Abstract
Background
Gastrointestinal stromal tumors (GISTs) are uncommon intestinal neoplasms in the dog. Literature regarding adjunctive therapy for GISTs in dogs is sparse. High‐risk GISTs in humans respond to tyrosine kinase inhibition in the adjuvant setting.
Objectives
To review cases of toceranib phosphate use in dogs with GISTs and provide initial assessment of possible biological activity. A secondary aim was to evaluate patient and tumor characteristics for possible prognostic value.
Animals
Twenty‐seven dogs with confirmed GISTs based on histopathology and immunohistochemistry treated with toceranib.
Methods
Retrospective study in which cases of toceranib use in dogs with GIST were solicited using the American College of Veterinary Internal Medicine Oncology and Small Animal Internal Medicine listservs.
Results
Five of 7 dogs with gross disease experienced clinical benefit (71%; 3 complete responses, 1 partial response, 1 stable disease). These included 2 dogs with durable responses after toceranib discontinuation. Median progression‐free interval (PFI) in dogs with gross disease was 110 weeks (range, 36‐155 weeks). Median PFI in dogs with microscopic disease was 67 weeks (range, 9‐257 weeks). Metastasis at diagnosis (P = 0.04) and high mitotic index (P < 0.001) were associated with shorter PFI in toceranib‐treated dogs.
Conclusions and Clinical Importance
Biological activity of toceranib is evident in dogs with gross disease. Metastasis of GIST at diagnosis, as well as high tumor mitotic index, was associated with shorter PFI in toceranib‐treated dogs. Larger studies are needed to define postsurgical risk and refine the use of toceranib in dogs with gross and microscopic GIST.
Keywords: DOG1, immunohistochemistry, KIT, mutation, progression‐free interval, tyrosine kinase inhibitor
Abbreviations
- AE
adverse event
- CB
clinical benefit
- CR
complete response
- DOG1
discovered‐on‐GIST 1
- FFPE
formalin‐fixed paraffin‐embedded
- GIST
gastrointestinal stromal tumor
- ICC
interstitial cell of Cajal
- IHC
immunohistochemistry
- NED
no evidence of disease
- NSAID
nonsteroidal anti‐inflammatory drug
- PD
progressive disease
- PDGFR
platelet‐derived growth factor receptor
- PFI
progression‐free interval
- PR
partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- RTK
receptor tyrosine kinase
- SD
stable disease
- TKI
tyrosine kinase inhibitor
- VCOG‐CTCAE
Veterinary Comparative Oncology Group Common Terminology Criteria for Adverse Events
- VEGFR
vascular endothelial growth factor receptor
1. INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are uncommon mesenchymal neoplasms arising from interstitial cells of Cajal (ICCs). They are rare in humans, but their true incidence in dogs is unknown.1, 2 Differentiating GISTs from other gastrointestinal sarcomas is challenging because of their identical appearance under light microscopy. However, routine use of immunohistochemistry (IHC) has improved the ability to diagnose GISTs. In humans, IHC for c‐Kit, a growth factor receptor, has been used to distinguish GISTs from other gastrointestinal sarcomas.3 Of note, 80%‐95% of GISTs in humans express c‐Kit, and mutation results in the receptor's constitutive activation in approximately 75%.4 The presence of discovered‐on‐GIST 1 (DOG1), a calcium‐dependent chloride channel important in the generation of ICCs' “slow waves,” has been determined to ubiquitously define GIST in humans, regardless of c‐Kit expression.5, 6 Although previous publications have defined GISTs in dogs as mesenchymal intestinal neoplasms expressing c‐Kit, recent literature suggests that a combination of c‐Kit and DOG1 IHC is most sensitive for diagnosis of GISTs in dogs.7
The KIT gene is a protooncogene encoding the c‐Kit receptor, mutation of which is known to drive a subset of mast cell tumors in dogs, conferring a more aggressive phenotype and worse prognosis.8 In addition to expressing c‐Kit and DOG1, GISTs in dogs also have been shown to have similar KIT mutations to GISTs in humans.9 Whether the aggressive biologic behavior pattern holds true for GISTs in dogs that express KIT mutations is unknown, but GISTs in humans with particular KIT mutations demonstrate malignant behavior.4, 10 Imatinib mesylate (Gleevec; Novartis, Basel, Switzerland), a tyrosine kinase inhibitor (TKI) that competitively inhibits phosphorylation of c‐Kit, has revolutionized the treatment of high‐risk GISTs in humans (Table 1) and has become the standard‐of‐care for these individuals.11 Sunitinib maleate (Sutent; Pfizer, New York, New York), a closely related TKI also active at c‐Kit, provides survival benefit in human patients who develop imatinib resistance or intolerance.12
Table 1.
Modified NIH consensus criteria for defining postsurgical risk in humans with GIST11
| Risk category | Tumor longest diameter (cm) | Mitotic index, per 50 hpf | Primary tumor |
|---|---|---|---|
| Very low | <2 | ≤5 | Any |
| Low | 2–5 | ≤5 | Any |
| Intermediate | 2–5 | >5 | Gastric |
| <5 | 6‐10 | Any | |
| 5‐10 | ≤5 | Gastric | |
| High | Any | Any | Tumor rupture |
| >10 | Any | Any | |
| Any | >10 | Any | |
| >5 | >5 | Any | |
| 2–5 | >5 | Non‐gastric | |
| 5–10 | ≤5 | Non‐gastric |
Abbreviation: hpf, high power field.
Little is known regarding treatment for GISTs in dogs. When surgical excision is possible, it is the treatment of choice.1 The utility of cytotoxic chemotherapy is unknown,1 although anecdotally response rates are low. Two case reports detail positive response to treatment with imatinib in the unresectable gross disease setting.13, 14 Unlike the well‐defined risk categories in humans, how to define postsurgical risk for metastasis or recurrence in dogs with GISTs is unknown.
