Abstract
Schiff bases of 3,4-dimethoxybenzenamine 1–25 were synthesized and evaluated for their antioxidant activity. All the synthesized compounds were characterized by various spectroscopic techniques. In addition, the characterizations of compounds 13, 15 and 16 were supported by crystal X-ray determinations and their geometrical parameters were compared with theoretical DFT calculations at the B3LYP level of theory. Furthermore, the X-ray crystal data of two non-crystalline compounds 8 and 18 were theoretically calculated and compared with the practical values of compounds 13, 15, 16 and found a good agreement. The compounds showed good DPPH scavenging activity ranging from 10.12 to 84.34 μM where compounds 1–4 and 6 showed stronger activity than the standard n-propyl gallate. For the superoxide anion radical assay, compounds 1–3 showed better activity than the standard.
Keywords: 3,4-dimethoxybenzenamine Schiff bases; crystal structure; DFT calculation; antioxidant activity
1. Introduction
Schiff bases are a class of compounds with unique biological [1], analytical and industrial properties [2]. A number of Schiff bases have been reported to possess antiglycation [3,4,5,6], antioxidant [7,8,9,10], antileishmanial [11], antifungal [12], anticancer [13], anticonvulscent [14], analgesic [15], antituberclotic [16], and diuretic [17] activities. Heterocyclic Schiff bases with various activities e.g., antibacterial, antifungal and anticancer have also been reported [18,19,20]. The active pharmacophore (-N=CH-) of Schiff bases plays a major role in these significant biological activities. However, the attached neighbouring groups may also affect the activity [21]. Biological activities of Schiff bases metal complexes have also been reported [22,23]. The copper (II) complexes of Schiff bases showed antitumor activity and the lanthanide complexes showed significant antioxidant activity [24,25,26,27,28]. Antioxidants can prevent injury to vessel membranes aiding appropriate blood circulation and are useful for the prevention of cardiovascular diseases. They provide protection against cancer-causing radicals and DNA damages [29]. The action of antioxidants is credited to their ability to convert free radicals to stable molecules. Antioxidants therefore, guard cells from the oxidative damage which leads to aging and diseases [30,31,32]. The free radicals were also reported to play a role in the pathology of arteriosclerosis, malaria and rheumatoid arthritis [33,34].
As for polyphenolic compounds, the phenolic Schiff bases (ArOH) scavenge free radicals (R•) by their ability to donate hydrogen atoms from hydroxyl groups through one of the following mechanisms:
(i) Proton Coupled-Electron Transfer (PC-ET) versus Hydrogen atom transfer (HAT)
| ArOH + R• → ArO• + RH |
In this mechanism, the electron and proton are transferred from the active phenolic group to the free radical in a single step. This type of reactions can be subdivided into two distinct subclasses, hydrogen atom transfer (HAT) and proton coupled electron transfer (PC-ET) [35,36,37,38]. In HAT, the proton and electron are transferred together, as a hydrogen atom, while in PC-ET mechanism, the proton and electron are transferred between different sets of orbitals [35].
(ii) Electron Transfer-Proton Transfer (ET-PT)
| ArOH + R• →ArOH+•+ R− → ArO• + RH |
The ET-PT mechanism consists of two steps. In the first step, an electron transfer (ET) from the phenolic compound to the free radical. In the second step, a heterolytic O-H bond dissociation of the radical cation (ArOH+•) leads to the formation of a phenoxyl radical (ArOH•).
(iii) Sequential Proton Loss Electron Transfer (SPLET)
|
ArOH → ArO− + H+ ArO− + R• → ArO• + R− R− + H+ → RH |
The above mechanism consists of three steps. In the first step, a heterolytic bond dissociation of a phenolic hydroxyl group leads to the formation of a phenoxyl anion and the release of a proton. In the second step, an electron transfer from the phenoxyl anion to the free radical leads to the formation of a phenoxyl radical and an anion (R−). In the end, the protonation of R− leads to the formation of RH. This mechanism is strongly favored under alkaline conditions (e.g., high pH), which may help in the proton of the first step [39,40].
(iv) Adduct formation (AF)
| ArOH + R• → [ArOH-R]• → stable adducts |
The AF mechanism is more specific and is observed between (a) carbon centered radicals and double bonds; or (b) hydroxyl radicals and aromatic rings. Numerous side reactions may occur that lead to stable adducts from [ArOH-R]•.
In continuation of our research on the synthesis of bioactive small molecules [41,42,43], we synthesized a series of 3,4-dimethoxybenzenamine Schiff bases (Scheme 1) and evaluated their antioxidant potential in the search of the potential antioxidant leads.
Scheme 1.
Synthesis of 3,4-dimethoxybenzenamine Schiff bases.
2. Results and Discussion
2.1. Chemistry
The 3,4-dimethoxybenzenamine Schiff bases were prepared by condensing 3,4-dimethoxy-benzenamine with several aromatic aldehydes by refluxing in ethanol for 3 to 4 h (Scheme 1). The crude products were further recrystallized from methanol and in most of the cases needle-like crystals were obtained (yields 81%–92%). The structural confirmation of the dimethoxybenzenamine Schiff bases was done by various spectroscopic techniques including 1H-NMR, IR and mass spectroscopy. All synthetic compounds were established as having E configuration [44,45,46]. The compounds 8, 9, 10, 15, 22, 24 and 25 are known [47,48,49,50] but compound 13 has only a CAS registry number 1002275–90–2 with no reference. Compounds 1–7, 11, 12, 14, 16, 18–21, 23 are new.
2.2. Antioxidant Activities
2.2.1. DPPH Scavenging Activity
The synthesized compounds 1–25 showed activity in the range of 10.12–84.34 μM (Table 1). Compound 1 (IC50 = 10.12 ± 0.54 μM) showed highest activity, three times more active than the standard (IC50 = 30.30 ± 0.2 μM). This is due to the ortho-trihydroxyl group which is known to show very good activity [51,52]. Compound 2 is a meta-trihydroxyl analogue but showed slightly less activity than compound 1. This may be due to the ortho-trihydroxyl groups of compound 1 which is similar to the catecholic moeity known to exhibit good antioxidant activities [53,54,55,56].
Table 1.
In vitro DPPH activity and % yield of compounds 1–25.
| N° | Yield (%) | IC50 (μM ± SEM a) | N° | Yield (%) | IC50 (μM ± SEM a) |
|---|---|---|---|---|---|
| 1 | 84 | 10.12 ± 0.54 | 14 | 87 | 42.80 ± 2.80 |
| 2 | 82 | 15.6 ± 0.06 | 15 | 82 | NA b |
| 3 | 78 | 19.2 ± 0.70 | 16 | 82 | NA b |
| 4 | 84 | 28.14 ± 0.86 | 17 | 84 | NA b |
| 5 | 85 | 30.45 ± 0.82 | 18 | 83 | NA b |
| 6 | 86 | 28.10 ± 1.30 | 19 | 85 | NA b |
| 7 | 81 | 33.02 ± 1.20 | 20 | 87 | NA b |
| 8 | 83 | 34.14 ± 1.50 | 21 | 88 | NA b |
| 9 | 92 | 40.01 ± 1.80 | 22 | - | NA b |
| 10 | 88 | NA b | 23 | 90 | NA b |
| 11 | 90 | 50.01 ± 2.20 | 24 | 84 | NA b |
| 12 | 87 | 38.16 ± 2.10 | 25 | 92 | NA b |
| 13 | 90 | NA b | n-propyl gallate c | - | 30.30 ± 0.2 |
SEM a is the standard error of the mean, NA b = Not active, n-propyl gallate c was the standard drug for the DPPH assays.
Incidently, compounds 1 and 2 which have an additional hydroxyl group as compared to compounds 4 and 6, showed stronger antioxidant activities than the latter two compounds. Among the five dihydroxyl analogues, compound 3, 4 and 6 showed better activity than standard. The activity of compound 3 is due to the 2', 5' positions of the dihydroxyl groups, favorable for stabilization of the free radical. The catecholic moeity in compounds 4 and 6 is well known structural feature for good activity [53,54,55,56]. Other meta-dihydroxyl analogues 5 and 7 also showed very close activity as compared to the standard. Compound 8 showed good activity due to adjacent 3-methoxyl and 4-hydroxyl positions. However, its other analogue 11 with reversed arrangement showed moderate activity.
For the mono-hydroxyl series, compound 9 having hydroxyl at 4' position showed good activity while its other analogues 10 and 15 showed no activity, due to the lack of free radical stabilizing capability. The presence of a bromo substituent further increases the capability to stabilize radicals as illustrated by compound 12. meta-arranged methoxyl and hydroxyl groups contributed to the good activity of compound 14. The remaining compounds do not possess functional groups to help stabilize free radicals and are therefore inactive.
