Abstract
Background
Naturally occurring hypothyroidism in adult cats is rare, with only 4 cases reported.
Objectives
To describe the historical, clinical, laboratory, and scintigraphic features of adult cats with spontaneous hypothyroidism.
Animals
Seven adult cats referred for suspected hypothyroidism.
Methods
Prospective case series. We collected data on cats’ signalment, clinical signs, results of physical examination, routine laboratory and thyroid hormone testing, and thyroid imaging (thyroid scintigraphy or ultrasound). We subsequently treated cats with levothyroxine and evaluated their response to treatment.
Results
Cats ranged from 3.5 to 11 years, with no apparent breed predilection; 6/7 cats were male. Only 2/7 cats were initially tested because of signs of hypothyroidism (hair‐coat changes, lethargy, obesity); others were tested for routine thyroid monitoring or palpable thyroid nodules. Four were azotemic (serum creatinine, 2.2‐3.4 mg/dL). Six of the cats had low serum thyroxine (T4) and free T4 (fT4) concentrations, whereas all 7 cats had high thyroid‐stimulating hormone (TSH) concentrations. In 6/7 cats, thyroid scintigraphy revealed bilateral goiter with intense radionuclide uptake; imaging showed no visible thyroid tissue in the other. After levothyroxine treatment, serum concentrations of T4 and fT4 increased and TSH fell; high serum creatinine normalized in azotemic cats; and repeat imaging showed reduction in goiter size.
Conclusions and Clinical Importance
Primary hypothyroidism develops in adult cats, with a higher prevalence than previously thought. Most cats appear to develop a goitrous form of hypothyroidism associated with thyroid hyperplasia, whereas thyroid atrophy appears to be less common. With levothyroxine replacement, clinical and laboratory abnormalities improve or resolve.
Keywords: atrophic, feline, goiter, goitrous, thyroid gland, thyroxine, TSH
Abbreviations
- cTSH
canine TSH
- fT4
free T4
- SDMA
serum symmetric dimethylarginine
- T4
thyroxine
- TcTU
thyroidal uptake of 99mTc‐pertechnetate
- T/S
thyroid‐to‐salivary ratio
- TSH
thyroid stimulating hormone
1. INTRODUCTION
Spontaneous primary hypothyroidism appears to be an extremely rare clinical disorder in adult cats, with only 4 reported cases over the last 25 years.1, 2, 3, 4 Most hypothyroid cats are younger (generally kittens, aged 2–4 months) and suffer from congenital hypothyroidism, which typically results in disproportionate dwarfism (cretinism). Of ∼60 cats with congenital hypothyroidism that have been reported,5, 6, 7, 8, 9 only 3 were older than 12 months of age at time of diagnosis, but these 3 cats were stunted since kittenhood, consistent with the diagnosis of congenital hypothyroidism.
In dogs and humans, almost all naturally occurring hypothyroidism is attributable to irreversible destruction of the thyroid gland. Histologically, primary hypothyroidism in humans and dogs presents as either lymphocytic thyroiditis or idiopathic thyroid degeneration (idiopathic follicular atrophy).10, 11, 12 Of the 4 reported adult cats with naturally occurring primary hypothyroidism, 2 had lymphocytic thyroiditis,1, 4 1 had idiopathic atrophy,2 and 1 had a goitrous form of hypothyroidism associated with diffuse thyroid follicular hyperplasia of both thyroid lobes.3 Goitrous hypothyroidism is extremely rare in dogs, with iatrogenic, drug‐induced thyroid goiter being the most common cause.13, 14 However, a subset of adult hypothyroid humans have goiter secondary to iodine deficiency, environmental goitrogens, or ineffective thyroid hormone synthesis caused by biosynthetic defect (dyshormonogenesis).15, 16, 17, 18, 19, 20
Given the paucity of data regarding adult‐onset feline hypothyroidism, we sought to describe the history, clinical features (including presence or absence of goiter), diagnostic testing, treatment, and long‐term outcome of 7 adult cats with spontaneous primary hypothyroidism. For these cats, we used the serum concentrations of T4, free T4 (fT4), and thyroid stimulating hormone (TSH), and results of thyroid scintigraphy to aid in both the diagnosis and long‐term monitoring of thyroid hormone replacement treatment.
2. MATERIALS AND METHODS
2.1. Case selection and data collection
This prospective case series included 7 adult cats with spontaneous hypothyroidism referred to the Animal Endocrine Clinic for evaluation over a 3.5‐year period from March 2014 to September 2017 and then followed until April 2018. Data collected for each cat included the following: age, breed, sex, reason for initial workup by referring veterinarian, clinical signs as reported by owners, dietary history, drugs and supplements administered, known concurrent illnesses, physical examination findings (including the presence or absence of thyroid goiter), routine laboratory findings (eg, complete blood count, serum biochemistry profile, symmetric dimethylarginine [SDMA] concentration,21 and urinalysis), complete serum thyroid panel (T4, fT4, and TSH concentrations), survey radiographs of the spine and limbs, and results of thyroid imaging studies (eg, thyroid scintigraphy, or ultrasound).
After collection of pretreatment data, all 7 cats were treated with levothyroxine (L‐T4), using an initial dosage of 100–150 µg/cat/day. Dose adjustments were titrated (by a 50 µg/cat/day increase or decrease as needed) based on follow‐up serum T4 and TSH concentrations, with the aim of restoring serum thyroid values to within reference intervals. Five cats with goitrous hypothyroidism also had follow‐up thyroid imaging (4 using scintigraphy and 1 with ultrasonography) to evaluate changes in thyroid function and volume after L‐T4 treatment.
Two of the 7 cats had thyroid aspirates collected for cytologic evaluation before any treatment, whereas a 3rd cat with persistent bilateral goiter despite treatment with L‐T4 underwent excisional biopsy of the left lobe of the thyroid gland for histopathologic examination.
2.2. Thyroid imaging
All cats underwent thyroid scintigraphy by injecting 111 MBq of sodium 99mTc‐pertechnetate (99m ) IV and imaging 1 hour later, as previously described.7, 22, 23 Thyroid activity was quantified by calculation of both the thyroid‐to‐salivary gland ratio (T/S) and the percent thyroidal uptake of the injected 99m (TcTU).7, 22, 23 The estimated thyroid volume was also calculated from the scintigraphic image, using the equation for a spheroid as previously described.23 In 1 cat, serial thyroid ultrasound imaging was used to estimate changes in goiter size by measuring the length and width of each lobe and then again calculating thyroid volume using the equation for a spheroid.24, 25
2.3. Assays for serum thyroid hormone and thyrotropin (TSH) Concentrations
Serum total T4, fT4, and TSH concentrations were measured by assays (DRI Thyroxine [T4] assay, Microgenics Corporation, Freemont, California; Free T4 ‐ by Equilibrium Dialysis, Antech Diagnostics, Irvine, California; Immulite Canine TSH, Siemens Healthcare Diagnostics Product, Tarrytown, New York) validated for use in cats, as previous described.26, 27 The reference intervals for these serum thyroid tests, previously established in clinically normal cats were as follows: total T4 = 0.9‐3.9 µg/dL; fT4 = 10–51 pmol/L; and cTSH = <0.03‐0.3 ng/mL.