Toceranib phosphate (Palladia; Zoetis, Parsippany, New Jersy), a TKI closely related to imatinib and sunitinib, and FDA‐approved for use in dogs, inhibits signaling at c‐Kit as well as several other tyrosine kinases.15 Although it might be anticipated that toceranib would have efficacy in dogs with GISTs, given its spectrum of action and the imatinib precedent in humans with GISTs, only a single case of a metastatic unresectable GIST in a dog with a positive response to toceranib has been reported.16 The purpose of our retrospective study was to solicit and compile data from practicing veterinary specialists characterizing their use of toceranib in dogs with GISTs and to provide initial assessment of possible biological activity. A secondary aim was to evaluate patient and tumor characteristics for possible prognostic value.
2. MATERIALS AND METHODS
2.1. Study design
The study was designed as a multicenter retrospective analysis. The American College of Veterinary Internal Medicine Oncology and Small Animal Internal Medicine listservs were used to solicit data from cases in which clinicians had treated dogs with GISTs with toceranib. To be eligible for analysis, the following data were required for each case: signalment (age, sex, breed), pathologist‐confirmed diagnosis of GIST, anatomic location of tumor, previous and concurrent treatment, toceranib dosage (mg/kg) and schedule, duration of treatment, best response, response duration, documentation of adverse events (AEs) (toxicity), and case outcomes to the best knowledge of the attending clinician. Toxicities were reported by the attending clinician according to the Veterinary Comparative Oncology Group Common Terminology Criteria for Adverse Events (VCOG‐CTCAE).17
Original histopathology reports were obtained for all cases. Individual laboratories were contacted regarding the availability of formalin‐fixed paraffin‐embedded (FFPE) specimens. Specimens that were available underwent review by a single pathologist (B.E. Powers). Additional IHC for c‐Kit and DOG1 was performed where adequate samples were available. Histologic evaluation and IHC staining were performed as previously described.7 Specimens with additional adequate samples available also were submitted for commercially available PCR‐based KIT mutation testing (c‐KIT Mutation Analysis; Clinical Immunology Laboratory, Colorado State University Veterinary Diagnostic Laboratories; Ft. Collins, Colorado). If dogs did not have specimens available for reevaluation, tumors were required to have been diagnosed as GIST with positive c‐Kit, positive DOG1, or positive c‐Kit and positive DOG1 IHC staining as determined by the reviewing pathologist.
Dogs were excluded if these data were not available or if they had received treatment other than surgery before toceranib treatment. Concurrent treatment with toceranib, including metronomic chemotherapy, was permitted.
Progression‐free interval (PFI) was calculated for all dogs and defined as the time from the day of first toceranib treatment to the time of disease progression (defined as local recurrence, local progression, metastasis, or some combination of these), the time of death from any cause, or the time of data collection.18 For dogs with gross (measurable) disease, as in previous publications describing the use of toceranib in dogs, clinical benefit (CB) was determined by best response to treatment and was defined as complete response (CR) or partial response (PR) of any duration, or stable disease (SD) of at least 10 weeks in duration.19 Animals experiencing SD < 10 weeks in duration or progressive disease (PD) as their best response to toceranib did not achieve CB. Individual responses were defined by the attending clinician using the Response Evaluation Criteria in Solid Tumors (RECIST v1.120; Table 2). Responses were evaluated by the attending clinician by physical examination and various imaging modalities at their discretion and with the approval of the pet owners, including abdominal radiographs, abdominal ultrasound examination, computed tomography, or some combination of these. Dogs in which all measurable disease was removed were classified as receiving treatment in the microscopic disease setting, regardless of the assessed histologic margin. Because the behavior of GISTs in dogs after surgery is unknown, all dogs were assumed to be at risk of recurrence. Their response was recorded as no evidence of disease (NED) at the time of treatment initiation for purposes of determining PFI. These dogs were not assessed for CB because of their lack of measurable disease.
Table 2.
Summary of Response Evaluation Criteria in Solid Tumors v1.120
| Complete response | Complete disappearance of all target lesions, lymph node diameter < 10 mm |
| Partial response | ≥30% decrease in sum diameter of target lesions or lymph nodes |
| Stable disease | Insufficient shrinkage to qualify for PR (<30% decrease in sum diameter) and insufficient increase to qualify for PD (<20% increase in sum diameter) |
| Progressive disease | ≥20% increase in sum diameter of target lesions or lymph nodes with absolute sum increase ≥5 mm or the appearance of one or more new lesions |
Abbreviations: PR, partial response; PD, progressive disease.
2.2. Statistical analysis
The relationship between a patient's tumor characteristics at diagnosis and treatment allocation (toceranib alone versus toceranib + adjunct therapy) was first tested using generalized linear models. All independent variables with a P‐value ≤0.05 were considered statistically significant. In a subsequent step, the influence of treatment and tumor characteristics at diagnosis on PFI and survival was tested using Cox/log‐rank tests for both PFI and survival data. First, a preliminary analysis was performed to identify potentially significant predictors with a P‐value ≤0.25. A multiple regression analysis was then performed with all potentially significant predictors to determine final statistical significance for a P‐value <0.05. The log‐rank test was used for the analysis of survival data with categorical predictors. Interactions among predictors were further tested in the statistical model, and the likelihood ratio test was used for final model selection. All analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Demographic information and GIST diagnosis
Data for 32 cases from 17 sites were received. After evaluation, 5 dogs were excluded. Two of these dogs had tumors which, after pathologist review, were inconsistent with GIST (final diagnoses included undifferentiated sarcoma and leiomyosarcoma). The third dog was treated with both radiotherapy and chemotherapy before toceranib treatment. The remaining 2 dogs had insufficient pathology information available to confirm GIST diagnosis. These exclusions resulted in the final evaluation of 27 dogs.