2.2.2. Superoxide Scavenging Activity
Compounds 1, 2 and 3 showed better activity than the standard drug n-propylgallate (Table 2). Compound 4 showed good activity. Compounds 5, 6, 7, 8, 9 and 12 showed moderate activities, while compound 14, 20 and 21 showed weak activities. The good activity of compounds 1–3 may be due to more stabilizing potential of these compounds to stabilize free radicals generated during bioassay. DPPH scavenging activity and superoxide scavenging activity mainly depend on the hydroxyl position as well as number of hydroxyl groups present in the molecule [57,58].
Table 2.
In vitro superoxide anion radical scavenging activity of compounds 1–25.
| Comp. No. | IC50 (μM ± SEM a) | Comp. No | IC50 (μM ± SEM a) |
|---|---|---|---|
| 1 | 85.03 ± 1.20 | 14 | 260.3 ± 6.4 |
| 2 | 90.60 ± 1.50 | 15 | NA b |
| 3 | 98.60 ± 1.70 | 16 | NA b |
| 4 | 145 ± 2.1 | 17 | NA b |
| 5 | 170.2 ± 3.2 | 18 | NA b |
| 6 | 175.0 ± 3.5 | 19 | NA b |
| 7 | 180.1 ± 3.8 | 20 | 315.1 ± 8.4 |
| 8 | 190.1 ± 3.9 | 21 | 320.1 ± 6.3 |
| 9 | 208.9 ± 5.4 | 22 | NA b |
| 10 | NA b | 23 | NA b |
| 11 | NA b | 24 | NA b |
| 12 | 210.1 ± 4.4 | 25 | NA b |
| 13 | NA b | n-propyl gallate c | 106.34 ± 1.6 |
SEM a is the standard error of the mean, NA b = Not active, n-propyl gallate c was the standard drug for the superoxide anionradical scavenging assays.
2.3. X-ray Crystallography Studies
2.3.1. Compound 13
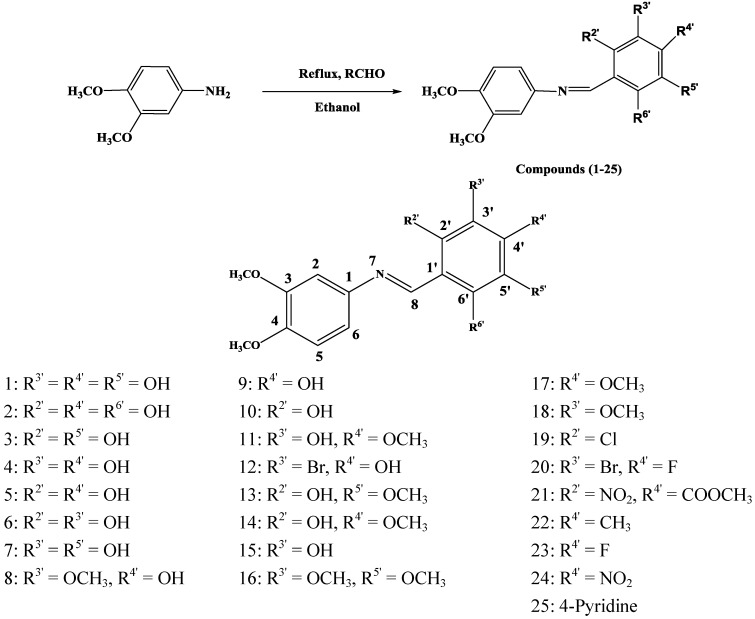
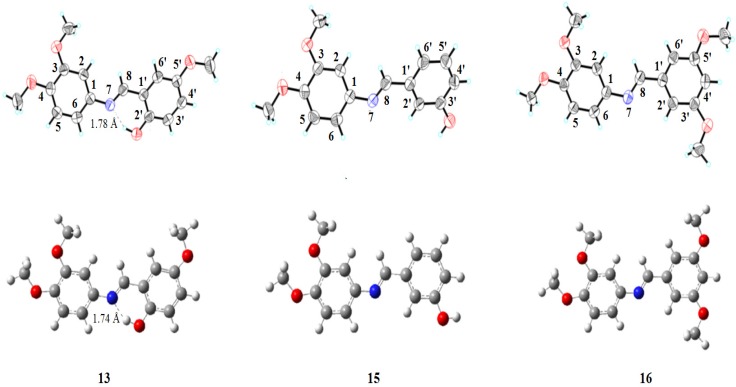
The structure of compound 13 is composed of a dimethoxybenzene moiety link with methoxyphenol moiety via azomethin bridge which adopts an E configuration (Figure 1). The dimethoxy-substituted planar benzene moiety (C1-C6) is oriented at a dihedral angle of 29.33(9)° with respect to the methoxy-substituted planar phenol moiety (C1'–C6') with standard deviation of 0.016(2)° for C2' atom from root mean square plane. In the crystal lattice (Figure 2), molecules are linked via C–H···O hydrogen bonding and form three dimensional consolidated network of mirror imaged sets running along the b axis where each set contains four molecule.
Figure 1.
X-ray and optimized structures of compounds 13, 15 and 16.
Figure 2.
Crystal packing diagram for compounds 13, 15 and 16.
Crystallographic data of compound 13 (CCDC 980015), can be obtained from Cambridge Crystallographic Data Center without any cost. Crystal and experimental data of compound 13 are presented in Table 3 and the hydrogen bonding data in Table 4.
Table 3.
The crystal X-ray and experimental data of compounds 13, 15 and 16.
| Compound 13 | Compound 15 | Compound 16 | |
|---|---|---|---|
| Empirical formula | C16H17NO4 | C15H15NO3 | C17H19NO4 |
| Formula weight | 287.31 | 257.28 | 301.33 |
| Temperature | 273(2)K | 273(2)K | 273(2)K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | Pna2(1) | P2(1)2(1)2(1) | P2(1)/c |
| a | 9.7203(7) Å | 5.3625(2)Å | 11.1898(4) Å |
| b | 30.576(2) Å | 11.1755(5) Å | 17.5567(6) Å |
| c | 4.8328(3) Å | 21.9532(10)Å | 8.1013(3) Å |
| α | 90° | 90° | 90° |
| β | 90° | 90° | 98.0720(10)° |
| γ | 90° | 90° | 90° |
| Volume | 1436.36(17)A3 | 1315.63(10)A3 | 1575.78(10) A3 |
| Z | 4 | 4 | 4 |
| Calculated density | 1.329 mg/m3 | 1.299 mg/m3 | 1.270 mg/m3 |
| Absorption coefficient | 0.096 mm−1 | 0.091 mm−1 | 0.091 mm−1 |
| F(000) | 608 | 544 | 640 |
| Crystal size | 0.67 × 0.16 × 0.14 mm | 0.77 × 0.49 × 0.45 mm | 0.46 × 0.44 × 0.42 mm |
| θ range | 1.33 to 25.50 ° | 1.86 to 25.50° | 1.84 to 25.50 |
| Reflections Collected | 8206 | 7839 | 9213 |
| Reflections Unique | 2629 | 2426 | 2934 |
| (Rint) | 0.0216 | 0.0165 | 0.0148 |
| R1 with I > 2σ(I) | 0.0347 | 0.0369 | 0.0352 |
| R2 with I > 2σ(I) | 0.0808 | 0.1066 | 0.0967 |
| R1 for all data | 0.0412 | 0.0386 | 0.0399 |
| R2 for all data | 0.0855 | 0.1086 | 0.1012 |
| Goodness of fit | 1.059 | 1.091 | 1.046 |
| max/min ρ eA°−3 | 0.107 and −0.129 | 0.349 and −0.295 | 0.140 and −0.141 |
| CCDC number | CCDC 980015 | CCDC 980014 | CCDC 980016 |
Table 4.
Hydrogen bonding data for compound 13.
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| O1' | H1A' | N7 | 0.94(3) | 1.78(3) | 2.628(2) | 149(2) |
| C5 | H5A | O2 a | 0.93 | 2.53 | 3.319(2) | 143 |
Symmetry codes: a 1/2+x,1/2-y,z.
2.3.2. Compound 15
Structurally, compound 15 is similar to compound 13 with the difference that the planar phenyl ring (C1'-C6') has only one hydroxyl group at the C3' position. The dihedral angle between the two planar benzene ring (C1'-C6' and C1-C6) was found to be 44.35(7)° with standard deviation of 0.009(2)° for C5 atom from root mean square plane.
The molecule does not possess any intramolecular interactions in the crystal lattice, molecules are packed in a series and form a consolidated network running along c-axis, joining through intermolecular O1---H1A'...N7, C6---H6'A...O2, C5---H5A...O1 hydrogen bonds. Crystallographic data of compound 15 (CCDC 980014), can be obtained from Cambridge Crystallographic Data Center without any cost. Crystal and experimental data of compound 15 presented in Table 3 and hydrogen bonding data in Table 5.
