Abstract
Curcumin belongs to the family of natural compounds collectively called curcuminoids and it possesses remarkable beneficial anti-oxidant, anti-inflammatory, anti-cancer, and neuroprotective properties. Moreover it is commonly assumed that curcumin has also been suggested as a remedy for digestive diseases such as inflammatory bowel diseases (IBD), a chronic immune disorder affecting the gastrointestinal tract and that can be divided in two major subgroups: Crohn’s disease (CD) and Ulcerative Colitis (UC), depending mainly on the intestine tract affected by the inflammatory events. The chronic and intermittent nature of IBD imposes, where applicable, long-term treatments conducted in most of the cases combining different types of drugs. In more severe cases and where there has been no good response to the drugs, a surgery therapy is carried out. Currently, IBD-pharmacological treatments are generally not curative and often present serious side effects; for this reason, being known the relationship between nutrition and IBD, it is worthy of interesting the study and the development of new dietary strategy. The curcumin principal mechanism is the suppression of IBD inflammatory compounds (NF-κB) modulating immune response. This review summarizes literature data of curcumin as anti-inflammatory and anti-oxidant in IBD, trying to understand the different effects in CD e UC.
Keywords: curcumin, inflammatory bowel disease (IBD), inflammation, innovative treatments
1. Introduction
Curcumin, the active yellow pigment of the turmeric spice, is an herb belonging to the ginger family native to India and Southeast Asia. Curcumin is commonly used in Indian traditional cusine and medicine, especially in the treatment of biliary disorders, rheumatism and diabetic ulcers [1].
In more recent years, curcumin has regained interest due to its pharmacological actions and anti-inflammatory, anti-oxidant, anti-tumor, and anti-proliferative properties [1,2,3,4,5,6]. In addition, it is also known for its beneficial effects in neurological diseases by acting as a neuroprotective agent [7]. The principal mechanism, by which curcumin mediates these effects, is connected to the activity of suppression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Furthermore, curcumin activity includes suppression of interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), two main cytokines that play important roles in the regulation of inflammatory responses [6].
For these important activities, curcumin is considered as a valid potential drug treatment in inflammatory bowel disease (IBD). IBD are chronic progressive diseases defined as autoimmune diseases implicated in aberrant and persistent inflammation of the bowel; the two main forms are the Crohn’s disease (CD) and the ulcerative colitis (UC). Although IBD is normally manifested in adulthood, it could onset in childhood even before 2 years of age (early onset child disease, EOCD). This early onset is typically more extensive and characterized by rapid progression, leading to severe repercussion on disease course.
Nowadays there are no therapeutic strategies able to significantly alter the natural history of IBD; instead, the nutritional therapy holds interesting possibilities for the treatment especially considered that conventional pharmacological treatments are discussed for the side-effects and/or adverse events particularly in early onset patients. In this review we summarized literature data of curcumin principal activity, trying to investigate its possible role in the treatment of IBD and to understand the different activities performed in CD and UC.
2. Generalities on Inflammatory Bowel Disease
IBD is a multifactorial disorder in which complex interactions among genetic, immune, and environmental factors are involved and represent a group of inflammatory intestinal idiopathic and chronic diseases [8]. The two main forms, CD and UC, overlap as intestinal disease and differ precisely in the clinical, pathogenic and biomolecular features.
UC is a chronic inflammatory disorder restricted to the colon, characterized by abdominal pain, mucosal ulceration, hematochezia and diarrhea. Pediatric patients may present macroscopic skin lesions in the colon, blackwash ileitis and extensive colitis and periappendiceal inflammation [9].
CD is an inflammatory disorder affecting the gastrointestinal tract in both children and adults. All layers of the intestine may be involved and normal healthy bowel can be found between sections of diseased bowel. The inflammation can affect the entire stretch starting from the mouth to the anus and in more complicated cases perianal and strictures fistulas may also arise. Given the variability of the disease localization the possibility to make a rapid and correct diagnosis is not so easy; initially the diagnosis could be done by performing the endoscopic analysis of biopsies from the patient’s gastrointestinal tract; then, a diagnostic workup for staging the disease especially when the onset is very early in pediatric patients is also very important [10]. From the literature it also appears that in a group of pediatric patients with EOCD an upper gastrointestinal and isolated colonic involvement is more frequent and more evident, compared to those patients in whom the disease occurs later [11].
The molecular pathogenesis of IBD is not completely understood, but among contributing factors may be included the bacterial translocation across a defective mucosal barrier and the imbalanced regulation of the intestinal immune response. The patients with IBD are defined considering the parameters of Montreal Classification: the disease onset, the location and the behavior. On the other hand, however, this classification had some limiting criteria for the pediatric patients classification. On this purpose on the classification of Paris has been added, for pediatric classification the growth failure, and the disease onset before 10 years of age (Table 1) [12].
Table 1.
Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification.
| Classification | Montreal | Paris |
|---|---|---|
| Age at Diagnosis |
A1 <17 year A2 17–40 year A3 above 40 year |
A1a 0–10 year A1b 10–17 year A2 17–40 year A3 > 40 year |
| Location |
L1 terminal ileal ± limited cecal disease L2 colonic L3 ileocolonic L4 only upper disease |
L1 distal 1/3 ileum ± limited cecal disease L2 colonic L3 ileocolonic L4a upper disease proximal to ligament of Treitz L4b upper disease distal to ligament of Treitz and proximal to distal 1/3 ileum |
| Behavior |
B1 non stricturingnon penetrating B2 stricturing B3 penetrating P perianal disease modifier |
B1 non stricturingnon penetrating B2 stricturing B3 penetrating B2B3 both penetrating and stricturing disease, either at the same or different times P perianal disease modifier |
| Growth | not classified |
G0 no evidence G1 growth delay |
2.1. Environmental Exposure and Lifestyle
The continuing changes in environmental factors, such as lifestyle, hygiene, medication, diet, affect the IBD onset differently and its course and prevalence is increasing worldwide [13]. The incidence is low in the Pacific region, Asia, South America Africa and Eastern Europe, while is very high in both Northern and Western Europe and in North America. Recent studies, however, have shown an increase of IBD in developing countries and among emigrant populations moving to industrialized societies [14,15]. The interaction between environmental exposure and life styles and IBD is observable, e.g., in the role of hygienic conditions suggesting that less hygienic situations play a protective role and cleaner life conditions are associated with increased rates of IBD, because in rich countries there is a lower rate of enteric pathogens, hot water and sanization resources [16,17]. The role of the breast-feeding leads as well to different conclusions as to whether it plays a protective role or increases the risk by altering the gut microbiota of breast-fed infants [18,19,20,21].
The exposure to antibiotics may influence the risk of IBD development affecting the mechanism that alters the gut microbiome, especially in children [22,23]. In a study of Canadian adults the relationship between the onset of IBD and the amount of antibiotics taken has been confirmed [24]. The lifestyle factors include the living setting and the dietary choices and both may play the role of risk or protective factors on IBD development. In the last decades much attention has also been paid to the living setting, analyzing the effects of the difference between living in an urban setting or in a rural setting on the onset and course of IBD [25]. Exposure to industrial agents and pollution may play an important role increasing the risk of early onset UC and DC [26]. A dietary intake rich in meats and fatty foods increases the risk of CD and a diet based on vegetables, fruits, fish and olive oil was inversely associated with CD. The different aliments can affect the gut permeability and the autoinflammatory response of the mucosa through microbiota alterations [27,28].
All findings evaluated indicate that the relationship between the different environmental factors and lifestyle that can affect CD and UC and the disease onset and course is complicated and the relationship between environment and genetic susceptibility is still unclear.
2.2. Genetic Involvement in IBD
The literature increasingly suggests that IBD develops in patients with a certain genetic predisposition and the localization, disease progressions and response to treatments have characteristics that strongly depend on the age of onset [29,30,31,32]. A difficult issue is that the functional relevance of most of the susceptibility genes is unclear and the total of loci identified until now do not account for the total hereditability of IBD. Even given the unproven assumptions that these loci are individually causal and collectively additive, the relative risk conferred by each locus is very small and their overall contribution would account for only 13.6% of CD and 7.5% of UC hereditability [33]. This difficult issue is a problem referred to the missing heritability. In addition to the variants found with the GWAS studies, other genes may be involved and they could be associated with the disease as monogenic pattern of hereditability and this could escape the association analysis [34,35]. There is in addition also the problem of the missing intelligibility, as it is very difficult to define and to make immunological sense of all these loci and pathways, both at the cellular and molecular level. Pediatric IBD onset is increasing; about 20%–25% of IBD patients develop intestinal inflammation during childhood and adolescence and approximately 1% of children below one year develop the disease. Moreover very early onset IBD has an estimated incidence of 4.37/100,000 children and a prevalence of 14/100,000 children [36].
