights. Bile acid data were reported in μg/mg of lyophilized fecal content in addition to being expressed as a percent of total BAs measured. The deconjugated primary BAs CA and CDCA were combined to represent total primary BAs measured, and LCA, DCA, and UDCA were combined to represent total secondary BAs.
2.6. Fecal dysbiosis index
The fecal DI was assessed in 11 dogs with CIE with available feces and 11 control dogs. The DNA was extracted from samples of 100 mg of feces using the MoBio Power soil DNA isolation kit (QIAGEN Inc., Germantown, Maryland) according to the manufacturer's instructions. A quantitative polymerase chain reaction (qPCR) panel consisting of 8 bacterial groups: total bacteria, Faecalibacterium, Turicibacter, Escherichia coli, Streptococcus, Blautia, Fusobacterium, and Clostridium hiranonis was performed as previously described.28 The qPCR data were expressed as the log amount of DNA (fg) for each particular bacterial group/10 ng of isolated total DNA. Results were imported into a mathematical algorithm for the calculation of a single numerical value, the DI. A negative DI indicates normobiosis, whereas a positive number indicates dysbiosis.28
2.7. Statistical analyses
The datasets were tested for normality and equality of variances using a Shapiro Wilk's test and the Brown‐Forsythe test, respectively. The labeled area on immunohistochemistry, ISH scores, fecal BA concentrations and percentages, and DI in dogs with CIE and controls were compared using a Mann‐Whitney U test or Student's t test where appropriate. A nonparametric Spearman rank correlation coefficient ρ was calculated to test for possible correlations between ASBT protein and mRNA expression, CIBDAI scores, cumulative histopathological scores, serum albumin concentrations, serum cobalamin concentrations, percentage of individual and combined fecal BAs, fecal DI, and individual log values for analyzed bacteria in dogs with CIE. Tests were performed by the JMP software (JMP 13, SAS software Inc., Cary, North Carolina), with a significant P value or ρ correlation coefficient set as ≤.05.
3. RESULTS
3.1. Study population
The CIE group consisted of 24 dogs (13 males/11 females; median age, 4.7 years; age range, 0.5‐10 years). Breeds most commonly represented included German shepherd (n = 4), Poodle (n = 2), and mixed breed (n = 2). Median body weight was 21.1 kg (range, 2.6‐66.8 kg). Median CIBDAI score was 5.5 (range, 2‐11.5). Median serum albumin concentration was 2.8 g/dL (range, 1.6‐3.71 g/dL), with serum albumin concentration <2.0 g/dL in 2/24 dogs. Median serum cobalamin concentration was 356 ng/L (range, 150‐1000 ng/L; reference interval, 251‐908 ng/L); 2/11 dogs were hypocobalaminemic. The median histopathological score was 4 out of 30 (range, 0‐11) for the ileum and 3 out of 24 (range, 1‐8) for the colon.
The control group was composed of 11 dogs (6 males/5 females; median age, 5.5 years; range, 1‐13 years). Breeds most commonly represented were mixed breed (n = 3) and Dachshund (n = 2). Median body weight was 15.8 kg (range, 2.3‐32 kg). The ages (P = .81) and body weights (P = .30) of control dogs were not statistically different from dogs with CIE.
3.2. ASBT mRNA and protein expression in the intestinal tract of control dogs
On immunohistochemistry, ASBT protein was identified in the ileum, cecum, and colon. The duodenum and jejunum were negative. The ileum had the highest levels of ASBT immunolabeling, followed by the colon and cecum. Within the cellular compartment, immunolabeling was located within the apical membrane of enterocytes. In the ileum (Figure 1A), the labeling was continuous and homogeneous in enterocytes of the villi, but absent in the crypts. In the colon (Figure 2A) and cecum, the labeling was multifocal and restricted to superficial enterocytes.
Figure 1.
