Abstract
A novel dimer of piceatannol glycoside, named rheumaustralin (1) was isolated from the underground parts of the ethnomedicinal plant Rheum austral (Polygonaceae) collected from Tibet together with 17 known compounds, including rheumin (2), 2,5-dimethyl-7-hydroxychromone (3), 2,5-dimethylchromone-7-O-β-d-glucopyranoside (4), 7-hydroxy-2-(2'-hydroxypropyl)-5-methylchromone (5), torachrysone (6) torachrysone-8-O-β-d-glucopyranoside (7), 4-(4'-hydroxyphenyl)-2-butanone-4'-O-β-d-glucopyranoside (8), amabiloside (9), N-trans-feruloyl tyramine (10), chrysophanol (11), aloe-emodin (12), emodin (13), physcion (14), physcion-1-O-β-d-glucopyranoside (15), emodin-8-O-β-d-glucopyranoside (16), d-catechin (17) and gallic acid (18). Their structures were determined by combined spectroscopic methods and by comparison of their spectral data with those reported in literature. Compounds 1–10 were tested for their ability to scavenge 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical.
Keywords: Rheum austral D. Don, piceatannol dimer, rheumaustralin, DPPH radical
1. Introduction
Rheum australe D. Don (syn. Rheum emodi Wall. ex Meissn., Polygonaceae) is a robust, perennial herb with stout rhizomes. The distribution of this plant is confined to the Himalayan region, covering the areas of India (Kashmir and Sikkim), Bhutan, Nepal, Pakistan, Myanmar, and China [1]. The roots of R. australe are widely used in Ayurvedic and Chinese folk medicine as a purgative, stomachic, astringent and tonic and for piles, chronic bronchitis and asthma, as well as in certain skin diseases. R. australe plants produce diverse phenolic metabolites. More than 56 compounds, belonging to anthraquinones, stilbenes, anthrones, oxanthrone ethers and esters, chromones, flavonoids, carbohydrates, lignans, phenols, and sterols have been identified or characterized from the roots and rhizomes of this plant collected from Nepal, India, Czech Republic (cultivated), and China [2,3,4]. Previous investigations on the constituents of R. australe collected from Tibet, locally known as “Zang Bian Dahuang”, led to the isolation of a series of piceatannol glycosides and anthraquinones as its major components [5,6]. Some of these compounds demonstrated a wide range of biological and pharmacological properties such as antioxidant [7,8], antifungal [9], cytotoxic [10], hypoglycemic [11], anti-tuberculosis [12], neuron protective [13] and antiviral [14] activities. As a part of a program to study the antioxidant secondary metabolits of Rheum plants from the Qinghai-Tibetan region of China [15], a novel dimer of piceatannol-4'-O-β-d-glucopyranoside, named rheumaustralin (1), was obtained from the underground parts of this ethnomedicinal plant, together with 17 phenolic compounds including pyranones, naphthalenes, chromones, anthraquinones, flavonoids, phenolic amides and some simple aromatic compounds. Herein, we describe the isolation, structural elucidation of these compounds, as well as the DPPH free radical scavenging activities of compounds 1–10.
2. Results and Discussion
2.1. Structural Elucidation of the New Compound
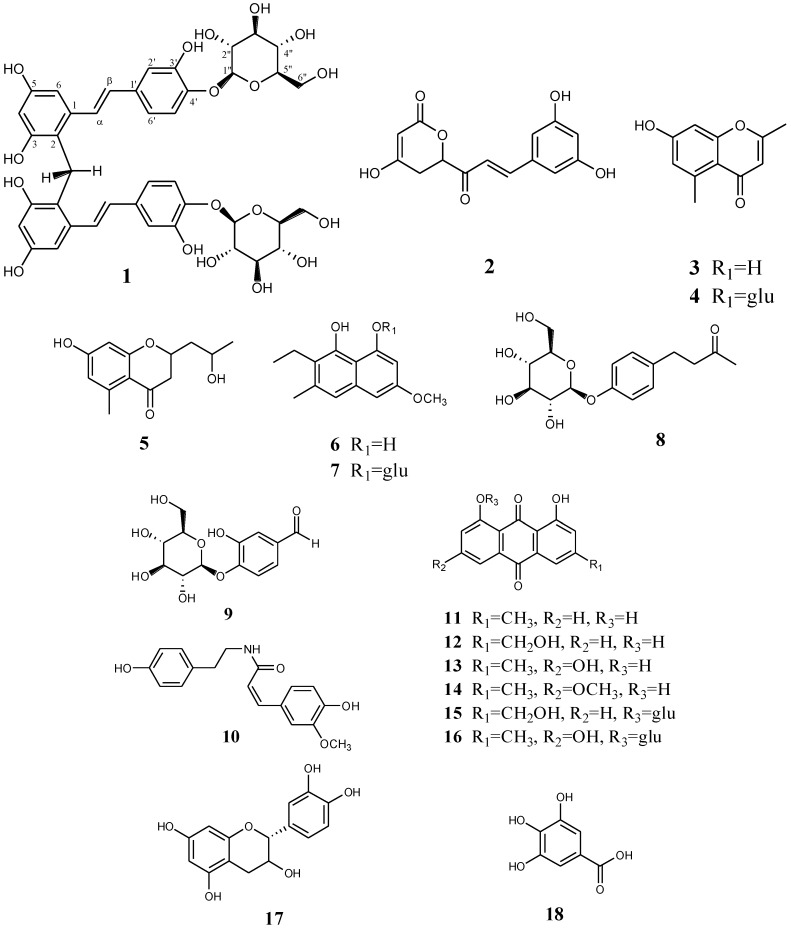
The isolated compounds were identified by different spectroscopic analyses, including the extensive use of HR-ESI-MS, 1D (1H and 13C) and 2D-NMR techniques (HSQC, HMBC), and by comparing the experimental NMR data to values reported in the literature. The structures of the isolated compounds are shown in Figure 1.