2.3. Immune Response and IBD
From different studies can be inferred that an imbalance of pro- and anti-inflammatory factors plays an important role in the pathogenesis of IBD [37,38]. Both disorders, CD and UC, are characterized by immunological responses against (bacterial) antigens but the type of the inflammatory reaction appears to be distinct. Given that in UC there exists neither predominance of interferon gamma (IFN-γ) nor IL-4, while it is clear the up-regulation of IL-5, we can consider it more as a Type 2 Immunity (TH2) disease. On the other hand, as in CD areas of active inflammation with elevated IFN-γ, IL-12 and tumor necrosis factor (TNF) levels are observed, CD is considered a Type 1 Immunity (TH1) disease prototype [39].
It is commonly assumed that T helper cells comprise two subsets, each having different patterns of cytokine production in immune responses: the TH1 and the TH2, both implicated in the regulation of many immune response. The TH1 cells secrete in particular IFNγ, TNFα and TNFβ and IL-2, while the TH2 cells secrete IL-4, IL-5, IL-6, IL-10 and IL-13 [40].
In their study, Pastorelli and colleagues showed an increased expression of IL-33 and IL-1 receptor ST2 in the serum and in the inflamed mucosa of the IBD patients. ST2 exists in two different splice variants leading to the synthesis of ST2L, a transmembrane receptor that confers IL-33’s biologic effects, and sST2, a soluble molecule that likely serves as a decoy receptor for IL-33. From their research it is clear that the system IL-33/ST2 is strongly activated and plays an important role in the pathogenesis of the IBD, also evident in patients with UC. Specifically, it was observed that during active UC, the accumulation of the intraepithelial and intracellular IL-33 as well as the decrease of the ST2L were regulated by TNF. In fact, the anti-TNF treatment of IBD patients, particularly those suffering from UC, modulates the levels of IL-33 and of sST2 [37].
A very recent study also shows that blockage of the IL-33 signal may help in the treatment of UC patients. Specifically this study demonstrates that IL-33 is able to induce the intestinal GATA-3 (master regulatory gene) in the mucosa T cells, thus entrusting to IL-33 a mediating role in the intestinal inflammatory TH2 responses (pathological or not) [41].
By studying how genetic and epigenetic factors influence the age at onset and other clinical features of CD, it is possible to improve the understanding of the disease pathogenesis, in particular through the development of in vitro and ex vivo models reproducing the interaction between epithelial cells and microbiota. These models are fundamental to study also the development of novel therapeutic approaches aimed to restore a normal balance between immunity and environment. These data contribute to develop disease-specific cellular models based on induced pluripotent stem cells.
Once the disease is started, complete healing is an exceptional outcome, suggesting that some epigenetic changes, such as DNA methylation, may stably affect the way that mucosal immunity respond to intestinal microbiota. Focusing on immune defects as well as excesses in the pathogenesis of IBD may be relevant for therapeutic approaches [42,43].
3. IBD and Pharmacological Treatments
The chronic and intermittent nature of inflammation in IBD requires long-term drug treatments in combination or alternation to different drugs. The first aim in treatment is to reduce symptoms and to induce the remission and then to maintain this remission for as long as possible.
The severity, the presence of complications and the goal of the treatment (induction or maintenance of the remission) determine the choice of therapeutic line: aminosalicylates are the first line therapy for mild to moderate IBD; corticosteroids are preferred to use for moderate to severe disease, but are ineffective in the maintenance of remission [44] and then immuno-modulators which generally are not the elective choice due to their slow onset of action and toxicity [45]. Numerous studies have reported on the use of biological factors capable of manipulating the immune and inflammatory responses: inhibitors of T-Cell activation, anti-inflammatory cytokines (IL-10 or IL-11) and inhibitors of pro-inflammatory cytokines including TNF antagonists (infliximab, certolizumab pegol, etanercept, onercept, adalimumab, mitogen-activated protein kinases), inhibitors of PPARs, inhibitors of pro-inflammatory cytokine receptors (anti IL-6 receptor), inhibitors of TH1 polarization (anti-IL-2R antibodies, anti-IL-12, IL-18 and IFNγ) and adhesion molecule inhibitors (natalizumab) [46].
Although there are several potential therapeutic targets considered for the treatment of IBD, currently only a few biologic drugs have achieved any significant results and are approved for treatment, such as infliximab that has been introduced into clinical routine in the United States in 1988 and continues to be effective.
Adverse Effects of IBD Treatments
The aminosalicylates are effective in controlling the inflammation, but may have adverse effects such as nausea, vomiting, heartburn, diarrhea and headache. The side effects of the corticosteroids are weight gain, acne, facial hair, hypertension, diabetes, bone mass loss and increased risk of infections.
The use of immunosuppressant drugs (thiopurines, methotrexate, tacrolimus, thalidomide, cyclosporine and infliximab) is effective in the treatment of IBD (active or quiescent CD or in cases of steroid dependent UC), as well in pediatric patients [47,48], but these drugs are not without adverse effects, sometimes also serious as the case of methotrexate, which may cause dyspepsia, alopecia, myelosuppression, abdominal pain, headache and arthralgia [49]. Thalidomide, that acts as an inhibitor of TNFα synthesis, besides being a teratogen, could lead to peripheral neuropathy, dizziness and allergic reactions [47,50]. The use of tacrolimus has shown an elevated risk to develop adverse effects in UC patients, including the most common, finger tremors. The anti-TNFα molecules, including infliximab and adalimumab, cause reactivation of latent infections, cutaneous reactions (skin eruptions and macules), systemic and hematological complications, allergic events and local side effects [51,52]. Recently Lakatos et al., suggested that the use of biological drugs for a long time may increase the risk to develop malignancies such as the non-Hodgkins’s lymphoma [53].
It has also been seen that therapy with biological agents (anti-TNFα), as well as the use of immunosuppressants and/or the repeated use of corticosteroids, make patients much more susceptible to endemic and opportunistic infections by bacteria, fungi, parasites or pathogens viruses [54].
Cyclosporine acts by inhibiting the production of IL-2 by activated T lymphocytes and it is used in the case of severe UC, but it is related to manifestations such as hypertension, impaired renal function and neurotoxicity (tremor or paresthesia), as well as minor adverse effect such as fever, headaches and diabetes mellitus [55].
As regards children and adolescents, researchers must bear in mind that the disease involves the physical but also the psychological level and drug treatments can affect the quality of life. Even if the strategies that involve the steroids use in children are preferable, one study performed in children suffering from Crohn’s disease has shown that a short course of polymeric diet was more effective then corticosteroid treatments [56]. Conventional medications used in the treatment of symptoms of IBD consist of anti-inflammatory and immuno-modulator drugs as summarized in Table 2 [44,45,46,47,48,49,50,51,52,53,54].
Table 2.
Characteristics of inflammatory bowel disease.
| Localization | Symptoms | Cytokine Inflammation | TH1/TH2 | Treatments | |
|---|---|---|---|---|---|
| Crohn’s Disease (CD) | Deep layers of the intestinal wall, the ileum, the first part of the colon, esophagus, stomach and duodenum | Pain in the abdomen, diarrhoea, weight loss, rectal bleeding and fever | Interferon gamma (IFN-Y), Interlukin 12 (IL-12), Tumor Necrosis Factor (TNF) | TH1 disease | Anti-inflammation drugs, corticosteroids, immunomodulators and biologic treatments |
| Ulcerative Colitis (UC) | Inner lining of the colon (large interstine) and rectum | Diarrhoea, abdominal cramps, rectal bleeding, frequent fever and nausea | Interlukin 5 (IL-5), Interlukin 33/Interlukin 1, Receptor ST2 (IL-33/ST2) | TH2 disease | Aminosalicylates, corticosteroids, immunomodulators and biological treatments |
4. Curcumin: Potential and Limits
Curcumin (diferuloymethane) is the most active component of the plant Curcuma longa, belonging to the family Zingiberaceae also known as turmeric, commonly employed as a natural food additive. It is an indigenous plant of India, but is also cultivated in other countries such as China and Sri Lanka [57]. Used in Indian and Chinese traditional medicine, it has been described as an anti-inflammatory, antioxidant, pro-apoptotic, chemopreventive, antitumor and antimicrobial compound, as reported in Table 3 [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140].
Table 3.