Table 5.
Hydrogen bonding data for compound 15.
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| O1' | H1A' | N7 a | 0.939(18) | 1.891(18) | 2.7841(16) | 158.1(16) |
| C6' | H6'A | O2 b | 0.93 | 2.58 | 3.2572(17) | 130 |
| C5 | H5A | O1 c | 0.93 | 2.57 | 3.1655(18) | 123 |
Symmetry codes: a 1/2+x,1/2-y,-z ; b 1-x,1/2+y,1/2-z; c -3/2+x,1/2-y,-z.
2.3.3. Compound 16
Compound 16 is structurally similar to Compound 13 and Compound 15 with the only difference that all four substituents on the aromatic skeleton are methoxyl groups. In this molecule the two benzene rings and the azomethine group are practically coplanar and the molecule adopts an E configuration about the C8-N7 bond. The dihedral angle between the two planar benzene rings (C1'-C6' and C1-C6) was found to be 40.56(6)° with standard deviation of −0.019(1)° for C1 atom from root mean square plane. All the bond distances are within normal range comparable to those of similar compounds. No chemical intramolecular interaction was observed. However in the crystal structure, molecules were linked via C2-H2'A...O1 and C3-H3A...O1 intermolecular interactions to form R22 (20) ring motive running along c-axis.
The crystallographic data of compound 16 (CCDC 980016), can be obtained from Cambridge Crystallographic Data Center. Crystal and experimental data of compound 16 presented in Table 3 and hydrogen bonding data in Table 6.
Table 6.
Hydrogen bonding data for compound 16.
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| C2 | H2’A | O1 a. | 0.93 | 2.57 | 3.4945(16) | 176 |
| C3 | H3A | O1 b | 0.93 | 2.52 | 3.4418(16) | 170 |
Symmetry codes: a 1-x,-y,2-z; b x,1/2-y,-1/2+z.
2.4. DFT Calculations
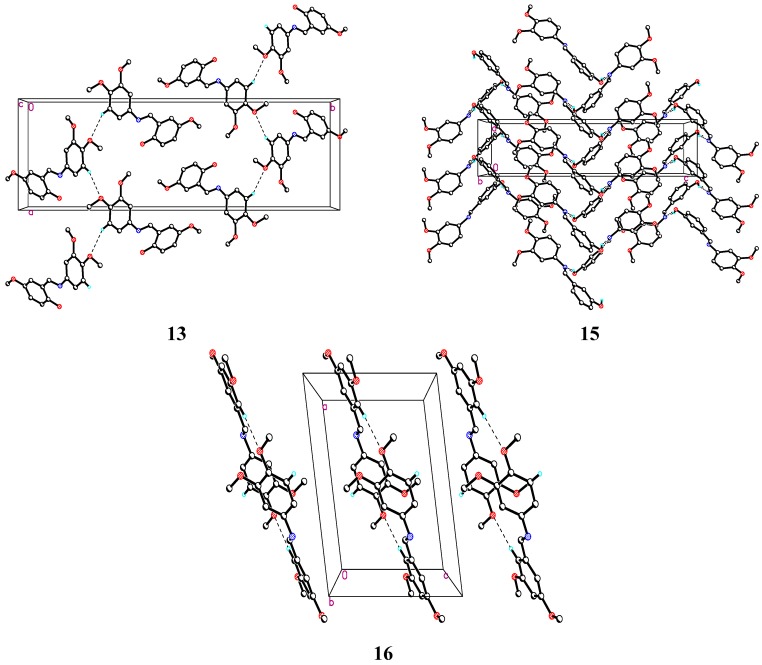
Crystal structures of compounds 13, 15 and 16 were compared to their optimized minima (Figure 3). The initial geometrical structures were obtained from the molden files of the X-ray solved structures. The optimization has been carried out at the B3LYP/6-311+G(d,p) level of theory by using Gaussian 09 package [7].
Figure 3.
Optimized structures of compounds 1, 2, 3, 8 and 18.
The minima of the optimized structures were confirmed by the absence of imaginary frequencies. The experimental and calculated bond lengths, bond and dihedral angles of the compounds are presented in Table 7.
Table 7.
The calculated and experimental values of the bond lengths, bond angles and torsion angles of compounds 13, 15, and 16.
| 13 | 15 | 16 | Calculated | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal | Exp | Cal | Exp | Cal | Exp | 1 | 2 | 3 | 8 | 18 | |
| Bond lengths (Å) | |||||||||||
| C1-N7 | 1.41 | 1.413 | 1.40 | 1.4243 | 1.41 | 1.4181 | 1.40 | 1.41 | 1.41 | 1.40 | 1.41 |
| N7-C8 | 1.29 | 1.276 | 1.28 | 1.2673 | 1.28 | 1.2677 | 1.28 | 1.29 | 1.29 | 1.27 | 1.28 |
| C8-C1' | 1.45 | 1.452 | 1.47 | 1.4680 | 1.47 | 1.4650 | 1.47 | 1.44 | 1.45 | 1.46 | 1.47 |
| C2'-O2' | 1.34 | 1.358 | - | - | - | - | - | 1.37 | 1.35 | - | - |
| C3'-O3' | 1.37 | - | 1.37 | 1.3601 | 1.36 | 1.3673 | 1.36 | - | - | 1.37 | 1.36 |
| C5'-O4' | - | - | - | - | - | - | 1.37 | 1.36 | - | 1.36 | - |
| C5'-O5' | 1.37 | 1.382 | - | - | 1.37 | 1.3694 | 1.38 | - | 1.37 | - | - |
| C6'-O6' | - | - | - | - | - | - | - | 1.34 | - | - | - |
| Bond angles (°) | |||||||||||
| C2-C1-N7 | 125 | 123.31 | 126 | 122.15 | 126 | 122.79 | 123 | 123 | 123 | 123 | 123 |
| C1-N7-C8 | 124 | 121.07 | 123 | 119.72 | 123 | 118.20 | 121 | 122 | 122 | 120 | 121 |
| N7-C8-C1’ | 122 | 122.43 | 122 | 123.96 | 123 | 124.02 | 123 | 122 | 122 | 123 | 123 |
| Torsion angles (°) | |||||||||||
| C2-C1-N7-C8 | 0 | −155.52 | 0 | −43.09 | 0 | 34.70 | 37 | 32 | 34 | 36 | 30 |
| C1-N7-C8-C1' | −180 | −175.32 | −180 | 174.26 | −180 | −179.25 | −177 | −177 | −177 | −177 | −177 |
| N7-C8-C1'-C2’ | 0 | 0 | 0 | −1.1 | 0 | −174.83 | 2 | 0 | 1 | 1 | 1 |
The experimental bond lengths and bond angles z-matrix coordinates are well reproduced theoretically. On the other hand, the dihedral angles are well reproduced, except for the dihedral angle between the 3,4-dimethoxybenzenyl and the azo group where a slight deviation was observed between the crystal structure compared to the optimized conformation. The hydrogen bonding between 2'-OH group and the azo group in Compound 13 is well reproduced theoretically, with a difference of 0.04 Å (Figure 1). In order to generalize the comparison between structural X-ray and calculated results, the optimized structures of compounds 1, 2, 3, 8 and 18 were obtained at the same level of theory (Table 7 and Figure 3). The structural parameters (bond, angles, and torsion angles) for the optimized structures of 1, 2, 3, 8 and 18 are very similar to the optimized and X-ray parameters of 13, 15 and 16.
3. Experimental
3.1. General Information
NMR experiments were performed in DMSO-d6 on a Bruker Ultra Shield 500 MHz FT NMR (Wissembourg, Switzerland). CHN analysis was performed on a Carlo Erba Strumentazione-Mod-1106 (Milan, Italy). Electron impact mass spectra (EI-MS) were recorded on a Finnigan MAT-311A instrument (Bremen, Germany). Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
3.2. DPPH (1,1-Diphenyl-2-picryl hydrazyl) Free Radical Scavenging Activity
The free radical scavenging activity was measured by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay using literature protocols. The reaction mixture contained test sample (5 μL, 1 mM in DMSO) and DPPH (Sigma, 95 μL, 300 μM) in ethanol. The reaction mixture was taken into a 96-well microtiter plate and incubated at 37 °C for 30 min. The absorbance was measured at 515 nm using microtitre plate reader (Molecular Devices, Sunnyvale, CA, USA). Percent radical scavenging activity was determined in comparison with DMSO containing control (Table 1). IC50 values represent the concentration of compounds able to scavenge 50% of DPPH radicals. Propyl gallate was used as positive control. All chemicals used were of analytical grade (Sigma, Ronkonkoma, NY, USA).
