Abstract
Several pleuromutilin derivatives possessing thiadiazole moieties were synthesized via acylation reactions under mild conditions. The in vitro antibacterial activities of the derivatives against methicillin-resistant S. aureus, methicillin-resistant S. epidermidis, S. aureus, S. epidermidis, E. coli, and B. cereus were tested by the agar dilution method and Oxford cup assay. All the screened compounds displayed potent activity. Compound 6d was the most active antibacterial agent because of its lowest MIC value and largest inhibition zone. Docking experiments were performed to understand the possible mode of the interactions between the derivatives and 50S ribosomal subunit. Moreover, the absorption, distribution, metabolism, excretion and toxicity properties of the synthesized compounds were analyzed after prediction using the Advanced Chemistry Development/Percepta Platform available online.
Keywords: pleuromutilin derivatives, antibacterial activity, synthesis, molecular docking, ADMET properties
1. Introduction
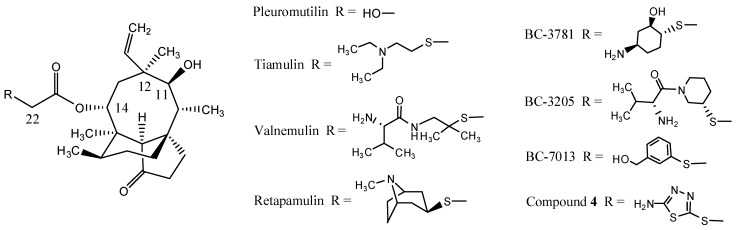
The natural compound pleuromutilin (Figure 1) was first discovered and isolated in a crystalline form from Pleurotus mutilus and P. passeckerianus in 1951 [1]. Pleuromutilin is a diterpene, constituted of a rather rigid 5-6-8 tricyclic carbon skeleton with eight stereogenic centers [2,3] and a glycolic acid chain at C-14 which was considered as the main molecular modification point [4]. Although pleuromutilin has a modest antibacterial activity, the modifications of the C-14 position have led to three drugs: tiamulin, valnemulin, and retapamulin (Figure 1). Tiamulin and valnemulin are used in veterinary medicine for pigs and poultry. Retapamulin was approved as a topical antimicrobial agent for the treatment of human skin infections in 2007 by FDA [5,6]. Extensive efforts were made to formulate BC-3781, BC-3205 and BC-7013 (Figure 1) for human use [7,8] after the success of retapamulin.
Figure 1.
Structural formulas of pleuromutilin, tiamulin, valnemulin, retapamulin, BC-3781, BC-3205 and BC-7013.
The interaction modes that pleuromutilin derivatives selectively inhibit bacterial proteins synthesis were demonstrated as early as 1974 [9]. Crystallography data, utilizing a structure of 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin, demonstrated that the interactions of tricyclic core of the tiamulin are mediated through hydrophobic interactions and hydrogen bonds, which are formed mainly by the nucleotides of the domain V of 23S rRNA at the peptidyl transferase center (PTC) [10,11]. The C-11 hydroxyl group and the C-21 keto group of pleuromutilin derivatives are located in a position suitable for hydrogen bonding to G-2505 phosphate and G-2061, respectively, while the C-14 side chain prevents the peptidyl transferase rRNA bases U-2584 and U-2585 from tRNA binding to the P-site [11]. This effect is of particular importance as Long et al. suggestion that the nature of the pleuromutilin side chain is potentially critical for overcoming resistance mediated by the mutation of the ribosomal protein [9,12].
Compounds containing a 1,3,4-thiadiazole moieties exhibit a wide range of biological activities such as antibacterial [13], antitumor [14] and antifungal activities [15]. Recently, we described a series of pleuromutilin derivatives with potent antibacterial activities from a 1,3,4-thiadiazole-based lead, 14-O-[(2-amino-1,3,4-thiadiazol-5-yl) thioacetyl] mutilin (4, Figure 1). Two compounds with anilino substituents in their side chains showed stronger antibacterial activities [16]. However, they also showed low chemical stabilities in the subsequent research because the amino group on the phenyl ring is easily oxidized in the air. To resolve this problem, the amino or phenyl ring was replaced by other substituents and some new pleuromutilin derivatives bearing 1,3,4-thiadiazoles were designed and synthesized. Moreover, detailed antibacterial activities and molecular docking studies of all synthesized compounds were performed to explore their binding models with 50S ribosomal subunit.
2. Results and Discussion
2.1. Chemistry
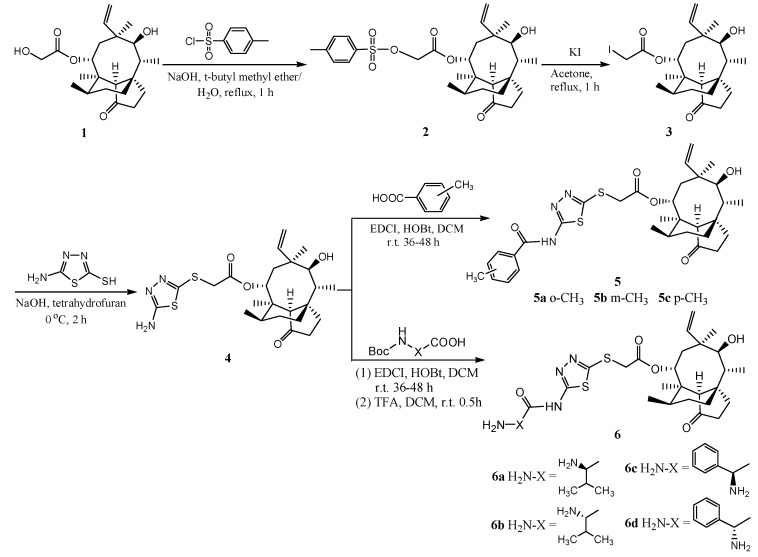
Pleuromutilin derivatives 5a–c and 6a–d were synthesised from the lead compound 14-O-[(2-amino-1,3,4-thiadiazol-5-yl) thioacetyl] mutilin (4) which was synthesized in turn from 22-O-tosylpleuromutilin (2) according to previous literature [4,16]. Compounds 5a–c and 6a–d were directly obtained by condensation reactions between the amino group of compound 4 and the carboxyl group of methyl-substituted aryl carboxylic acids or amino acids which amino groups were protected by tert-butoxycarbonyl (BOC) groups. The reactions were performed at room temperature in the presence of 1-ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBt) which was used to suppress racemization and improve the efficiency of the amide synthesis (Scheme 1). The protected amino groups were hydrolyzed with TFA for 30 min and the compounds 6a–d were obtained. The synthesis and the IR, 1H-NMR, 13C-NMR and HRMS spectra of all the new compounds were reported in the Supplementary data.