2.4. Data and statistical analyses
All statistical analyses were performed by proprietary statistical software (GraphPad Prism, version 7.0; GraphPad Software, La Jolla, California). Most data are presented with descriptive and summary statistics (median, range, proportions). For select comparisons, such as reduction of goiter size after thyroid hormone treatment, a paired T test was used to compare the measured thyroid volumes before and after L‐T4 replacement. Similarly, before and after comparisons of serum T4, fT4, TSH, and creatinine concentrations in the hypothyroid cats were also analyzed by the paired T test. For all analyses, statistical significance was defined as P < .05.
3. RESULTS
3.1. Signalment of hypothyroid cats
The 7 hypothyroid cats ranged in age from 3.5 to 11.0 years (median, 7 years). When age was classified according to the cats’ life stage,28 2 were in prime (3–6 years), 4 were in mature (7–10 years), and 1 was in the senior life stage (11–14 years). Breeds included domestic shorthair in 6 cats and American shorthair in 1. Six of the 7 hypothyroid cats were male and 1 was female; all were castrated or spayed.
3.2. History, clinical presentation, and physical examination findings
Cats were initially tested for thyroid disease by the referring veterinarians for 1 of 3 reasons: (1) routine annual thyroid monitoring (n = 3); (2) palpable goiter (thyroid nodules) detected on physical examination (n = 2); and (3) clinical signs suggestive of hypothyroidism (n = 2). Of the 2 cats with clinical features suggestive of hypothyroidism (ie, hair loss, lethargy, and obesity), owners had first noted clinical signs 2 and 8 months before examination.
The most common clinical signs reported by owners included polyuria and polydipsia, mild changes in hair coat (unkempt appearance, increased shedding, dandruff, or hair thinning), and weight gain (Table 1). Two of the owners reported no overt clinical signs for their cats. Diet fed to the 7 cats consisted of a variety of both moist and dry commercial cat foods to 5, moist only to 1, and dry only to 1 cat. None of the cats were being administered any drugs or supplements associated with changes in thyroid function or goiter (eg, sulfonamide antibiotics or iodine/iodide).
Table 1.
Clinical features, routine laboratory findings, and serum thyroid hormone concentrations in 7 adult cats with naturally occurring primary hypothyroidism
| Historical, owner‐reported signs | |
| Polyuria and polydipsia | 4 |
| Hair coat changes | 4 |
| Weight gain | 4 |
| Lethargy/mental dullness | 3 |
| Poor appetite | 1 |
| Cold intolerance | 1 |
| Physical examination findings | |
| Palpable goiter | 6 |
| Unkempt, dull coat | 3 |
| Hair thinning/ hypotrichosis | 3 |
| Overweight/obesity | 3 |
| Dandruff, flaking dry skin | 2 |
| Bradycardia (≤150 bpm) | 1 |
| Routine laboratory findings | |
| Azotemia (serum creatinine >2.0 mg/dL) | 4 |
| Urine specific gravity <1.035 | 2 |
| Anemia | 2 |
| Hypercholesterolemia (>300 mg/dL) | 1 |
| High serum creatine kinase | 1 |
| High serum SDMA | 1 |
| Serum thyroid hormone concentrations | |
| Low serum T4 (< 0.9 µg/dL) | 6 |
| Low serum fT4 (<10 pmol/L) | 6 |
| High serum TSH (>0.3 ng/mL) | 7 |
On physical examination, symmetric enlargement of both thyroid lobes (goiter) could be palpated in 6 of the 7 cats. The median weight was 5.3 kg (range 3.6–7.3 kg). Three of the 7 cats weighed >6.0 kg and were considered obese (body condition scores, 6/9 to 7/9), whereas the body condition in the remaining 4 cats was considered ideal. All 7 cats had normal muscle condition. Sinus bradycardia (heart rate, 120 bpm) was auscultated in 1 cat. Relatively mild changes in hair coat (unkempt appearance, increased shedding, or hair thinning), were identified in 4 cats (Table 1). None of these cats had overt or complete areas of alopecia.
3.3. Routine laboratory findings
The most frequent abnormality on routine laboratory testing was azotemia (defined as serum creatinine concentrations >2.0 mg/dL; Table 1). Serum creatinine concentrations in the 4 azotemic cats ranged from 2.2 to 3.4 mg/dL; concomitant urine specific gravities were less‐than‐maximally concentrated <1.035 in 2 cats (1.015 and 1.034), and well concentrated in 2 (1.047 and 1.050). Only 1 of the 4 azotemic cats had a slightly high serum SDMA concentration (15 µg/dL); the other 3 had SDMA values (11, 12, and 13 µg/dL) that were in the upper tertile of the reference interval (≤14 µg/dL21). In the 3 nonazotemic cats, serum creatinine concentrations were in upper half of the reference interval (1.5, 1.7, and 1.9 mg/dL). Two cats were mildly anemic, as reflected by a low RBC count, hemoglobin concentration, or hematocrit (Table 1).
3.4. Serum thyroid hormone and TSH concentrations
Serum T4 and fT4 concentrations were low in 6 of the 7 cats (Table 1; Figure 1A,B). One cat had low‐normal concentrations of both T4 and fT4 (within the lower tertile of the reference interval). Serum TSH concentrations were high in all cats (Figure 1C), ranging from 2.1 to 20 ng/mL (median, 10.1 ng/mL).
Figure 1.

Serum concentrations of (A) total T4, (B) fT4, (C) TSH, and (D) creatinine in 7 hypothyroid cats, measured before and after treatment with levothyroxine. Serum T4 and fT4 concentrations increase, while TSH and creatinine concentrations decrease after administration of thyroid replacement treatment. The shaded area represents the reference interval. To convert T4 concentration from µg/dL to nmol/L, multiply by 12.87. To convert fT4 concentration from pmol/L to ng/dL, divide by 12.87
3.5. Survey radiographs of the spine and limbs
Ossification centers of the long bones and vertebrae were evaluated with survey radiographs. All 7 cats had complete closure of the physes (ossification centers) of long bones and vertebrae.