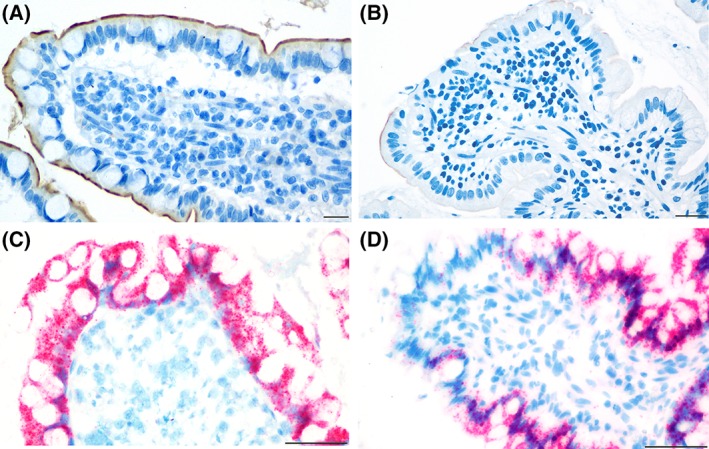
Distribution of immunolabeling (in brown) for the apical sodium‐dependent bile acid transporter (ASBT) protein (A and B) and in situ hybridization (in red) for ASBT mRNA (C and D) in the ileum. In control dogs (A), immunolabeling in the apical membrane of the enterocytes was continuous. ASBT was minimally expressed in dogs with chronic inflammatory enteropathy (CIE) (B). ASBT mRNA expression was observed in both the nucleus and cytoplasm of enterocytes of control dogs (C) and dogs with CIE (D). Scale bar is equal to 20 μm, magnification of ×400 (A and B) or 50 μm, magnification of ×400 (C and D)
Figure 2.
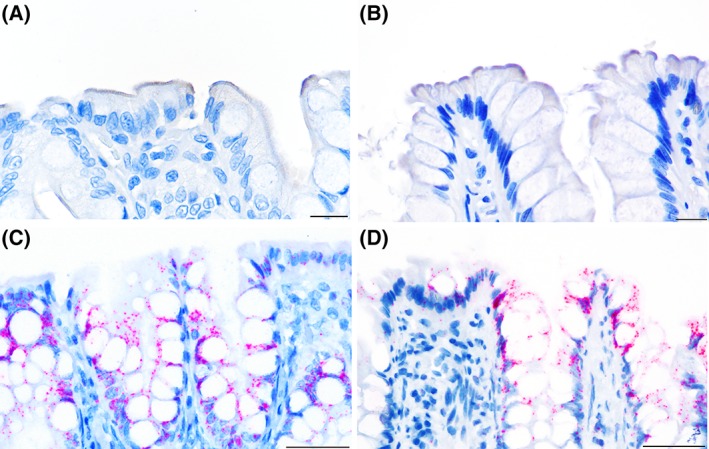
Distribution of immunolabeling (in brown) for the ASBT protein (A and B) and in situ hybridization (in red) for ASBT mRNA (C and D) in the colon. In both control dogs (A) and dogs with CIE (B), the immunolabeling in the apical membrane of the superficial colonocytes was multifocal. ASBT mRNA expression was observed in both the nucleus and cytoplasm of superficial and cryptal colonocytes and was similar between control dogs (C) and dogs with CIE (D). Scale bar is equal to 15 μm, magnification of ×600 (A and B) or 50 μm, magnification of ×400 (C and D)
On ISH, ASBT mRNA expression was distributed in enterocytes throughout the villi in the small intestine, in the mucosa of the large intestine, and in the crypts of all intestinal segments. Expression of ASBT mRNA was minimal in the duodenum and jejunum, marked to diffuse in the ileum (Figure 1C), and mild to moderate in the cecum and colon (Figure 2C). Within the cell compartment, ASBT mRNA was located both in the nucleus and cytoplasm of enterocytes.
3.3. ASBT mRNA and protein expression in the ileum and colon of CIE dogs
The location and distribution of the immunolabeling and mRNA expression in CIE dogs were the same as described in the control dogs. However, CIE dogs had significantly decreased immunolabeling for ASBT protein in the ileum (median, 206 pixels; range, 0‐17 809 pixels; P < .001; Figures 1B and 3A) compared to control dogs (median, 6191 pixels; range, 800‐21 955 pixels; Figure 1A). At mRNA levels, ASBT expression in the ileum of CIE dogs (Figures 1D and 3B) was numerically lower (median score, 3.21; range, 0.8‐4.85), but not significantly different from control dogs (median score, 4.35; range, 3‐4.95; P = .06; Figure 1C).