Molecular targets of curcumin and relative effects and diseases involved.
| Targets of Curcumin | Effects of Curcumin | Diseases Involved |
|---|---|---|
| Activation of redox-regulated transcription factor Nrf2 that induces heme oxygenase 1 (HO1) paraoxonase 1 (PON1) and GSH 3 [58,60,61,62] | Free-radical-scavenging activity | Chronic inflammatory diseases |
| Inhibition of DNA-binding of STAT3 3 [63] | Anti-inflammatory activity | Chronic inflammatory diseases |
| Reduced phosphorylation of cytosolic phospholipase A2 (cPLA2) limiting the arachidonic acid availability [64] | Anti-inflammatory activity | Chronic inflammatory diseases |
| Reduced phosphorylation of IκB [65] | Anti-inflammatory activity | Chronic inflammatory diseases Cancer |
| Inhibition of the transcription factor Nf-κB [65,66,67,68,69,70,71,72] | Anti-inflammatory activityAnti-oxidant activity Tumor suppressive activity |
Chronic inflammatory diseases Cancer |
| Inhibition of mRNA levels of COX2 and iNOS [73,74,75,76] | Anti-inflammatory activity Tumor suppressive activity |
Chronic inflammatory diseases Cancer |
| Inhibition of matrix metalloproteinases MMP-9 and MMP-2 [72,77,78, 79] | Tumor suppressive activity (Anti-inflammatory activity) |
Cancer (Chronic inflammatory diseases) |
| Inhibition of histone deacetylases (HDACs) and acetyltransferases (HATs) activity [80,81,82,83,84,85,86,87,88] | Gene regulation | Cancer |
| Up-regulation and down-regulation of micro RNA (22, 199, 186, 203) [80,89,90,91] | Pro-apoptotic activity Tumor suppressive activity |
Cancer |
| Activation of caspase 3, 7, 8 and caspase 9 [92,93,94] | Pro-apoptotic activity | Cancer |
| Increased cleavage of poly (ADP-ribose) polymerase (PARP) [67,95] | Pro-apoptotic activity | Cancer |
| Up-regulation of several tumor suppressor genes [95,96,97,98,99] | Tumor suppressive activity | Cancer |
| Up-regulation of different proapoptotic genes [100,101,102,103] | Pro-apoptotic activity Tumor suppressive activity |
Cancer |
| Inactivation of several oncogenes [104,105,106,107,108,109,110,111,112] | Tumor suppressive activity | Cancer |
| Down-regulation of different antiapoptotic genes [113,114] | Pro-apoptotic activity Tumor suppressive activity |
Cancer |
| Inhibition of angiogenesis suppressing VEGF, Akt and PI3K [65,115,116,117,118] | Tumor suppressive activity | Cancer |
| Inhibition of enzymes of phase I reactions [119,120,121] | Tumor suppressive activity | Cancer |
| Activation of enzymes of phase II reactions [122,123,124,125,126,127,128] | Tumor suppressive activity | Cancer |
| Down-regulation of androgen receptor (AR) [110,129,130,131] | Tumor suppressive activity | Cancer |
| Repressed N-methyl-D-aspartate (NMDA) receptor-mediated Ca2+ [132,133,134,135,136] | Protection from excitotoxicity | Neurodegenerative diseases |
| Reduced oxidative mitochondrial damage [137,138,139,140] | Antioxidative activity | Neurodegenerative diseases |
Several studies have described the strongly link within these beneficial properties; indeed they don’t show different and separated effects, but they are related each other as a consequence of some specific characteristic. As an example, the anti-oxidant effect of curcumin and analogues was related to their anti-tumor and anti-inflammatory mechanism, as already described in recent studies [141,142,143].
Curcumin has been proposed to be a therapeutic molecule in various illnesses such as arthritis, cancer, diabetes, cardiovascular diseases, liver fibrosis, gall stone formation, neurological disease and inflammatory bowel disease [144,145]. The clinical development as a therapeutic drug is limited due to its poor aqueous solubility, poor absorption, biodistribution, rapid metabolism and fast elimination [146,147]. In the last years various natural and synthetic analogues of curcumin have been synthesized to improve bioavailability problems increasing its therapeutic potential, but their effectiveness is still controversial [148].
In spite of all this, the oral administration of the drug allows for an active level of curcumin in the gastrointestinal tract, making it a good candidate for the treatment of the diseases in this anatomical site [149,150,151].
How curcumin exerts its pleiotropic effects has been thoroughly investigated and a vast array of targets has been assumed to play a role in the disease pathogenesis. Some molecular targets include transcription factors, inflammatory cytokines, enzymes and the epigenetic modulation which modulate histone deacetylases, histone acetyltransferases, DNA methyltransferase I and miRNAs [80].
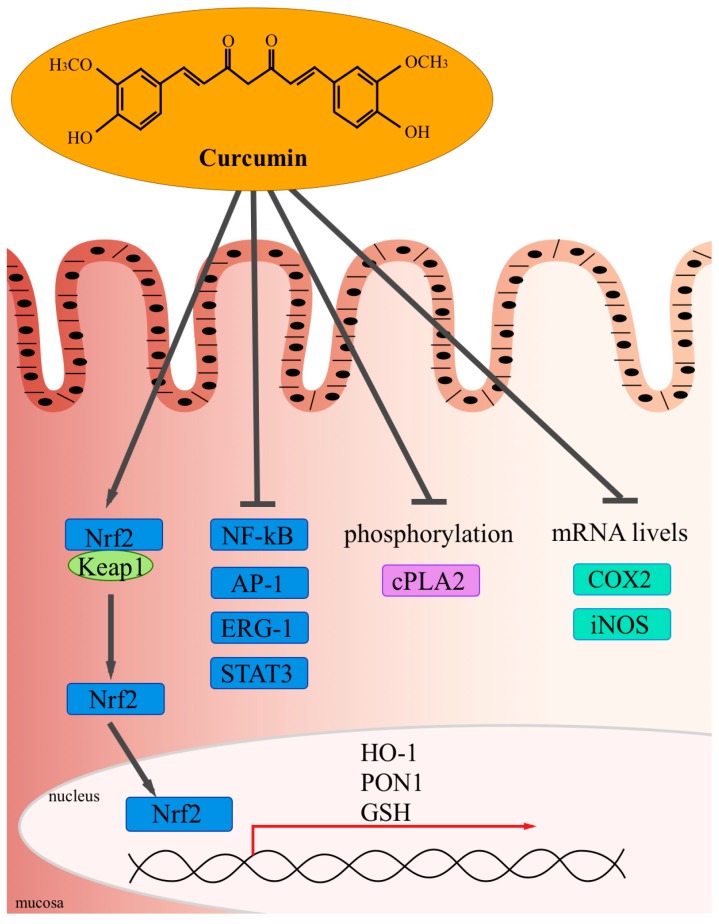
In idiopathic inflammatory bowel diseases the persistent inflammation depends partially on the activation of NF-κB signaling cascade level or other molecular targets, as indicated in Table 3, and part of the pleiotropic effect of curcumin seems to due to inhibition of this pathway. In vivo and in vitro studies exhibit a different reaction to curcumin in both IBD form, CD and UC, probably depending on the kind of immune dysregulation involved, as shown in Figure 1 [152,153,154,155].
Figure 1.
The curcumin activity in mucosal.
Experimental Studies and Clinical Trials
In their study Billerey-Larmonier et al., showed the effect of dietary curcumin in two mice strains (BALB/c and SJL/J) with chemically induced colitis. An improvement in the pathological condition of BALB/c mice, exhibiting a mixed TH1/TH2 response, was detected, while a lack of benefits was revealed in SJL/J mice characterized by TH1 response, suggesting a role of curcumin in regulating the immune response [152].
Other studies showed a weakened colonic inflammation in induced colitis mice and rats due to inhibition of NF-κB pathway, p38 mitogen-activated protein kinases (MAPK) activity and reduction of pro-inflammatory TH1 cytokine response, resulting in suppression of inducible nitric oxide synthase and lower neutrophil recruitment [156,157]. Furthermore, in mice with spontaneous development of intestinal inflammation the administration of the Indian spice showed a reduced inflammation in histological colonic pattern [158]. In the human colonic mucosa of IBD patients a reduced MAPK activity was detected, while IL-10 was increased and IL-1β reduced [154]. Although in mice experiments curcumin was administered intraparenterally, the oral administration in human patients highlighted improvements on the overall IBD situation, detecting however, a similar response to curcumin as adjuvant IBD therapy in patients with CD and UC [159,160]. The co-administration of curcumin with conventional drugs was also shown to be safe and well-tolerated in IBD pediatric patients, and in fact no clinically significant side effects were reported [161].
Keeping in mind that CD is associated with a TH1/TH17 cell mediated response, while UC is associated with atypical TH2 response, clinical trials, unlike in vivo studies, suggest that curcumin could act on a common pathway shared by the two immune responses, probably NF-κB.
To evaluate the curcumin efficacy in IBD disease a study on 10 IBD patients was conducted: five patients suffered from CD and five were affected by Ulcerative Proctitis, a mild form of UC. For this form of UC patients were administered for the first month of therapy 550 mg of curcumin twice daily and for the second month the same dose, but three times a day. After therapy a significant reduction of both the symptoms and the inflammatory indices was evident. To CD patients instead 360 mg were administered three times per day for the first month and four times daily for the second and third month. Only four patients completed the study and in them an evident reduction of both the CD activity index and some indicative parameters was observed [162].
Another study was carried out in an adult woman (60 years) suffering from UC and enteropathic arthropathy. After having tried all medications including all possible combinations and after refusing treatments with biological agents because of possible side effects, she agreed to also take 500 mg of curcumin daily with 40 mg of prendnisone. After a year of treatment, improvements were evident to the extent that there were no marked ulcerations and biopsies showed a chronic inactive UC [163].