Dogs were primarily mixed breeds (n = 9). Pure breeds represented included Golden Retriever (n = 4), Labrador Retriever (n = 3), Border Collie (n = 2), Standard Poodle (n = 2), Australian Cattle Dog (n = 1), Beagle (n = 1), Bouvier des Flandres (n = 1), English Bulldog (n = 1), Jack Russell Terrier (n = 1), Keeshond (n = 1), and Shih Tzu (n = 1). Case data from 17 castrated males and 10 spayed females were evaluated; no case data from sexually intact animals were submitted. The median age was 11 years (range, 4‐14 years).
Diagnosis of GIST was made in 23 dogs by definitive‐intent surgery and in 4 dogs by surgical biopsy in the face of unresectable gross disease. Cases with available FFPE specimens underwent additional pathologist review. Thirteen of 27 dogs had available FFPE specimens for additional histopathology analysis. All available specimens were evaluated by a single pathologist (B.E. Powers) as described earlier to confirm GIST diagnosis. In addition, 12 of 27 samples had sufficient additional FFPE specimen available for commercial KIT mutation analysis.
3.2. Disease characteristics at diagnosis
Dogs generally were presented for vague clinical signs such as lethargy, anorexia, and vomiting, but in 3 dogs were no gastrointestinal signs were reported. Weight loss was only appreciated in 3 dogs, illustrating the acute presentation of most cases. Three dogs had tumor rupture at diagnosis, resulting in septic peritonitis in 2 dogs and hemoperitoneum in 1 dog. Distribution of tumor anatomic location and summarized selected tumor characteristics are presented in Table 3; small intestinal and cecal sites were most prevalent. Eleven dogs had metastasis observed at diagnosis, with sites of metastasis including the mesentery (n = 5), liver (n = 3), abdominal lymph nodes (n = 2), and spleen (n = 1).
Table 3.
Selected tumor pathologic characteristics at diagnosis
| Characteristic | Number of cases |
|---|---|
| Total cases | 27 |
| Anatomic location | |
| Stomach | 3 |
| Small intestine | 11 |
| Cecum | 12 |
| Colon | 1 |
| Longest diameter | |
| <5 cm | 9 |
| 5‐10 cm | 13 |
| >10 cm | 1 |
| Not reported | 4 |
| Mitotic index (per 10 hpf) | |
| <5 | 9 |
| 5–10 | 7 |
| >10 | 6 |
| Not reported | 5 |
| Metastasis | |
| Present | 11 |
| Absent | 16 |
| Tumor rupture | |
| Present | 3 |
| Absent | 24 |
Abbreviation: hpf, high power field.
One of 12 dogs that had FFPE tissue available for KIT mutation analysis was positive for an internal tandem duplication in KIT exon 11. No internal tandem duplications of KIT exons 8 or 11 were detected in the remaining 11 samples. (See Table S1 in this article's supporting information for full description of included tumor pathologic characteristics.)
3.3. Toceranib treatment
In 7 dogs, toceranib treatment was initiated in the face of gross disease. In 4 of these dogs, toceranib was used to treat unresectable gross disease at initial diagnosis. In 1 dog, a primary gastrointestinal tumor could not be removed, whereas in the other 3 dogs, the primary tumor was completely excised, but ≥1 metastatic lesions could not be resected. In the remaining 3 dogs, toceranib treatment was initiated at the appearance of unresectable distant metastatic disease after definitive surgical removal of the primary tumor at a previous date. In these 3 dogs, times from initial diagnosis to start of toceranib treatment after diagnosis of distant metastasis were 33, 36, and 146 weeks.
In 20 dogs, toceranib treatment was initiated after definitive surgery in the microscopic disease setting. All of these dogs were classified as having NED at the time of toceranib treatment initiation for purposes of calculating PFI.
The median dose of toceranib used in studied dogs was 2.6 mg/kg (range, 0.6‐3.5 mg/kg). The majority of these dogs (81%, 22/27) received the drug 3 times per week. The remaining 5 dogs received toceranib q48h.
Concurrent medical treatment with toceranib was allowed (Table 4). Six dogs were treated with concurrent nonsteroidal anti‐inflammatory drugs (NSAIDs). In 3 of these, NSAIDs were reported as being used for conditions unrelated to the patient's GIST, such as osteoarthritis. No dog was treated with glucocorticoids concurrently. In the remaining 3 dogs that received NSAIDs, concurrent chemotherapy was also prescribed. Two dogs received metronomic cyclophosphamide, 1 at an unknown dosage and 1 at 15 mg/m2 q48h; both of these dogs had microscopic disease. One dog with gross disease received metronomic chlorambucil at a dosage of 3.8 mg/m2/d. Various supportive medications (eg, antiemetics, antimicrobials, and appetite stimulants) were administered at the discretion of the attending clinician.
Table 4.