Scheme 1.
Synthesis of compounds 5a–c and 6a–d.
2.2. Antibacterial Activity
The synthesized pleuromutilin derivatives 5a–c and 6a–d along with tiamulin used as reference drug were screened for their in vitro antibacterial activity against MRSA, MRSE, S. aureus, S. epidermidis, E. coli, and B. cereus. The antibacterial activities were reported in Table 1 as the minimum inhibitory concentration (MIC) using the agar dilution method and in Table 2 as the zones of inhibition using Oxford cup assay.
Table 1.
Antibacterial activity (MIC) of the synthesized pleuromutilin derivatives.
| Comp. | MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| MRSA | MRSE | S. aureus | S. epidermidis | E. coli | B. cereus | |
| 5a | 32 | 64 | 32 | 32 | ≥128 | 16 |
| 5b | 64 | ≥128 | 64 | 64 | ≥128 | 32 |
| 5c | 32 | 32 | 16 | 32 | 64 | 4 |
| 6a | 8 | 16 | 4 | 16 | 32 | 2 |
| 6b | 32 | 32 | 32 | 32 | 64 | 16 |
| 6c | 8 | 32 | 8 | 16 | 64 | 8 |
| 6d | 0.5 | 4 | 0.5 | 2 | 32 | 0.25 |
| Tiamulin | 0.5 | 2 | 0.5 | 2 | 16 | 0.25 |
Table 2.
Zone of Inhibition for MRSA, MRSE, S. aureus, S. epidermidis, E. coli and B. cereus in mm.
| Comp. | MRSA | MRSE | S. aureus | S. epidermidis | E. col | B. cereus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 320 | 160 | 320 | 160 | 320 | 160 | 320 | 160 | 320 | 160 | 320 | 160 | |
| 5a | 16.51 | 13.22 | 16.40 | 12.93 | 16.23 | 13.39 | 16.46 | 13.82 | 13.16 | 11.65 | 17.08 | 15.10 |
| 5b | 14.03 | 11.67 | 13.88 | 12.34 | 13.42 | 11.14 | 14.02 | 12.53 | 12.97 | 11.13 | 15.51 | 13.55 |
| 5c | 16.47 | 13.14 | 16.85 | 13.46 | 17.35 | 13.89 | 17.05 | 13.66 | 12.23 | 10.83 | 18.73 | 15.26 |
| 6a | 17.51 | 14.49 | 17.35 | 13.89 | 18.82 | 14.75 | 18.31 | 14.32 | 14.22 | 12.17 | 19.33 | 16.51 |
| 6b | 16.05 | 12.84 | 15.69 | 12.30 | 15.93 | 12.04 | 16.17 | 12.12 | 12.59 | 10.86 | 17.42 | 14.05 |
| 6c | 17.11 | 14.28 | 15.13 | 12.21 | 17.44 | 14.39 | 16.83 | 13.25 | 14.85 | 12.67 | 18.25 | 13.91 |
| 6d | 19.46 | 17.62 | 17.28 | 15.03 | 22.67 | 18.82 | 19.15 | 16.73 | 16.22 | 13.61 | 23.84 | 19.39 |
| Tiamulin | 20.35 | 17.84 | 17.93 | 15.75 | 22.23 | 19.04 | 20.58 | 16.05 | 17.84 | 15.29 | 23.18 | 20.57 |
All of screened compounds showed potent activity against all the strains except E. coli, and slightly less potent against resistant strains (MRSA and MRSE) than standard ones (S. aureus and S. epidermidis). In particular, compound 6d bearing the l-(‒)-phenylglycinamide group on the C-14 glycolic acid side chain, was found to have the most potent activities against MRSA (MIC = 0.5 μg/mL), MRSE (MIC = 4 μg/mL), S. aureus (MIC = 0.5 μg/mL), S. epidermidis (MIC = 2 μg/mL) and B. cereus (MIC = 0.25 μg/mL) comparable to tiamulin. However its stereoisomer, compound 6c showed moderate activities against MRSA, MRSE, S. aureus, S. epidermidis and B. cereus.
The results of Oxford cup assay correspond with that obtained by agar dilution method (MIC) as a whole. Compound 6d showed the best growth inhibition against the pathogens particularly MRSA, MRSE and B. cereus comparable to tiamulin. Compounds 6a and 6c showed moderate growth of MRSA, MRSE, S. aureus, S. epidermidis and B. cereus. However, all the compounds exhibited weak activity against the E. coli.
Compounds 6a–d with an amino group on the terminal C-14 glycolic acid side chain presented improved activity against all the strains except E.coli compared with the compounds 5a–c bearing the methylbenzene group on the terminal C-14 glycolic acid side chain. The results of antibacterial activities indicated that the introduction of the amino group into the C-14 glycolic acid side chain could enhance antibacterial activity, which was consistent with previous reported [4,16].
Among the above mentioned compounds, 6d was found inhibit the growth of the organisms at minimum concentration and found to possess good antibacterial activity. The MIC values and the observed growth inhibition have demonstrated that 6d might act as potent antibacterial agent.