Figure 1.
Chemical structures of compounds 1–18.
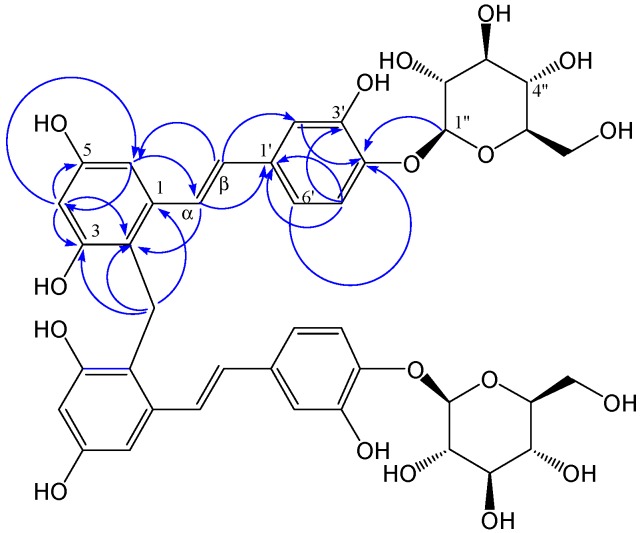
Compound 1 was obtained as a yellow powder with an optical rotation value 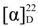 +50.3030 (c = 0.22, MeOH). The molecular formula of compound 1, C41H44O18, was deduced from the quasimolecular ion peak at m/z 823.2437 [M]− (calcd. for C41H43O18, 823.2449) in the negative HR-ESI-MS, indicating 20 double bond equivalents. The IR (KBr) spectrum showed characteristic absorption bands for hydroxyl groups (3,440 cm−1), methylene groups (2,923 and 1,443 cm−1), aromatic rings (1,514 cm−1) and olefinic groups (1,630 and 986 cm−1). The 1H-NMR spectrum (Table 1) of compound 1 showed two sets of signals. The former set of signals, between δ = 6.0 and 7.5 ppm, was assigned to the protons of a trans-olefinic group (δ = 7.22 and 6.60 ppm, d, J = 16.0 Hz), two aromatic rings with 1,3,5-trisubstituted (δ = 7.06 ppm, d, J = 8.4 Hz; 6.90, d, J = 1.9 Hz and 6.82, dd, J = 8.4, 1.9 Hz) and 1,2,3,4-tetrasubstituted (δ = 6.51 ppm, d, J = 2.3 Hz; 6.28 ppm, d, J = 2.3 Hz) systems. The HMBC (Figure 2) correlations between δH 6.51 (H-6) with δC 120.2 (C-2), 102.8 (C-4), 156.6 (C-5) and 128.1 (C-α); δH7.22 (H-α) with δC 140.1 (C-1), 120.2 (C-2), 105.0 (C-6), 135.4 (C-1') and 129.4 (C-β); δH 6.60 (Η-β) with δC 140.1 (C-1), 115.0 (C-2'), 120.0 (C-6'), 135.4 (C-1') and 128.1 (C-α); indicated the presence of a stilbene skeleton. The latter set of signals, between δ = 3.0 and 5.0 ppm, was assigned to the glycosyl protons and the methylene protons (δ = 4.11 ppm, s), consistent with the 13C-NMR spectrum along with the DEPT spectra of compound 1 (Table 1), which showed six signals characteristic of a glucosyl group (δ = 104.3, 74.9, 77.5, 71.3, 78.3 and 62.4 ppm) and a methylene carbon (δ = 21.3 ppm). The sugar residue was identified as a d-glucopyranosyl unit by gas chromatography of the hydrolyzed product. The mode of the glucosyl linkage was determined to be β from the coupling constant value (d, J = 7 Hz) of the anomeric proton signal. The location of the glucosyl group is suggested to be C-4' by HMBC, which displayed a correlation from δH 4.75 (H-1'') to δC 146.1 (C-4'). These moieties account for ten degrees of unsaturation, only half of those in the molecular formula of compound 1. This result indicated that the structure of compound 1 was symmetrical. In summary, detailed analysis of 1D and 2D-NMR spectra suggested that the structural features of the symmetrical moieties of 1 was very similar to those of piceatannol-4'-O-β-d-glucopyranoside, which was previously isolated from R.
australe as the main component, except for the presence of a CH2 group at position 2 (δ = 120.2 ppm). The connection of the symmetrical units was established by the HMBC experiment (Table 1), clearly indicating the correlation peaks from the methylene protons (δ = 4.11 ppm, s) to C-1 (δ = 140.1 ppm), C-2 (δ = 120.2 ppm) and C-3 (δ = 156.4 ppm), suggested that the connection group is the methylene at C-2. Therefore, the structure of compound 1 was established as shown in Figure 1. The 1H-NMR and 13C-NMR (100 MHz) spectral assignments performed by extensive 2D-NMR experiments (HSQC and HMBC) are summarized in Figure 2 and Table 1.