Dogs that received adjuvant chemotherapy, NSAID therapy, or both with toceranib treatment
| Case number | Toceranib treatment category | Chemotherapy | NSAID |
|---|---|---|---|
| 8 | Microscopic disease | … | Yes |
| 10 | Microscopic disease | Cyclophosphamide, unknown dose | Yes |
| 12 | Microscopic disease | Cyclophosphamide, 15 mg/m2 every 48 hours | Yes |
| 16 | Microscopic disease | … | Yes |
| 19 | Gross disease, unresectable at diagnosis | Chlorambucil, 3.8 mg/m2/day | Yes |
| 21 | Microscopic disease | … | Yes |
3.4. Response to treatment
Clinical benefit was observed in 5 of 7 (71%; 3 CR, 1 PR, 1 SD) dogs with gross disease (Table 5). These responses included 4 of 4 (100%; 2 CR, 1 PR, 1 SD) dogs that had unresectable disease at diagnosis and 1 of 3 (33%; 1CR) dogs that developed unresectable tumor recurrence or metastatic disease after definitive surgical removal of a primary GIST. In dogs that experienced CB, median toceranib treatment duration was 39 weeks (range, 24‐58 weeks). One of 5 dogs that experienced CB still was receiving toceranib at the time of data submission. The median PFI in dogs that experienced CB was 110 weeks (range, 36‐155 weeks). Two dogs experienced prolonged CB after discontinuation of toceranib treatment. Both dogs received a planned course of toceranib treatment; 1 received 24 weeks of treatment, 1 received 39 weeks of treatment, and both achieved CR. The former dog then experienced PD at 144 weeks and died of its GIST 175 weeks after starting treatment. The latter dog was still experiencing CR at week 155 after treatment initiation when it developed disseminated histiocytic sarcoma. Its death from this second neoplasm occurred at 157 weeks after treatment initiation. Of the 3 remaining dogs that experienced CB, 1 experienced PD while taking toceranib and consequently discontinued the treatment with a PFI of 36 weeks, and died of its GIST 37 weeks after treatment initiation. The treatment was discontinued in the second dog after 58 weeks because of financial constraints, with a PFI of 110 weeks, and death secondary to GIST 143 weeks after diagnosis. The third dog, which was on toceranib treatment at the time of data submission, had experienced CR with a PFI of 44 weeks.
Table 5.
Best responsea to toceranib treatment in studied dogs with measurable (gross) disease
| Clinical benefit | No clinical benefit | ||||
|---|---|---|---|---|---|
| CR | PR | SD, greater than 10 weeks | SD, less than 10 weeks | PD | |
| All dogs with measurable disease (n = 7) | 3 | 1 | 1 | 0 | 2 |
| Disease classification at treatment initiation | |||||
| Measurable disease at diagnosis (n = 4) | 2 | 1 | 1 | 0 | 0 |
| Late metastasis/recurrence (n = 3) | 1 | 0 | 0 | 0 | 2 |
Based on RECIST criteria.20
All 20 dogs that received toceranib in the microscopic disease setting were classified as having experienced NED at the start of the treatment. The median treatment duration in dogs with microscopic disease was 49 weeks (range, 1‐159 weeks). Seven dogs received a planned course of toceranib treatment, after which it was discontinued. Their treatment durations ranged from 24 to 159 weeks (6 months to 3 years). A single dog in this treatment group developed disease recurrence (pathologist‐confirmed hepatic metastasis) 1 year after discontinuation of a planned course of toceranib treatment that was successfully treated by resection of metastasis followed by reintroduction of toceranib. The median PFI in dogs with microscopic disease was 67 weeks (range, 9‐257 weeks). Three dogs with NED experienced PD (tumor recurrence, metastasis, or both) while receiving toceranib, and consequently the drug was discontinued. These dogs were treated for 22, 43, and 64 weeks, respectively.
3.5. Adverse events and case outcomes
Adverse events were observed in 64% of cases (18/28); most were low‐grade (grade 1 or 2) gastrointestinal toxicities. All reported AEs are presented in Table 6. No VCOG grade 4 or 5 AEs were reported. Eleven dogs that experienced suspected toxicity underwent dose adjustment, and treatment was discontinued in 6 dogs because of suspected toxicity. Gastrointestinal toxicity was the reported cause of toceranib discontinuation in 5 of 6 of these dogs. These dogs received a median toceranib dose of 2.7 mg/kg (range, 2.3‐2.8 mg/kg), and 4 of 5 dogs received the drug 3 days per week. The highest VCOG gastrointestinal toxicities experienced by these dogs were grade 2 vomiting, grade 1 diarrhea (n = 2), grade 3 anorexia, and grade 2 diarrhea with grade 2 weight loss. Toceranib treatment lasted for a median of 33 weeks (range, 1‐48 weeks) in these 5 dogs. In the remaining dog, toceranib was discontinued because of grade 3 syncope. This dog had received toceranib at a dosage of 3.5 mg/kg, 3 days per week for 49 weeks.
Table 6.
Reported adverse events observed in dogs that received toceranib
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Constitutional | |||
| Lethargy | 4 | … | … |
| Weight loss | … | 1 | 1 |
| Gastrointestinal | |||
| Inappetence | 3 | 2 | 1 |
| Diarrhea | 8 | 3 | … |
| Vomiting | 1 | 2 | 1 |
| Hematologic | |||
| Neutropenia | 1 | … | … |
| Lymphopenia | 1 | … | … |
| Anemia | 1 | … | … |
| Biochemical | |||
| Increased ALT activity | 2 | … | … |
| Increased ALP activity | 1 | … | … |
| Increased total bilirubin | 1 | … | … |
| Increased BUN | 1 | … | … |
| Renal | |||
| Proteinuria | … | … | 2 |
| Cardiovascular | |||
| Hypertension | … | 2 | … |
| Syncope | … | … | 1 |
| Dermatologic | |||
| Alopecia | … | 1 | … |
| Scaling | … | 1 | … |
| Rash: acneiform | … | 1 | … |
| Pruritus | … | 1 | … |
Abbreviations: ALT, alanine aminotransferase; ALP, alkaline phosphatase; BUN, blood urea nitrogen.
Fourteen dogs were alive at the time of data submission, whereas 10 had died. Seven of 10 dogs (70%) died or were euthanized as a consequence of their GIST. Dogs in which cause of death was not described were assumed to have died of GIST. Documented unrelated causes were available for 3 dogs and included bile peritonitis, disseminated histiocytic sarcoma, and hemopericardium. Three dogs were lost to follow‐up.