3.6. Pretreatment thyroid imaging
In 6 of the 7 cats, qualitative thyroid scintigraphy revealed bilateral thyroid lobe enlargement (goiter) and intense radionuclide thyroid uptake (Figures 2B and 3). All 6 of these cats with goitrous hypothyroidism had high values for T/S ratio, percent TcTU, and thyroid volume (Figure 4). In the 7th cat, thyroid imaging showed no visible thyroid tissue, neither in the normal cervical nor an ectopic location (Figure 2C). This cat with atrophic hypothyroidism had a low T/S ratio (0.1), percent TcTU (0.02%), and thyroid volume (0.05 g).
Figure 2.
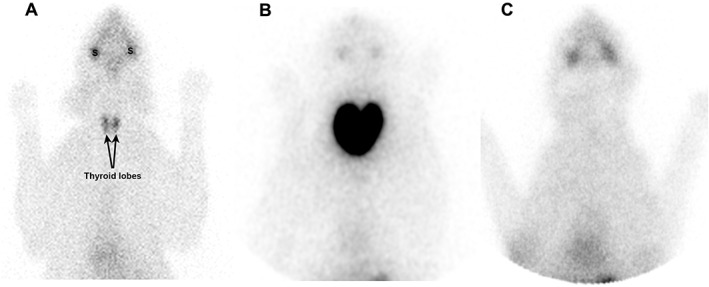
Thyroid scintigraphy illustrating scans of (A) clinically normal cat; (B) cat with goitrous hypothyroidism; and (C) cat with atrophic hypothyroidism. Radionuclide uptake in the normal thyroid closely approximates the uptake in the salivary glands (S), with an expected “intensity” ratio of 1 : 1. In contrast, the cat with goitrous hypothyroidism has bilaterally enlarged thyroid lobes, with a homogeneous pattern of increased and intense thyroid uptake of the injected radionuclide (as compared with the salivary glands). No thyroid tissue can be identified in the cat with atrophic hypothyroidism, leading to a decreased to nil intensity of uptake
Figure 3.

Thyroid scintigraphy in 3 cats with goitrous hypothyroidism, before and after L‐T4 treatment. A and B, 8‐year‐old, DSH male cat, before and after 22 months of treatment with L‐T4 (100 µg twice daily, PO). Goiter size and intensity of radionuclide uptake is reduced after thyroid hormone replacement. C and D, 7‐year‐old, DSH male cat, before and after 4 months of treatment with L‐T4 (100 µg twice daily, PO). Goiter size and intensity of radionuclide uptake is reduced after thyroid hormone replacement. E and F, 7‐year‐old, DSH male cat, before and after 9.5 months of treatment with L‐T4 (75 µg twice daily). Both thyroid lobes are now within reference limits for size, shape, position in the cervical area, and intensity of radionuclide uptake
Figure 4.

Results of quantitative thyroid scintigraphy in 4 cats with goitrous hypothyroidism, before and after L‐T4 treatment. Compared with high pretreatment values, (A) T/S ratio, (B) percent thyroidal uptake of 99mTcTU, and (C) thyroid volume all decreased after thyroid hormone replacement. The shaded area represents the reference interval
3.7. Thyroid aspiration cytology and biopsy
Before treatment, 2 cats had thyroid aspirates collected for cytology. In both cats, findings were consistent with thyroid hyperplasia, consisting of variably sized sheets and clusters of epithelial/neuroendocrine‐appearing cells ranging from round to polygonal in shape, with small to moderate amounts of light to dark blue cytoplasm. The cells were frequently associated with small amounts of pink globular to fibrillar material that was thought to represent colloid. The cells displayed minimal features of atypia and mitotic figures were not observed.
Histopathologic finding of the thyroid lobe excised from 1 cat with persistent goiter despite L‐T4 treatment (with normalization of serum TSH concentrations) for 22 months revealed that thyroid parenchyma was characterized by prominent coalescing nests of small follicles, which contained normal to decreased amounts of colloid (Figure 5). These extensive regions were interposed between areas containing larger thyroid follicles with abundant colloid lined by low cuboidal epithelial cells. The extensive regions of hypercellularity, small thyroid follicles, and diminished colloid were considered consistent with follicular hyperplasia of the thyroid gland.
Figure 5.

Photomicrograph of the thyroid gland from a hypothyroid cat that had persistent goiter despite treatment with L‐T4 for 22 months (see scintiscans in Figure 3A,B). At high magnification (Bar = 100 µm) the architecture of the gland is altered. The thyroid lobe is more densely cellular, and many follicular lumens are smaller than normal. These smaller follicles are irregularly shaped and contain decreased amounts of colloid, consistent with mild thyroid hyperplasia
3.8. Treatment with levothyroxine and outcome
After treatment with L‐T4 (median, 200 µg/cat/day [32.7 µg/kg/day]; range 100–200 µg/cat/day [16.7–55.6 µg/kg/day]) for 3 to 7 months (median time, 150 days), serum concentrations of T4 and fT4 both increased and TSH decreased, generally into the respective reference intervals (Figures 1A‐C). In 5 treated cats with goitrous hypothyroidism, repeat thyroid imaging demonstrated decreases in goiter size, as reflected by the reduced thyroid volumes (Figures 3 and 6). The intensity of scintigraphic thyroid uptake also fell, evidenced by the decreases in both T/S ratio and percent TcTU (Figure 4).
Figure 6.

Serial ultrasound measurements of thyroid volume in an 11‐year old, male DSH before and after L‐T4 treatment (see cat's pretreatment scintiscan in Figure 2B). Goiter size decreases progressively with thyroid hormone replacement
After L‐T4 treatment, serum creatinine concentrations decreased in all 7 cats (Figure 1D). In the 4 cats that were azotemic before L‐T4 treatment, serum creatinine concentrations normalized after L‐T4 supplementation (Figure 1D). Of the 2 azotemic cats that had urine specific gravity values that less‐than‐appropriately concentrated (<1.035) before L‐T4 treatment, 1 cat remained isosthenuric, whereas the other concentrated appropriately after treatment. Mild anemia also resolved in the 2 affected cats after L‐T4 treatment. By 2–4 months after treatment, owners reported that their cat's lethargy, hair coat changes, and obesity (if present before treatment) were greatly improved or had resolved. All cats are alive, well, and remain non‐azotemic on L‐T4 treatment at the time of manuscript preparation, with a median treatment time of 739 days (range, 233–1439 days).