+50.3030 (c = 0.22, MeOH). The molecular formula of compound 1, C41H44O18, was deduced from the quasimolecular ion peak at m/z 823.2437 [M]− (calcd. for C41H43O18, 823.2449) in the negative HR-ESI-MS, indicating 20 double bond equivalents. The IR (KBr) spectrum showed characteristic absorption bands for hydroxyl groups (3,440 cm−1), methylene groups (2,923 and 1,443 cm−1), aromatic rings (1,514 cm−1) and olefinic groups (1,630 and 986 cm−1). The 1H-NMR spectrum (Table 1) of compound 1 showed two sets of signals. The former set of signals, between δ = 6.0 and 7.5 ppm, was assigned to the protons of a trans-olefinic group (δ = 7.22 and 6.60 ppm, d, J = 16.0 Hz), two aromatic rings with 1,3,5-trisubstituted (δ = 7.06 ppm, d, J = 8.4 Hz; 6.90, d, J = 1.9 Hz and 6.82, dd, J = 8.4, 1.9 Hz) and 1,2,3,4-tetrasubstituted (δ = 6.51 ppm, d, J = 2.3 Hz; 6.28 ppm, d, J = 2.3 Hz) systems. The HMBC (Figure 2) correlations between δH 6.51 (H-6) with δC 120.2 (C-2), 102.8 (C-4), 156.6 (C-5) and 128.1 (C-α); δH7.22 (H-α) with δC 140.1 (C-1), 120.2 (C-2), 105.0 (C-6), 135.4 (C-1') and 129.4 (C-β); δH 6.60 (Η-β) with δC 140.1 (C-1), 115.0 (C-2'), 120.0 (C-6'), 135.4 (C-1') and 128.1 (C-α); indicated the presence of a stilbene skeleton. The latter set of signals, between δ = 3.0 and 5.0 ppm, was assigned to the glycosyl protons and the methylene protons (δ = 4.11 ppm, s), consistent with the 13C-NMR spectrum along with the DEPT spectra of compound 1 (Table 1), which showed six signals characteristic of a glucosyl group (δ = 104.3, 74.9, 77.5, 71.3, 78.3 and 62.4 ppm) and a methylene carbon (δ = 21.3 ppm). The sugar residue was identified as a d-glucopyranosyl unit by gas chromatography of the hydrolyzed product. The mode of the glucosyl linkage was determined to be β from the coupling constant value (d, J = 7 Hz) of the anomeric proton signal. The location of the glucosyl group is suggested to be C-4' by HMBC, which displayed a correlation from δH 4.75 (H-1'') to δC 146.1 (C-4'). These moieties account for ten degrees of unsaturation, only half of those in the molecular formula of compound 1. This result indicated that the structure of compound 1 was symmetrical. In summary, detailed analysis of 1D and 2D-NMR spectra suggested that the structural features of the symmetrical moieties of 1 was very similar to those of piceatannol-4'-O-β-d-glucopyranoside, which was previously isolated from R.
australe as the main component, except for the presence of a CH2 group at position 2 (δ = 120.2 ppm). The connection of the symmetrical units was established by the HMBC experiment (Table 1), clearly indicating the correlation peaks from the methylene protons (δ = 4.11 ppm, s) to C-1 (δ = 140.1 ppm), C-2 (δ = 120.2 ppm) and C-3 (δ = 156.4 ppm), suggested that the connection group is the methylene at C-2. Therefore, the structure of compound 1 was established as shown in Figure 1. The 1H-NMR and 13C-NMR (100 MHz) spectral assignments performed by extensive 2D-NMR experiments (HSQC and HMBC) are summarized in Figure 2 and Table 1.
Table 1.
1H, 13C-NMR and HMBC (500M Hz) data of 1 (CD3OD, δ in ppm).
| Position | δH (Mult., J in Hz) | δC | DEPT | HMBC (Selected) |
|---|---|---|---|---|
| 1 | 140.1 | C | ||
| 2 | 120.2 | C | ||
| 3 | 156.4 | C | ||
| 4 | 6.28 (d, 2.3) | 102.8 | CH | C-2, 3, 5, 6 |
| 5 | 156.6 | C | ||
| 6 | 6.51 (d, 2.4) | 105.0 | CH | C-2, 4, 5 |
| 1' | 135.4 | C | ||
| 2' | 6.90 (d, 1.9) | 115.0 | CH | C-3', 4', 6' |
| 3' | 147.9 | C | ||
| 4' | 146.1 | C | ||
| 5' | 7.06 (d, 8.4) | 118.5 | CH | C-1', 3', 4' |
| 6' | 6.82 (dd, 8.5, 1.9) | 120.0 | CH | C-2′, 4′ |
| α | 7.22 (d, 16.0) | 128.1 | CH | C-1, 2, 6, 1' |
| β | 6.60 (d, 16.0) | 129.4 | CH | C-1, 2', 6', 1' |
| 1'' | 4.75 (d, 7.6) | 104.3 | CH | C-4' |
| 2'' | 3.49 (m) | 74.9 | CH2 | |
| 3'' | 3.49(m) | 77.5 | CH2 | |
| 4'' | 3.42 (m) | 71.3 | CH2 | |
| 5'' | 3.42 (m) | 78.3 | CH2 | |
| 6'' | 3.91 (brd) | 62.4 | CH2 | C-4'', 5'' |
| 3.74 (dd, 11.4, 4.6) | ||||
| CH2 | 4.11 (s) | 21.3 | CH2 | C-1, 2, 3 |
Figure 2.
Main HMBC (indicated by blue arrows from 1H to 13C) of compound 1.
2.2. Antioxidant Activities by DPPH Scavenging Capacities
Aqueous and methanolic extracts of R. australe were reported to exhibit prominsing antioxidant activities in a previous study [16]. The most abundant stilbenoid of R. austral, piceatannol-4'-O-β-d-glucopyranoside, was suggested to be an important constituent responsible for the antioxidant potential of the extracts of plant materials collected from Tibet [7]. In order to evaluate the potentials of other types of phenolic constituents, compounds 1–10 were screened for their antioxidant activities by the DPPH free radical-scavenging assay that has been widely used for the evaluation of antioxidant activities of natural products. The results obtained in this study are summarized in Table 2. Among these compounds, rheumaustralin (1) displayed relatively strong antioxidant activity with an IC50 value of 2.3 µM, lower than piceatannol (IC50 = 0.14 μmol/L), and higher than resveratrol (IC50 = 15.6 μmol/L). This result was consistent with the concept that the antioxidant activity of stilbenoids depends on the position of the hydroxyl groups. The existence of para-hydroxyl groups significantly enhance antioxidant activity [17]. The glycosylation of hydroxyl groups, however, may decrease the antioxidant activity of stibenoid.