3.3. In Vitro Assay for Superoxide Anion Radical Scavenging Activity
The superoxide producing system was set up by mixing phenazinemethosulfate (PMS), NADH, and oxygen (air), and the production of superoxide was estimated by the nitroblue tetrazolium method. Measurement of superoxide radical scavenging activity was carried out on the basis of the method described by the modified method used by Ferda. In aerobic reaction mixtures containing NADH, phenazine methosulphate and nitro blue tetrazolium, PMS is reduced by NADH and then gave rise to O2−, which in turn reduced NBT. On the basis of this PMS has frequently been used to mediate O2−.
The reaction mixture comprised 100 µM β-nicotinamide adenine dinucleotide reduced form (NADH, 40 µL), 80 µM of nitro blue tetrazolium (NBT, 40 µL), 8 µM phenazine methosulphate (PMS, 20 µL), 1 mM sample (10 µL), and 0.1 M phosphate buffer (pH 7.4, 90 µL). The reagents were prepared in buffer and sample in DMSO. The reaction was performed in 96-well microtitre plate at room temperature and absorbance was measured at 560 nm. The formation of superoxide was monitored by measuring the formation of water soluble blue formosan dye. A lower absorbance of reaction mixture indicated a higher scavenging activity of the sample. Percent radical scavenging activity (% RSA) by samples was determined in comparison with a control using the following equation:
| %RSA = 100 − {(OD test compound/OD control) × 100 |
3.4. General Procedure for the Synthesis 3,4-Dimethoxybenzenamine Schiff Bases
The 3,4-dimethoxyanaline Schiff bases were synthesized by refluxing in ethanol (10 mL) for 3 h 3,4-dimethoxyanaline (2 mmol) and each pure aryl aldehyde (2 mmol). The progress of the reaction was monitored by TLC. After completion of reaction, the solvent was evaporated under vacuum to afford crude products which were further recrystallized from methanol to give needle-like pure products in good to excellent yields.
(E)-5-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,2,3-triol (1). 1H-NMR: δ 11.05 (s, 2H, OH), 9.61 (s, 1H, OH), 8.60 (s, 1H, N=CH-Ar), 7.05 (s, 2H), 6.95 (d, 1H, J = 8.0 Hz), 6.91 (d, 1H, J = 2.0 Hz), 6.82 (dd, 1H, J = 8.0, J = 2.0, Hz), 3.83 (s, 3H, OCH3), 3.77 (s, 3H, OCH3); 13C-NMR: δ 160.1, 150.1, 148.1, 146.3, 146.2, 146.2, 138.1, 134.2, 119.4, 114.2, 109.2, 108.8, 108.8, 56.3, 56.3; Anal. Calcd for C15H15NO5, C = 62.28, H = 5.23, N = 4.84 Found C = 62.29, H = 5.22, N = 4.85 EI MS m/z (% rel. abund.): 289 (M+, 10), 258 (12), 138 (20), 137 (100).
(E)-2-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,3,5-triol (2). 1H-NMR: δ 10.65 (s, 2H, OH), 9.23 (s, 1H, OH), 8.65 (s, 1H, N=CH-Ar), 7.15 (s, 2H), 6.94 (d, 1H, J = 8.0 Hz), 6.90 (d, 1H, J = 2.0 Hz), 6.81 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.82 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C-NMR: δ 163.7, 163.7, 163.4, 160.1, 146.4, 150.2, 148.1, 119.4, 114.3, 109.2, 106.1, 96.2, 96.2, 56.1, 56.1; Anal. Calcd for C15H15NO5, C = 62.28, H = 5.23, N = 4.84, Found C = 62.28, H = 5.23, N = 4.84; EI MS m/z (% rel. abund.): 289 (M+, 13), 258 (11), 138 (17), 137 (100).
(E)-2-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,4-diol (3). 1H-NMR: δ 12.49 (s, 1H, OH), 10.18 (s, 1H, OH), 9.19 (s, 1H, N=CH-Ar), 7.11 (d, 1H, J = 7.0 Hz), 7.04 (s, 1H), 7.00 (d, 1H, J = 7.0 Hz), 6.97 (d, 1H, J3/2 = 8.0 Hz), 6.86 (d, 1H, J = 8.0, Hz), 6.78 (s, 1H), 3.80 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 160.0, 153.6, 151.0, 150.1, 148.7, 146.3, 120.3, 119.4, 119.5, 118.4, 116.1, 114.2, 109.2, 56.1, 56.1; Anal. Calcd for C15H15NO4, C = 65.92, H = 5.53, N = 5.13, Found C = 65.91, H = 5.54, N = 5.12; EI MS m/z (% rel. abund.): 273 (M+, 60), 241 (8), 137 (100), 122 (20), 109 (30).
(E)-4-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,2-diol (4). 1H-NMR: δ 12.20(s, 1H, OH), 10.30 (s, 1H, OH), 8.40 (s, 1H, N=CH-Ar), 7.10 (d, 1H, J = 7.0 Hz), 7.06 (s, 1H), 7.02 (d, 1H, J = 7.0 Hz), 6.88 (d, 1H, J3/2 = 8.0 Hz), 6.82 (d, 1H, J = 8.0, Hz), 6.74 (s, 1H), 3.83 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C-NMR: δ 149.4, 146.2, 117.1, 116.1, 131.1, 123.1, 160.2, 146.4, 109.1, 114.2, 119.1, 148.1, 150.0, 56.1, 56.1; Anal. Calcd for C15H15NO4, C = 65.92, H = 5.53, N = 5.13, Found C = 65.90, H = 5.55, N = 5.11; EI MS m/z (% rel. abund.): 273 (M+, 42), 241 (12), 137 (100), 122 (15), 109 (28).
(E)-4-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,3-diol (5). 1H-NMR: δ 13.71 (s, 1H, OH), 10.18 (s, 1H, OH), 8.80 (s, 1H, N=CH-Ar), 7.40 (d, 1H, J = 8.0 Hz), 7.05 (d, 1H, J = 2.0 Hz), 6.99 (d, 1H, J = 7.0 Hz), 6.92 (dd, 1H, J = 8.0, J = 2.0, Hz), 6.40 (dd, 1H, J = 8.0, J = 2.0, Hz), 6.28 (d, 1H, J = 2.0 Hz),3.82 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 162.3, 162.1, 160.1, 150.2, 148.1, 146.4, 133.6, 119.4, 114.2, 113.0, 109.1, 108.5, 103.5, 56.1, 56.1; Anal. Calcd for C15H15NO4, C = 65.92, H = 5.53, N = 5.13 Found C = 65.93, H = 5.55, N = 5.12; EI MS m/z (% rel. abund.): 273 (M+, 70), 241 (17), 137 (100), 122 (22), 109 (38).
(E)-3-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,2-diol (6). 1H-NMR: δ 13.42 (s, 1H, OH), 9.11 (s, 1H, OH), 8.93 (s, 1H, N=CH-Ar), 7.72 (d, 1H, J = 8.0 Hz), 7.15 (d, 1H, J = 8.0 Hz), 7.10 (s, 1H), 7.07 (d, 1H, J = 2.0 Hz), 6.76 (dd, 1H, J = 8.0, J = 2.0, Hz), 7.65 (t, 1H, J = 8.0 Hz), 3.81 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C-NMR: δ 160.1, 151.5, 150.1, 148.2, 146.4, 146.0, 124.6, 122.7, 119.8, 119.6, 119.5, 114.2, 109.1, 56.1, 56.1; Anal. Calcd for C15H15NO4, C = 65.92, H = 5.53, N = 5.13, Found C = 65.91, H = 5.54, N = 5.12; EI MS m/z (% rel. abund.): 273 (M+, 78), 241 (16), 137 (100), 122 (11).
(E)-5-(((3,4-Dimethoxyphenyl)imino)methyl)benzene-1,3-diol (7). 1H-NMR: δ 9.47 (s, 2H, 2×OH), 8.45 (s, 1H, N=CH-Ar), 7.01 (d, 2H, J = 8.0 Hz), 6.84 (d, 1H, J = 2.0 Hz), 6.65 (d, 1H, J = 8.0 Hz), 6.40 (d, 1H, J = 2.0 Hz), 6.06 (dd, 1H, J = 8.0, J = 2.0, Hz), 3.81 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 160.1, 160.1, 160.0, 150.1, 148.1, 146.4, 141.3, 119.5, 114.3, 109.1, 107.4, 107.4, 105.8, 56.1, 56.1; Anal. Calcd for C15H15NO4, C = 65.92, H = 5.53, N = 5.13, Found C = 65.92, H = 5.54, N = 5.11; EI MS m/z (% rel. abund.): 273 (M+, 50), 241 (11), 137 (100), 122 (18).