4. DISCUSSION
Our results provide further information about spontaneous adult‐onset primary hypothyroidism in cats. Although the condition is rare, ∼2 cats are diagnosed with spontaneous adult‐onset hypothyroidism each year at the primary author's endocrine referral clinic. We suspect that this represents only “the tip of the iceberg” of cats affected with this syndrome, because of the mild clinical signs displayed by these cats, and the lack of awareness of this condition by first opinion clinicians. Heightened awareness that adult hypothyroidism can develop in cats, together with increased screening for the disorder, will ultimately determine if this condition is indeed rare or more common than currently thought (Supplemental Data S1).
All of our hypothyroid cats were adults, with 4/7 diagnosed in the “mature” life stage (age 7–10 years), 2 in the younger “prime” life stage (3–6 years) and 1 in the older “senior” life stage (11–14 years).28 Time of first onset of clinical disease was difficult to determine, but historically, none of our cats displayed any signs longer than 10 months before diagnosis. Almost all of our cats (6/7) were of mixed breeding (DSH), suggesting no specific breed predilection. Six of our 7 cats were castrated males; we have no explanation for this possible sex predilection, especially since human patients and dogs with hypothyroidism have a female, rather than a male, sex predilection.29, 30, 31 Obviously, more cats need to be diagnosed and studied to clarify sex distribution in feline hypothyroidism.
In contrast to previous reports, most cats in our case series had goitrous hypothyroidism, with only 1 having idiopathic thyroid atrophy. However, this preponderance of goitrous hypothyroidism in our cats could reflect the fact that one‐third of cats were referred primarily for evaluation of palpable bilateral thyroid nodules (together with unexpectedly low serum T4 concentrations). Cats without a palpable goiter might not have been as readily referred for thyroid evaluation with scintigraphy at our clinic, or screened for thyroid function on routine evaluation. A larger number of hypothyroid cats need to be diagnosed and evaluated with thyroid imaging or thyroid pathology (or both) to better determine the prevalence of atrophic versus goitrous hypothyroidism in cats.
Most of our cats had only mild clinical features of hypothyroidism and were initially tested for thyroid disease either because of routine thyroid monitoring (eg, annual screening for hyperthyroidism) or a palpable bilateral goiter detected on routine physical examination. Hypothyroidism was initially suspected in only 2 of our cats, both of which had hair thinning, lethargy, and obesity. Even when hair loss or other cutaneous changes were noted, these signs were relatively mild, with no cat having overt or complete areas of alopecia. The most common clinical signs were polydipsia and polyuria, associated with mild‐to‐moderate azotemia in 4 of our 7 cats (see below). This was followed by weight gain and obesity, signs that are also common in euthyroid, middle‐aged adult cats.32, 33 Because of the lack of overt clinical signs in many cats and the presence of only vague and nonspecific signs in others, hypothyroidism can easily be missed and may be misdiagnosed as “normal aging” or concurrent disease (eg, chronic kidney disease [CKD]).
In our 6 cats with goitrous hypothyroidism, the underlying cause of the bilateral thyroid lobe enlargement is not clear. In the 1 cat that had persistent goiter despite long‐term L‐T4 treatment, histopathology revealed changes consistent with thyroid hyperplasia, with no evidence of nodular/adenomatous changes, cellular atypia, or capsular invasion. Therefore, these changes are unlike the pathologic findings characteristic of feline adenoma or adenomatous hyperplasia seen in cats with hyperthyroidism,34, 35 but are similar to those found in the previously reported adult cat with goitrous hypothyroidism.3 Compared with that cat, the hyperplastic changes in our cat were milder; treating chronically with L‐T4 and normalizing high serum TSH concentrations likely led to partial regression of the thyroid hyperplasia. However, the fact that all 6 cats with goitrous hypothyroidism had bilateral, diffuse thyroid lobe enlargement on physical examination and imaging indicates the presence of intact thyroid tissue, and the massive sizes of these goiters suggest a process that was ongoing for months to years. Acquired causes for goitrous hypothyroidism, such as iodine deficiency or environmental goitrogens, were considered highly unlikely based on the cats’ history and diet of commercial canned food. In addition, all of our 6 affected cats lived in households with 1 or more other cats, none of which were hypothyroid or had palpable goiter. One goitrous cat lived in the same household with his littermate; evaluation of this unaffected cat revealed completely normal thyroid function, as documented by serum thyroid and TSH testing and qualitative and quantitative thyroid scintigraphy (data not shown).
The hypothesis that best fits our 6 cats with goitrous hypothyroidism is that these cats suffer from a hereditary defect or block in thyroid hormone production (dyshormonogenesis) by an anatomically intact thyroid gland.36 This would be similar to the defects reported in kittens with congenital goitrous hypothyroidism, which is most commonly associated with an impaired ability of the thyroid gland to organify iodide (ie, organification or peroxidase defect).7, 37, 38, 39, 40 In congenital, goitrous hypothyroidism, the inability to secrete adequate amounts of T4 and T3 leads to the loss of normal negative‐feedback inhibition on pituitary thyrotropes, resulting in persistent secretion of excessive amounts of TSH. The unrelenting stimulation of intact thyroid follicular cells by the high circulating concentrations of TSH results in thyroid hyperplasia, enlargement of the intact thyroid, and clinical palpable goiter. In humans, the clinical presentation of patients with dyshormonogenetic goiter depends on the severity of the inborn error in thyroid hormone metabolism. A severe defect will lead to neonatal or congenital hypothyroidism, goiter, mental retardation, and growth abnormalities (cretinism), similar to many of the reported hypothyroid kittens.7, 37, 38, 39, 40 Milder forms of congenital dyshormonogenesis can present later in life (ie, adolescence or young adulthood) as goiter and minimal, if any, signs of thyroid dysfunction.18, 41, 42, 43, 44 Such milder or partial defects in thyroid hormone production could explain the pathophysiology in our 6 goitrous cats, in which goiter was one of the major reasons for work‐up and few overt clinical signs of thyroid disease were present. If this is the case, our cats were able to compensate for the block in thyroid hormone secretion for many months to years, as evidenced by the cats’ normal rate of growth, body size, and closure of bone physes (ie, none of our adult cats were dwarfed or had open bone plates, both characteristic finding in kittens with congenital hypothyroidism).5, 6, 7 In addition, none of the 6 cats with goitrous hypothyroidism displayed signs of mental dullness, as reported in younger cats with untreated congenital hypothyroidism.5, 6, 7 Because severe hypothyroidism in early life leads to lack of bone growth and neurodevelopment (cretinism), moderate to severe intellectual deficits are common, at least in people.45, 46, 47
Cats in our study underwent thyroid scintigraphy to confirm the diagnosis and characterize the disease.2, 7, 48 Hypothyroid cats with thyroid atrophy have no detectable thyroid tissue on scintigraphy, and minimal or absent thyroid uptake of the injected radionuclide (99m ), as seen in the 1 cat with atrophic hypothyroidism (Figure 2C).2, 48 In contrast, high circulating TSH concentrations greatly increase 99m uptake into the anatomically intact thyroid gland in untreated cats suffering from goitrous hypothyroidism,48, 49 as evidenced in the 6 cats by the intense (“hot”) uptake of 99m into both thyroid lobes (Figures 2B and 3) and the high calculated values for both the T/S ratio and percent TcTU (Figure 4). The cats with goitrous hypothyroidism also had a marked increase in their measured thyroid volumes (2.5–8 fold higher than that of the upper reference interval).