Table 2.
Radical scavenging activities of the compounds 1–10.
| Compounds | DPPH Radical IC50 (μM) a |
|---|---|
| 1 | 2.3 ± 0.5 |
| 2 | 31.7 ± 1.1 |
| 3 | 25.7 ± 0.7 |
| 4 | 66.9 ± 1.3 |
| 5 | 21.7 ± 1.1 |
| 6 | 32.1 ± 1.5 |
| 7 | 56.4 ± 0.9 |
| 8 | 109.7 ± 2.1 |
| 9 | 69.7 ± 1.5 |
| 10 | 23.4 ± 0.8 |
| resveratrol b | 15.6 ± 0.7 |
| piceatannol b | 0.14 ± 0.05 |
| ascorbic acid b | 19.7 ± 0.8 |
| BHA b | 18.7 ± 0.9 |
| α-tocopherol b | 25.1 ± 1.1 |
a IC50 values were expressed as means ± standard deviation of three independent replicates; b Positive control substance.
Compounds 2, 3, 5, 6 and 10 exhibited promising antioxidant activities with IC50 values in the range of 20 to 35 µM, which was comparable to those of BHA, ascorbic acid and α-tocopherol; while 4, 7 and 9 showed moderate activities with IC50 values in the range of 50 to 70 µM. Compound 8 showed lowest activity with an IC50 value of 109.7 µM.
3. Experimental
3.1. General
The 1H-, 13C-, and 2D-NMR spectra were recorded on Bruker DRX-500 (500 MHz) spectrometer with TMS as internal standard. The ESI-MS and HR-ESI-MS spectra were recorded on VG AutoSpe 3000 and API Qstar P ulsar LC/TOF spectrometers, respectively. The UV spectra were measured by using a Shimadzu double-beam 210A spectrophotometer. The IR spectra were recorded on a Bio-Rad FTS-135 spectrometer, in KBr pellets. The optical rotations were measured by using a SEPA-3000 automatic digital polarimeter. The column chromatographic separations were performed on silica gel (200–300 mesh size; Qingdao Marine Chemical Inc., Qingdao, China), or Lichroprep RP-18 gel (40–63 µm mesh size; Merck, Darmstadt, Germany). The column fractions obtained were monitored by TLC, and spots were visualized by heating the silica gel plates after spraying with 15% H2SO4 in water. The TLC and PTLC separations were performed on silica gel Gf 254 pre-coated plates (Qingdao Marine Chemical Inc.). 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), hexamethyldisilazane and trimethyl-chlorosilane were purchased from TCI (Shanghai, China), Piceatannol and resveratrol were isolated from R. australe D. Don [18].
3.2. Plant Materials
The underground parts of R. australe were collected in August 2010 from Doilungdêqên County, Lhasa, China, and authenticated by Prof. Zheng-Dong Fang of Shangri-La Alpine Botanic Garden (Yunnan, China) and re-identified by co-author Dr. Qing-Song Yang. A voucher specimen (No. 2010080401) was deposited in the School of Chemistry and Biotechnology, Yunnan University of Nationalities, Yunnan, China.
3.3. Extraction and Isolation of the Compounds
The air-dried powder of the underground parts (1.2 kg) of R. australe were extracted exhaustively with 75% aqueous EtOH (25 L) at room temperature for 5 times. The EtOH extract was concentrated in vacuo to yield a semi-solid (338 g), which was suspended in water (1000 mL), and extracted successively with petroleum ether (3 × 1000 mL) and EtOAc (3 × 1000 mL). The EtOAc organic phase was concentrated to yield a residue (225 g), which was loaded on a silica gel (SiO2) column (4 kg) and eluted with CHCl3/MeOH gradient to give four fractions (1–4). Fraction 1 eluted with CHCl3 was partly subjected to repeated column chromatography (CC; SiO2; CHCl3/MeOH, 15:1) to afford 11 (125 mg), 12 (295 mg), 13 (255 mg) and 14 (235 mg). Fraction 2 eluted with CHCl3/MeOH (10:1) was subjected to repeated CC (SiO2; PE/acetone, 8:2) to afford 3 (25 mg), 5 (17 mg) 6 (155 mg) and 17 (16 mg). Fraction 3 eluted with CHCl3/MeOH (8:2) was subjected to repeated CC (SiO2; CHCl3/MeOH, 10:1–8:2) to afford 2 (995 mg), 10 (8 mg), 15 (595 mg) and 16 (418 mg). Fraction 4 eluted with CHCl3/MeOH (6:4) was subjected to repeated CC (SiO2; CHCl3/MeOH, 10:1–8:2) to afford three sub-fractions (F4a, F4b and F4c). Sub-fraction F4b was partly separated by PTLC (CHCl3/MeOH, 9:1) to afford 4 (27 mg) and 7 (15 mg). Sub-fraction F4c was subjected to repeated CC (SiO2; CHCl3/MeOH, 10:1–5:1) to afford 8 (30 mg) and a mixture containing 8 and 9. This mixture was subjected to repeated CC on RP18 gel eluted by MeOH/water (58:42) to afford 8 (10 mg) and 9 (12 mg). The remaining aqueous phase was subjected to CC on D101 macroporous resin and eluted with 30% MeOH to afford four main fractions (A–D). Fraction B (18 g) was subjected to CC on Sephadex LH-20 using MeOH as the elution solvent to afford five sub-fractions (B1–B5). Sub-fractions B3 and B4 was further separated by repeated CC on RP C-18 and eluted by 25% MeOH to afford compound 1 (627 mg) and 18 (21 mg).