Considering the positive effects of curcumin obtained in these studies described above and others performed in patients suffering from IBD, curcumin could be an alternative and/or an additional treatment in controlling both CD and UC disease [151]. We report in Table 4 the main clinical trials, ongoing and concluded, evaluating the efficacy of curcumin in IBD.
Table 4.
Clinical trials to assess the efficacy of curcumin in IBD.
| ClinicalTrials.gov Identifier | Number of Patients (Age) | Disease | Doses of Curcumin | Phase |
|---|---|---|---|---|
| NCT01320436 | 50 (18 to 70 years) | Ulcerative colitis (Disease activity score of >5 and ≤13 according to the Simple clinical colitis activity index (SCCAI) | Patients allocated for this arm will receive 5ASA medication (as advised by their treating physician) + 3 capsules (820 mg each) curcumin twice daily after meals. | 3 |
| NCT00889161 | 11 (8 to 18 years) | Inflammatory bowel disease (mild disease or in clinical remission) [160] | Initial dosage of 500 mg twice a day for 3 weeks. Using the forced dose titration design, dose will be titrated up to 1 g twice a day at week 3 for a total of three weeks and then titrated again to 2 g twice a day at week 6 for three weeks | 1 |
| NCT00793130 | 30 (18 to 75 years) | Mild or moderate Ulcerative Colitis | Dietary Supplement: Coltect Two tablets twice daily (BID) during the 2 months of the study. Each tablet contains 500 mg Curcumin, 250 mg Green tea and 100 μg Selenomethionine. |
Unknown |
| NCT01647412 | 40 (10 to 17 years) | Crohn’s Disease (Moderate to severely active CD, as defined by a PCDAI score >30 and | The experimental group will receive the exclusion diet and nutraceutical therapy (DNT) and daily subcutaneously administered recombinant human growth hormone (rhGH) for the first 26 weeks. After 26 weeks this group will continue on the exclusion diet nutraceutical therapy for the remaining 26 weeks of the study. | 2 |
| 89 (13 to 65 years) |
Ulcerative Colitis (patients in remission of disease) [148] | Oral curcumin (2 g/day; 1 g morning and evening, after meals) | Concluded |
5. Curcumin and Inflammation
The NF-κB transcription factor family plays a key role in several cellular functions (inflammation, apoptosis, cell survival, proliferation, angiogenesis and innate and acquired immunity) as well as in regulating the expression of more than 500 different genes involved in inflammatory and immune responses [155,164]. Many molecules are involved in the inflammatory response regulated by NF-κB pathway (e.g., TNF, IL-1, IL-6, IL-8, IL-10) as well as tissue destructive enzymes (e.g., matrix metalloproteinases and prostaglandins). TNF is the most promptly released cytokine upon injury and, through the interaction with its receptors, it regulates the production of pro-inflammatory and anti-inflammatory cytokines [165]. Therapies that target rate-limiting steps like TNF (such as infliximab), have markedly improved the autoimmune disease progression in IBD. Moreover NF-κB plays a pivotal role in the regulation of the adaptive phase and in the resolution of the inflammation through apoptosis mechanisms, including the pyroptosis IL-1β dependent one [166,167].
All these findings considered, the activity of curcumin in this context is very important, because it mediates its effects through modulation NF-κB and pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6 [168,169]. The transcriptional factors NF-κB as well as growth factor and growth factor receptors, protein kinases, adhesion molecules and enzymes are molecular targets for curcumin activity [170].
6. Curcumin Analogues and Nanoformulations
This natural bioactive component has shown a wide range of biological properties and pharmacological actions, suggesting interesting clinical applications, but researchers must face the other side of the coin, that is all those factors contributing to the low bioavailability, the poor solubility (i.e., 0.4 mg/mL at pH 7.3) and absorption, and the rapid metabolic elimination by reduction and conjugation [171] causing limitations to the clinical applications. In order to solve this problem, there is a need to develop curcumin analogues and nanoformulations with higher metabolic stability than the original compound [172,173,174].
6.1. Curcumin Analogues
Recently several studies, as those experimental in vivo evaluating the tumor cell viability, have demonstrated that compounds analogous to curcumin have the same beneficial properties of the original compound and, at the same concentration, even a better effect [175].
Curcumin analogues, such as dimethoxycurcumin or novel water-soluble curcumin derivatives have shown good pharmacological effects in metabolic disorders and in diabetes mellitus [176,177]. The major biochemical characteristics needed in analogues are stability, good pharmacokinetic properties, drug release in the correct site and decreased fluctuations [178]. Moreover recent studies support previous evidence that the biological activity of analogues of curcumin (as an example, 2,5-bis(4-hydroxy-3-methoxybenzylidene)cyclopentanone) was better than that of curcumin: the antioxidant and anti-cyclooxygenase activities of this compound are 2- and 7-times higher, and the anti-inflammatory activity 5-times higher than those of curcumin at a dose of 20 mg/kg, p.o. This compound, indeed, potently inhibits histamine release by altering some intracellular signaling events in mast cells and will be a good candidate for an anti-allergic and anti-inflammatory drug [179,180,181,182].
6.2. Nanoformulations
Furthermore, to overcome the low aqueous solubility of curcumin as a therapeutic agent, many technologies have been developed and applied. In particular several studies have described the positive results obtained from the design and the development of nano-sized delivery systems for curcumin, including liposomes, polymeric nanoparticles and micelles, conjugates, peptide carriers, cyclodextrins, solid dispersions, lipid nanoparticles and emulsions [183,184].
Literature data report preliminary promising results obtained by experimental in vitro and in vivo studies, through the development of specific curcumin delivery systems protecting against the fast degradation and targeting the inflamed colon. The compound was encapsulated in polymeric pH-sensitive nanoparticles to obtain a selective and specific delivery to the inflamed mucosa. Nano-sized drug delivery systems represent an efficacious strategy against the inflammatory system in IBD treatment [185,186]. In particular recent studies show the effective anti-inflammatory properties (myeloperoxidase activity, a measure of neutrophil infiltration, and TNFα secretion), in vitro and in vivo, obtained by curcuma encapsulated in polymeric pH-sensitive nanoparticles for a selective and specific delivery of curcumin to the inflamed mucosa [186,187].
The nanoformulation that seems to be the better solution as methods to deliver the curcumin in IBD condition is cyclodextrin-curcumin complex: the results obtained in vitro and in vivo confirmed that hydroxypropyl-β-cyclodextrin-curcumin complex represents a valuable innovative therapeutic approach for IBD treatment [187]. Although the nanoformulations have shown a good level of safety it is necessary to pay attention to their potential toxicity, especially with repeated administrations at high dosage [176].
7. Conclusions
Curcumin is a natural compound that reduces the development of chronic experimental colitis and alleviates the inflammatory response whose precise modes of action is still unclear, and it seems likely that its molecular targets differ according to cell and disease system. Several studies have demonstrated the promising role of curcumin as a novel therapy for children and adults with IBD.
To date a precise understanding of the effective dose, safe regimental therapy, and mechanism of action for the use of curcumin in the treatment of IBD is unknown, but there is abundant evidence proving its effects on the NF-κB pathway and p38 MAPK in the intestinal mucosa.
The key role played by curcumin in the diet and its implications for the quality of life of IBD patients should be studied because preliminary data obtained in clinical trials are very encouraging. The pleiotropic role of curcumin in IBD pathogenesis and range severity of phenotype is very remarkable.
We conclude that large-scale, double-blind trials need to be conducted to establish the role of curcumin in the treatment of IBD. The parameters crucial to be included in the study are disease onset, age of patients, pharmacological assumptions and diet interaction, administration with respect to the inflammation phase (acute or in regression). In case of nanoformulations, clinical trials are also required to establish not only the efficacy, but also the safety in case of repeated use. In conclusion we think that it is necessary to deepen if, how and how much curcumin is useful for preventing the recurrence of IBD by modifying the patient’s diet in remission periods and/or for decreasing the mucosal inflammation in the acute phase.
Acknowledgments
This study was supported by a grant from the Institute for Maternal and Child Health—IRCCS “Burlo Garofolo”—Trieste, Italy (RC 42/2011).
Author Contributions
L.V.B. and A.M. conceived and wrote, revised and assembled the paper. M.G. wrote “IBD and pharmacological treatments” and “Adverse effects of IBD treatments”. P.M.T. wrote “Introduction” and “Curcumin and inflammation”. V.Z. wrote “Curcumin: potential and limits” and “Experimental studies and clinical trials”. A.M.B. wrote “Generalities on Inflammatory Bowel Disease and Inflammation” and “Curcumin analogues and nanoformulations”. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples are not available from authors.