Our results indicate that measurement of serum TSH concentration is the most sensitive diagnostic test for naturally occurring hypothyroidism in cats, similar to our previous findings in cats with iatrogenic (131I‐induced) hypothyroidism.27, 50 All 7 hypothyroid cats inthe current study had extremely high serum TSH concentrations (7–40 times higher than the upper reference interval). Serum concentrations of T4 and fT4 were low in 6/7 cats, diagnostic for overt hypothyroidism. One cat maintained normal serum concentrations of T4 and fT4 despite high TSH values, diagnostic for subclinical (mild) hypothyroidism,51, 52, 53, 54 a pattern similar to that of 131I‐treated cats that develop iatrogenic hypothyroidism.27, 50 In addition, recent prevalence studies of human hypothyroidism reveal that most people (up to 95% in some reports) have subclinical hypothyroidism, with overt hypothyroidism being less common.55, 56, 57 Additionally, serum TSH concentration is the most specific diagnostic test for hypothyroidism: low serum T4 and fT4 concentrations commonly develop in cats with nonthyroidal illness,50, 58 but high values for TSH have not been reported in these sick, euthyroid cats.50, 59 However, some human patients with nonthyroidal illness will develop slightly increased serum TSH concentrations, especially during the recovery phase of their illness.60, 61 In addition, human patients treated with some medications (eg, metoclopramide, amiodarone) can develop slightly increased serum TSH concentrations.62, 63, 64 Similarly, transiently high serum TSH concentrations can develop in dogs with nonthyroidal disease (with hypoadrenocorticism being the best characterized example),65, 66, 67, 68 as well as during treatment with certain drugs.13, 14, 69, 70 Therefore, it is clear that more studies evaluating serum TSH concentrations in a larger population of cats are needed—especially in cats with nonthyroidal illness during the recovery phase of illness, as well as after treatment with medications known to increase serum TSH in other species.
After levothyroxine supplementation to our hypothyroid cats, high serum TSH concentrations decreased to within the reference interval, as the negative feedback effects of higher circulating T4 and T3 concentrations suppressed excessive TSH secretion.7, 50 In our cats, we used serum TSH concentrations for long‐term monitoring, with daily L‐T4 doses titrated to maintain TSH concentrations within its reference interval (<0.3 ng/mL) while maintaining serum T4 and fT4 concentrations within their respective reference intervals. With that regime, our cats needed a final daily L‐T4 dose ranging from 100 to 200 µg/cat/day (16.7–55.6 µg/kg/day). Our median dose administered per kg (32.7 µg/kg/day) was higher than that generally reported for thyroid hormone replacement in dogs (≈20 µg/kg/day).71, 72, 73
Treatment with levothyroxine improved or resolved clinical signs, when present, in all of our hypothyroid cats. Thyroid hormone replacement also led to shrinkage of the bilateral goiter; this might be expected after lowering high serum TSH concentrations to within the reference range, especially if TSH‐induced, diffuse thyroid hyperplasia was responsible for the goiter.7 As circulating TSH concentrations fell on levothyroxine replacement, scintigraphic evidence of increased thyroid uptake, such as the intensity of 99m accumulation and calculated values for T/S and percent TcTU, also decreased in our goitrous hypothyroid cats (Figure 4). In 1 of our goitrous cats, both the thyroid gland size and uptake values normalized after L‐T4 replacement (Figure 3E,F). However, thyroid gland size and radionuclide uptake failed to completely normalize in all of our goitrous cats despite long‐term, adequate thyroid hormone replacement, similar to findings reported in younger cats with congenital hypothyroidism,74 as well as humans with dyshormonogenetic goiter.44
One of the major indications for workup in our cats was the finding of high serum creatinine concentrations, consistent with IRIS Stage 2 to Stage 3 CKD75 in 4/7 cats. Azotemia is a well‐recognized complication of hypothyroidism in humans,76, 77, 78, 79, 80, 81 developing in over half of patients in some reports.82, 83 The pathophysiology of hypothyroid‐induced azotemia is complex but is related in large part to decreases in cardiac output, renal blood flow, and glomerular filtration rate.84, 85 Unless patients have concurrent, primary kidney disease, azotemia is reversible with adequate thyroid hormone replacement treatment, leading to a consistent fall in serum creatinine concentrations.76, 77, 78, 79, 80, 81, 82, 83, 84, 85 Cats in our study demonstrated similar decreases in serum creatinine concentrations after treatment, with serum creatinine normalizing (falling back into within the reference interval) in the 4 azotemic cats. Like the hypothyroid cats of this case series, cats with iatrogenic (131I‐induced) hypothyroidism commonly become azotemic after treatment, and serum creatinine decreases or normalizes in many of those cats after thyroid hormone replacement.21, 50 Overall, the fact that the azotemia can resolve in hypothyroid cats after thyroid hormone replacement implies that these cats likely did not have CKD or that it was not as severe as the high serum creatinine concentration might imply.
Hypothyroidism can also impair urine concentrating ability, an effect that can also be reversed with thyroid hormone treatment.84, 85, 86, 87 Cats in our study mostly concentrated their urine (urine specific gravities > 1.035), but 2 of the azotemic cats had values that were less‐than‐appropriately concentrated. After L‐T4 treatment, 1 cat remained isosthenuric, but the other began to concentrate appropriately. This impaired urine concentrating ability likely contributed to the polyuria and polydipsia reported in 4 of our hypothyroid cats, since these signsimproved (2 cats) or resolved (2 cats) after thyroid hormone replacement.