3.6. Statistical analysis
Tumor length (P = 0.014) and mitotic index (P = 0.004) were found to have a significant effect on treatment allocation. Specifically, dogs with larger tumors and those with a higher mitotic index were more likely to receive toceranib treatment alone, without adjuvant treatment (ie, NSAIDs, chemotherapy, or both). No statistically significant differences in PFI, however, were identified between dogs that received only toceranib (median, 70 weeks; range, 4‐199 weeks) and dogs that received toceranib with adjuvant treatment (median, 92 weeks; range, 36‐257; P = 0.577). When evaluating PFI, the presence of metastases at diagnosis (P = 0.04; Figure 1) and high mitotic index (P < 0.001) were associated with worse outcome. However, no other patient or tumor characteristics examined (number of clinical signs at diagnosis, presence of gastrointestinal‐specific clinical signs at diagnosis, presence of weight loss at diagnosis, tumor location, whether or not definitive‐intent surgery was performed, tumor length, and tumor rupture at diagnosis; data not shown) were significantly associated with shorter PFI in studied dogs.
Figure 1.
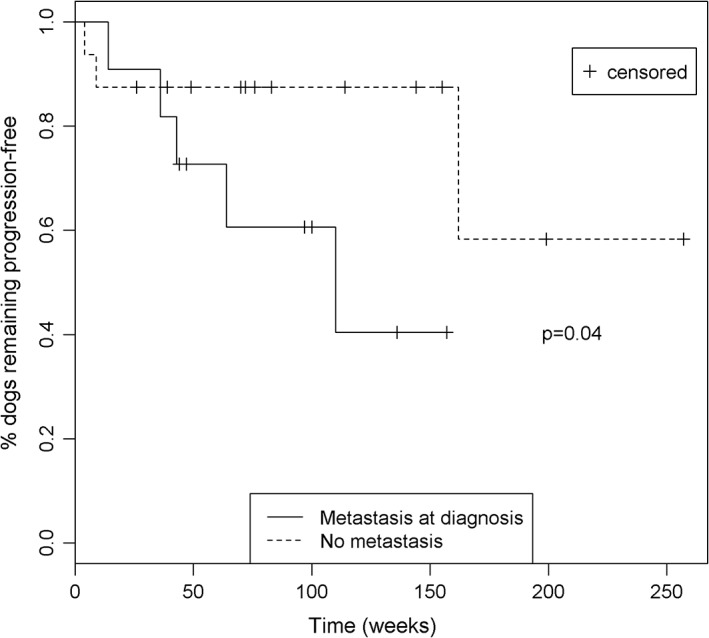
Kaplan‐Meier plot showing PFI for dogs with and without metastasis at diagnosis. Dogs with metastasis at diagnosis had a significantly shorter PFI (P = 0.04)
4. DISCUSSION
The aim of our retrospective study was to assess dogs with pathologist‐confirmed GIST that received toceranib for possible biological activity. Although the retrospective nature of our study precludes direct comparison of response rates or durations to those reported elsewhere, more limiting is the lack of literature discussing GIST in dogs. Complicating this situation is the fact that earlier reports in the veterinary literature do not differentiate between GIST and other mesenchymal gastrointestinal neoplasms.21 Consequently, the incidence of GIST in dogs may be higher than previously believed, because reports that retrospectively evaluated gastrointestinal mesenchymal tumors by the use of IHC reclassified 66%‐85% of leiomyomas and leiomyosarcomas as GISTs.22, 23
Little data are available about the response of GISTs in dogs to any medical treatment; literature pertaining to the use of cytotoxic chemotherapy is particularly sparse. A single case series of gastrointestinal leiomyosarcomas, published before the routine use of c‐Kit IHC, described the use of adjuvant chemotherapy in 2 dogs. One dog that received doxorubicin as a single‐agent treatment died 4 months after surgery. The other dog received combination chemotherapy consisting of doxorubicin, cyclophosphamide, and vincristine, and was alive when lost to follow‐up >2 years after surgery.24
The use of TKIs has been reported in the treatment of a few GISTs in dogs. Imatinib use has been reported in 2 dogs with unresectable GIST. One dog was treated for multiple late‐occurring intra‐abdominal metastatic lesions and achieved CR. At its death from pneumonia >4 years after initiating imatinib therapy, no clinical evidence of GIST recurrence was found.14 The second dog had an unresectable GIST at diagnosis and achieved a PR after 67 days of imatinib treatment; response duration was 140 days at the time of the report's publication.13 Toceranib treatment previously was reported in 21 GISTs of 1243 canine tumors treated by veterinary oncologists during an online clinical experience program (Johannes CM et al. Palladia year one clinical experience: online case entry summary. Veterinary Cancer Society Conference; San Diego, CA; 29 Oct ‐ 1 Nov 2010. p. 72, Abstract.). Of the 9 dogs in this group for which follow‐up data were available, treatment duration ranged from 3 to 36 weeks (median, 8 weeks) (Johannes CM, unpublished data). Because of the nature of this clinical experience survey, whether the treated dogs had gross or microscopic disease is unknown. Finally, a single case report of toceranib treatment in a dog with metastatic, unresectable GIST recently has been reported. This dog with an unresectable cecal GIST and metastasis to the liver, abdominal lymph nodes, mesentery, and peritoneum, achieved CR after 9 months of treatment.16
Given preliminary evidence of TKI efficacy in dogs with GISTs, testing surgically resected tumors for KIT mutations appears helpful. However, in our study, only 1 of 12 samples tested was positive for a KIT mutation when using a commercially available PCR‐based assay. In addition, in the single reported case of toceranib use in a dog with GIST in which CR in the gross disease setting was reported, no KIT mutation was found using a commercially available PCR‐based assay from a different laboratory than utilized in our study.16 In humans, the most common KIT mutations in GISTs are in‐frame deletions in exon 11 (juxtamembrane domain). Other less common KIT mutations include single nucleotide substitutions in exons 9 and 11 and duplications in exon 11.10, 25 Small studies have investigated KIT mutations in dogs with GIST. In 1 study of 17 GIST samples from dogs, 6 showed in‐frame deletions in exon 11.9 In another study of 46 GIST samples from dogs that examined only exon 11, exon 11 KIT mutations were detected in 34 samples, the most common of which were deletions (n = 20) and single nucleotide substitutions (n = 4).26 Because the commercial assay utilized in our study tests only for internal tandem duplications in KIT exons 8 and 11, it is not surprising that only 1 tested tumor was positive for a KIT mutation, given the variety of types of KIT mutations previously identified. The results reported here support the need for more broad KIT mutation analysis when investigating aberrations in this gene before targeted therapy in dogs with GIST.