2.3. Molecular Docking Study
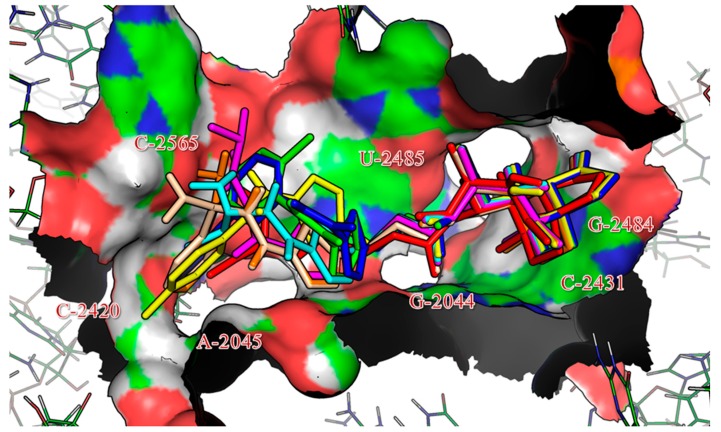
To investigate the binding mode of the series of pleuromutilin derivatives, docking simulations were performed with Homdock software [17]. The re-docking of tiamulin 2 into 1XBP12 placed the drug in the same conformation as that in the X-ray structure (RMSD 0.7 Å). The docking results for the seven compounds revealed a similar binding pattern within the binding site, with a RMSD range of 1.00 to 1.23 Å, as documented for tiamulin and presented in Figure 2 which shows a superimposition of tiamulin and the seven docked compounds. Furthermore, the docking results are in good agreement with the interactions highlighted in the crystal structure of 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin.
Figure 2.
Superimposition of native ligand, tiamulin (colored by red) and the best conformations of 5a (green), 5b (blue), 5c (yellow), 6a (magenta), 6b (cyan), 6c (orange) and 6d (wheat) docked to the binding pocket of ribosome (1XBP).
The docking simulations revealed hydrogen bonding played the most important role in the binding of the compounds to 1XBP (Table 3; Figure 3). However, other interactions, such as hydrophobic ones are not shown in Table 3 or Figure 3. The seven compounds exhibited multiple binding modes into 1XBP with the binding free energies (ΔGb) in the range of −10.42 to −15.09 kcal/mol.
Table 3.
Binding free energy, number of noncovalent molecular interactions and RMSD.
| Compound | ΔGb (kcal/mol) | Noncovalent Molecular Interaction | RMSD(Å) | |||
|---|---|---|---|---|---|---|
| Hydro I Interaction | Atom of Compound | Residue | Distance (Å) | |||
| 5a | −10.42 | H-bonding | OH (8-membered ring) | G-2484 | 2.0 | 1.21 |
| H-bonding | N (thiadiazole) | G-2044 | 2.4 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.7 | |||
| 5b | −10.56 | H-bonding | OH (8-membered ring) | G-2484 | 2.0 | 1.23 |
| H-bonding | N (thiadiazole) | C-2044 | 2.1 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.2 | |||
| 5c | −11.50 | H-bonding | OH (8-membered ring) | G-2484 | 2.1 | 1.08 |
| H-bonding | C=O (ester) | G-2044 | 2.7 | |||
| H-bonding | C=O (ester) | G-2044 | 2.8 | |||
| 6a | −12.21 | H-bonding | OH (8-membered ring) | G-2484 | 1.9 | 1.00 |
| H-bonding | C=O (ester) | G-2044 | 2.5 | |||
| H-bonding | C=O (ester) | G-2044 | 2.5 | |||
| H-bonding | NH2 (terminal) | C-2565 | 2.1 | |||
| H-bonding | NH2 (terminal) | C-2565 | 2.7 | |||
| 6b | −11.69 | H-bonding | OH (8-membered ring) | G-2484 | 1.9 | 1.00 |
| H-bonding | C=O (ester) | G-2044 | 2.5 | |||
| H-bonding | C=O (ester) | G-2044 | 2.5 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.1 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.3 | |||
| H-bonding | NH2 (terminal) | C-2565 | 2.6 | |||
| 6c | −14.14 | H-bonding | OH (8-membered ring) | G-2484 | 2.1 | 1.02 |
| H-bonding | C=O (ester) | G-2044 | 2.3 | |||
| H-bonding | C=O (ester) | G-2044 | 2.3 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.3 | |||
| H-bonding | NH2 (phenylglycinamide) | C-2565 | 1.6 | |||
| 6d | −15.09 | H-bonding | OH (8-membered ring) | G-2484 | 2.1 | 1.02 |
| H-bonding | C=O (ester) | G-2044 | 2.3 | |||
| H-bonding | C=O (ester) | G-2044 | 2.3 | |||
| H-bonding | N (thiadiazole) | G-2044 | 2.2 | |||
| H-bonding | NH2 (phenylglycinamide) | C-2565 | 2.3 | |||
| Cation–π interaction | N (pyrrolidine) | G-2045 | 3.7 | |||
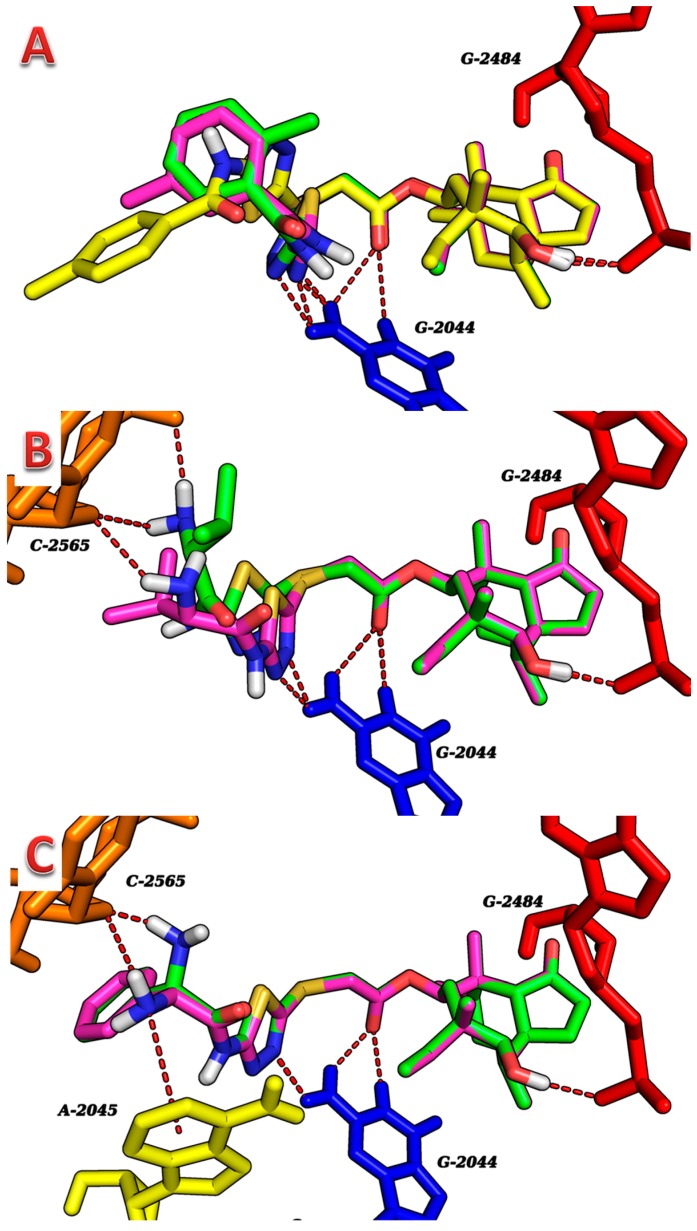
Figure 3.