This reversible form of azotemia in hypothyroid cats suggests that clinicians should include hypothyroidism as a differential for all adult cats that develop azotemia. This is especially true in relatively young or middle‐aged cats that lack other evidence for CKD, such as low urine specific gravity, high serum SDMA concentrations, or small kidney size. These cats should have serum T4 and TSH concentrations measured; finding a low to low‐normal serum T4 concentration (ie, in the lower third of the reference interval; generally <1.5 µg/dL) together with a clearly high serum TSH concentration (>1.0 ng/mL) is diagnostic for hypothyroidism and confirms the need for thyroid hormone treatment. Similarly, given the subtlety of clinical signs in cats with spontaneous adult‐onset hypothyroidism, clinicians should consider measuring serum TSH concentration as part of routine thyroid monitoring in adult cats, especially in those that have a palpable thyroid nodule(s) but do not show classical clinical features of hyperthyroidism. Finding an undetectable TSH concentration with high‐normal to high serum T4 concentrations is consistent with hyperthyroidism26 and certainly excludes hypothyroidism; a high serum TSH concentration with low‐normal T4 concentrations is diagnostic for hypothyroidism and justifies the need for thyroid imaging, L‐T4 treatment, or both.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ACKNOWLEDGEMENT
Work was performed at the Animal Endocrine Clinic, New York, USA.
Peterson ME, Carothers MA, Gamble DA, Rishniw M. Spontaneous primary hypothyroidism in 7 adult cats. J Vet Intern Med. 2018;32:1864–1873. 10.1111/jvim.15239
REFERENCES
- 1. Rand JS, Levine J, Best SJ, Parker W. Spontaneous adult‐onset hypothyroidism in a cat. JVet Intern Med. 1993;32:1864–1873. [DOI] [PubMed] [Google Scholar]
- 2. Blois SL, Abrams‐Ogg AC, Mitchell C, et al. Use of thyroid scintigraphy and pituitary immunohistochemistry in the diagnosis of spontaneous hypothyroidism in a mature cat. JFeline Med Surg. 2010;12:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galgano M, Spalla I, Callegari C, et al. Primary hypothyroidism and thyroid goiter in an adult cat. JVet Intern Med. 2014;28:682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kent A, Constantino‐Casas F, Herrtage ME. Naturally occurring acquired primary hypothyroidism in a cat due to lymphocytic thyroiditis. Vet Rec Case Rep. 2016;4:e000282 https://doi.org/000210.001136/vetreccr-002015-000282. [Google Scholar]
- 5. Daminet S. Feline hypothyroidism In: Mooney CT, Peterson ME, eds. Manual of Canine and Feline Endocrinology. 4th ed Quedgeley, Gloucester: British Small Animal Veterinary Association; 2012:1–5. [Google Scholar]
- 6. Baral R, Peterson ME. Thyroid gland disorders In: Little SE, ed. The Cat: Clinical Medicine and Management. Philadelphia: Elsevier Saunders; 2012:571–592. [Google Scholar]
- 7. Peterson ME. Primary goitrous hypothyroidism in a young adult domestic longhair cat: Diagnosis and treatment monitoring. JFMS Open Rep. 2015;1:205511691561515 https://doi.org/2055116915615110.2055116915611177/2055116915615153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowe A. Congenital hypothyroidism in a cat. Can Vet J. 2004;45:168, 170. [PMC free article] [PubMed] [Google Scholar]
- 9. Szabo SD, Wells KL. What is your diagnosis? Congenital hypothyroidism. JAm Vet Med Assoc. 2007;230:29–30. [DOI] [PubMed] [Google Scholar]
- 10. Graham PA, Refsal KR, Nachreiner RF. Etiopathologic findings of canine hypothyroidism. Vet Clin North Am Small Anim Pract. 2007;37:617–631. [DOI] [PubMed] [Google Scholar]
- 11. Mooney CT. Canine hypothyroidism: A review of aetiology and diagnosis. N Z Vet J. 2011;59:105–114. [DOI] [PubMed] [Google Scholar]
- 12. Carle A, Pedersen IB, Knudsen N, et al. Thyroid volume in hypothyroidism due to autoimmune disease follows a unimodal distribution: Evidence against primary thyroid atrophy and autoimmune thyroiditis being distinct diseases. JClin Endocrinol Metab. 2009;94:833–839. [DOI] [PubMed] [Google Scholar]
- 13. Seelig DM, Whittemore JC, Lappin MR, Myers AM, Avery PR. Goitrous hypothyroidism associated with treatment with trimethoprim‐sulfamethoxazole in a young dog. JAm Vet Med Assoc. 2008;232:1181–1185. [DOI] [PubMed] [Google Scholar]
- 14. Taeymans O, O'Marra SK. Imaging diagnosis–acquired goitrous hypothyroidism following treatment with trimethoprim sulfamethoxazole. Vet Radiol Ultrasound. 2009;50:442–444. [DOI] [PubMed] [Google Scholar]
- 15. Bloomfield SS. Goitrous hypothyroidism. Can Med Assoc J. 1962;86:535–536. [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy JS. The pathology of dyshormonogenetic goitre. JPathol. 1969;99:251–264. [DOI] [PubMed] [Google Scholar]
- 17. Harvey RF, Doniach D. Dyshormonogenetic goitre with high circulating levels of thyroid‐stimulating hormone. Proc R Soc Med. 1971;64:299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghossein RA, Rosai J, Heffess C. Dyshormonogenetic goiter: A clinicopathologic study of 56 cases. Endocr Pathol. 1997;8:283–292. [DOI] [PubMed] [Google Scholar]
- 19. Braham E, Ben Rejeb H, Marghli A, Kilani T, El Mezni F. A rare and particular form of goiter to recognize. Ann Transl Med. 2013;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer PA. Primary hypothyroidism due to other causes In: Braverman LE, Cooper DS, eds. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. 10 ed Philadelphia: Lippincott Williams & Wilkins; 2013:552–560. [Google Scholar]
- 21. Peterson ME, Varela FV, Rishniw M, Polzin DJ. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats With hyperthyroidism. JVet Intern Med. 2018;32:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson ME, Broome MR. Thyroid scintigraphy findings in 2,096 cats with hyperthyroidism. Vet Radiol Ultrasound. 2015;56:84–95. [DOI] [PubMed] [Google Scholar]
- 23. Peterson ME, Guterl JN, Rishniw M, Broome MR. Evaluation of quantitative thyroid scintigraphy for diagnosis and staging of disease severity in cats with hyperthyroidism: comparison of the percent thyroidal uptake of pertechnetate to the thyroid‐to‐salivary ratio and thyroid‐to‐background ratios. Vet Radiol Ultrasound. 2016;57:427–440. [DOI] [PubMed] [Google Scholar]
- 24. Ueda D. Sonographic measurement of the volume of the thyroid gland in healthy children. Acta Paediatr Jpn. 1989;31:352–354. [DOI] [PubMed] [Google Scholar]
- 25. Weisstein EW. Volume. MathWorld—A Wolfram Web Resource. http://mathworld.wolfram.com/Volume.html. Accessed April 10, 2018.