References
- 1.Gupta S.C., Kismali G., Aggarwal B.B. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A., Ahuja A., Ali J., Baboota S. Conundrum and therapeutic potential of curcumin in drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2010;27:279–312. doi: 10.1615/CritRevTherDrugCarrierSyst.v27.i4.10. [DOI] [PubMed] [Google Scholar]
- 3.Ammon H.P., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 4.Lev-Ari S., Strier L., Kazanov D., Elkayam O., Lichtenberg D., Caspi D., Arber N. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology. 2006;45:171–177. doi: 10.1093/rheumatology/kei132. [DOI] [PubMed] [Google Scholar]
- 5.Neerati P., Devde R., Gangi A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with Type-2 Diabetes Mellitus. Phytother. Res. 2014 doi: 10.1002/ptr.5201. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.S., Young M.R., Bobe G., Colburn N.H., Milner J.A. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev. Res. (Phila) 2009;2:200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J., Lee H.J., Lee K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010;112:1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 8.Abraham C., Cho J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009;19:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bie C.I., Paerregaard A., Kolacek S., Ruemmele F.M., Koletzko S., Fell J.M., Escher J.C., EUROKIDS Porto IBD Working Group of ESPGHAN Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-Year analyses of the EUROKIDS Registry. Inflamm. Bowel Dis. 2013;19:378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 10.Maccioni F., Ansari N.A., Mazzamurro F., Civitelli F., Viola F., Cucchiara S., Catalano C. Detection of Crohn Disease Lesions of the Small and Large Bowel in Pediatric Patients: Diagnostic Value of MR Enterography Versus Reference Examinations. Am. J. Roentgenol. 2014;203:W533–W542. doi: 10.2214/AJR.13.11792. [DOI] [PubMed] [Google Scholar]
- 11.Aloi M., Lionetti P., Barabino A., Guariso G., Costa S., Fontana M., Romano C., Lombardi G., Miele E., Alvisi P., et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2014;20:597–605. doi: 10.1097/01.MIB.0000442921.77945.09. [DOI] [PubMed] [Google Scholar]
- 12.Levine A., Griffiths A., Markowitz J., Wilson D.C., Turner D., Russell R.K., Fell J., Ruemmele F.M., Walters T., Sherlock M., et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm. Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 13.Ponder A., Long M.D. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin. Epidemiol. 2013;5:237–247. doi: 10.2147/CLEP.S33961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ng S.C., Bernstein C.N., Vatn M.H., Lakatos P.L., Loftus E.V., Jr., Tysk C., O’Morain C., Moum B., Colombel J.F., Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD) Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 16.Molodecky N.A., Kaplan G.G. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. (N. Y.) 2010;6:339–346. [PMC free article] [PubMed] [Google Scholar]
- 17.Castiglione F., Diaferia M., Morace F., Labianca O., Meucci C., Cuomo A., Panarese A., Romano M., Sorrentini I., D’Onofrio C., et al. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: A case-control, multi-centre, prospective study in Southern Italy. J. Crohn’s Colitis. 2012;6:324–329. doi: 10.1016/j.crohns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Baron S., Turck D., Leplat C., Merle V., Gower-Rousseau C., Marti R., Yzet T., Lerebours E., Dupas J.L., Debeugny S., et al. Environmental risk factors in paediatric inflammatory bowel diseases: A population based case control study. Gut. 2005;54:357–363. doi: 10.1136/gut.2004.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klement E., Cohen R.V., Boxman J., Joseph A., Reif S. Breastfeeding and risk of inflammatory bowel disease: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2004;80:1342–1352. doi: 10.1093/ajcn/80.5.1342. [DOI] [PubMed] [Google Scholar]
- 20.Barclay A.R., Russell R.K., Wilson M.L., Gilmour W.H., Satsangi J., Wilson D.C. Systematic review: The role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 2009;155:421–426. doi: 10.1016/j.jpeds.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Khalili H., Ananthakrishnan A.N., Higuchi L.M., Richter J.M., Fuchs C.S., Chan A.T. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm. Bowel Dis. 2013;19:542–547. doi: 10.1097/MIB.0b013e31828132f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronman M.P., Zaoutis T.E., Haynes K., Feng R., Coffin S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics. 2012;130:794–803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw S.Y., Blanchard J.F., Bernstein C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010;105:2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- 24.Shaw S.Y., Blanchard J.F., Bernstein C.N. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am. J. Gastroenterol. 2011;106:2133–2142. doi: 10.1038/ajg.2011.304. [DOI] [PubMed] [Google Scholar]
- 25.Soon I.S., Molodecky N.A., Rabi D.M., Ghali W.A., Barkema H.W., Kaplan G.G. The relationship between urban environment and the inflammatory bowel diseases: A systematic review and meta-analysis. BMC Gastroenterol. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan G.G., Hubbard J., Korzenik J., Sands B.E., Panaccione R., Ghosh S., Wheeler A.J., Villeneuve P.J. The inflammatory bowel diseases and ambient air pollution: A novel association. Am. J. Gastroenterol. 2010;105:2412–2419. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Souza S., Levy E., Mack D., Israel D., Lambrette P., Ghadirian P., Deslandres C., Morgan K., Seidman E.G., Amre D.K. Dietary patterns and risk for Crohn’s disease in children. Inflamm. Bowel Dis. 2008;14:367–373. doi: 10.1002/ibd.20333. [DOI] [PubMed] [Google Scholar]
- 28.Chapman-Kiddell C.A., Davies P.S., Gillen L., Radford-Smith G.L. Role of diet in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2010;16:137–151. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 30.Heyman M.B., Kirschner B.S., Gold B.D., Ferry G., Baldassano R., Cohen S.A., Winter H.S., Fain P., King C., Smith T., et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J. Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 31.Bianco A.M., Zanin V., Girardelli M., Magnolato A., Martelossi S., Tommasini A., Marcuzzi A., Crovella S. A common genetic background could explain early-onset Crohn’s disease. Med. Hypotheses. 2012;78:520–522. doi: 10.1016/j.mehy.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Cannioto Z., Berti I., Martelossi S., Bruno I., Giurici N., Crovella S., Ventura A. IBD and IBD mimicking enterocolitis in children younger than 2 years of age. Eur. J. Pediatr. 2009;168:149–155. doi: 10.1007/s00431-008-0721-2. [DOI] [PubMed] [Google Scholar]
- 33.Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y., Lee J.C., Schumm L.P., Sharma Y., Anderson C.A., et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhlig H.H. Monogenic diseases associated with intestinal inflammation: Implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795–1805. doi: 10.1136/gutjnl-2012-303956. [DOI] [PubMed] [Google Scholar]
- 35.Bianco A.M., Girardelli M., Vozzi D., Crovella S., Kleiner G., Marcuzzi A. Mevalonate kinase deficiency and IBD: Shared genetic background. Gut. 2014;63:1367–1368. doi: 10.1136/gutjnl-2013-306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlig H.H., Schwerd T., Koletzko S. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastorelli L., Garg R.R., Hoang S.B., Spina L., Mattioli B., Scarpa M., Fiocchi C., Vecchi M., Pizarro T.T. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl. Acad. Sci. USA. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cominelli F. Cytokine-based therapies for Crohn’s disease-new paradigms. N. Engl. J. Med. 2004;351:2045–2048. doi: 10.1056/NEJMp048253. [DOI] [PubMed] [Google Scholar]
- 39.Bamias G., Martin C., Mishina M., Ross W.G., Rivera-Nieves J., Marini M., Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 40.Wallace K.L., Zheng L.B., Kanazawa Y., Shih D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidelin J.B., Coskun M., Kvist P.H., Holm T.L., Holgersen K., Nielsen O.H. IL-33 promotes GATA-3 polarization of gut-derived T cells in experimental and ulcerative colitis. J. Gastroenterol. 2014 doi: 10.1007/s00535-014-0982-7. [DOI] [PubMed] [Google Scholar]
- 42.Karatzas P.S., Gazouli M., Safioleas M., Mantzaris G.J. DNA methylation changes in inflammatory bowel disease. Ann. Gastroenterol. 2014;27:125–132. [PMC free article] [PubMed] [Google Scholar]
- 43.Orel R., Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014;20:11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faubion W.A., Jr., Loftus E.V., Jr., Harmsen W.S., Zinsmeister A.R., Sandborn W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 45.Present D.H., Meltzer S.J., Krumholz M.P., Wolke A., Korelitz B.I. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann. Intern. Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 46.Ardizzone S., Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- 47.Lazzerini M., Martelossi S., Magazzù G., Pellegrino S., Lucanto M.C., Barabino A., Calvi A., Arrigo S., Lionetti P., Lorusso M., et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: A randomized clinical trial. JAMA. 2013;27:2164–2173. doi: 10.1001/jama.2013.280777. [DOI] [PubMed] [Google Scholar]