(E)-4-(((3,4-Dimethoxyphenyl)imino)methyl)-2-methoxyphenol (8). 1H-NMR: δ 9.65 (s, 1H, OH), 8.49 (s, 1H, N=CH-Ar), 7.51 (d, 1H, J = 2.0 Hz), 7.32 (dd, 1H, J = 8.0, J = 2.0, Hz), 6.96 (d, 1H, J = 8.0 Hz), 6.93 (d, 1H, J = 2.0 Hz), 6.89 (d, 1H, J = 8.0 Hz), 6.06 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.85 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.76 (s, 3H, OCH3); 13C-NMR: δ 160.1, 151.1, 150.1, 149.1, 148.2, 146.3, 130.8, 122.8, 119.5, 117.1, 114.2, 112.0, 109.1, 56.1, 56.1, 55.9; Anal. Calcd for C16H17NO4, C = 66.89, H = 5.96, N = 4.88, Found C = 65.90, H = 5.95, N = 4.90; EI MS m/z (% rel. abund.): 287 (M+, 100), 255 (13), 137 (84), 122 (25).
(E)-4-(((3,4-Dimethoxyphenyl)imino)methyl)phenol (9). 1H-NMR: δ 10.04 (s, 1H, OH), 8.50 (s, 1H, N=CH-Ar), 7.77 (d, 2H, J = 8.0 Hz), 6.96 (d, 1H, J = 8.0 Hz), 6.92 (d, 1H, J = 2.0 Hz), 6.88 (d, 2H, J = 8.0 Hz), 6.81 (dd, 1H, J = 8.0, J = 2.0, Hz), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3); 13C-NMR: δ 160.5, 160.0, 150.0, 148.1, 146.4, 130.4, 130.4, 129.2, 119.4, 116.0, 116.0, 114.2, 109.1, 56.1, 56.1; Anal. Calcd for C15H15NO3, C = 70.02, H = 5.88, N = 5.44, Found C = 70.01, H = 5.89, N = 5.43; EI MS m/z (% rel. abund.): 257 (M+, 100), 225 (11), 137 (68), 105 (20).
(E)-2-(((3,4-Dimethoxyphenyl)imino)methyl)phenol (10). 1H-NMR: δ 13.32 (s, 1H, OH), 8.93 (s, 1H, N=CH-Ar), 7.63 (dd, 1H, J = 8.0, J = 2.0 Hz), (dt, 1H, J = 8.0, J = 2.0 Hz), 6.93 (d, 1H, J = 2.0 Hz), 6.80-6.72 (m, 4H), 3.83 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 161.1, 160.0, 150.1, 148.1, 146.4, 132.2, 132.0, 121.3, 120.4, 119.4, 117.6, 114.2, 109.1, 56.1, 56.1; Anal. Calcd for C15H15NO3, C = 70.02, H = 5.88, N = 5.44, Found C = 70.03, H = 5.88, N = 5.43; EI MS m/z (% rel. abund.): 257 (M+, 100), 225 (15), 137 (80), 93 (30).
(E)-5-(((3,4-Dimethoxyphenyl)imino)methyl)-2-methoxyphenol (11). 1H-NMR: δ 9.28 (s, 1H, OH), 8.48 (s, 1H, N=CH-Ar), 7.42 (d, 1H, J = 2.0 Hz), 7.30 (dd, 1H, J = 8.0, J = 2.0, Hz), 7.04 (d, 1H, J = 8.0 Hz), 6.97 (d, 1H, J = 8.0 Hz), 6.93 (d, 1H, J = 2.0 Hz), 6.82 (dd, 1H, J = 8.0, J = 2.0, Hz), 3.84 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.77 (s, 3H, OCH3); 13C-NMR: δ 160.0, 152.1, 150.1, 148.2, 147.2, 146.4, 147.1, 131.0, 125.4, 119.4, 115.8, 114.2, 109.1, 56.1, 56.1, 55.8; Anal. Calcd for C16H17NO4, C = 66.89, H = 5.96, N = 4.88, Found C = 66.88, H = 5.95, N = 4.89; EI MS m/z (% rel. abund.): 287 (M+, 100), 255 (18), 137 (69), 122 (20).
(E)-2-Bromo-4-(((3,4-dimethoxyphenyl)imino)methyl)phenol (12). 1H-NMR): δ 12.68 (s, 1H, OH), 8.94 (s, 1H, N=CH-Ar), 8.04 (d, 1H, J = 2.0 Hz), 7.76 (dd, 1H, J = 8.0, J = 2.0, Hz), 7.07 (d, 1H, J = 8.0 Hz), 6.98 (d, 1H, J = 8.0 Hz), 6.95 (d, 1H, J = 2.0 Hz), 6.84 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.80 (s, 3H, OCH3), 3.77 (s, 3H, OCH3); 13C-NMR (DMSO-d6): δ 160.1, 158.6, 150.1, 148.2, 146.4, 130.2, 129.5, 128.4, 119.5, 118.1, 114.3, 113.8, 109.2, 56.1, 56.1; Anal. Calcd for C15H14BrNO3, C = 53.59, H = 4.20, N = 4.17, Found C = 53.60, H = 4.21, N = 4.18; EI MS m/z (% rel. abund.): 337 (M+2 , 61), 335 (M+ , 64), 255 (30), 137 (100).
(E)-2-(((3,4-Dimethoxyphenyl)imino)methyl)-4-methoxyphenol (13). 1H-NMR): δ 10.91 (s, 1H, OH), 8.52 (s, 1H, N=CH-Ar), 7.22 (d, 1H, J = 2.0 Hz), 7.12 (d, 1H, J = 2.0 Hz), 7.03–6.97 (m, 3H), 6.90 (d, 1H, J = 8.0 Hz), 3.84 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 3.76 (s, 3H, OCH3); 13C-NMR: δ 160.1, 153.2, 153.1, 150.1, 148.2, 146.4, 119.5, 118.2, 118.0, 117.1, 114.2, 113.4, 109.2, 56.1, 56.1, 55.8; Anal. Calcd for C16H17NO4, C = 66.89, H = 5.96, N = 4.88, Found C = 66.91, H = 5.95, N = 4.91; EI MS m/z (% rel. abund.): 287 (M+, 100), 255 (19), 137 (80), 122 (25).
(E)-2-(((3,4-Dimethoxyphenyl)imino)methyl)-5-methoxyphenol (14). 1H-NMR): δ 13.84 (s, 1H, OH), 8.88 (s, 1H, N=CH-Ar), 7.51 (d, 1H, J = 7.5 Hz), 7.09 (d, 1H, J = 2.0 Hz), 7.01 (d, 1H, J = 8.0 Hz), 6.96 (dd, 1H, J = 8.0, J = 2.0 Hz), 6.57 (dd, 1H, J = 7.5, J = 2.0 Hz), 6.48 (dd, 1H, J = 2.0 Hz), 3.83 (s, 3H, OCH3), 3.81(s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 164.2, 162.0, 160.1, 1501, 148.2, 146.4, 133.2, 119.5, 114.2, 112.6, 109.1, 107.1, 103.3, 56.1, 56.1, 55.7; Anal. Calcd for C16H17NO4, C = 66.89, H = 5.96, N = 4.88, Found C = 66.90, H = 5.95, N = 4.92; EI MS m/z (% rel. abund.): 287 (M+, 100), 255 (30), 137 (85), 122 (19).
(E)-3-(((3,4-Dimethoxyphenyl)imino)methyl)phenol (15). 1H-NMR): δ 9.64 (s, 1H, OH), 8.57 (s, 1H, N=CH-Ar), 7.35 (d, 1H, J = 2.0 Hz), 7.31-7.30 (m, 2H), 7.31–7.30 (m, 2H), 6.98–6.97 (m, 1H), 6.92 (dd, 1H, J = 8.0, J = 2.0 Hz), 6.86 (dd, 1H, J = 7.5, J = 2.0 Hz), 3.82 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR (DMSO-d6): δ 160.1, 158.4, 150.1, 148.2, 146.4, 138.6, 130.1, 121.6, 119.5, 118.1, 114.8, 114.3, 109.2, 56.1, 56.1; Anal. Calcd for C15H15NO3, C = 70.02, H = 5.88, N = 5.44, Found C = 70.03, H = 5.91, N = 5.42; EI MS m/z (% rel. abund.): 257 (M+, 100), 225 (21), 137 (60), 93 (20).
(E)-N-(3,5-Dimethoxybenzylidene)-3,4-dimethoxyaniline (16). 1H-NMR): δ 8.60 (s, 1H, N=CH-Ar), 7.10 (d, 2H, J = 2.0 Hz), 7.00 (d, 1H, J = 2.0 Hz), 6.98 (d, 1H, J = 8.0 Hz), 6.96 (dd, 1H, J = 8.0, J = 2.0 Hz), 6.86 (t, 1H, J = 5.5 Hz), 3.82 (s, 9H, 3×OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 161.5, 161.5, 160.1, 150.1, 148.2, 146.4, 140.5, 119.5, 114.3, 109.2, 103.4, 103.4, 102.6, 56.1, 56.1, 55.8, 55.8; Anal. Calcd for C17H19NO4, C = 67.76, H = 6.36, N = 4.65, Found C = 67.77, H = 6.35, N = 4.66; EI MS m/z (% rel. abund.): 301 (M+, 40), 269 (45), 149 (21), 137 (100).