- 26. Peterson ME, Guterl JN, Nichols R, Rishniw M. Evaluation of serum thyroid‐stimulating hormone concentration as a diagnostic test for hyperthyroidism in cats. JVet Intern Med. 2015;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lucy JM, Peterson ME, Randolph JF, et al. Efficacy of low‐dose (2 millicurie) versus standard‐dose (4 millicurie) radioiodine treatment for cats with mild‐to‐moderate hyperthyroidism. JVet Intern Med. 2017;31:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogt AH, Rodan I, Brown M, et al. AAFP‐AAHA: Feline life stage guidelines. JAm Anim Hosp Assoc. 2010;46:70–85. [DOI] [PubMed] [Google Scholar]
- 29. Wiersinga WM. Adult Hypothyroidism In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth (MA): MDText.com, Inc; 2014. [Google Scholar]
- 30. Milne KL, Hayes HM Jr. Epidemiologic features of canine hypothyroidism. Cornell Vet. 1981;71:3–14. [PubMed] [Google Scholar]
- 31. Dixon RM, Reid SW, Mooney CT. Epidemiological, clinical, haematological and biochemical characteristics of canine hypothyroidism. Vet Rec. 1999;145:481–487. [DOI] [PubMed] [Google Scholar]
- 32. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Intern J Appl Res Vet Med. 2005;3:88–96. [Google Scholar]
- 33. Colliard L, Paragon BM, Lemuet B, Bénet JJ, Blanchard G. Prevalence and risk factors of obesity in an urban population of healthy cats. JFeline Med Surg. 2009;11:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerber H, Peter H, Ferguson DC, Peterson ME. Etiopathology of feline toxic nodular goiter. Vet Clin North Am Small Anim Pract. 1994;24:541–565. [DOI] [PubMed] [Google Scholar]
- 35. Peterson ME. Animal models of disease: Feline hyperthyroidism: an animal model for toxic nodular goiter. JEndocrinol. 2014;223:T97–114. [DOI] [PubMed] [Google Scholar]
- 36. Medeiros‐Neto GA, Billerbeck AE, Wajchenberg BL, Targovnik HM. Defective organification of iodide causing hereditary goitrous hypothyroidism. Thyroid. 1993;3:143–159. [DOI] [PubMed] [Google Scholar]
- 37. Arnold U, Opitz M, Grosser I, Bader R, Eigenmann JE. Goitrous hypothyroidism and dwarfism in a kitten. JAm Anim Hosp Assoc. 1984;20:753–758. [Google Scholar]
- 38. Sjollema BE, den Hartog MT, de Vijlder JJ, van Dijk JE, Rijnberk A. Congenital hypothyroidism in two cats due to defective organification: Data suggesting loosely anchored thyroperoxidase. Acta Endocrinol (Copenh). 1991;125:435–440. [DOI] [PubMed] [Google Scholar]
- 39. Jones BR, Gruffydd‐Jones TJ, Sparkes AH, Lucke VM. Preliminary studies on congenital hypothyroidism in a family of Abyssinian cats. Vet Rec. 1992;131:145–148. [DOI] [PubMed] [Google Scholar]
- 40. Mazrier H, French A, Ellinwood NM, et al. Goitrous congenital hypothyroidism caused by thyroid peroxidase deficiency in a family of domestic shorthair cats (abstract). JVet Intern Med. 2003;17:395–396. [Google Scholar]
- 41. Perez‐Cuvit E, Crigler JF Jr, Stanbury JB. Partial and total iodide organification defect in different sibships in a kindred. Am J Hum Genet. 1977;29:142–148. [PMC free article] [PubMed] [Google Scholar]
- 42. Vittal S, Chandrasekaran M, Kumar KB, Sucharitha V, Jeevaratinam R. Dyshormonogenetic goitre. JR Coll Surg Edinb. 1993;38:205–207. [PubMed] [Google Scholar]
- 43. Thompson L. Dyshormonogenetic goiter of the thyroid gland. Ear Nose Throat J. 2005;84:200. [PubMed] [Google Scholar]
- 44. Perry KD, Hope J, Yang J. Dyshormonogenetic goiter‐like changes in a child with congenital hypothyroidism and a euthyroid adult. Diagn Cytopathol. 2013;41:720–724. [DOI] [PubMed] [Google Scholar]
- 45. Gardiner‐Hill H. Cretinism and myxoedema. Br Med J. 1937;1:132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Song SI, Daneman D, Rovet J. The influence of etiology and treatment factors on intellectual outcome in congenital hypothyroidism. JDev Behav Pediatr. 2001;22:376–384. [DOI] [PubMed] [Google Scholar]
- 47. Srivastav A, Maisnam I, Dutta D, Ghosh S, Mukhopadhyay S, Chowdhury S. Cretinism revisited. Indian J Endocrinol Metab. 2012;16:S336–S337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peterson ME. Feline focus: Diagnostic testing for feline thyroid disease: Hypothyroidism. Compendium. 2013;35:E4. [PubMed] [Google Scholar]
- 49. Quante S, Fracassi F, Gorgas D, et al. Congenital hypothyroidism in a kitten resulting in decreased IGF‐I concentration and abnormal liver function tests. JFeline Med Surg. 2010;12:487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peterson ME, Nichols R, Rishniw M. Serum thyroxine and thyroid‐stimulating hormone concentration in hyperthyroid cats that develop azotaemia after radioiodine therapy. JSmall Anim Pract. 2017;58:519–530. [DOI] [PubMed] [Google Scholar]
- 51. Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med. 2001;345:260–265. [DOI] [PubMed] [Google Scholar]
- 52. Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12:839–847. [DOI] [PubMed] [Google Scholar]
- 53. Fatourechi V. Subclinical hypothyroidism: An update for primary care physicians. Mayo Clin Proc. 2009;84:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peeters RP. Subclinical Hypothyroidism. N Engl J Med. 2017;376:2556–2565. [DOI] [PubMed] [Google Scholar]
- 55. Canaris GJ, Tape TG, Wigton RS. Thyroid disease awareness is associated with high rates of identifying subjects with previously undiagnosed thyroid dysfunction. BMC Public Health. 2013;13:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). JClin Endocrinol Metab. 2002;87:489–499. [DOI] [PubMed] [Google Scholar]