It is difficult to say definitively whether or not studied dogs treated in the microscopic disease setting achieved CB from toceranib. Twelve of 20 dogs (60%) treated in the microscopic disease setting were still alive at study conclusion. Ten of these dogs (10/12, 83%) were still experiencing NED at the time of data collection and the remaining 2 (2/12, 17%) had experienced PD. Seven of the 10 dogs that still experienced NED were no longer taking toceranib, whereas 3 of the dogs that still experienced NED remained on toceranib. Whether or not these 10 dogs would have experienced NED for these durations in the absence of toceranib treatment is unknown. Two of 12 dogs that were still alive at the time of data collection experienced PD while being treated in the microscopic disease setting (2/12, 17%). The first did so 14 weeks after starting treatment and was alive 36 weeks after diagnosis at the time of data collection. However, the second of these 2 dogs had discontinued the treatment (planned) after 108 weeks and experienced PD 161 weeks after diagnosis (53 weeks after cessation of treatment). This dog was treated successfully by metastatic resection, and toceranib was reinitiated in the microscopic disease setting. This dog was alive at the time of data collection, >225 weeks after diagnosis, on its second course of toceranib. This latter patient's clinical course, as well as the 3 dogs described earlier that experienced PD while taking toceranib, suggest that in a subset of dogs, GIST will behave aggressively and recur or metastasize after initial surgical treatment. This observation emphasizes the need to better understand adjuvant therapy in dogs with GIST.
Conversely, it is clear that studied dogs with gross disease benefited from toceranib treatment. All dogs with unresectable disease at initial diagnosis experienced CB. The rate of CB was low in dogs that did not begin toceranib until tumor recurrence or late metastasis; however, 1 dog in this group experienced CB consisting of CR. Given a previous report of the use of imatinib in the treatment of late metastasis of GIST in a dog with a durable positive response,14 the low CB rate in the few dogs treated late in their disease course discussed here should not discourage toceranib use in similar cases.
A secondary aim of our study was to examine patient and tumor characteristics for possible prognostic value. Unfortunately, how to predict which dogs, after definitive‐intent surgery, are at risk for tumor recurrence or late metastasis and therefore might benefit from c‐Kit inhibition with toceranib, is an unanswered question. In human beings, risk strata previously have been well‐defined (Table 1), and it is recommended that individuals with high‐risk GISTs should receive imatinib for a minimum of 3 years after surgical resection.11, 27 Interestingly, in our study, dogs with metastasis at diagnosis and dogs in which tumors had a high mitotic index demonstrated shorter PFI. These findings are consistent with observations in human patients with GISTs.11 In human patients with GISTs, additional factors such as tumor location and oncogenic mutation genotype also are correlated with tumor behavior and outcome,28, 29 but similar data do not exist in dogs. In our study, no statistical difference in PFI was noted when considering any of the remaining examined tumor characteristics. This lack of significance could be attributed to type II error secondary to the small sample size of our preliminary study.
Finally, when assessing CB of toceranib in dogs with GISTs, the influence of oncogenic mutations is unknown. In addition to the KIT mutations described earlier, GISTs in humans uncommonly have platelet‐derived growth factor receptor alpha (PDGFRA) mutations, a target inhibited by toceranib, and these mutations are associated with a more favorable prognosis.10, 15 To the authors' knowledge, no mutation of PDGFRA has been reported in a GIST from a dog. Toceranib inhibits additional members of the split kinase family, including vascular endothelial growth factor receptor,15 and it is possible that anti‐angiogenic effects could contribute to CB in dogs with GISTs. Overall, further prospective research with a larger study population, genomic analysis, and advanced mathematical modeling30, 31 is needed to clearly define risk strata in dogs with GISTs.
Toceranib was well tolerated in these patients, with AE types and rates consistent with those of previous studies of toceranib use in dogs.8, 32 Gastrointestinal AEs, in particular diarrhea, were most common; this finding may reflect drug effects or the nature of these patients' underlying disease. One dog experienced dermatologic AEs (Table 6), which was not suspected to be related to toceranib treatment by the attending clinician. Similarly, although the single dog that experienced syncope was lost to follow‐up after toceranib discontinuation, this AE was not suspected to be related to toceranib treatment by the attending clinician.
Our study had several limitations common to retrospective analyses. Given the way case data were acquired, clinicians who responded to the call for data might preferentially have recalled responding patients. In addition, examining only patients undergoing adjuvant therapy could introduce bias, resulting in the selection of cases not representative of the at‐large population of dogs with GIST. Both could result in a falsely increased rate of CB or falsely long PFI. Although dogs most often received well‐tolerated and biologically effective doses of toceranib based on current literature,32 no standardized toceranib dosage or administration schedule was utilized.