3.4. Acid Hydrolysis of Compound 1
A solution of compound 1 (7 mg) in 5% aqueous sulfuric acid (2 mL) was heated in a water bath (80 °C) for 4 h. The solution, after cooling, was diluted with H2O (2 mL), neutralized with 5% NaOH solution and then extracted with EtOAc (5 × 3 mL). The aqueous layer was concentrated under a stream of nitrogen. The residue was then dissolved in anhydrous pyridine (0.8 mL), followed by the addition of trimethysilylation reagent hexamethyldisilazane/trimethylchlorosilane/pyridine (HMDS/TMCS/pyridine, 3:1:8). It was then stirred at 60 °C for an additional 30 min. The solution was analyzed by GC for sugar identification. GC analyses were performed using an Agilent 5890 instrument on an Agilent HP-1 column (0.25 mm, 30 m, i.d., 0.25 µm). Temperatures of both the injector and detector were 200 °C. A temperature gradient system was used for the oven, starting at 150 °C and increasing up to 250 °C at a rate of 8 °C/min. D-Glucose was confirmed by comparison with the retention time of an authentic standard.
3.5. Spectroscopic Data
Rheumaustralin (1). Yellow powder; 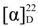 = + 50.3030ο (c = 0.22, MeOH); IR (KBr) νmax 3440, 2922, 1630, 1514, 1443, 1349, 1272, 1090, 986, 803 cm−1; UV (MeOH) λmax (log ε) 223 (4.3), 325 (4.1) nm; positive ESI-MS [M+Na]+ at m/z 847; negative HR-ESI-MS [M−H]− at m/z 823.2437 (calcd for C41H43O18 823.2449); 1H- and 13C-NMR data (Table 1).
= + 50.3030ο (c = 0.22, MeOH); IR (KBr) νmax 3440, 2922, 1630, 1514, 1443, 1349, 1272, 1090, 986, 803 cm−1; UV (MeOH) λmax (log ε) 223 (4.3), 325 (4.1) nm; positive ESI-MS [M+Na]+ at m/z 847; negative HR-ESI-MS [M−H]− at m/z 823.2437 (calcd for C41H43O18 823.2449); 1H- and 13C-NMR data (Table 1).
Rheumin (2). Yellow powder; EI-MS [M]+ at m/z 276; 1H-NMR (400 MHz, DMSO-d6) δ = 7.04 ppm (1H, d, J = 16.6 Hz, H-9), 6.92 (1H, d, J = 16.6 Hz, H-8), 6.46 (2H, d, J = 2.0 Hz, H-2', 6'), 6.24 (1H, d, J = 2.0 Hz, H-4'), 6.19 (1H, s), 5.63 (1H, d, J = 9.1 Hz, H-3), 2.72 (1H, dd, J = 2.5, 16.2 Hz, H-4), 2.18 (1H, dd, J = 9.3, 16.2 Hz, H-4); 13C-NMR (100 MHz, DMSO-d6) δ = 174.5 ppm (C-7), 172.7 (C-2), 158.8 (C-3', 5'), 138.9 (C-9), 137.1 (C-1'), 117.9 (C-8), 113.8 (C-6), 105.7 (C-2', 6'), 104.3 (C-4'), 80.6 (C-3) and 41.9 (C-4). These data are in accordance with those reported in the literature [19].
2,5-Dimethyl-7-hydroxychromone (3). Yellow needles, 1H-NMR (400 MHz, DMSO-d6) δ = 10.51 ppm (Ar-OH), 6.58 (1H, brs, H-8), 6.52 (1H, brs, H-6), 5.96 (1H, s, H-3), 2.59 (3H, s, 5-CH3), 2.25 (3H, s, 2-CH3); 13C-NMR (100 MHz, DMSO-d6) δ = 187.0 ppm (C-4), 165.7 (C-2), 163.7 (C-7), 159.2 (C-9), 141.0 (C-5), 118.5 (C-10), 111.1 (C-6), 101.7 (C-8), 98.5 (C-3), 22.9 (2-CH3), 14.8 (5-CH3). These data are in accordance with those reported in the literature [20].
2,5-Dimethylchromone-7-O-β-D-glucopyranoside (4). White powder, positive ESI-MS [M+Na]+ at m/z: 375; 1H-NMR (400 MHz, DMSO) δ = 6.85 ppm (1H, brs, H-8), 6.70 (1H, brs, H-6), 6.07 (1H, s, H-3), 4.63 (1H, s, anomeric H), 3.79–3.66 (2H, d, J = 10.4 Hz; H-6'), 3.32–3.13 (4H, t, J = 8.5 Hz; H-2'–H-5'), 2.73–2.67 (3H, d, J = 1.4 Hz, 5-CH3), 2.35–2.27 (3H, d, J = 1.6 Hz, 2-CH3); 13C-NMR (100 MHz, CDCl3) δ = 178.8 ppm (C-4), 164.9 (C-2), 160.4 (C-7), 159.3 (C-9), 141.8 (C-5), 117.1 (C-10), 116.6 (C-6), 111.5 (C-8), 101.9 (C-3), 100.3 (C-1'), 77.6 (C-2'), 76.9 (C-5'), 73.6 (C-3'), 70.1 (C-4'), 61.0 (C-6'), 22.8 (2-CH3), 19.9 (5-CH3). These data are in accordance with those reported in the literature [21].