(E)-3,4-Dimethoxy-N-(4-methoxybenzylidene)aniline (17). 1H-NMR): δ 8.79 (s, 1H, N=CH-Ar), 8.10 (d, 2H, J = 8.5 Hz), 8.06 (d, 2H, J = 8.5 Hz),7.07 (d, 1H, J = 2.0 Hz), 7.02 (d, 1H, J = 8.0 Hz), 6.97 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.89 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3); 13C-NMR (DMSO-d6): δ 162.7, 160.1, 150.1, 148.3, 146.4, 130.1, 130.1, 128.5, 119.5, 114.3, 114.3, 114.2, 109.2, 56.1, 56.1; Anal. Calcd for C16H17NO3, C = 70.83, H = 6.32, N = 5.16, Found C = 70.84, H = 6.33, N = 5.15; EI MS m/z (% rel. abund.): 271 (M+, 26), 151 (11), 138 (17), 137 (100).
(E)-3,4-Dimethoxy-N-(3-methoxybenzylidene)aniline (18). 1H-NMR): δ 8.46 (s, 1H, N=CH-Ar), 7.46 (d, 1H, J = 8.0 Hz), 7.43 (d, 1H, J = 7.5 Hz),7.31-7.24 (m, 2H), 7.04 (d, 1H, J = 8.0 Hz), 6.98 (dd, 1H, J = 8.0, J = 2.0 Hz), 6.72 (d, 1H, J = 8.0 Hz), 3.84 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 3.80 (s, 3H, OCH3); 13C-NMR: δ 160.5, 160.1, 150.1, 148.3, 146.4, 138.1, 129.7, 121.4, 119.5, 116.4, 114.3, 111.3, 109.2, 56.1, 56.1; Anal. Calcd for C16H17NO3, C = 70.83, H = 6.32, N = 5.16, Found C = 70.82, H = 6.32, N = 5.15; EI MS m/z (% rel. abund.): 271 (M+, 100), 239 (20), 137 (60), 105 (24).
(E)-N-(2-Chlorobenzylidene)-3,4-dimethoxyaniline (19). 1H-NMR): δ 8.90 (s, 1H, N=CH-Ar), 8.16 (dd, 1H, J = 6.0, J = 2.0 Hz), 7.60 (dd, 1H, J = 6.0, J = 2.0 Hz), 7.56 (ddd, 1H, J = 6.0, J = 2.0, J = 2.0 Hz), 7.46 (t, 1H, J = 8.0 Hz), 7.02 (d, 1H, J = 7.5 Hz), 7.00 (s, 1H), 6.93 (dd, 1H, J = 6.0, J = 2.0 Hz), 3.84 (s, 3H, OCH3), 3.80 (s, 3H, OCH3); 13C-NMR: δ 157.1, 150.0, 148.2, 146.4, 133.8, 133.2, 132.2, 130.0, 127.1, 126.8, 119.5, 114.3, 109.2, 56.1, 56.1; Anal. Calcd for C15H14ClNO2, C = 65.34, H = 5.12, N = 5.08, Found C = 65.33, H = 5.13, N = 5.09; EI MS m/z (% rel. abund.): 277 (M+2, 31), 275 (M+, 100), 244 (15), 239 (17), 137 (40).
(E)-N-(4-Bromo-3-fluorobenzylidene)-3,4-dimethoxyaniline (20). 1H-NMR): δ 8.92 (s, 1H, N=CH-Ar), 7.90 (d, 1H, J = 8.0 Hz), 7.60 (dd, 1H, J = 7.0, J = 4.0 Hz), 7.20 (d, 1H, J = 8.0 Hz), 7.04 (d, 1H, J = 2.0 Hz), 6.98 (s, 1H), 6.93 (dd, 1H, J = 7.0, J = 2.0 Hz), 3.95 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3); 13C-NMR: δ 165.4, 160.1, 148.2, 150.1, 146.4, 134.2, 133.2, 124.0, 119.5, 116.1, 114.2, 112.3, 109.2, 56.1, 56.1; Anal. C15H13BrFNO2, C = 53.27, H = 3.87, N = 4.14, Found C = 53.29, H = 3.89, N = 4.15; EI MS m/z (% rel. abund.): 339 (M+2, 46), 337 (M+, 49), 257 (25), 137 (100).
(E)-Methyl 4-(((3,4-dimethoxyphenyl)imino)methyl)-3-nitrobenzoate (21). 1H-NMR): δ 8.92 (s, 1H, N=CH-Ar), 8.70 (d, 1H, J = 2.0 Hz), 8.25 (dd, 1H, J = 7.0, J = 2.0 Hz), 8.21 (d, 1H, J = 8.0 Hz), 7.06 (d, 1H, J = 2.0 Hz), 7.01 (s, 1H), 6.96 (dd, 1H, J = 7.0, J = 2.0 Hz), 3.95 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.80 (s, 3H, OCH3); 13C-NMR: δ 165.8, 160.2, 150.1, 148.3, 147.6, 146.4, 136.1, 132.6, 131.6, 130.1, 123.4, 119.5, 114.2, 109.2, 56.1, 56.1, 52.1; Anal. Calcd for C17H16N2O6, C = 59.30, H = 4.68, N = 8.14, Found C = 59.31, H = 4.69, N = 8.15; EI MS m/z (% rel. abund.): 344 (M+, 100), 297 (22), 284 (30), 137 (100).
(E)-3,4-Dimethoxy-N-(4-methylbenzylidene)aniline (22). 1H-NMR): δ 8.61 (s, 1H, N=CH-Ar), 7.82 (d, 2H, J = 8.0 Hz), 7.33 (d, 2H, J = 8.5 Hz), 6.98 (d, 1H, J = 2.0 Hz), 6.97 (d, 1H, J = 8.0 Hz), 6.87 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.81 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 2.38 (s, 3H, CH3); 13C-NMR: δ 160.1, 150.1, 148.2, 146.4, 140.5, 133.2, 129.2, 129.2, 129.0, 129.0, 119.6, 114.3, 109.2, 56.1, 56.1, 21.2; Anal. Calcd for C16H17NO2, C = 75.27, H = 6.71, N = 5.49, Found C = 75.29, H = 6.72, N = 5.50; EI MS m/z (% rel. abund.): 255 (M+, 100), 223 (18), 137 (80), 103 (28).
(E)-3,4-Dimethoxy-N-(pyridin-4-ylmethylene)aniline (23). 1H-NMR): δ 9.02 (s, 1H, N=CH-Ar), 8.61 (d, 2H, J = 8.0 Hz), 7.91 (d, 2H, J = 8.5 Hz), 7.03 (d, 1H, J = 2.0 Hz), 6.99 (d, 1H, J = 8.0 Hz), 6.91 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.84 (s, 3H, OCH3), 3.79 (s, 3H, OCH3); 13C-NMR: δ 160.1, 150.2, 149.2, 148.3, 149.2, 144.2, 142.1, 120.3, 120.3, 119.5, 114.2, 109.3, 56.1, 56.1; Anal. Calcd for C14H14N2O2, C = 69.41, H = 5.82, N = 11.56, Found C = 69.40, H = 5.83, N = 11.57; EI MS m/z (% rel. abund.): 242 (M+, 100), 210 (20), 137 (50), 91 (50).
(E)-N-(4-Fluorobenzylidene)-3,4-dimethoxyaniline (24). 1H-NMR): δ 8.67 (s, 1H, N=CH-Ar), 8.00 (dd, 2H, J = 8.5, J = 4.0 Hz), 7.91 (t, 2H, J = 8.5 Hz), 7.00 (d, 1H, J = 2.0 Hz), 6.98 (d, 1H, J = 8.0 Hz), 6.94 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.82 (s, 3H, OCH3), 3.78 (s, 3H, OCH3); 13C-NMR: δ 165.1, 160.1, 150.2, 148.3, 146.3, 132.1, 130.7, 130.7, 119.4, 115.5, 115.5, 114.3, 109.2, 56.1, 56.1; Anal. Calcd for C15H14FNO2, C = 69.49, H = 5.44, N = 5.40, Found C = 69.48, H = 5.43, N = 5.42; EI MS m/z (% rel. abund.): 259 (M+, 100), 227 (11), 137 (100), 95 (20).