Docking modes of the synthesized compounds into 1XBP. (A) 5a (green), 5b (magenta) and 5c (yellow); (B) 6a (green) and 6b (magenta) to 1XBP; (C) 6c (green) and 6d (magenta). Important residues are drawn in stick and different color. Hydrogen bonds and cation–π interaction are showed as dashed red lines.
Compounds 5a and 5b exhibited the similar docking mode with three hydrogen bonds between their OH (8-membered ring) and N (thiadiazole) with G-2484 and G-2044 (Table 3; Figure 3A). Compound 5c showed a different docking mode, it exhibited two hydrogen bonds between its C=O (ester) group with G-2044 except one hydrogen bond between its OH (8-membered ring) with G-2484. The higher binding affinity of 5c compared to 5a and 5b may be explained by the terminal methylbenzene group protruding into a hydrophobic area formed by resides of A-2420, C-2046 and A-2045. Although compound 6a is a stereoisomer of 6b, it showed a slight different docking mode compared to 6b (Table 3; Figure 3B). Compounds 6c and 6d adopted very similar conformations and hydrogen bonds interactions except for terminal phenylglycinamide group. However, compound 6d displayed a higher binding affinity (ΔGb = −15.09) than 6c, perhaps because of a cation-π interaction formed between the N (pyrrolidine) and G-2045 (Table 3; Figure 3C). Because the difference of the binding free energies caused by different binding modes for the stereoisomers, their showed significant difference in antibacterial activities.
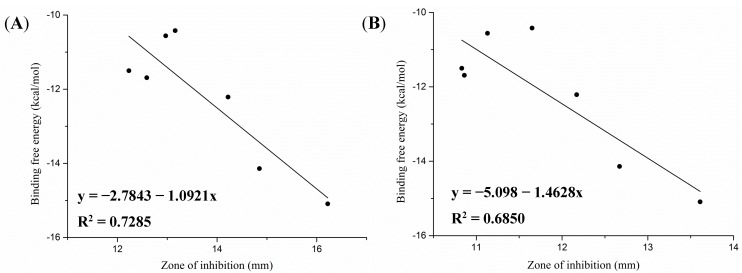
The molecular docking results revealed a rational correlation between the predicted binding affinities (binding free energies) and the antibacterial activity. Because X-ray structures of the 50S ribosomal subunits are available only for E. coli (PDB ID: 2AW4), we investigated the linear relationships between the binding free energy (ΔGb, kcal/mol) and the zone of inhibition (mm) for 320 and 160 μg/mL concentrations of E. coli based on the high similarity in domain V of 23S rRNA at the PTC between 1XBP and 2AW4 [16]. The results revealed a direct reasonable correlation between the binding free energy and the zone of inhibition, with correlation coefficients (R2) of 0.6626 and 0.7658 for the screened compounds as illustrated in Figure 4.
Figure 4.
Correlation between binding free energy (ΔGb, kcal/mol) and antibacterial activity (zone of inhibition; mm) against E. coli. for scereened compounds with 320 μg/mL (A) and 160 μg/mL (B).
2.4. Prediction of ADMET Properties
The prediction of absorption, distribution, metabolism, excretion and toxicity properties (ADMET) facilitates the lead compound discovery process, which is crucial for reducing ADMET problems in the later stage by optimization of those properties during the early stages of drug discovery. The ADMET properties of the synthesized compounds were predicted, including absorption, extent of blood-brain barrier permeation (Log BB), rate of passive diffusion/permeability (Log PS), plasma protein binding (PPB), volume of distribution (Vd) and median lethal dose (LD50), as well as acid dissociation constant (pKa) and the value of the octanol-water partitioning coefficient as the logarithmic ratio (Log P) using the Advanced Chemistry Development (ACD)/Percepta Platform available online.
According to the values shown in Table 4, the compounds with similar side chains had the same ADMET properties because of their similar pKa and Log p values predicted by ACD/Lab. It is well known that the log p value can be used to assess the hydrophilicity of a compound. A high log p value is associated with poor absorption or permeation, and the ideal value should be below about 5 [18]. Our study suggested that compounds 6a–d had log p values of 3.88–4.64, while 5a–c were slightly above this limit. Compounds 5a–c can be more efficiently absorbed in the human intestine with the highest jejunal permeability coefficient (Peff) at pH 6.5 than the compounds 6a–b. Log PS and Log BB are main parameters characterizing different aspects of the analyzed process when the blood-brain barrier (BBB)-transport potential of drugs or other molecules is measured experimentally [19]. The higher lipophilicity and lower protonation state made compounds 5a–c permeate the membranes of endothelial cells more easily (Log BB = 0.29; Log PS = −1.7). PPB values represent the overall fraction of drug bound in human plasma such as albumin, α-1-acid glycoprotein, lipoproteins, and transcortin. All of the compounds were likely to be bound to serum proteins with a high degree of combination (PPB > 95%) which led to their lower volume of distribution (Vd < 5 L/kg). All of the compounds were mildly toxic with LD50 in the range of 730–1,400 mg/kg predicted for mouse after oral administration.