Concurrent use of metronomic chemotherapy was allowed but occurred in only a few dogs. Limited data are available regarding the efficacy of metronomic cyclophosphamide combined with piroxicam in incompletely excised soft tissue sarcomas,33 and no data exist regarding its use in intestinal neoplasms to the authors' knowledge. Although preliminary data suggest toceranib combined with metronomic cyclophosphamide may have immunomodulatory effects in tumor‐bearing dogs,34 none of the dogs in that small study had an intestinal neoplasm. The dogs in our study that received concurrent toceranib and metronomic chemotherapy also received NSAID therapy. In addition, a few dogs received toceranib concurrent with NSAIDs alone, but the NSAIDs were reported to be prescribed for problems unrelated to their GIST (Table 4). Although cyclooxygenase‐2 (COX‐2) is upregulated in humans with GISTs and some literature suggests an association with a malignant tumor phenotype, this relationship has not been definitively established.35, 36, 37, 38 Whether humans with GISTs that express high COX‐2 activity have a worse outcome, or benefit from COX‐2 inhibition, are also unanswered questions.37, 39 To our knowledge, no literature exists examining COX‐2 expression in GISTs in dogs, although its expression has been demonstrated in a variety of carcinomas in dogs, including intestinal and colorectal adenocarcinomas.40, 41 In our study, high mitotic index (P = 0.004) and large tumor size (P = 0.014) were significantly associated with the use of toceranib alone, without adjuvant therapy (NSAID, metronomic chemotherapy, or both). These findings are unexpected and their clinical relevance is unknown. They may represent type I (false‐positive) error, and the association might disappear with a larger sample size. Only a few dogs in our study received adjuvant therapy of any kind, and dogs given NSAID therapy alone generally were reported to have received this treatment for problems such as osteoarthritis, unrelated to their GIST. Overall, further studies are required to evaluate the utility of metronomic chemotherapy with and without NSAIDs in the treatment of dogs with GIST and other intestinal neoplasms.
In our study, no standardization of staging tests or toceranib treatment monitoring diagnostic tests took place. This situation might have resulted in skewing of metastasis rates or when PD was detected. In addition, we were unable to utilize a contemporary control group and the small sample size likely led to an underpowered study. Despite these limitations, and given the response rates observed here, especially in dogs with gross disease, toceranib merits consideration in the treatment of at least a subset of dogs with GIST. Metastasis at diagnosis and high tumor mitotic index were associated with shorter PFI in toceranib‐treated dogs, but these dogs still may benefit from toceranib treatment, and thus our results should not preclude toceranib use in patients with metastatic or mitotically active tumors.
In conclusion, biological activity of toceranib was evident in studied dogs with gross disease. Metastasis at diagnosis and high tumor mitotic index were associated with a shorter PFI. Larger prospective studies are needed to determine prognostic factors and the role of toceranib in the treatment of dogs with GIST in the microscopic and gross disease settings.
CONFLICT OF INTEREST DECLARATION
Chad M. Johannes is a former employee of Pfizer Animal Health and serves as a member of the Zoetis Inc. (formerly Pfizer Animal Health) Oncology Advisory Board and occasionally receives honoraria for these activities. The remaining authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Supporting information
Table S1 Cases of canine GIST and pathologic characteristics at diagnosis.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the clinicians who contributed case data: Suzanne Rau, Kenneth Rassnick, Jennifer Hofer, Christine Oakley, Paulo Vilar Saavedra, Yoshimi Iwaki, Katie Curran, Sarah Vidal, Brenda Phillips, Theresa Arteaga, Stephen Atwater, Lindsay Thalheim, Martin Crawford‐Jakubiak, Merrianne Burtch, JA Impellizeri, Sarah Gillings, Gerald Post, Gillian Rothchild, Keith Payton, and Stuart Gluckman. They acknowledge Sarah Marnin for her invaluable assistance in obtaining pathology samples. This study was funded by the Iowa State University College of Veterinary Medicine Oncology Department and performed primarily at Iowa State University College of Veterinary Medicine. A portion of this work was presented as an oral abstract at the 2017 ACVIM Forum, National Harbor, MD.
OFF‐LABEL ANTIMICROBIAL DECLARATION
The authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Berger EP, Johannes CM, Jergens AE, et al. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of gastrointestinal stromal tumors of dogs. J Vet Intern Med. 2018;32:2045–2053. 10.1111/jvim.15335
Funding information Iowa State University College of Veterinary Medicine Oncology section, Department of Veterinary Clinical Sciences
REFERENCES
- 1. Selting KA. Intestinal tumors In: Withrow SJ, Vail DM, Page RL, eds. Small Animal Clinical Oncology. 5th ed. St. Louis, MO: Elsevier; 2013:412‐423. [Google Scholar]
- 2. Streutker C, Huizinga J, Driman D, Riddell R. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology. 2007;32:2045‐2053. [DOI] [PubMed] [Google Scholar]
- 3. Novelli M, Rossi S, Rodriguez‐Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57:259‐270. [DOI] [PubMed] [Google Scholar]
- 4. Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol. 2014;27:S1‐S16. [DOI] [PubMed] [Google Scholar]
- 5. West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1):107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon S, Grabellus F, Ferrera L, et al. DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors. Cancer Res. 2013;73(13):3661‐3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dailey DD, Ehrhart E, Duval DL, et al. DOG1 is a sensitive and specific immunohistochemical marker for diagnosis of canine gastrointestinal stromal tumors. J Vet Diagn Invest. 2015;27(3):268‐277. [DOI] [PubMed] [Google Scholar]