(E)-3,4-Dimethoxy-N-(4-nitrobenzylidene)aniline (25). 1H-NMR): δ 8.88 (s, 1H, N=CH-Ar), 8.37 (d, 2H, J = 8.0 Hz), 8.18 (d, 2H, J = 8.5 Hz), 7.11 (d, 1H, J = 2.0 Hz), 7.03 (d, 1H, J = 8.0 Hz), 7.01 (dd, 1H, J = 8.0, J = 2.0 Hz), 3.84 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 2.38 (s, 3H, CH3); 13C-NMR: δ 160.1, 150.3, 150.1, 148.2, 146.4, 142.4, 127.6, 127.6, 124.1, 124.1, 119.5, 114.2, 109.2 Anal. Calcd for C15H14N2O4, C = 62.93, H = 4.93, N = 9.79, Found C = 62.94, H = 4.92, N = 9.80; EI MS m/z (% rel. abund.): 286 (M+, 100), 254 (17), 239 (20), 137 (100), 122 (16).
3.5. Theoretical Calculations
The optimization of the synthesized Schiff bases were performed at the B3LYP/6-311++G(d,p) level of theory [59]. The minima were confirmed by vibrational frequency analysis (i.e., no imaginary frequency were found). All theoretical calculations were carried out using Gaussian09 package [60].
4. Conclusions
In conclusion, compounds having hydroxyl groups at suitable places as well as number of hydroxyl groups play a key role in the antioxidant activity. Three crystal structures along with its theoretical calculations are also reported with experimental value well correlated.
Acknowledgments
Authors would like to acknowledge The Ministry of Education Malaysia and Universiti Teknologi MARA for the financial support under CIFI grant 600-RMI/DANA/5/3/CIFI (69/2013), and Ahmad Nazif Aziz would like to express his gratitude to Universiti Malaysia Terengganu for granting him study leave.
Author Contributions
All authors contributed equally to the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Khan K.M., Shah Z., Ahmad V.U., Khan M., Taha M., Rahim F., Jahun H., Perveen S., Choudhary M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011;7:572–580. doi: 10.2174/157340611797928415. [DOI] [PubMed] [Google Scholar]
- 2.Musharraf S.G., Bibi A., Shahid N., Najam-ul-Haq M., Khan M., Taha M., Mughal U.R., Khan K.M. Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am. J. Anal. Chem. 2012;3:779–789. doi: 10.4236/ajac.2012.312104. [DOI] [Google Scholar]
- 3.Khan K.M., Khan M., Ali M., Taha M., Rasheed S., Perveen S., Choudhary M.I. Synthesis of bis-Schiff bases of isatins and their antiglycation activity. Bioorg. Med. Chem. 2009;17:7795–7780. doi: 10.1016/j.bmc.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Khan K.M., Rahim F., Ambreen N., Taha M., Khan M., Jahan H., Najeebullah U., Shaikh A., Iqbal S., Perveen S., et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013;9:588–595. doi: 10.2174/1573406411309040013. [DOI] [PubMed] [Google Scholar]
- 5.Taha M., Naz H., Rasheed S., Ismail N.H., Rahman A.A., Yousuf S., Choudhary M.I. Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules. 2014;19:1286–1301. doi: 10.3390/molecules19011286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan K.M., Taha M., Rahim F., Fakhri M.I., Jamil W., Khan M., Rasheed S., Karim A., Perveen S., Choudhary M.I. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Pak. Chem. Soc. 2013;35:929–937. [Google Scholar]
- 7.Anouar E.H., Raweh S., Bayach I., Taha M., Baharudin M.S., Meo F.D., Hasan M.H., Adam A., Ismail N.H., Weber J.F., et al. Antioxidant properties of phenolic Schiff bases: Structure-activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013;27:951–964. doi: 10.1007/s10822-013-9692-0. [DOI] [PubMed] [Google Scholar]
- 8.Taha M., Ismail N.H., Jamil W., Yousuf S., Jaafar F.M., Ali M.I., Kashif S.M., Hussain E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules. 2013;18:10912–10929. doi: 10.3390/molecules180910912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan K.M., Shah Z., Ahmad V.U., Khan M., Taha M., Ali S., Perveen S., Choudhary M.I., Voelter W. 2,4,6-Trichlorophenylhydrazine Schiff bases as dpph radical and super oxide anion scavengers. Med. Chem. 2012;8:452–461. doi: 10.2174/1573406411208030452. [DOI] [PubMed] [Google Scholar]
- 10.Khan K.M., Taha M., Naz F., Ali S., Perveen S., Choudhary M.I. Synthesis of acylhydrazide Schiff bases and their anti-oxidant activity. Med. Chem. 2012;8:705–710. doi: 10.2174/157340612801216111. [DOI] [PubMed] [Google Scholar]
- 11.Taha M., Baharudin M.S., Ismail N.H., Khan K.M., Jaafar F.M., Samreen, Siddiqui S., Choudhary M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013;23:3463–3466. doi: 10.1016/j.bmcl.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Sundriyal S., Sharma R.K., Jain R. Current advances in antifungal targets and drug development. Curr. Med. Chem. 2006;13:1321–1335. doi: 10.2174/092986706776873023. [DOI] [PubMed] [Google Scholar]
- 13.Popp F.D., Kirsch W.J. Synthesis of potential anticancer agents. V. Schiff bases and related compounds. J. Org. Chem. 1961;26:3858–3860. doi: 10.1021/jo01068a056. [DOI] [Google Scholar]
- 14.Jain J.S., Srivastava R.S., Aggarwal N., Sinha R. Synthesis and evaluation of Schiff bases for anticonvulsant and behavioural depressant properties. Cent. Nerv. Syst. Agents Med. Chem. 2007;7:200–204. doi: 10.2174/187152407781669143. [DOI] [Google Scholar]
- 15.Chinnasamy R.P., Sundararagan R., Govindaraj S. Synthesis, characterization and analgesic activity of novel Schiff base isatin derivatives. Soc. Pharm. Edu. Res. 2010;1:342–347. doi: 10.4103/0110-5558.72428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A., Dewangan D., Verma S., Mishra A., Dubey R.D. Synthesis of Schiff bases of 2-amino-5-aryl-1, 3,4-thiadiazole and its analgesic, anti-inflammatory, antibacterial and antitubercular activity. Int. J. Chem. Tech. Res. 2011;3:178–184. [Google Scholar]
- 17.Mishra P., Gupta P.N., Shakya A.K., Shukla R., Srimal R.C. Anti-inflammatory and diuretic activity of a new class of compounds-Schiff bases of 3-amino-2-methylquinazolin 4(3H)-ones. Indian J. Physiol. Pharmacol. 1995;39:169–172. [PubMed] [Google Scholar]
- 18.Vicini P., Geronikaki A., Incerti M., Busonera B., Poni G., Cabras C.A., Colla P.L. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003;11:4785–4789. doi: 10.1016/S0968-0896(03)00493-0. [DOI] [PubMed] [Google Scholar]
- 19.Andreani A., Rambaldi M., Bonazzi D., Greci L., Andreani F. Study on compounds with potential antitumor activity. III. Hydrazone derivatives of 5-substituted 2-chloro-3-formyl-6-methylindole. Farmaco Sci. 1979;34:132–138. doi: 10.1002/chin.197924211. [DOI] [PubMed] [Google Scholar]
- 20.Gemi M.J., Biles C., Keiser B.J., Poppe S.M., Swaney S.M., Tarapley W.G., Romeso D.L., Yage Y. Novel 1,5-diphenylpyrazole nonnucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: Lead identification and SAR of 3- and 4-substituted derivatives. J. Med. Chem. 2000;43:1034–1040. doi: 10.1021/jm990383f. [DOI] [PubMed] [Google Scholar]
- 21.Satyanarayana V.S.V., Sivakumar A., Ghosh A.R. Synthesis, characterization of some new five membered heterocycles based on imidazole moiety and their applications on therapeutics. Lett. Drug. Des. Discov. 2011;8:276–283. doi: 10.2174/157018011794578196. [DOI] [Google Scholar]
- 22.Walcourt A., Loyevsky M., Lovejoy D.B., Gordeuk V.R., Richardson D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004;36:401–407. doi: 10.1016/S1357-2725(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 23.Gaur S. Physico-chemical and Biological properties of Mn(II), Co(II), Ni(II) and Cu(II) chelates of Schiff Bases. Asian J. Chem. 2003;15:250–254. [Google Scholar]
- 24.Nehru K., Athappan P., Rajagopal G. Ruthenium(II)/(III) complexes of bidentate acetyl hydrazide Schiff bases. Transition Met. Chem. 2001;26:652–656. doi: 10.1023/A:1012060428697. [DOI] [Google Scholar]