7-Hydroxy-2-(2'-hydroxypropyl)-5-methylchromone (5). Yellow powder, positive ESI-MS [M+H]+ at m/z: 233; 1H-NMR (400 MHz, CDCl3) δ ppm 6.56 (1H, d, J = 2.0 Hz, H-8), 6.53 (1H, d, J = 2.0 Hz, H-6), 5.96 (1H, s, H-3), 4.10 (1H, m, H-2'), 2.62 (1H, dd, J = 5.1, 14.5 Hz, H-1'a), 2.62 (3H, s, 5-CH3), 2.55 (1H, dd, J = 8.0, 14.5 Hz, H-1'b), 1.18 (3H, d, J = 6.2 Hz, C-3'); 13C-NMR (100 MHz, CDCl3) δ = 182.0 ppm (C-4), 167.1 (C-9), 163.2 (C-7), 161.5 (C-2), 143.6 (C-5), 118.1 (C-3), 115.7 (C-10), 112.5 (C-6), 101.7 (C-8), 66.3 (C-2'), 44.2 (C-1'), 23.5 (C-3'), 23.2 (5-CH3). These data are in accordance with those reported in the literature [22].
Torachrysone (6). Yellow powder; positive FAB-MS [M+H]+ at m/z 247; 1H-NMR (500 MHz, CDCl3) δ = 7.09 ppm (1H, s, H-4), 6.83 (1H, d, J = 2.4 Hz, H-7), 6.78 (1H, d, J = 2.4 Hz, H-5), 3.78 (3H, s, OCH3), 2.38 (3H, s, COCH3), 2.17 (3H, s, CH3); 13C-NMR (125 MHz, CDCl3) δ = 204.7 ppm (COCH3), 158.1 (C-8), 154.7 (C-6), 150.8 (C-1), 134.9 (C-3), 133.8 (C-9), 122.6 (C-2), 118.1 (C-4), 107.9 (C-10), 102.9 (C-7), 102.5 (C-5), 55.8 (6-OCH3), 32.5 (COCH3), 19.8 (3-CH3). These data are in accordance with those reported in the literature [23].
Torachrysone-8-O-β-D-glucopyranoside (7). Yellow powder; positive FAB-MS [M+H]+ at m/z 409; 1H-NMR (500 MHz, DMSO-d6) δ = 9.47 ppm (1H, s, 1-OH), 7.12 (1H, s, H-4), 6.95 (1H, d, J = 2.5 Hz, H-7), 6.84 (1H, d, J = 2.5Hz, H-5), 5.12 (1H, d, J = 8.0 Hz, anomeric-H), 3.81 (3H, s, OCH3), 3.15–3.83 (6H, m, sugar-H), 2.48 (3H, s, COCH3), 2.21 (3H, s, CH3); 13C-NMR (125 MHz, DMSO-d6) δ = 205.1 ppm (COCH3), 158.3 (C-8), 155.4 (C-6), 151.2 (C-1), 136.9 (C-3), 133.7 (C-9), 123.2 (C-2), 118.8 (C-4), 108.7 (C-10), 103.1 (C-7), 102.7 (C-5), 101.2 (C-1'), 78.2 (C-5'), 76.7 (C-3'), 73.8 (C-2'), 70.4 (C-4'), 61.2 (C-6'), 55.8 (6-OCH3), 32.6 (COCH3), 20.1 (3-CH3). These data are in accordance with those reported in the literature [24].
4-(4'-Hydroxyphenyl)-2-butanone-4'-O-β-D-glucopyranoside (8). Colorless needles, negative ESI-MS [M−H]− at m/z 325; 1H-NMR (400 MHz, CDCl3) δ ppm 7.14 (2H, d, J = 8.5 Hz; H-7, 9), 7.03 (2H, d, J = 8.5 Hz; H-6, 10), 4.88 (1H, d, J = 7.0 Hz, H-1'), 3.88–3.94 (2H, d, J = 10.8 Hz; H-6'), 3.80–3.40 (5H, m, H-2', 5'), 2.80 (4H, m, H-2, 3), 2.13 (3H, s, H-1). 13C-NMR (100 MHz, CDCl3) δ = 209.8 ppm (C-2), 156.1 (C-8), 135.0 (C-5), 128.9 (C-6,10), 116.4 (C-7,9), 101.1 (C-1'), 76.7 (C-3'), 76.6 (C-5'), 73.5 (C-2'), 70.0 (C-4'), 61.3 (C-6'), 44.6 (C-4), 25.6 (C-1, 3). These data are in accordance with those reported in the literature [25].
Amabiloside (9). Colorless needles, positive ESI-MS [M+H]+ at m/z: 300; 1H-NMR (400 MHz, CDCl3) δ = 9.79 ppm (1H, s, 1-CHO), 7.39 (1H, dd, J = 8.0, 2.0 Hz, H-6), 7.34 (1H, d, J = 2.0 Hz, H-2), 7.32 (1H, d, J = 8.0 Hz, H-5), 4.89 (1H, d, J = 7.6 Hz, H-1'), 3.55 (1H, dd, J = 9.5, 7.6 Hz, H-2'), 3.50 (1H, dd, J = 9.5, 7.6 Hz, C-3'), 3.48 (1H, m, C-5'), 3.41 (1H, dd, J = 9.5, 9.5 Hz, C-4'), 3.91 (1H, brd, J = 11.4 Hz, H-6'a), 3.74 (1H, dd, J = 11.4, 4.6 Hz, H-1'b); 13C-NMR (100 MHz, CDCl3) δ = 193.2 ppm (CHO), 152.4 (C-4), 149.0 (C-3), 133.4 (C-1), 125.0 (C-6), 117.1 (C-5), 116.3 (C-2), 102.8 (C-1'), 78.5 (C-3'), 77.5 (C-5'), 74.1 (C-2'), 71.2 (C-4'), 62.4 (C-6'). These data are in accordance with those reported in the literature [26].