- 57. Hennessey JV, Espaillat R. Subclinical hypothyroidism: A historical view and shifting prevalence. Int J Clin Pract. 2015;69:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Peterson ME, Melian C, Nichols R. Measurement of serum concentrations of free thyroxine, total thyroxine, and total triiodothyronine in cats with hyperthyroidism and cats with nonthyroidal disease. JAm Vet Med Assoc. 2001;218:529–536. [DOI] [PubMed] [Google Scholar]
- 59. Davignon D, Lucy J, Randolph JF, Scarlett‐Kranz JM, Peterson ME. Effect of non‐thyroidal illness on serum concentrations of T4, free T4, and thyroid stimulating hormone in cats (abstract). JVet Intern Med. 2015;29:1174–1175. [Google Scholar]
- 60. Lee S, Farwell AP. Euthyroid sick syndrome. Compr Physiol. 2016;6:1071–1080. [DOI] [PubMed] [Google Scholar]
- 61. Ganesan K, Wadud K. Thyroid, euthyroid sick syndrome. Treasure Island (FL): StatPearls; 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482219/ [PubMed] [Google Scholar]
- 62. Dong BJ. How medications affect thyroid function. West J Med. 2000;172:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cohen‐Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol. 2010;6:34–41. [DOI] [PubMed] [Google Scholar]
- 64. Dai Q, Kuang A. The relationship between metoclopramide and hypothalamus‐pituitary‐thyroid axis and it's clinical application. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2004;21:164–168. [PubMed] [Google Scholar]
- 65. Scott‐Moncrieff JC, Nelson RW, Bruner JM, Williams DA. Comparison of serum concentrations of thyroid‐stimulating hormone in healthy dogs, hypothyroid dogs, and euthyroid dogs with concurrent disease. JAm Vet Med Assoc. 1998;212:387–391. [PubMed] [Google Scholar]
- 66. Kantrowitz LB, Peterson ME, Melian C, Nichols R. Serum total thyroxine, total triiodothyronine, free thyroxine, and thyrotropin concentrations in dogs with nonthyroidal disease. JAm Vet Med Assoc. 2001;219:765–769. [DOI] [PubMed] [Google Scholar]
- 67. Mooney CT, Shiel RE, Dixon RM. Thyroid hormone abnormalities and outcome in dogs with non‐thyroidal illness. JSmall Anim Pract. 2008;49:11–16. [DOI] [PubMed] [Google Scholar]
- 68. Reusch CE, Fracassi F, Sieber‐Ruckstuhl NS, et al. Altered serum thyrotropin concentrations in dogs with primary hypoadrenocorticism before and during treatment. JVet Intern Med. 2017;31:1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Daminet S, Ferguson DC. Influence of drugs on thyroid function in dogs. JVet Intern Med. 2003;17:463–472. [DOI] [PubMed] [Google Scholar]
- 70. Hume KR, Rizzo VL, Cawley JR, Balkman CE. Effects of toceranib phosphate on the hypothalamic‐pituitary‐thyroid axis in tumor‐bearing dogs. JVet Intern Med. 2018;32:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hulter HN, Gustafson LE, Bonner EL Jr., Toto RD, Mackie S. Thyroid replacement in thyroparathyroidectomized dogs. Miner Electrolyte Metab. 1984;10:228–232. [PubMed] [Google Scholar]
- 72. Dixon RM, Reid SW, Mooney CT. Treatment and therapeutic monitoring of canine hypothyroidism. JSmall Anim Pract. 2002;43:334–340. [DOI] [PubMed] [Google Scholar]
- 73. Le Traon G, Brennan SF, Burgaud S, et al. Clinical evaluation of a novel liquid formulation of L‐thyroxine for once daily treatment of dogs with hypothyroidism. JVet Intern Med. 2009;23:43–49. [DOI] [PubMed] [Google Scholar]
- 74. Mazrier H, French A, Ellinwood NM, et al. Goitrous congenital hypothyroidism caused by thyroid peroxidase deficiency in a family of domestic shorthair cats (abstract). JVet Intern Med. 2003;17:395–396. [Google Scholar]
- 75. Elliott J, Watson A. Chronic kidney disease: International Renal Interest Society staging and management In: Bonagura J, Twedt D, eds. Current Veterinary Therapy XV. St Louis, MO: Saunders‐Elsevier; 2014:857–863. [Google Scholar]
- 76. Steiger MJ, Watson AR, Morgan AG. Hypothyroidism and renal impairment. JR Soc Med. 1991;84:688–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lafayette RA, Costa ME, King AJ. Increased serum creatinine in the absence of renal failure in profound hypothyroidism. Am J Med. 1994;96:298–299. [DOI] [PubMed] [Google Scholar]
- 78. van Welsem ME, Lobatto S. Treatment of severe hypothyroidism in a patient with progressive renal failure leads to significant improvement of renal function. Clin Nephrol. 2007;67:391–393. [DOI] [PubMed] [Google Scholar]
- 79. Chakera A, Paul HJ, O'Callaghan CA. Reversible renal impairment caused by thyroid disease. Scand J Urol Nephrol. 2010;44:190–192. [DOI] [PubMed] [Google Scholar]
- 80. El Ters M, Patel SM, Norby SM. Hypothyroidism and reversible kidney dysfunction: An essential relationship to recognize. Endocr Pract. 2014;20:490–499. [DOI] [PubMed] [Google Scholar]
- 81. Vikrant S, Chander S, Kumar S, Gupta D. Hypothyroidism presenting as reversible renal impairment: An interesting case report. Ren Fail. 2013;35:1292–1294. [DOI] [PubMed] [Google Scholar]
- 82. Montenegro J, Gonzalez O, Saracho R, Aguirre R, González O, Martínez I. Changes in renal function in primary hypothyroidism. Am J Kidney Dis. 1996;27:195–198. [DOI] [PubMed] [Google Scholar]
- 83. Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med. 1999;159:79–82. [DOI] [PubMed] [Google Scholar]
- 84. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review. Gen Comp Endocrinol. 2009;160:205–215. [DOI] [PubMed] [Google Scholar]
- 85. Rhee CM. The interaction between thyroid and kidney disease: An overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab. 2012;16:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mansourian AR. A literature review on the adverse effects of hypothyroidism on kidney function. Pak J Biol Sci. 2012;15:709–719. [DOI] [PubMed] [Google Scholar]