Figure 3.
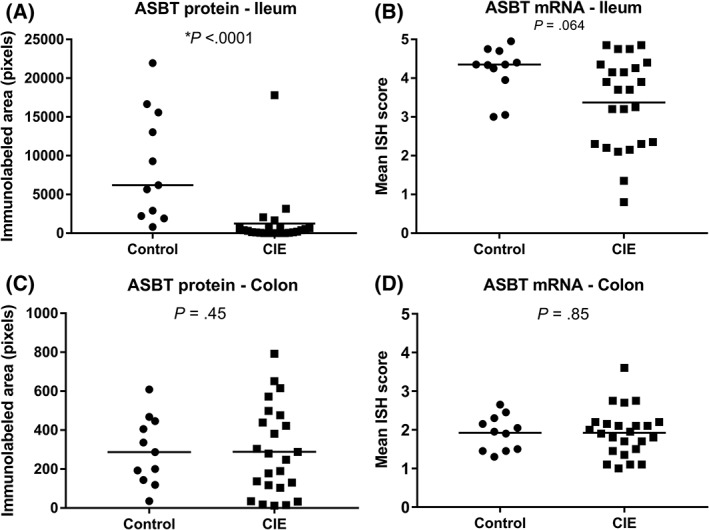
Comparison of ASBT protein and mRNA expression in the ileum and colon between control dogs and dogs with CIE. In the ileum, the median immunolabeled area for ASBT protein (A) was significantly decreased in dogs with CIE (P < .001) when compared to control dogs. ASBT mRNA expression in the ileum (B) was not significantly higher in control dogs than in CIE dogs (P = .06). In the colon, ASBT protein (C) (P = .45) and mRNA (D) (P = .85) expression was similar for dogs with CIE and controls. Bars represent the median. *Significantly different
In the colon, the protein levels of ASBT in CIE dogs (median, 189 pixels; range, 12‐792 pixels; Figures 2A,B and 3C) did not differ from controls (median, 287 pixels; range, 35‐608 pixels; P = .45). Similarly, no differences were detected for mRNA expression of ASBT in the colon between control (median score, 1.9; range 1.3‐2.45) and CIE dogs (median score, 1.9; range 1‐3.6; P = .85; Figures 2C,D and 3D).
3.4. Fecal bile acids
The total concentration of BAs in feces was similar between control (median, 5.8 μg/mg; range, 1.69‐46.14 μg/mg) and CIE dogs (median, 10 μg/mg; range, 2.71‐18.55 μg/mg; P = .35; Figure 4A). The CIE dogs had an increased percentage of fecal primary BAs (median, 30.5%; range, 1.7%‐99%) when compared to control dogs (median, 8.2%; range, 1.5%‐95%; P = .04; Figure 4B). The proportion of CDCA levels was increased (median, 7%; range, 0.4%‐18.8% P = .005; Figure 4C) in CIE dogs when compared to control dogs (median, 2.5%; range, 0.1%‐6.4%). Dogs with CIE had a decreased percentage of fecal LCA (median, 4.7%; range, 0.1%‐17.5%; P = .03;Figure 4D) compared to control dogs (median, 11.8%; range, 0.1%‐21.8%).
Figure 4.