- 48.Renna S., Cottone M., Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J. Gastroenterol. 2014;20:9675–9690. doi: 10.3748/wjg.v20.i29.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feagan B.G., Rochon J., Fedorak R.N., Irvine E.J., Wild G., Sutherland L., Steinhart A.H., Greenberg G.R., Gillies R., Hopkins M. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N. Engl. J. Med. 1995;332:292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 50.Felipez L.M., Gokhale R., Tierney M.P., Kirschner B.S. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J. Pediatr. Gastroenterol. Nutr. 2012;54:28–33. doi: 10.1097/MPG.0b013e318228349e. [DOI] [PubMed] [Google Scholar]
- 51.Denadaia R., Vieira Teixeiraa F., Steinwurzb F., Romitic R., Saad-Hossnea R. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: A systematic literature review based on 222 cases. J. Crohn’s Colitis. 2013;7:517–524. doi: 10.1016/j.crohns.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Uyanikoglu A., Ermis F., Akyuz F., Pinarbasi B., Baran B., Aydogan T., Demir K., Besisik F., Kaymakoglu S. Infliximab in inflammatory bowel disease: Attention to adverse events. Eur. Rev. Med. Pharmacol. Sci. 2014;18:2337–2342. [PubMed] [Google Scholar]
- 53.Lakatos P.L., Miheller P. Is there an increased risk of lymphoma and malignancies under anti-TNF therapy in IBD? Curr. Drug Targets. 2010;11:179–186. doi: 10.2174/138945010790309867. [DOI] [PubMed] [Google Scholar]
- 54.Stallmach A., Hagel S., Bruns T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010;24:167–182. doi: 10.1016/j.bpg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Van Assche G., D’haens G., Noman M., Vermeire S., Hiele M., Asnong K., Arts J., D’hoore A., Penninckx F., Rutgeerts P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. doi: 10.1016/S0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 56.Rabizadeh S., Dubinsky M. Update in pediatric inflammatory bowel disease. Rheum. Dis. Clin. North Am. 2013;39:789–799. doi: 10.1016/j.rdc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Esatbeyoglu T., Huebbe P., Ernst I.M.A., Chin D., Wagner A.E., Rimbach G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. 2012;51:5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 59.Rahmani A.H., al Zohairy M.A., Aly S.M., Khan M.A. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. Biomed. Res. Int. 2014;2014:761608. doi: 10.1155/2014/761608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrader C., Schiborr C., Frank J., Rimbach G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2011;105:167–170. doi: 10.1017/S0007114510004356. [DOI] [PubMed] [Google Scholar]
- 62.Rahman I. Antioxidant therapeutic advances in COPD. Ther. Adv. Respir. Dis. 2008;2:351–374. doi: 10.1177/1753465808098224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J.Y., Zhong X., Yum H.W., Lee H.J., Kundu J.K., Na H.K., Surh Y.J. Curcumin Inhibits STAT3 Signaling in the Colon of Dextran Sulfate Sodium-treated Mice. J. Cancer Prev. 2013;18:186–191. doi: 10.15430/JCP.2013.18.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong J., Bose M., Ju J., Ryu J.H., Chen X., Sang S., Lee M.J., Yang C.S. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 65.Singh S., Aggarwal B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J. Biol. Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 66.Plummer S.M., Holloway K.A., Manson M.M., Munks R.J., Kaptein A., Farrow S., Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 67.Bharti A.C., Donato N., Singh S., Aggarwal B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 68.Lin J.K. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch. Pharm. Res. 2004;27:683–692. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 69.Shishodia S., Amin H.M., Lai R., Aggarwal B.B. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem. Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 70.Aggarwal S., Ichikawa H., Takada Y., Sandur S.K., Shishodia S., Aggarwal B.B. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 71.Mackenzie G.G., Queisser N., Wolfson M.L., Fraga C.G., Adamo A.M., Oteiza P.I. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin’s lymphoma cells. Int. J. Cancer. 2008;123:56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 72.Aggarwal B.B., Shishodia S., Takada Y., Banerjee S., Newman R.A., Bueso-Ramos C.E., Price J.E. Curcumin suppresses the paclitaxel-induced nuclear factor NF-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 73.Wang H.M., Zhao Y.X., Zhang S., Liu G.D., Kang W.Y., Tang H.D., Ding J.Q., Chen S.D. PPAR gamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J. Alzheimer Dis. 2010;20:1189–1199. doi: 10.3233/JAD-2010-091336. [DOI] [PubMed] [Google Scholar]
- 74.Jin C.Y., Lee J.D., Park C., Choi Y.H., Kim G.Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007;28:645–651. doi: 10.1111/j.1745-7254.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 75.Goel A., Boland C.R., Chauhan D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111–118. doi: 10.1016/S0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- 76.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 77.Woo M.S., Jung S.H., Kim S.Y., Hyun J.W., Ko K.H., Kim W.K., Kim H.S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 78.Hassan Z.K., Daghestani M.H. Curcumin effect onMMPs and TIMPs genes in a breast cancer cell line. Asian Pac. J. Cancer Prev. 2012;13:3259–3264. doi: 10.7314/APJCP.2012.13.7.3259. [DOI] [PubMed] [Google Scholar]
- 79.Lee K.W., Kim J.H., Lee H.J., Surh Y.J. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid. Redox Signal. 2005;7:1612–1620. doi: 10.1089/ars.2005.7.1612. [DOI] [PubMed] [Google Scholar]
- 80.Reuter S., Gupta S.C., Park B., Goel A., Aggarwal B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bora-Tatar G., Dayangaç-Erden D., Demir A.S., Dalkara S., Yelekçi K., Erdem-Yurter H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorg. Med. Chem. 2009;17:5219–5228. doi: 10.1016/j.bmc.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 82.Liu H.L., Chen Y., Cui G.H., Zhou J.F. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol. Sin. 2005;26:603–609. doi: 10.1111/j.1745-7254.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Shu W., Chen W., Wu Q., Liu H., Cui G. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin. Pharmacol. Toxicol. 2007;101:427–433. doi: 10.1111/j.1742-7843.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 84.Lee S.J., Krauthauser C., Maduskuie V., Fawcett P.T., Olson J.M., Rajasekaran S.A. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011;11:144. doi: 10.1186/1471-2407-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marcu M.G., Jung Y.J., Lee S., Chung E.J., Lee M.J., Trepel J., Neckers L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006;2:169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 86.Kang J., Chen J., Shi Y., Jia J., Zhang Y. Curcumin-induced histone hypoacetylation: The role of reactive oxygen species. Biochem. Pharmacol. 2005;69:1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 87.Balasubramanyam K., Varier R.A., Altaf M., Swaminathan V., Siddappa N.B., Ranga U., Kundu T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 88.Morimoto T., Sunagawa Y., Kawamura T., Takaya T., Wada H., Nagasawa A., Komeda M., Fujita M., Shimatsu A., Kita T., et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Investig. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saini S., Arora S., Majid S., Shahryari V., Chen Y., Deng G., Yamamura S., Ueno K., Dahiya R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev. Res. (Phila) 2011;4:1698–1709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun M., Estrov Z., Ji Y., Coombes K.R., Harris D.H., Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., Zhang T., Ti X., Shi J., Wu C., Ren X., Yin H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010;399:1–6. doi: 10.1016/j.bbrc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Jiang A.J., Jiang G., Li L.T., Zheng J.N. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Biol. Rep. 2014 doi: 10.1007/s11033-014-3769-2. [DOI] [PubMed] [Google Scholar]
- 93.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mezzanotte L., An N., Mol I.M., Löwik C.W., Kaijzel E.L. A new multicolor bioluminescence imaging platform to investigate NF-κB activity and apoptosis in human breast cancer cells. PLoS One. 2014;9:e85550. doi: 10.1371/journal.pone.0085550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sa G., Das T. Anticancer effects of curcumin: Cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park M.J., Kim E.H., Park I.C., Lee H.C., Woo S.H., Lee J.Y., Hong Y.J., Rhee C.H., Choi S.H., Shim B.S., et al. Curcumin inhibits cell cycle progression of immortalized human umbilical vein endothelial (ECV304) cells by up-regulating cyclin-dependent kinase inhibitor, p21WAF1/CIP1, p27KIP1 and p53. Int. J. Oncol. 2002;21:379–383. [PubMed] [Google Scholar]