Table 4.
Prediction ADMET properties for examined pleuromutilin derivatives.
| Comp. | ADMET Parameter | pKa | c Log p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Peff (cm/s) a | Log BB b | Log PS c | PPB (%) d | Vd (L/kg) e | LD50 (mg/kg) f | Acid | Base | ||
| 5a | 6.39 × 10−4 | 0.29 | −1.7 | 99.27 | 4.15 | 1400 | 10.40 | - | 6.29 |
| 5b | 6.39 × 10−4 | 0.29 | −1.7 | 99.27 | 4.15 | 1400 | 10.40 | - | 6.29 |
| 5c | 6.39 × 10−4 | 0.29 | −1.7 | 99.27 | 4.15 | 1400 | 10.40 | - | 6.29 |
| 6a | 5.12 × 10−4 | 0.02 | −2.3 | 97.14 | 3.15 | 730 | 10.30 | 7.70 | 3.88 |
| 6b | 5.12 × 10−4 | 0.02 | −2.3 | 97.14 | 3.15 | 730 | 10.30 | 7.70 | 3.88 |
| 6c | 5.41 × 10−4 | 0.2 | −2.4 | 98.44 | 1.60 | 970 | 7.80 | 11.80 | 4.64 |
| 6d | 5.41 × 10−4 | 0.2 | −2.4 | 98.44 | 1.60 | 970 | 7.80 | 11.80 | 4.64 |
a Jejunal permeability coefficients at pH 6.5 that depends on paracellular and transcellural transport routes and unstirred water layer resistance; b Extent of brain penetration determined by ratio of total drug concentrations in tissue and plasma at steady-state conditions; c Rate of brain penetration. PS stands for Permeability-Surface area product and is defined from the kinetic equation of capillary transport; d The cumulative percentage of a compound bound to human plasma proteins, such as albumin, alpha1-acid glycoprotein and others (RI ≥ 0.30); e Prediction (probably 90%) of Volume of Distribution (Vd) regarding the effect of physicochemical properties (Log p and ionization) on drug distribution in the body; f Acute toxicity (LD50) for mouse after oral administration (RI ≥ 0.46).
As we know, toluene group noticeably increased the lipophilicity compare to amino group, while the compound lipophilicity can more or less affect their ADMET properties. Compounds 5a–c with toluene group to the side chain showed slightly better ADMET properties than compounds 6a–d. However, compound 6d may serve as a possible drug-like compound by comprehensive considering its antibacterial activity and ADMET properties.
Based on the structural fragment and atomic contributions which are associated with data drawn from quantitative structure activity relationship (QSAR) studies, the predictions for ADMET properties using ACD/Percepta Platform can be efficiently evaluated in silico approaches, thereby accelerating the drug discovery process [20].
3. Experimental Section
3.1. General
All reagents and solvents were of analytical grade and used without further purification. All reactions were monitored by TLC on 0.2-mm-thick silica gel GF254 pre-coated plates. After elution, the plates were visualized under UV illumination at 254 nm for UV active materials. Further visualization was achieved by staining with a 0.05% KMnO4 aqueous solution. All column chromatography purifications were carried out on silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd, Qingdao, China) with conventional methods. The melting points of the synthesized compounds were determined on a Tianda Tianfa YRT-3 apparatus (Tianjin, China) with open capillary tubes and were uncorrected. IR spectra were obtained on a NEXUS-670 spectrometer (Nicolet Thermo, Edina, MN, USA) using KBr thin films and the absorptions are reported in cm−1. NMR spectra were recorded in appropriate solvents using a Bruker-400 MHz spectrometer (Bruker BioSpin, Zürich, Zürich State, Switzerland). The chemical shifts (δ) were expressed in parts per million (ppm) relative to tetramethylsilane. The multiplicities of the NMR signals were designated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad), etc. High-resolution mass spectra (HRMS) were obtained on a Bruker Daltonics APEX II 47e mass spectrometer equipped with an electrospray ion source.
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Compounds 5a–c
A mixture of methyl-substituted benzoic acid (2.2 mmol), compound 4 (0.98 g, 2 mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.42 g, 2.2 mmol), 1-hydroxy-benzotriazole (0.30 g, 2.2 mmol) and dichloromethane (50 mL) was stirred at room temperature for 28 h. The mixture was washed with saturated aqueous NaHCO3 and water, dried with anhydrous MgSO4 overnight and rotary evaporated to dryness. The crude residue thus obtained was purified by silica gel column chromatography (petroleum ether-ethyl acetate 2:1 v/v) to afford the pure desired compounds 5a–5c.
14-O-[(2-Methylbenzamide-1,3,4-thiadiazol-5-yl) thioacetyl] mutilin (5a): Compound 5a was prepared according to the general procedure from 14-O-[(2-amino-1,3,4-thiadiazol-5-yl)thioacetyl] mutilin (4) and o-methylbenzoic acid. The crude product was purified by silica gel column chromatography to give 0.64 g (yield 52%) of compound 5a, mp 113–115 °C. IR (KBr): υmax cm−1 3448, 2931, 1733, 1676, 1533, 1458, 1302, 1151, 1116, 1049, 893, 737, 666 cm−1. 1H-NMR (CDCl3) δ ppm 7.74 (d, J = 7.3 Hz, 1H), 7.48–7.42 (m, 1H), 7.34 (s, 2H), 6.41 (q, J = 17.4, 11.0 Hz, 1H), 5.76 (d, J = 8.4 Hz, 1H), 5.30 (q, J = 11.0, 1.1 Hz, 1H), 5.15 (q, J = 17.4, 1.2 Hz, 1H), 3.99–3.88 (t, 2H), 3.33 (d, J = 6.3 Hz, 1H), 2.53 (s, 3H), 2.33–2.12 (m, 3H), 2.08–2.01 (m, 2H), 1.75 (q, J = 14.4, 2.4 Hz, 1H), 1.62 (m, J = 17.5, 11.1, 3.8 Hz, 3H), 1.54–1.49 (m, 1H), 1.45–1.36 (m, 4H), 1.25 (m, J = 14.1, 12.9, 9.4 Hz, 3H), 1.17–1.07 (m, 4H), 0.86 (d, J = 7.0 Hz, 3H), 0.70 (d, J = 7.0 Hz, 3H). 13C-NMR (CDCl3) δ ppm 217.03, 167.11, 166.78, 160.81, 158.32, 138.92, 138.58, 131.89, 128.51, 126.24, 117.47, 74.75, 70.53, 58.56, 58.24, 45.57, 44.84, 44.07, 42.00, 36.82, 36.10, 34.56, 30.52, 26.96, 26.52, 24.95, 20.60, 18.54, 16.87, 14.97, 14.32, 11.59. HRMS (ESI) of C32H41N3O5S2 [M+H]+ calcd, 612.2527; Found, 612.2524.