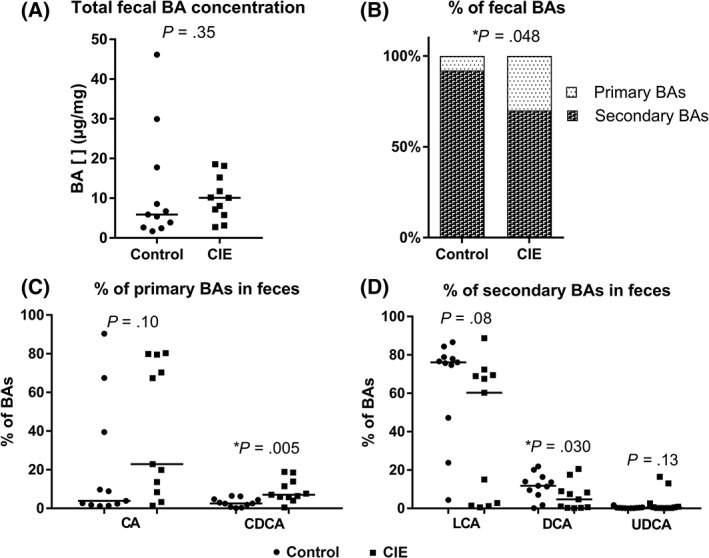
Composition of the bile acid (BA) pool in the feces of control dogs (circles) and dogs with CIE (squares). (A) The total fecal BA concentration is similar between the 2 groups (P = .35). (B) The percentage of primary BAs is significantly higher in dogs with CIE (P = .04) than in control dogs. (C) The percentage of chenodeoxycholic acid (CDCA) is significantly higher in CIE dogs (P = .005) than in control dogs. (D) The percentage of litocholic acid (LCA), deoxycholic acid (DCA) is significantly lower CIE dogs (P = .03) than in control dogs. All results are expressed as the median. [], concentration; ns, non‐significant; CA, cholic acid; LCA, litocholic acid; UDCA, ursodeoxycholic acid. *Significantly different
3.5. Fecal dysbiosis index
Dogs with CIE had a higher fecal DI (mean ± SD, 1.77 ± 4.46, P = .01) than did the control dogs (mean ± SD, −2.17 ± 1.7; Figure 5). However, the individual log values for each bacterial group included in the DI were not significantly different between the 2 groups (Table 1).
Figure 5.
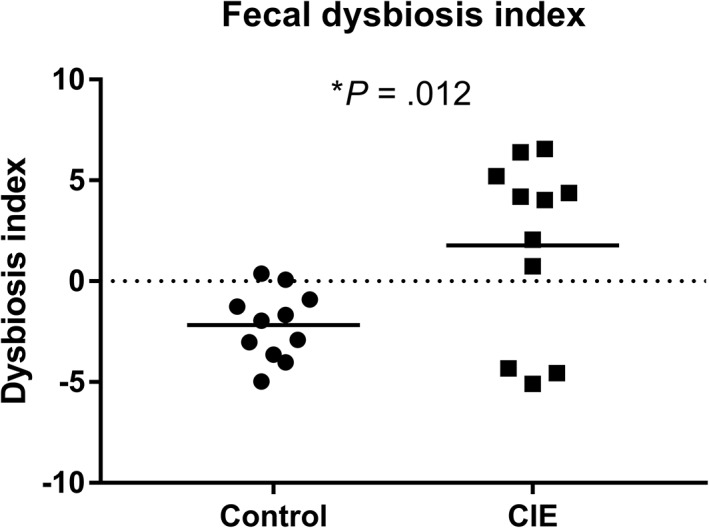
The composition of the fecal microbiota is represented as a dysbiosis index (DI). The median DI for dogs with CIE was significantly higher than for controls (P = .01). Bars represent the mean. *Significantly different
Table 1.
Dysbiosis index (DI) and qPCR results for its 8 components. This table shows a comparison of the DI and the abundances of its 8 components between control dogs and dogs with chronic inflammatory enteropathy (CIE). The abundances are based on quantitative polymerase chain reaction (qPCR) assays and are expressed as the mean values of the log10 value ± SD
| All bacteria | Faecalibacterium | Turicibacter | Streptococcus | E. coli | Blautia | Fusobacterium | C. hiranonis | DI | |
|---|---|---|---|---|---|---|---|---|---|
| CIE | 10.79 ± 0.60 | 5.07 ± 0.92 | 5.65 ± 1.01 | 6.12 ± 1.96 | 7.18 ± 1.52 | 9.87 ± 1.22 | 8.22 ± 1.23 | 3.95 ± 2.78 | 1.77 ± 4.46* |
| Control | 10.77 ± 0.31 | 6.02 ± 1.29 | 6.33 ± 1.29 | 4.98 ± 1.29 | 6.07 ± 1.59 | 10.34 ± 0.57 | 9.02 ± 0.89 | 6.06 ± 1.66 | −2.17 ± 1.7 |
Significantly different, P = .01.