N-trans-feruloyl tyramine (10). Amorphous solid, positive ESI-MS [M+Na]+ at m/z: 336; 1H-NMR (400 MHz, CD3OD) δ = 7.45 ppm (1H, d, J = 16.0 Hz), 7.14 (1H, d, J = 8.0 Hz), 7.08 (2H, d, J = 8.0 Hz), 7.03 (1H, d, J = 12.0 Hz), 6.81 (1H, dd, J = 8.0, 12.0 Hz), 6.74 (2H, d, J = 8.0 Hz), 6.43 (1H, d, J = 16 Hz), 3.88 (3H, s), 3.41 (2H, t, J = 8.0 Hz), 2.74 (2H, t, J = 8.0 Hz); 13C-NMR (100 MHz, CD3OD) δ = 169.1 ppm (C-1), 118.6 (C-2), 142.0 (C-3), 131.2 (C-1'), 130.7 (C-2',6'), 156.9 (C-4'), 116.2 (C-3',5'), 128.2 (C-1'''), 111.4 (C-2'''), 149.9 (C-3'''), 149.2 (C-4'''), 116.4 (C-5'''), 123.2 (C-6'''), 56.3 (OCH3), 42.5 (C-2''), 35.8 (C-3''). These data are in accordance with those reported in the literature [27].
3.6. DPPH Assays
The DPPH antioxidant assay was performed with slight modification from that reported previously [28]. Sample stock solution (1 mM) of rheumaustralin (1) was diluted to concentrations of 1.0, 3.0, 5.0, 7.0, 9.0 and 11.0 µM in methanol. Sample stock solution (10 µM) of piceatannol was diluted to concentrations of 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30 µM in methanol.Sample stock solutions (1 mM) of resveratrol, ascorbic acid, butylated hydroxyanisole (BHA), (±)-α-tocopherol and compounds 2–10 were diluted to concentrations of 10, 30, 50, 70, 100 and 120 µM in MeOH. Two milliliter of DPPH methanol solution (100 µM, final concentration = 50 µM) was added to 2.0 mL of a methanol solution of various sample concentrations. The mixtures were shaken vigorously and then kept in dark at room temperature. After 30 min, the absorbance values were measured at 517 nm and converted into the percentage inhibition of DPPH (Ip) using the following formula:
| Ip = [(Abssample − Abscontrol)/Abscontrol] × 100 | (1) |
A mixture of DPPH solution (2.0 mL, 100 µM) and methanol (2.0 mL) was used as the negative control.The IC50 values obtained represent the concentrations of the tested samples that caused 50% inhibition of DPPH radicals. The experiments were performed in triplicate, and the results are given as mean ± standard deviation (SD).
4. Conclusions
An unusual piceatannol dimer named rheumaustralin (1) was isolated from the underground parts of R. australe collected from Tibet, together with 17 known phenolic compounds 2−18. Compounds 9 and 10 were isolated from Rheum plants for the first time. Stilbenoids such as resveratrol and piceatannol are widely distributed in higher plants as phytoalexins [29]. Some of their natural derivatives occur in oligomeric forms. The increasing degrees of polymerization and intriguing variety of polymerization patterns provide stilbene oligomers with dazzling chemical diversities. According to the patterns of oligomer construction and biosynthesis of stilbene oligomers reviewed recently [30,31], rheumaustralin (1), in which the stilbene units are connected only through a methylene (CH2) group, may represent a new connectivity pattern for these stilbene dimers. The discovery of this novel dimer further demonstrates the diversity of the stilbenoids from the genus Rheum. In addition, the free radical scavenging activities of compounds 1–10 against DPPH radicals have been evaluated in this study. All tested compounds showed bioactivites against DPPH radicals. Among them, rheumaustralin exhibited appreciable scavenging activity, with an IC50 value of 2.3 μM. Compounds 2, 3, 5, 6 and 10 showed promising activities with IC50 values in the range of 20 to 35 µM, which was comparable to those of BHA, ascorbic acid and α-tocopherol. These phenolic compounds may have therapeutic potential and deserve further study.
Acknowledgments
The financial support from Yunnan Natural Science Foundation (2012FB172) and Kunming Science and Technology Project (11H010401, 2013-02-06-A-H-02-3054) is gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/8/11453/s1..
Supplementary Files
Author Contributions
L.H. and Q.H. designed research. L.H., N.-N.C., C.Y., Q.-S.Y. and F.-F.W. performed research and analyzed the data. L.H., N.-N.C. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–16 are available from the authors.