14-O-[(3-Methylbenzamide-1,3,4-thiadiazol-5-yl) thioacetyl] mutilin (5b): Compound 5a was prepared according to the general procedure from 4 and m-methylbenzoic acid. The crude product was purified over silica gel column chromatography to give 5b (0.83 g, yield 68%), mp 107–110 °C. IR (KBr): υmax cm−1 3448, 2931, 1732, 1671, 1533, 1458, 1303, 1152, 1116, 1017, 735, 674 cm−1. 1H-NMR (CDCl3) δ ppm 8.03–7.90 (m, 2H), 7.44 (d, J = 6.0, 2H), 6.38 (q, J = 17.4, 11.0, 1H), 5.74 (d, J = 8.4, 1H), 5.30–5.24 (m, 1H), 5.12 (d, J = 17.4, 1H), 4.04–3.89 (m, 2H), 3.32 (d, J = 6.4, 1H), 2.45 (s, 3H), 2.33–2.17 (m, 3H), 2.01 (t, J = 16.0, 8.2, 2H), 1.74 (q, J = 14.4, 2.5, 1H), 1.63 (t, J = 13.0, 7.2, 2H), 1.51–1.30 (m, 6H), 1.27–1.22 (m, 1H), 1.15–1.05 (m, 4H), 0.85 (d, J = 7.0, 3H), 0.68 (d, J = 7.0, 3H). 13C-NMR (CDCl3) δ ppm 217.33, 167.05, 165.88, 161.89, 158.89, 139.25, 134.68, 131.30, 129.58, 129.23, 126.08, 117.78, 75.06, 70.90, 58.55, 45.88, 45.11, 44.37, 42.30, 37.13, 36.52, 34.88, 30.84, 27.26, 26.80, 25.28, 21.81, 17.17, 15.26, 11.92. HRMS (ESI) of C32H41N3O5S2 [M+H]+ calcd, 612.2515; Found, 612.1506.
14-O-[(4-Methylbenzamide-1,3,4-thiadiazol-5-yl) thioacetyl] mutilin (5c): Compound 5c was prepared according to the general procedure from 4 and p-methylbenzoic acid. The crude product was purified over silica gel column chromatography to give 5c (0.67 g, yield 55%), mp 82–85 °C. IR (KBr): υmax cm−1 3447, 2931, 1733, 1669, 1612, 1540, 1457, 1375, 1299, 1187, 1116, 1019, 836, 744 cm−1. 1H-NMR (CDCl3) δ ppm 8.10 (d, J = 8.2, 2H), 7.34 (d, J = 8.1, 2H), 6.37 (q, J = 17.4, 11.0, 1H), 5.74 (d, J = 8.4, 1H), 5.25 (d, J = 11.1, 1H), 5.11 (d, J = 17.4, 1H), 4.06–3.90 (m, 2H), 3.31 (s, 1H), 3.20 (s, 1H), 2.45 (s, 3H), 2.30–2.14 (m, 3H), 2.04 (d, J = 7.0, 1H), 2.02–1.94 (m, 1H), 1.73 (q, J = 14.4, 2.1, 1H), 1.61 (d, J = 10.2, 2H), 1.51–1.33 (m, 6H), 1.25 (d, J = 15.8, 1H), 1.18 (s, 4H), 1.09 (d, J = 6.0, 4H), 0.85 (d, J = 6.9, 3H), 0.69 (d, J = 7.0, 3H). 13C-NMR (CDCl3) δ ppm 217.02, 166.73, 165.36, 161.57, 158.45, 144.36, 138.89, 129.69, 128.78, 128.28, 127.52, 117.41, 74.72, 70.55, 58.21, 45.55, 44.04, 41.97, 36.81, 36.10, 34.55, 30.51, 27.02, 26.49, 24.95, 21.85, 21.61, 16.83, 14.93, 11.58. HRMS (ESI) of C32H41N3O5S2 [M+H]+ calcd, 612.2524; Found, 612.2517.
3.2.2. General Procedure for the Synthesis of Compounds 6a–d
Amino acid derivative (5 mmol) was dissolved in a mixture of tetrahydrofuran (50 mL) and water (20 mL). A 1 N NaOH (6 mL) was added followed by the addition of 1.1 g di-tert-butyl dicarbonate (5 mmol) dropwise at room temperature. After stirring for 4 h, the tetrahydrofuran was evaporated under vacuum from the reaction mixture. To the residue was added ethyl acetate (50 mL) and 5% citric acid (30 mL). The organic layer was separated, washed with water (20 mL × 3), dried with anhydrous MgSO4 and rotary evaporated to dryness. The crude residue was used the next reaction without purification. A mixture of the above N-Boc protected amino acids (2.2 mmol), compound 4 (0.98 g, 2 mmol), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (0.42 g, 2.2 mmol), 1-hydroxybenzotriazole (0.30 g, 2.2 mmol), triethylamine (0.30 g, 3.0 mmol) and dichloromethane (50 mL) was stirred at room temperature for 36 h. The mixture was washed with saturated aqueous NaHCO3 and water then, evaporated under vacuum and the residue was treated with a mixture of trifluoroacetic acid (TFA, 10 mL) and dichloromethane (10 mL) at room temperature for 30 min. The reaction mixture was quenched with 25% aqueous NaHCO3 (30 mL) and washed with water, dried with anhydrous Na2SO4 overnight and rotary evaporated to dryness. The crude residue thus obtained was purified by silica gel column chromatography (petroleum ether-ethyl acetate 1:2 v/v) to afford the desired compounds.