- 25.Wen X., Hua-Xin Z., Zhong.-Lin L., Cheng.-Yong S., Bei.-Sheng K. Classification, coordination and properties of acylhydrazone compounds. Zhongshan Daxue Xuebao. 2001;40:39–43. [Google Scholar]
- 26.Buu-Hoï Ng.Ph., Xuong Ng.D., Ham Ng.H., Binon F., Roger R. Tuberculostatic hydrazides and their derivatives. J. Chem. Soc. 1953 doi: 10.1039/JR9530001358. [DOI] [Google Scholar]
- 27.Ainscough E.W., Brodie A.M., Dobbs A.J., Ranford J.D., Waters J.M. Antitumour copper(II) salicylaldehyde benzoyhydrazone (H2sb) complexes. Inorg. Chim. Acta. 1998;267:27–38. doi: 10.1016/S0020-1693(97)05548-5. [DOI] [Google Scholar]
- 28.Zhang L., Tang N., Fang J.-G., Tan M.-Y. Synthesis, characterization and antioxidative activity of lanthanide complexes with 3,5-dibenz-yloxybenzoyl-2,4-dihydroxybenzaldehyde hydrazone. J. Chin. Rare Earth Soc. 2003;21:595–597. [Google Scholar]
- 29.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 30.Moure A., Cruz J., Franco D., Dominguez M., Sineiro J., Dominguez H., Nunez J. Natural antioxidants from residual sources, a review. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- 31.Hollman P.C., Hertog M.G., Katan M.B. Analysis and health effects of flavonoids. Food Chem. 1996;57:43–46. doi: 10.1016/0308-8146(96)00065-9. [DOI] [Google Scholar]
- 32.Schmidley J.W. Free radicals in central nervous system ischemia. Stroke. 1990;21:1086–1090. doi: 10.1161/01.str.21.7.1086. [DOI] [PubMed] [Google Scholar]
- 33.Meyer A.S., Heiononen M., Frankel E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998;61:71–75. doi: 10.1016/S0308-8146(97)00100-3. [DOI] [Google Scholar]
- 34.Hunt E.J., Lester C.E., Lester P.A., Tackett R.L. Effect of St. John's wort on free radical production. Life Sci. 2001;69:181–190. doi: 10.1016/S0024-3205(01)01102-X. [DOI] [PubMed] [Google Scholar]
- 35.Mayer J.M., Hrovat D.A., Thomas J.L., Borden W.T. Proton-Coupled Electron Transfer versus Hydrogen Atom Transfer in Benzyl/Toluene, Methoxyl/Methanol, and Phenoxyl/Phenol Self-Exchange Reactions. J. Am. Chem. Soc. 2002;124:11142–11147. doi: 10.1021/ja012732c. [DOI] [PubMed] [Google Scholar]
- 36.Hammes-Schiffer S. Proton-coupled electron transfer: Classification scheme and guide to theoretical methods. Energy Environ. Sci. 2012;5:7696–7703. doi: 10.1039/c2ee03361e. [DOI] [Google Scholar]
- 37.Hammes-Schiffer S. Theoretical perspectives on proton-coupled electron transfer reactions. Acc. Chem. Res. 2001;34:273–281. doi: 10.1021/ar9901117. [DOI] [PubMed] [Google Scholar]
- 38.Anouar E.H., Shah S.A.A., Hassan N.B., Moussaoui N.E., Ahmad R., Zulkefeli M., Weber J.-F.F. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules. 2014;19:3489–3507. doi: 10.3390/molecules19033489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Meo F., Lemaur V., Cornil J.R.M., Lazzaroni R., Duroux J.-L., Olivier Y., Trouillas P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A. 2013;117:2082–2092. doi: 10.1021/jp3116319. [DOI] [PubMed] [Google Scholar]
- 40.Musialik M., Litwinienko G. Scavenging of dpph* radicals by vitamin E is accelerated by its partial ionization: The role of sequential proton loss electron transfer. Org. Lett. 2005;7:4951–4954. doi: 10.1021/ol051962j. [DOI] [PubMed] [Google Scholar]
- 41.Khan K.M., Naz F., Taha M., Khan A., Perveen S., Choudhary M.I., Voelter W. Synthesis and in vitro urease inhibitory activity of N,N'-disubsituted thioureas. Eur. J. Med. Chem. 2014;74:314–323. doi: 10.1016/j.ejmech.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Khan K.M., Jamil W., Ambreen N., Taha M., Perveen S., Morales G.A. An Expeditious synthetic approach towards the synthesis of bis-Schiff bases (aldazines) using ultrasound. Ultrason. Sonochem. 2014;21:1200–1205. doi: 10.1016/j.ultsonch.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Khan K.M., Khan M., Ambreen N., Taha M., Rahim F., Rasheed S., Saied S., Shafi H., Perveen S., Choudhary M.I. Oxindole derivatives: Synthesis and antiglycation activity. Med. Chem. 2013;9:681–688. doi: 10.2174/1573406411309050007. [DOI] [PubMed] [Google Scholar]
- 44.Taha M., Ismail N.H., Jaafar F.M., Aziz A.N., Yousuf S. (E)-N′-(3,4-Dihydroxybenzylidene)-2,4-dimethylbenzohydrazide monohydrate. Acta Cryst. 2013;E69:o490. doi: 10.1107/S1600536813005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taha M., Baharudin M.S., Ismail N.H., Shah S.A.A., Yousuf S. (E)-2-Methoxy-N′-(2,4,6-trihydroxybenzylidene)Benzohydrazide. Acta Cryst. 2013;E69:o277. doi: 10.1107/S1600536813001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taha M., Ismail N.H., Jaafar F.M., Khan K.M., Yousuf S. (E)-2,4-Dimethyl-N′-(2-methylbenzylidene) benzohydrazide. Acta Cryst. 2013;E69:o400. doi: 10.1107/S1600536813004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csaszar J. Spectral studies of molecular complexes of aromatic Schiff bases with picric acid. Acta Chim. Hung. 1990;127:277–286. [Google Scholar]
- 48.Csaszar J., Balog J. Spectra of aromatic Schiff bases. Acta Chim. Hung. 1975;86:100–116. [Google Scholar]
- 49.Akkurt M., Jarrahpour A., Aye M., Gencaslan M., Buyuekgungor O. 3,4-Dimethoxy-N-(4-nitrobenzylidene)aniline. Acta Cryst. E. 2008;64:o2087. doi: 10.1107/S1600536808032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H., Carter R.G. Asymmetric construction of nitrogen-containing [2.2.2] Bicyclic Scaffolds Using N-(p-Dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide. J. Org. Chem. 2009;74:5151–5156. doi: 10.1021/jo9009062. [DOI] [PubMed] [Google Scholar]
- 51.Nanjo F., Goto K., Seto R., Suzuki M., Sakai M., Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 52.Thangapandiyan S., Miltonprabu S. An in vivo and in vitro studies on the antioxidant property of epigallocatechin gallate on sodium fluoride induced toxicity in rats. Int. J. Phytopharmacol. 2013;4:245–254. [Google Scholar]
- 53.Barontini M., Bernini R., Crisante F., Fabrizi G. Selective and efficient oxidative modifications of flavonoids with 2-iodoxybenzoic acid (IBX) Tetrahedron. 2010;66:6047–6053. doi: 10.1016/j.tet.2010.06.014. [DOI] [Google Scholar]
- 54.Bernini R., Fabrizi G., Pouysegu L., Deffieux D., Quideau S. Synthesis of biologically active catecholic compounds via ortho-selective oxygenation of phenolic compounds using hypervalent iodine(V) reagents. Curr. Org. Synth. 2012;9:650–669. doi: 10.2174/157017912803251792. [DOI] [Google Scholar]
- 55.Bernini R., Crisante F., Fabrizi G., Gentili P. Convenient synthesis of 1-aryl-dihydroxyisochromans exhibiting antioxidant activity. Curr. Org. Chem. 2012;16:1051–1057. doi: 10.2174/138527212800194700. [DOI] [Google Scholar]
- 56.Barontini M., Bernini R., Carastro R., Gentili P., Romani A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014;38:809–816. doi: 10.1039/c3nj01180a. [DOI] [Google Scholar]
- 57.Perez-Gonzalez A., Galano A., Alvarez-Idaboy J.R. Dihydroxybenzoic acids as free radical scavengers: Mechanisms, kinetics, and trends in activity. New J. Chem. 2014;38:2639–2652. doi: 10.1039/c4nj00071d. [DOI] [Google Scholar]
- 58.Marković Z., Đorović J., Dimitrić Marković J., Živić M., Amić D. Investigation of the radical scavenging potency of hydroxybenzoic acids and their carboxylate anions. Monatsh. Chem. Chem. Mon. 2014;145:953–962. doi: 10.1007/s00706-014-1163-3. [DOI] [Google Scholar]
- 59.Becke A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- 60.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2009. Revision A.02. [Google Scholar]