- 97.Weinberg R.A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 98.Srivastava R.K., Chen Q., Siddiqui I., Sarva K., Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1. Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 99.Mukhopadhyay A., Banerjee S., Stafford L.J., Xia C., Liu M., Aggarwal B.B. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 100.Shankar S., Srivastava R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 101.Tourkina E., Gooz P., Oates J.C., Ludwicka-Bradley A., Silver R.M., Hoffman S. Curcumin-induced apoptosis in scleroderma lung fibroblasts: Role of protein kinase cepsilon. Am. J. Respir. Cell Mol. Biol. 2004;31:28–35. doi: 10.1165/rcmb.2003-0354OC. [DOI] [PubMed] [Google Scholar]
- 102.Yu J., Zhou X., He X., Dai M., Zhang Q. Curcumin induces apoptosis involving bax/bcl-2 in human hepatoma SMMC-7721 cells. Asian Pac. J. Cancer Prev. 2011;12:1925–1929. [PubMed] [Google Scholar]
- 103.Choudhuri T., Pal S., Agwarwal M.L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 104.Elamin M.H., Shinwari Z., Hendrayani S.F., Al-Hindi H., Al-Shail E., Khafaga Y., Al-Kofide A., Aboussekhra A. Curcumin inhibits the sonic hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol. Carcinog. 2010;49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 105.Limtrakul P., Anuchapreeda S., Lipigorngoson S., Dunn F.W. Inhibition of carcinogen induced c-Ha-ras and cfos proto-oncogenes expression by dietary curcumin. BMC Cancer. 2001;1:1. doi: 10.1186/1471-2407-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seol D.W., Chen Q., Zarnegar R. Transcriptional activation of the hepatocyte growth factor receptor (c-met) gene by its ligand (hepatocyte growth factor) is mediated through AP-1. Oncogene. 2000;19:1132–1137. doi: 10.1038/sj.onc.1203404. [DOI] [PubMed] [Google Scholar]
- 107.Bangaru M.L.Y., Chen S., Woodliff J., Kansra S. Curcumin (diferuloylmethane) induces apoptosis and blocks migration of humanmedulloblastoma cells. Anticancer Res. 2010;30:499–504. [PubMed] [Google Scholar]
- 108.Chen A., Xu J., Johnson A.C. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 109.Korutla L., Cheung J.Y., Mendelsohn J., Kumar R. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995;16:1741–1745. doi: 10.1093/carcin/16.8.1741. [DOI] [PubMed] [Google Scholar]
- 110.Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000;4:1–6. [PubMed] [Google Scholar]
- 111.Hong R.L., Spohn W.H., Hung M.C. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin. Cancer Res. 1999;5:1884–1891. [PubMed] [Google Scholar]
- 112.Camacho-Barquero L., Villegas I., Sáànchez-Calvo J.M., Talero E., Sánchez-Fidalgo S., Motilva V., Alarcón de la Lastra C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007;7:333–342. doi: 10.1016/j.intimp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Catz S.D., Johnson J.L. Transcriptional regulation of bcl-2 by nuclear factor kappaB and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 114.Herrmann J.L., Briones F., Jr., Brisbay S., Logothetis C.J., McDonnell T.J. Prostate carcinoma cell death resulting from inhibition of proteasome activity is independent of functionalBcl-2 and p53. Oncogene. 1998;17:2889–2899. doi: 10.1038/sj.onc.1202221. [DOI] [PubMed] [Google Scholar]
- 115.Kunnumakkara A.B., Guha S., Krishnan S., Diagaradjane P., Gelovani J., Aggarwal B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 116.Chua C.C., Hamdy R.C., Chua B.H.L. Mechanism of transforming growth factor-beta1-induced expression of vascular endothelial growth factor in murine osteoblastic MC3T3-E1 cells. Biochim. Biophys. Acta. 2000;1497:69–76. doi: 10.1016/S0167-4889(00)00040-9. [DOI] [PubMed] [Google Scholar]
- 117.Chadalapaka G., Jutooru I., Chintharlapalli S., Papineni S., Smith R., 3rd, Li X., Safe S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu S., Shen G., Khor T.O., Kim J.H., Kong A.N.T. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thapliyal R., Maru G.B. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem. Toxicol. 2001;39:541–547. doi: 10.1016/S0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 120.Firozi P.F., Aboobaker V.S., Bhattacharya R.K. Action of curcumin on the cytochrome P450-system catalyzing the activation of aflatoxin B1. Chem. Biol. Interact. 1996;100:41–51. doi: 10.1016/0009-2797(95)03684-9. [DOI] [PubMed] [Google Scholar]
- 121.Ciolino H.P., Daschner P.J., Wang T.T.Y., Yeh G.C. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 inMCF-7 human breast carcinoma cells. Biochem. Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 122.Singh S.V., Hu X., Srivastava S.K., Singh M., Xia H., Orchard J.L., Zaren H.A. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998;19:1357–1360. doi: 10.1093/carcin/19.8.1357. [DOI] [PubMed] [Google Scholar]
- 123.Thapliyal R., Deshpande S.S., Maru G.B. Mechanism(s) of turmeric-mediated protective effects against benzo(a)pyrenederived DNA adducts. Cancer Lett. 2002;175:79–88. doi: 10.1016/S0304-3835(01)00675-9. [DOI] [PubMed] [Google Scholar]
- 124.Sharma R.A., Ireson C.R., Verschoyle R.D., Hill K.A., Williams M.L., Leuratti C., Manson M.M., Marnett L.J., Steward W.P., et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: Relationship with drug levels. Clin. Cancer Res. 2001;7:1452–1458. [PubMed] [Google Scholar]
- 125.Nishinaka T., Ichijo Y., Ito M., Kimura M., Katsuyama M., Iwata K., Miura T., Terada T., Yabe-Nishimura C. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol. Lett. 2007;170:238–247. doi: 10.1016/j.toxlet.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 126.Piper J.T., Singhal S.S., Salameh M.S., Torman R.T., Awasthi Y.C., Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998;30:445–456. doi: 10.1016/S1357-2725(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 127.Valentine S.P., le Nedelec M.J., Menzies A.R., Scandlyn M.J., Goodin M.G., Rosengren R.J. Curcumin modulates drug metabolizing enzymes in the female SwissWebster mouse. Life Sci. 2006;78:2391–2398. doi: 10.1016/j.lfs.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 128.Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 129.Guo H., Xu Y.M., Ye Z.Q., Yu J.H., Hu X.Y. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IkappaBalpha, c-Jun and androgen receptor. Pharmazie. 2013;68:431–434. [PubMed] [Google Scholar]
- 130.Nakamura K., Yasunaga Y., Segawa T., Ko D., Moul J.W., Srivastava S., Rhim J.S. Curcumin downregulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002;21:825–380. [PubMed] [Google Scholar]
- 131.Tsui K.H., Feng T.H., Lin C.M., Chang P.L., Juang H.H. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J. Androl. 2008;29:661–668. doi: 10.2164/jandrol.108.004911. [DOI] [PubMed] [Google Scholar]
- 132.Braidy N., Grant R., Adams S., Guillemin G.J. Neuroprotective effects of naturally occurring polyphenols on quinolinic acid-induced excitotoxicity in human neurons. FEBS J. 2010;277:368–382. doi: 10.1111/j.1742-4658.2009.07487.x. [DOI] [PubMed] [Google Scholar]
- 133.Matteucci A., Cammarota R., Paradisi S., Varano M., Balduzzi M., Leo L., Bellenchi G.C., de Nuccio C., Carnovale-Scalzo G., Scorcia G., et al. Curcumin protects against NMDA-induced toxicity: A possible role for NR2A subunit. Investig. Ophthalmol. Vis. Sci. 2011;52:1070–1077. doi: 10.1167/iovs.10-5966. [DOI] [PubMed] [Google Scholar]
- 134.Matteucci A., Frank C., Domenici M.R., Balduzzi M., Paradisi S., Carnovale-Scalzo G., Scorcia G., Malchiodi-Albedi F. Curcumin treatment protects rat retinal neurons against excitotoxicity: Effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Exp. Brain Res. 2005;167:641–648. doi: 10.1007/s00221-005-0068-0. [DOI] [PubMed] [Google Scholar]
- 135.Wang R., Li Y.B., Li Y.H., Xu Y., Wu H.L., Li X.J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008;1210:84–91. doi: 10.1016/j.brainres.2008.01.104. [DOI] [PubMed] [Google Scholar]
- 136.Yazawa K., Kihara T., Shen H., Shimmyo Y., Niidome T., Sugimoto H. Distinct mechanisms underlie distinct polyphenol-induced neuroprotection. FEBS Lett. 2006;580:6623–6628. doi: 10.1016/j.febslet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 137.Zhu Y.G., Chen X.C., Chen Z.Z., Zeng Y.Q., Shi G.B., Su Y.H., Peng X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004;25:1606–1612. [PubMed] [Google Scholar]
- 138.Wang Q., Sun A.Y., Simonyi A., Jensen M.D., Shelat P.B., Rottinghaus G.E., MacDonald R.S., Miller D.K., Lubahn D.E., Weisman G.A., et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 139.Rastogi M., Ojha R.P., Rajamanickam G.V., Agrawal A., Aggarwal A., Dubey G.P. Curcuminoids modulates oxidative damage and mitochondrial dysfunction in diabetic rat brain. Free Radical Res. 2008;42:999–1005. doi: 10.1080/10715760802571988. [DOI] [PubMed] [Google Scholar]
- 140.Sood P.K., Nahar U., Nehru B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotoxic. Res. 2011;20:351–361. doi: 10.1007/s12640-011-9249-8. [DOI] [PubMed] [Google Scholar]