14-O-[(3-Methyl-2-(l)-amino-butyrylamide-1,3,4-thioacetyl-5-yl)thioacetyl] mutilin (6a): Compound 6a was prepared according to the general procedure from 4 and l-valine. The crude product was purified by silica gel column chromatography to give 6a (0.87 g, yield 72%), mp 91–93 °C. IR (KBr): υmax cm−1 3422, 2932, 1733, 1521, 1458, 1285, 1152, 1117, 1053, 1018, 981, 916, 698. 1H-NMR (CDCl3) δ ppm 6.41 (dd, J = 17.4, 11.0, 1H), 5.74 (d, J = 8.5, 1H), 5.30 (dd, J = 11.0, 1.3, 1H), 5.16 (dd, J = 17.4, 1.4, 1H), 4.11 (q, J = 7.1, 1H), 4.00 (s, 1H), 3.54 (d, J = 4.0, 1H), 3.34 (d, J = 6.4, 1H), 2.40–2.16 (m, 4H), 2.11–1.98 (m, 4H), 1.73 (d, J = 2.6, 1H), 1.69–1.56 (m, 3H), 1.55–1.29 (m, 8H), 1.24 (t, J = 7.1, 2H), 1.18–1.10 (m, 4H), 1.04 (d, J = 7.0, 4H), 0.86 (dd, J = 6.9, 1.6, 5H), 0.71 (d, J = 7.0, 3H). 13C-NMR (CDCl3) δ ppm 217.02, 173.25, 166.81, 159.10, 138.94, 117.43, 74.74, 70.58, 60.51, 60.03, 58.24, 45.56, 44.79, 44.10, 41.99, 36.83, 36.22, 34.57, 31.33, 30.53, 26.96, 26.54, 24.95, 19.62, 16.88, 16.39, 14.93, 14.32, 11.58. HRMS (ESI) of C30H46N4O5S2 [M+H]+ calcd, 593.2831; Found, 593.2827.
14-O-[(3-Methyl-2-(d)-aminobutyrylamide-1,3,4-thioacetyl-5-yl)thioacetyl] mutilin (6b). Compound 6b was prepared according to the general procedure from 4 and d-valine. The crude product was purified by silica gel column chromatography to give 6b (0.80 g, yield 66%), mp 82–85 °C. IR (KBr): υmax cm−1 3419, 2935, 1731, 1541, 1456, 1398, 1296, 1153, 1118, 1058, 1018, 980, 915. 1H-NMR (DMSO) δ ppm 6.49–6.37 (m, 1H), 5.77 (d, J = 8.4 Hz, 1H), 5.32 (dd, J = 11.0, 5.9 Hz, 1H), 5.18 (dd, J = 17.4, 5.0 Hz, 1H), 4.18–4.08 (m, 1H), 4.02 (d, J = 2.7 Hz, 1H), 3.59 (dd, J = 42.1, 4.2 Hz, 1H), 3.35 (d, J = 6.3 Hz, 1H), 2.37–2.14 (m, 4H), 2.07 (d, J = 16.4 Hz, 3H), 1.93 (s, 1H), 1.82–1.73 (m, 1H), 1.70–1.63 (m, 3H), 1.54 (d, J = 11.6 Hz, 1H), 1.50–1.33 (m, 6H), 1.27 (t, J = 7.1 Hz, 1H), 1.21–1.13 (m, 5H), 1.04 (dd, J = 18.6, 6.9 Hz, 4H), 0.88 (dd, J = 6.9, 2.2 Hz, 4H), 0.76–0.67 (m, 3H). 13C-NMR (DMSO) δ ppm 216.79, 175.22, 168.65, 166.36, 158.38, 138.44, 116.94, 74.23, 70.08, 61.50, 60.10, 57.74, 45.48, 45.09, 44.21, 43.60, 41.50, 36.34, 35.67, 34.12, 30.03, 26.47, 26.10, 24.46, 20.70, 16.43, 14.46, 13.87, 11.18. HRMS (ESI) of C30H46N4O5S2 [M+H]+ calcd, calcd, 593.2833; Found, 593.2828.
14-O-[(2-d(−)Phenylglycinamide-1,3,4-thioacetyl-5-yl)thioacetyl] mutilin (6c): Compound 6c was prepared according to the general procedure from 4 and d-phenylglycine. The crude product was purified by silica gel column chromatography to give 6c (0.72 g, yield 58%), mp 89–91 °C. IR (KBr): υmax cm−1 3448, 2927, 1730, 1522, 1457, 1285, 1153, 1116, 1017, 937, 916, 698. 1H-NMR (CDCl3) δ ppm 7.43 (d, J = 7.2 Hz, 2H), 7.36–7.27 (m, 3H), 6.42 (dd, J = 17.3, 11.0 Hz, 1H), 5.72 (d, J = 8.4 Hz, 1H), 5.20 (dd, J = 26.9, 14.2 Hz, 2H), 4.58 (s, 1H), 3.35 (d, J = 6.4 Hz, 1H), 3.27–3.04 (m, 5H), 2.37–2.28 (m, 1H), 2.22 (dd, J = 17.2, 9.5 Hz, 2H), 2.11–2.01 (m, 4H), 1.76 (t, J = 12.9 Hz, 1H), 1.64 (dd, J = 22.0, 11.3 Hz, 2H), 1.56–1.35 (m, 4H), 1.26 (dd, J = 15.6, 10.8 Hz, 2H), 1.19–1.09 (m, 7H), 0.89 (d, J = 7.0 Hz, 3H), 0.70 (t, J = 8.9 Hz, 3H). 13C-NMR (CDCl3) δ ppm 217.18, 173.23, 170.02, 139.19, 129.07, 128.30, 127.19, 117.48, 74.81, 69.97, 60.02, 58.39, 47.77, 47.40, 45.67, 45.07, 44.17, 42.02, 36.96, 36.24, 34.67, 31.68, 30.66, 27.09, 26.54, 25.07, 17.11, 15.12, 14.41, 11.76. HRMS (ESI) of C32H42N4O5S2 [M+H]+ calcd, 627.2663; found, 627.2659.