References
- 1.Bao B., Alisa E.G. RHEUM Linnaeus. In: Li A., Bao B., Alisa E.G., Suk-pyo H., John M., Sergei L.M., Hideaki O., Chong-wook P., editors. Flora of China. Volume 5. Science Press & Missouri Botanical Garden; St. Louis, MO, USA: 2003. pp. 277–350. [Google Scholar]
- 2.Xiao P., He L., Wang L. Ethnopharmacologic study of chinese rhubarb. J. Ethnopharmacol. 1984;10:275–293. doi: 10.1016/0378-8741(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 3.Rokaya M.B., Münzbergová Z., Timsina B., Bhattarai K.R. Rheum australe D. Don: A review of its botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2012;141:761–774. doi: 10.1016/j.jep.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Zargar B.A., Masoodi M.H., Ahmed B., Ganie S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011;128:585–589. doi: 10.1016/j.foodchem.2011.03.083. [DOI] [Google Scholar]
- 5.Liu B., Yang J., Wang S. The chemical constituents in rhubarb rhizomes and roots derived from Rheum emodi Wall. Huaxi Yaoxue Zazhi. 2007;22:33–35. [Google Scholar]
- 6.Wang A.Q., Li J.L., Li J.S. Chemical constituents of Rheum emodi. Zhong Cao Yao. 2010;41:343–346. [Google Scholar]
- 7.Chai Y.Y., Wang F., Li Y.L., Liu K., Xu H. Antioxidant activities of stilbenoids from Rheum emodi Wall. Evid. Based Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda H., Morikawa T., Toguchida I., Park J.Y., Harima S., Yoshikawa M. Antioxidant constituents from rhubarb: Structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg. Med. Chem. Lett. 2001;9:41–50. doi: 10.1016/S0968-0896(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S.K., Singh S.S., Verma S., Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000;72:43–46. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y.Q., Fukai T., Sakagami H., Kuroda J., Miyaoka R., Tamura M., Nomura T. Cytotoxic and DNA damage-inducing activities of low molecular weight phenols from rhubarb. Anticancer Res. 2001;21:2847–2853. [PubMed] [Google Scholar]
- 11.Suresh B.K., Tiwari A.K., Srinivas P.V., Ali A.Z., China R.B., Rao J.M. Yeast and mammalian α-glucosidase inhibitory constituents from Himalayan rhubarb Rheum emodi Wall ex Meisson. Bioorg. Med. Chem. Lett. 2004;14:3841–3845. doi: 10.1016/j.bmcl.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 12.Liang H.X., Dai H.Q., Fu H.A., Dong X.P., Adebayo A.H., Zhang L.X., Cheng Y.X. Bioactive compounds from Rumex plants. Phytochem. Lett. 2010;3:181–184. doi: 10.1016/j.phytol.2010.05.005. [DOI] [Google Scholar]
- 13.Xiang L., Lei F., Xing D., Wang W., Zheng J. Neuron protective constituents from Rheum nanum and Rheum sublanceolatum. Tsinghua Sci. Technol. 2005;10:426–429. doi: 10.1016/S1007-0214(05)70094-8. [DOI] [Google Scholar]
- 14.Andersen D.O., Weber N.D., Wood S.G., Hughes B.G., Murray B.K., North J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991;16:185–196. doi: 10.1016/0166-3542(91)90024-L. [DOI] [PubMed] [Google Scholar]
- 15.Liu W.B., Hu L., Hu Q., Chen N.N., Yang Q.S., Wang F.F. New resveratrol oligomer derivatives from the roots of Rheum lhasaense. Molecules. 2013;18:7093–7102. doi: 10.3390/molecules18067093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar V., Guha G., Ashok Kumar R. Antioxidant and anti-cancer potentials of Rheum emodi rhizome extracts. Evid. Based Complement. Alternat. Med. 2011 doi: 10.1093/ecam/neq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J.G., Lu M., Chen Z.H., Zhu H.H., Li Y., Yang L., Wu L.M., Liu Z.L. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chem. Eur. J. 2002;8:4191–4198. doi: 10.1002/1521-3765(20020916)8:18<4191::AID-CHEM4191>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Hu L., Chen N.N., Feng L., Hu Q., Liu W.B., Yang Q.S., Wang F.F. Piceatannol derivatives from Rheum austral D. Don and their chemotaxonomic significance. Biochem. Syst. Ecol. 2014;55:369–373. doi: 10.1016/j.bse.2014.06.007. [DOI] [Google Scholar]
- 19.Li. J.L., Li. J.S., He. W.Y., Kong. M. Studies on the non-anthraquiones of Rheum hotaoense. Zhong Cao Yao. 1998;29:721–723. [Google Scholar]
- 20.Kjer J., Wray V., Edrada-Ebel R., Ebel R., Pretsch A., Lin W., Proksch P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009;72:2053–2057. doi: 10.1021/np900417g. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H.P., Wang Z.Y., Chen J.R., Li R.M., Wang Z.Q. New chromone glucoside from roots of Rumex gmelini. Nat. Prod. Res. Dev. 2009;21:189–191. [Google Scholar]
- 22.Xu J., Kjer J., Sendker J., Wray V., Guan H., Edrada R., Proksch P. Chromones from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. J. Nat. Prod. 2009;72:662–665. doi: 10.1021/np800748u. [DOI] [PubMed] [Google Scholar]
- 23.Mei R., Liang H., Wang J., Zeng L., Lu Q., Cheng Y. New seco-anthraquinone glucosides from Rumex nepalensis. Planta Med. 2009;75:1162. doi: 10.1055/s-0029-1185467. [DOI] [PubMed] [Google Scholar]
- 24.Demirezer Ö, Kuruüzüm A., Bergere I., Schiewe H.J., Zeeck A. Five naphthalene glycosides from the roots of Rumex patientia. Phytochemistry. 2001;56:399–402. doi: 10.1016/S0031-9422(00)00368-X. [DOI] [PubMed] [Google Scholar]
- 25.Shikishima Y., Takaishi Y., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O. Phenylbutanoids and stilbene derivatives of Rheum maximowiczii. Phytochemistry. 2001;56:377–381. doi: 10.1016/S0031-9422(00)00370-8. [DOI] [PubMed] [Google Scholar]
- 26.Likhitwitayawuid K., Ruangrungsi N., Cordell G.A. Amabiloside, a new glycoside from Crinum amabile. Nat. Prod. Lett. 1993;3:1–4. doi: 10.1080/10575639308043830. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H., Nakamura T., Ichino K., Ito K. A phenolic amide from Actinodaphne longifolia. Phytochemistry. 1989;28:2516–2517. doi: 10.1016/S0031-9422(00)98022-1. [DOI] [Google Scholar]
- 28.Sharma O.P., Bhat T.K. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- 29.Rivière C., Pawlus A.D., Mérillon J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012;29:1317–1333. doi: 10.1039/c2np20049j. [DOI] [PubMed] [Google Scholar]
- 30.Xiao K., Zhang H.J., Xuan L.J., Zhang J., Xu Y.M., Bai D.L. Stilbenoids: Chemistry and bioactivities. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Volume 34. Elsevier Science; Amsterdam, The Netherlands: 2008. pp. 453–646. [Google Scholar]
- 31.Shen T., Wang X.N., Lou H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009;26:916–935. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.