- 141.Bhullar K.S., Jha A., Youssef D., Rupasinghe H.P. Curcumin and its carbocyclic analogs: Structure-activity in relation to antioxidant and selected biological properties. Molecules. 2013;18:5389–5404. doi: 10.3390/molecules18055389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Somchit M., Changtam C., Kimseng R., Utaipan T., Lertcanawanichakul M., Suksamrarn A., Chunglok W. Demethoxycurcumin from Curcuma longa rhizome suppresses iNOS induction in an in vitro inflamed human intestinal mucosa model. Asian Pac. J. Cancer Prev. 2014;15:1807–1810. doi: 10.7314/APJCP.2014.15.4.1807. [DOI] [PubMed] [Google Scholar]
- 143.Li Y.B., Gao J.L., Zhong Z.F., Hoi P.M., Lee S.M., Wang Y.T. Bisdemethoxycurcumin suppresses MCF-7 cells proliferation by inducing ROS accumulation and modulating senescence-related pathways. Pharmacol. Rep. 2013;65:700–709. doi: 10.1016/S1734-1140(13)71048-X. [DOI] [PubMed] [Google Scholar]
- 144.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou H., Beevers C.S., Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kurien B.T., Singh A., Matsumoto H., Scofield R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 147.Chignell C.F., Bilski P., Reszka K.J., Motten A.G., Sik R.H., Dahl T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994;59:295–302. doi: 10.1111/j.1751-1097.1994.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 148.Rajasekaran S.A. Therapeutic potential of curcumin in gastrointestinal diseases. World J. Gastrointest. Pathophysiol. 2011;15:1–14. doi: 10.4291/wjgp.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Taylor R.A., Leonard M.C. Curcumin for inflammatory bowel disease: A review of human studies. Altern. Med. Re. 2011;16:152–156. [PubMed] [Google Scholar]
- 150.Hanai H., Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr. Pharm. Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 151.Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama K., Mitsuyama K., Sata M., et al. Curcumin maintenance therapy for ulcertive colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 152.Billerey-Larmonier C., Uno J.K., Larmonier N., Midura A.J., Timmermann B., Ghishan F.K., Kiela P.R. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm. Bowel Dis. 2008;14:780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Epstein J., Sanderson I.R., MacDonald T.T. Curcumin as a therapeutic agent: The evidence from in vitro animal and human studies. Br. J. Nutr. 2010;103:407–412. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 154.Epstein J., Docena G., MacDonald T.T., Sanderson I.R. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1B and matrix metalloproteinase-3 and anhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br. J. Nutr. 2010;103:824–832. doi: 10.1017/S0007114509992510. [DOI] [PubMed] [Google Scholar]
- 155.Holt P.R., Katz S., Kirshoff R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 156.Salh B., Assi K., Templeman V., Parhar K., Owen D., Gómez-Muñoz A., Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2003;285:G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 157.Ukil A., Maity S., Karmakar S., Datta N., Vedasiromoni J.R., Das P.K. Curcumin, the major component of food flavour turmetic, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br. J. Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nones K., Dommels Y.E., Martell S., Butts C., McNabb W.C., Park Z.A., Zhu S., Hedderley D., Barnett M.P., Roy N.C. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdrla-/-) mice, a model of inflammatory bowel diseases. Br. J. Nutr. 2009;101:169–181. doi: 10.1017/S0007114508009847. [DOI] [PubMed] [Google Scholar]
- 159.Kang B.Y., Song Y.J., Kim K.M., Choe Y.K., Hwang S.Y., Kim T.S. Curcumin inhibits Th1 cytokine profile in CD4 T cells by suppressing interleukin-12 production in macrophages. Br. J. Pharmacol. 1999;128:380–384. doi: 10.1038/sj.bjp.0702803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang M., Deng C.S., Zheng J.J., Xia J. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol. Sin. 2006;27:1071–1077. doi: 10.1111/j.1745-7254.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 161.Suskind D.L., Wahbeh G., Burpee T., Cohen M., Christie D., Webwe W. Tolerability of curcumin in pediatric inflammatory bowel disease: A forced-dose titration study. J. Pediatr. Gastroneterol. Nutr. 2013;56:277–279. doi: 10.1097/MPG.0b013e318276977d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lahiff C., Moss A.C.M. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm. Bowel Dis. 2011;17:E66. doi: 10.1002/ibd.21710. [DOI] [PubMed] [Google Scholar]
- 163.Gasparini C., Celeghini C., Monasta L., Zauli G. NF-κB pathways in hematological malignancies. Cell. Mol. Life Sci. 2014;71:2083–2102. doi: 10.1007/s00018-013-1545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghosh S., Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/S0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 165.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 166.Lawrence T., Fong C. The resolution of inflammation: Anti-inflammatory roles for NF-kappaB. Int. J. Biochem. Cell Biol. 2010;42:519–523. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 167.Lawrence T., Gilroy D.W., Colville-Nash P.R., Willoughby D.A. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 168.Buhrmann C., Mobasheri A., Matis U., Shakibaei M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010;12:R127. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shakibaei M., John T., Schulze-Tanzil G., Lehmann I., Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 170.Buhrmann C., Mobasheri A., Busch F., Aldinger C., Stahlmann R., Montaseri A., Shakibaei M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011;286:28556–28566. doi: 10.1074/jbc.M111.256180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee K.H. Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. J. Nat. Prod. 2010;73:500–516. doi: 10.1021/np900821e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 173.Zhongfa L., Chiu M., Wang J., Chen W., Yen W., Fan-Havard P., Yee LD., Chan K.K. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother. Pharmacol. 2012;69:679–689. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52:S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 175.Xia Y.Q., Wei X.Y., Li W.L., Kanchana K., Xu C.C., Chen D.H., Chou P.H., Jin R., Wu J.Z., Liang G. Curcumin Analogue A501 induces G2/M Arrest and Apoptosis in Non-small Cell Lung Cancer Cells. Asian Pac. J. Cancer Prev. 2014;15:6893–6898. doi: 10.7314/APJCP.2014.15.16.6893. [DOI] [PubMed] [Google Scholar]
- 176.Son Y., Lee J.H., Cheong Y.K., Chung H.T., Pae H.O. Antidiabetic potential of the heme oxygenase-1 inducer curcumin analogues. Biomed. Res. Int. 2013;2013:918039. doi: 10.1155/2013/918039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan X., Li H., Bai H., Su Z., Xiang Q., Wang C., Zhao B., Zhang Y., Zhang Q., Chu Y., et al. Synthesis of novel curcumin analogues for inhibition of 11β-hydroxysteroid dehydrogenase type 1 with anti-diabetic properties. Eur. J. Med. Chem. 2014;77:223–230. doi: 10.1016/j.ejmech.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 178.Anthwal A., Thakur B.K., Rawat M.S., Rawat D.S., Tyagi A.K., Aggarwal B.B. Synthesis, Characterization and in Vitro Anticancer Activity of C-5 Curcumin Analogues with Potential to Inhibit TNF-α-Induced NF-κB Activation. BioMed Res. Int. 2014;2014:524161. doi: 10.1155/2014/524161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sardjiman S.S., Reksohadiprodjo M.S., Hakim L., van der Goot H., Timmerman H. 1,5-Diphenyl-1,4-pentadiene-3-ones and cyclic analogues as antioxidative agents. Synthesis and structure-activity relationship. Eur. J. Med. Chem. 1997;32:625–630. [Google Scholar]
- 180.Di Bello M.G., Masini E., Ioannides C., Ndisang J.F., Raspanti S., Sacchi T.B., Mannaioni P.F. Histamine release from rat mast cells induced by the metabolic activation of drugs of abuse into free radicals. Inflamm. Res. 1998;47:122–130. doi: 10.1007/s000110050299. [DOI] [PubMed] [Google Scholar]
- 181.Mannaioni P.F., Bello M.G.D., Raspanti S., Mugnai L., Romano V., Masini E. Free radical mediated release of histamine from rat mast cells induced by drugs of abuse. Inflamm. Res. 1996;45:S25–S26. doi: 10.1007/BF03354072. [DOI] [PubMed] [Google Scholar]
- 182.Nugroho A.E., Ikawati Z., Sardjiman, Maeyama K. Effects of benzylidenecyclopentanone analogues of curcumin on histamine release from mast cells. Biol. Pharm. Bull. 2009;32:842–849. doi: 10.1248/bpb.32.842. [DOI] [PubMed] [Google Scholar]
- 183.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 184.Ghalandarlaki N., Alizadeh A.M., Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. BioMed Res. Int. 2014;2014:394264. doi: 10.1155/2014/394264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beloqui A., Coco R., Memvanga P.B., Ucakar B., des Rieux A., Préat V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014;473:203–212. doi: 10.1016/j.ijpharm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 186.Gugulothu D., Kulkarni A., Patravale V., Dandekar P. pH-sensitive nanoparticles of curcumin-celecoxib combination: Evaluating drug synergy in ulcerative colitis model. J. Pharm. Sci. 2014;103:687–696. doi: 10.1002/jps.23828. [DOI] [PubMed] [Google Scholar]
- 187.Yadav V.R., Suresh S., Devi K., Yadav S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech. 2009;10:752–762. doi: 10.1208/s12249-009-9264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]