3.6. Correlation between ASBT expression, histopathological scores, fecal BA composition, DI, serum cobalamin concentration and serum albumin concentration
The strongest associations found included a positive correlation between the percentage of primary BAs in the feces of CIE dogs with the fecal DI (ρ = 0.65; P corr = .02), and between the log values of C. hiranonis and the percentage of secondary BAs (ρ = 0.75; P corr = .007). A positive relationship was found between the ASBT protein and mRNA expression levels in the ileum of CIE dogs (ρ = 0.41; P corr = .04). The cumulative histopathological score in the ileum was negatively correlated with the protein expression of ASBT in the ileum (ρ = −0.40; P corr = .04). No significant correlation was found between clinical disease severity (CIBDAI scores) and ASBT protein expression in the ileum or colon, respectively (ρ = 0.27, P corr = .19; ρ = 0.21, P corr = .31). Similarly, no relationship was observed between the ASBT protein expression in the ileum of CIE dogs and the serum concentrations of cobalamin or albumin, respectively (ρ = −0.004, P corr = .98; ρ = 0.25, P corr = .24; Supporting Information Table S1).
4. DISCUSSION
Our study indicated that the ileum is the intestinal segment with the highest expression of ASBT mRNA and protein in dogs. Dogs with CIE had significantly decreased protein levels of ASBT in the ileum when compared to control dogs. In addition, bacterial dysbiosis and an increased percentage of primary BAs were identified in the feces of dogs with CIE.
The ASBT distribution in the control dogs was similar to that described in humans16 and rodents.22, 29 As in other species, the ileum appears to be the main intestinal segment responsible for BA uptake in dogs. In most laboratory species, including the mouse,29 rat,30 and hamster,31 the distribution of ASBT in the intestine is limited to the terminal ileum. In humans, however, ASBT also is expressed in the duodenum32 and colon33 at both mRNA and protein levels. In our study, low mRNA levels of ASBT were observed in the proximal small intestine (ie, duodenum and jejunum), but ASBT protein was not detected in these segments. These findings may be related to the fact that gene expression techniques, such as ISH, are more sensitive than immunohistochemistry or because the levels of the expressed gene are not high enough for protein translation.34 Alternatively, ASBT may be transcribed, but not translated, in the proximal small intestine of dogs.
In our study, protein levels of ASBT in the ileum of dogs with CIE correlated inversely with histopathological scores, indicating low ASBT expression in cases with severe mucosal inflammation and morphologic disruption of enterocytes. This observation can be explained by inhibition of ASBT gene expression by inflammatory cytokines,22 but also may reflect cell damage and loss of transporters. The inflammatory process is considered a major mechanism for clinical signs in human patients with IBD and dogs with CIE.1 However, it has been hypothesized that poor BA absorption because of ASBT inhibition may directly contribute to diarrhea in people with IBD.9 Lower ASBT expression is reported primarily in human patients with ileal inflammation, but also can be observed in patients with inflammation limited to the colon.21, 35 The method of sample collection (ie, endoscopic or full‐thickness biopsies) did not interfere with the analysis of ASBT expression because the distribution of ASBT mRNA and protein was limited to epithelial cells within the mucosa. Although a positive correlation was observed between mRNA expression and protein expression of ASBT in the ileum, the difference in mRNA levels between dogs with CIE and control dogs did not reach significance. This finding could be explained by post‐transcriptional modifications of mRNA36 or could reflect protein loss associated with epithelial injury secondary to inflammation.37