- 8. London CA, Malpas PB, Wood‐Follis SL, et al. Multi‐center, placebo‐controlled, double‐blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856‐3865. [DOI] [PubMed] [Google Scholar]
- 9. Gregory‐Bryson E, Bartlett E, Kiupel M, Hayes S, Yuzbasiyan‐Gurkan V. Canine and human gastrointestinal stromal tumors display similar mutations in c‐KIT exon 11. BMC Cancer. 2010;10:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245‐266. [DOI] [PubMed] [Google Scholar]
- 11. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411‐1419. [DOI] [PubMed] [Google Scholar]
- 12. Demetri GD, Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi M, Kuroki S, Ito K, et al. Imatinib‐associated tumour response in a dog with a non‐resectable gastrointestinal stromal tumour harbouring a c‐kit exon 11 deletion mutation. Vet J. 2013;198:271‐274. [DOI] [PubMed] [Google Scholar]
- 14. Irie M, Takeuchi Y, Ohtake Y, et al. Imatinib mesylate treatment in a dog with gastrointestinal stromal tumors with a c‐kit mutation. J Vet Med Sci. 2015;77(11):1535‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. London CA, Hannah AL, Zadovoskaya R, et al. Phase I dose‐escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755‐2768. [PubMed] [Google Scholar]
- 16. Elliott J, Swinbourne F, Parry A, Baines L. Successful treatment of a metastatic, gastrointestinal stromal tumour in a dog with toceranib phosphate (Palladia). J Small Anim Pract. 2017;58:416‐418. [DOI] [PubMed] [Google Scholar]
- 17. Veterinary cooperative oncology group—common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016;14:417–446. [DOI] [PubMed] [Google Scholar]
- 18. Bellera C, Penel N, Ouali M, et al. Guidelines for time‐to‐event end point definitions in sarcomas and gastrointestinal stromal tumors (GIST) trials: results of the DATECAN initiative (Definition for the Assessment of Time‐to‐event Endpoints in CANcer trials). Ann Oncol. 2015;26:865‐872. [DOI] [PubMed] [Google Scholar]
- 19. London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet Comp Oncol. 2012;10(3):194‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 21. LaRock R, Ginn P. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol. 1997;34:303‐311. [DOI] [PubMed] [Google Scholar]
- 22. Russell KN, Mehler SJ, Skorupski KA, Baez JL, Shofer FS, Goldschmidt MH. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990–2003). J Am Vet Med Assoc. 2007;230(9):1329‐1333. [DOI] [PubMed] [Google Scholar]
- 23. Maas CP, Haar GT, Gaag I, Kirpensteijn J. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation. Vet Surg. 2007;36:302‐313. [DOI] [PubMed] [Google Scholar]
- 24. Cohen M, Post GS, Wright JC. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107‐110. [DOI] [PubMed] [Google Scholar]
- 25. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466‐1478. [DOI] [PubMed] [Google Scholar]
- 26. Takanosu M, Amano S, Kagawa Y. Analysis of c‐KIT exon 11 mutations in canine gastrointestinal stromal tumours. Vet J. 2016;207:118‐123. [DOI] [PubMed] [Google Scholar]
- 27. Joensuu H, Eriksson M, Hall KS, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. J Am Med Assoc. 2012;307(12):1265‐1272. [DOI] [PubMed] [Google Scholar]
- 28. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long‐term follow‐up. Am J Surg Pathol. 2005;29(1):52‐68. [DOI] [PubMed] [Google Scholar]
- 29. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long‐term follow‐up. Am J Surg Pathol. 2006;30(4):477‐489. [DOI] [PubMed] [Google Scholar]
- 30. Mochel JP, Gabrielsson J, Fink M, et al. Animal Health Modeling & Simulation Society: a new society promoting model‐based approaches in veterinary pharmacology. J Vet Pharmacol Ther. 2013;36:417‐419. [DOI] [PubMed] [Google Scholar]
- 31. Riviere JE, Gabrielsson J, Fink M, Mochel JP. Mathematical modeling and simulation in animal health. Part I: moving beyond pharmacokinetics. J Vet Pharmacol Ther. 2016;39(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 32. Bernabe LF, Portela R, Nguyen S, et al. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet Res. 2013;9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elmslie R, Glawe P, Dow S. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissues sarcomas. J Vet Intern Med. 2008;22:1373‐1379. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell L, Thamm D, Biller B. Clinical and immunomodulatory effects of toceranib combined with low‐dose cyclophosphamide in dogs with cancer. J Vet Intern Med. 2012;26:355‐362. [DOI] [PubMed] [Google Scholar]
- 35. Gumurdulu D, Erdogan S, Kayaselcuk F, et al. Expression of COX‐2, PCNA, Ki‐67 and p 53 in gastrointestinal stromal tumors and its relationship with histopathological parameters. World J Gastroenterol. 2007;13(3):426‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Türköz HK, Alkan I, Sişman S, Ozcan D. Cyclooxygenase‐2 expression and connection with tumor recurrence and histopathologic parameters in gastrointestinal stromal tumors. APMIS. 2009;117(11):825‐830. [DOI] [PubMed] [Google Scholar]
- 37. Jiang J, Jin MS, Suo J, Wang YP, He L, Cao XY. Evaluation of malignancy using Ki‐67, p 53, EGFR and COX‐2 expressions in gastrointestinal stromal tumors. World J Gastroenterol. 2012;18(20):2569‐2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu N, Huang J, Sun S, et al. Expression of matrix metalloproteinase‐9, cyclooxygenase‐2 and vascular endothelial growth factor are increased in gastrointestinal stromal tumors. Int J Clin Exp Med. 2015;8(4):6495‐6501. [PMC free article] [PubMed] [Google Scholar]
- 39. Stewart AE, Heslin MH, Arch J, et al. Cyclooxygenase‐2 expression and clinical outcome in gastrointestinal stromal tumors. J Gastrointest Surg. 2006;10(2):315‐319. [DOI] [PubMed] [Google Scholar]
- 40. Doré M. Cyclooxygenase‐2 expression in animal cancers. Vet Pathol. 2011;48(1):254‐265. [DOI] [PubMed] [Google Scholar]
- 41. McEntee M, Cates J, Neilsen N. Cyclooxygenase‐2 expression in spontaneous intestinal neoplasia of domestic dogs. Vet Pathol. 2002;39:428‐436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Cases of canine GIST and pathologic characteristics at diagnosis.