Because the dogs with CIE had decreased ASBT protein expression in the ileum, one might expect BA malabsorption with increased loss of fecal BAs, as described in people with irritable bowel syndrome38 or Crohn's disease.39 However, in our study, the total concentration of fecal BAs in CIE dogs was similar to that of the controls. In humans, various methods have been used to diagnose BA malabsorption, including measurement of fecal BA concentrations, determination of retention of labeled BA analogs such as selenium homotaurocholic acid, and measurement of plasma concentrations of metabolites from BA synthesis such as lathosterol, 7a‐hydroxycholesterol, or 7a‐hydroxycholest‐4‐en‐3one (C4).40, 41 To date, only 1 report has addressed this issue in dogs with CIE, in which serum C4 concentrations suggested BA malabsorption in 3/17 dogs.42 Although our study did not identify overt BA malabsorption in dogs with CIE, the increased percentage of primary BAs in feces does suggest BA dysmetabolism. This also was identified in dogs with CIE in another recent study.8 Similar to our findings, increased proportions of CDCA and decreased proportions of DCA have been reported in fecal samples of human patients with irritable bowel syndrome.43 The increased proportion of primary BAs could be explained by a compensatory increase in de novo synthesis of BAs by hepatocytes43 or by decreased bacterial biotransformation.11
The intestinal microbiota is the sole metabolic pathway for BA transformation.43 Thus, intestinal dysbiosis with a decrease in bacteria with bile salt hydroxylase activity can lead to decreased deconjugation and dehydroxylation of BAs.11 Although the individual values for the bacterial taxa analyzed in this study were not significantly different, the DI was a reliable indicator of dysbiosis in the dogs with CIE. The abundance of a single taxon may not consistently distinguish between health and disease,28 and for this reason the DI is preferred. The positive correlation between the abundance of C. hiranonis and the percentage of secondary BAs in feces suggests that this bacterium might play an important role in BA dehydroxylation in dogs.28, 44 In a previous study, C. hiranonis was found to be decreased in the feces of dogs with CIE.28
Because dogs with CIE have decreased ASBT protein levels in the ileum, therapies that reestablish ASBT expression might be beneficial.21 Corticosteroids are used routinely in the treatment of humans with IBD and dogs with CIE10, 45 and have been demonstrated to restore ASBT expression in people,21 either by decreasing the levels of inflammatory cytokines that repress ASBT expression or by direct induction of ASBT transcription via activation of glucocorticoid receptors.10 Further studies are needed to better characterize ASBT expression in different types of CIE in dogs (ie, steroid‐responsive, food‐responsive, and antibiotic‐responsive). In our study, fecal BAs and DI were evaluated only in 11 dogs with CIE and 11 control dogs, and further studies with larger numbers of animals are needed to confirm these findings.
In conclusion, our study indicates that levels of ASBT protein are downregulated in the ileum of dogs with CIE, most likely as a consequence of sustained inflammation. Additionally, we established a relationship among ASBT expression, fecal BA dysmetabolism, and fecal dysbiosis in dogs with CIE.
CONFLICT OF INTEREST DECLARATION
The authors Blake C. Guard, Amanda B. Blake, Jonathan A. Lidbury, Jörg M. Steiner, and Jan S. Suchodolski are affiliated with the Gastrointestinal Laboratory, Texas A&M University, which offers gastrointestinal assays on a fee‐for‐service basis.
Supporting information
Supporting Information Table S1 Correlation between ASBT expression, clinical, laboratory, and histopathological findings in dogs with CIE.
ACKNOWLEDGMENTS
Paula R. Giaretta has a fellowship from the Brazilian National Council for Scientific and Technological Development (CNPq). The authors thank the Finnish Center for Laboratory Animal Pathology (FCLAP) for providing unstained histologic slides. The technical assistance of So Young Park, Michelle Jonika, and Laura Parikka is gratefully acknowledged. Dogs with CIE were enrolled in the study and the samples collected at the Faculty of Veterinary Medicine at University of Helsinki in Finland, at the Gastrointestinal Laboratory at Texas A&M University in the United States, or at one of several other referral hospitals across the United States. Sample analyses were performed at Texas A&M University and University of Helskinki. Data analysis and manuscript writing were done at Texas A&M University. Part of these data will be presented as a research report at the 2018 American College of Veterinary Pathologists Annual Meeting, Washington, DC.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study design was reviewed and approved by the IACUC (animal use protocols 2012‐083 and 2015‐0069) or by the Finnish National Animal Experiment Board (ESAVI/6973/04.10.03/2011 and ESAVI/10384/04.10.07/2014), for dogs enrolled at Texas A&M University or the University of Helsinki, respectively. The post‐euthanasia collection of samples from control dogs was exempted by the Texas A&M IACUC.
Giaretta PR, Rech RR, Guard BC, et al. Comparison of intestinal expression of the apical sodium‐dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J Vet Intern Med. 2018;32:1918–1926. 10.1111/jvim.15332
Funding information: Texas A&M University; University of Helsinki
REFERENCES
- 1. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;32:1918‐1926. [DOI] [PubMed] [Google Scholar]
- 2. Dandrieux JR. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract. 2016;57:589‐599. [DOI] [PubMed] [Google Scholar]
- 3. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178:368‐369. [DOI] [PubMed] [Google Scholar]
- 4. Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile‐acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531‐539. [DOI] [PubMed] [Google Scholar]
- 5. Lenicek M, Duricova D, Komarek V, et al. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis. 2011;17:1322‐1327. [DOI] [PubMed] [Google Scholar]
- 6. Honneffer J, Guard B, Steiner JM, et al. Untargeted metabolomics reveals disruption within bile acid, cholesterol, and tryptophan metabolic pathways in dogs with idiopathic inflammatory bowel disease. 2015 digestive diseases week abstracts. Gastroenterology. 2015; 148:S‐715 (abstract). [Google Scholar]
- 7. Guard BC, Honneffer JB, Jonika MM, et al. Longitudinal characterization of dysbiosis and unconjugated bile acid profiles in the feces of dogs with inflammatory bowel disease. 2017 digestive diseases week abstracts. Gastroenterology. 2017; 152:S992 (abstract). [Google Scholar]
- 8. Guard BC. Microbial Characterization, Metabolomic Profiling, and Bile Acid Metabolism in Healthy Dogs and Dogs with Chronic Enteropathy [PhD thesis], College Station, TX: Texas A &M University; 2017.
- 9. Vitek L. Bile acid malabsorption in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:476‐483. [DOI] [PubMed] [Google Scholar]
- 10. Dawson PA. Bile formation and the enterohepatic circulation In: Johnson LR, ed. Physiology of the Gastrointestinal Tract. 5th ed. Washington, D.C.: Academic Press; 2012:2308. [Google Scholar]
- 11. Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batta AK, Salen G, Arora R, Shefer S, Batta M, Person A. Side chain conjugation prevents bacterial 7‐dehydroxylation of bile acids. J Biol Chem. 1990;265:10925‐10928. [PubMed] [Google Scholar]
- 13. Vázquez‐Baeza Y, Hyde ER, Suchodolski JS, Knight R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. 2016;1:16177. [DOI] [PubMed] [Google Scholar]
- 14. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. Gastroenterology. 1972;62:918‐934. [PubMed] [Google Scholar]
- 16. Meier Y, Eloranta JJ, Darimont J, et al. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590‐594. [DOI] [PubMed] [Google Scholar]
- 17. Alpini G, Glaser SS, Rodgers R, et al. Functional expression of the apical Na+−dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734‐1740. [DOI] [PubMed] [Google Scholar]
- 18. Christie DM, Dawson PA, Thevananther S, et al. Comparative analysis of the ontogeny of a sodium‐dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:377‐385. [DOI] [PubMed] [Google Scholar]
- 19. Wojtal KA, Eloranta JJ, Hruz P, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871‐1877. [DOI] [PubMed] [Google Scholar]
- 20. Jahnel J, Fickert P, Hauer AC, Hogenauer C, Avian A, Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42:1423‐1431. [DOI] [PubMed] [Google Scholar]
- 21. Jung D, Fantin AC, Scheurer U, Fried M, Kullak‐Ublick GA. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53:78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen F, Ma L, Sartor RB, et al. Inflammatory‐mediated repression of the rat ileal sodium‐dependent bile acid transporter by c‐fos nuclear translocation. Gastroenterology. 2002;123:2005‐2016. [DOI] [PubMed] [Google Scholar]
- 23. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291‐297. [DOI] [PubMed] [Google Scholar]
- 24. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138(Suppl 1):S1‐S43. [DOI] [PubMed] [Google Scholar]
- 25. Shu J, Dolman GE, Duan J, Qiu G, Ilyas M. Statistical colour models: an automated digital image analysis method for quantification of histological biomarkers. Biomed Eng Online. 2016;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J Mol Diagn. 2012;14:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batta AK, Salen G, Rapole KR, et al. Highly simplified method for gas‐liquid chromatographic quantitation of bile acids and sterols in human stool. J Lipid Res. 1999;40:1148‐1154. [PubMed] [Google Scholar]
- 28. AlShawaqfeh MK, Wajid B, Minamoto Y, et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol Ecol. 2017;93. doi: 10.1093/femsec/fix136. [DOI] [PubMed] [Google Scholar]
- 29. Dawson PA, Haywood J, Craddock AL, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920‐33927. [DOI] [PubMed] [Google Scholar]
- 30. Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium‐dependent bile acid transporter. J Clin Investig. 1995;95:745‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium‐dependent bile acid transporter. J Biol Chem. 1994;269:1340‐1347. [PubMed] [Google Scholar]
- 32. Hruz P, Zimmermann C, Gutmann H, et al. Adaptive regulation of the ileal apical sodium dependent bile acid transporter (ASBT) in patients with obstructive cholestasis. Gut. 2006;55:395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Milkiewicz M, Klak M, Kempinska‐Podhorodecka A, et al. Impaired hepatic adaptation to chronic cholestasis induced by primary sclerosing cholangitis. Sci Rep. 2016;6:39573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pascal LE, True LD, Campbell DS, et al. Correlation of mRNA and protein levels: cell type‐specific gene expression of cluster designation antigens in the prostate. BMC Genomics. 2008;9:246‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montagnani M, Love MW, Rossel P, et al. Absence of dysfunctional ileal sodium‐bile acid cotransporter gene mutations in patients with adult‐onset idiopathic bile acid malabsorption. Scand J Gastroenterol. 2001;36:1077‐1080. [DOI] [PubMed] [Google Scholar]
- 36. Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535‐550. [DOI] [PubMed] [Google Scholar]
- 37. Walker D, Knuchel‐Takano A, McCutchan A, et al. A comprehensive pathological survey of duodenal biopsies from dogs with diet‐responsive chronic enteropathy. J Vet Intern Med. 2013;27:862‐874. [DOI] [PubMed] [Google Scholar]
- 38. Smith MJ, Cherian P, Raju GS, Dawson BF, Mahon S, Bardhan KD. Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond. 2000;34:448‐451. [PMC free article] [PubMed] [Google Scholar]
- 39. Borghede MK, Schlütter JM, Agnholt JS, Christensen LA, Gormsen LC, Dahlerup JF. Bile acid malabsorption investigated by selenium‐75‐homocholic acid taurine (75SeHCAT) scans: causes and treatment responses to cholestyramine in 298 patients with chronic watery diarrhoea. Eur J Intern Med. 2011;22:e137‐e140. [DOI] [PubMed] [Google Scholar]
- 40. Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11:1232‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Färkkilä MA, Kairemo KJ, Taavitsainen MJ, Strandberg TA, Miettinen TA. Plasma lathosterol as a screening test for bile acid malabsorption due to ileal resection: correlation with 75SeHCAT test and faecal bile acid excretion. Clin Sci. 1996;90:315‐319. [DOI] [PubMed] [Google Scholar]
- 42. Kent AC, Cross G, Taylor DR, et al. Measurement of serum 7alpha‐hydroxy‐4‐cholesten‐3‐one as a marker of bile acid malabsorption in dogs with chronic diarrhoea: a pilot study. Vet Rec. 2016;3:e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea‐predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513‐520. e246‐e247. [DOI] [PubMed] [Google Scholar]
- 44. Kitahara M, Takamine F, Imamura T, et al. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha‐dehydroxylating activity. Int J Syst Evol Microbiol. 2001;51:39‐44. [DOI] [PubMed] [Google Scholar]
- 45. Hall EJ, Day MJ. Diseases of the small intestine In: Ettinger SJ, Feldman EC, Cote E, eds. Textbook of Veterinary. Internal Medicine. 8th ed. Philadelphia, PA: Elsevier; 2017:1545‐1558. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Correlation between ASBT expression, clinical, laboratory, and histopathological findings in dogs with CIE.