14-O-[(2-l(−)Phenylglycinamide-1,3,4-thioacetyl-5-yl)thioacetyl] mutilin (6d): Compound 6d was prepared according to the general procedure from 4 and l-phenylglycine. The crude product was purified over silica gel column chromatography to give 6d (0.79 g, yield 63%), mp 86–89 °C. IR (KBr): υmax cm−1 3447, 2927, 1731, 1522, 1457, 1284, 1153, 1117, 1018, 980, 938, 916, 699. 1H-NMR (CDCl3) δ ppm 7.36 (m, J = 13.5, 9.7, 4.9 Hz, 5H), 6.39 (dd, J = 17.4, 11.0 Hz, 1H), 5.73 (d, J = 8.5 Hz, 1H), 5.27 (d, J = 10.9 Hz, 1H), 5.13 (dd, J = 17.4, 1.5 Hz, 1H), 4.85 (s, 1H), 4.10 (t, J = 7.1 Hz, 1H), 3.98 (s, 2H), 3.33 (d, J = 6.4 Hz, 1H), 2.31–2.14 (m, 3H), 2.09–1.98 (m, 4H), 1.72 (d, J = 2.5 Hz, 1H), 1.67–1.57 (m, 2H), 1.52–1.39 (m, 5H), 1.33 (d, J = 13.6 Hz, 1H), 1.25 (t, J = 7.1 Hz, 2H), 1.15–1.07 (m, 4H), 0.85 (d, J = 7.0 Hz, 3H), 0.70 (dd, J = 7.0, 2.1 Hz, 3H). 13C-NMR (CDCl3) δ ppm 217.43, 172.14, 171.69, 167.20, 139.71, 139.35, 129.74, 129.24, 127.40, 117.84, 77.50, 77.26, 75.14, 71.00, 60.93, 59.96, 58.64, 45.97, 45.18, 44.51, 42.41, 37.24, 36.63, 34.99 (s), 30.94, 27.37, 26.92, 25.37, 21.58, 17.30, 15.36, 14.74, 12.01. HRMS (ESI) of C32H42N4O5S2 [M+H]+ calcd, 627.2669; Found, 627.2661.
3.3. Antibacterial Activity
3.3.1. MIC Determination
The MIC studies were performed on MRSA, MRSE, S. aureus CVCC 1882, S. epidermidis CMCC 26069, E. coli CVCC 1570, and B. cereus CMCC (B) 63302 using the agar dilution method according to the National Committee for Clinical Laboratory Standards (NCCLS). Compounds were dissolved in 25%–45% DMSO in water to prepare a solution that had a concentration of 1.28 mg/mL. Tiamulin fumarate used as reference drug was directly dissolved in 10 mL distilled water. All the solutions were then diluted two-fold with distilled water to provide 11 dilutions down to the final concentration (0.625 μg/mL). The dilutions (2 mL) of each test compound/drug were incorporated into 18 mL hot Mueller-Hinton agar medium, which resulted in the final concentration of each dilutions decreasing tenfold.
Inoculums of MRSA, MRSE, S. aureus, S. epidermidis, E. coli, and B. cereus were prepared from blood slants and adjusted to approximately 105–106 CFU/mL with sterile saline (0.90% NaCl). A 10 μL amount of bacterial suspension was spotted onto Mueller-Hinton agar plates containing serial dilutions of the compounds/drugs. The plates were incubated at 36.5 °C for 24–48 h. The MIC was defined as the minimum concentration of the compound needed to completely inhibit bacterial growth. The same procedure was repeated in triplicate.
3.3.2. Oxford Cup Assay
Oxford cup assays were performed to evaluate the rate of inhibition of bacterial growth. Inoculums were prepared in 0.9% saline using McFarland standard and spread uniformly on Mueller-Hinton agar plates. All the compounds were diluted to 320 and 160 μg/mL and the resulting solutions were added to the Oxford cups which were placed at equal distances above agar surfaces. The zone of inhibition for each concentration was measured after 24 h incubation at 37 °C. The same procedure was repeated in triplicate.
3.4. Molecular Modeling Studies
The crystal structure of 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin (PDB ID: 1XBP) [10] was used for all simulations with Homdock software in the Chil2 package [17] combining of a Graph based molecular alignment (GMA) tool and a Monte-Carlo/Simulated Annealing (MC/SA) algorithm based docking (GlamDock) tool. Tiamulin was the template for flexible molecular alignment, and the interaction was optimized by GlamDock according to the ChillScore scoring function based on ChemScore with a smooth, improved potential. All the compounds were prepared with Avogadro software [21], including a 5,000 steps Steepest Descent and 1000 steps Conjugate Gradients geometry optimization based on the MMFF94 force field. The 50S ribosomal subunit was extracted from the crystal structure of 1XBP and transformed to mol2 format. The docking position was set to the binding site of tiamulin. All the compounds were superposed to tiamulin as the template by the GMA, and the placement of compounds were subjected to a 200 steps’ gradient optimization by Glamdock engine. All the other parameters were set to be default.
As a result of calculations, each compound, which has the best RMSD compared to tiamulin conformation, was kept for binding affinity comparison. The binding affinity between compounds and receptor were estimated by ChillScore. Hydrogen bonds and other interactions were detected by PoseView [22] and all the figures were generated by PyMol 1.5.03 [23].
3.5. ADMET Prediction
The prediction of ADMET properties is important in the drug selection and promotion process so as to avoid inappropriate compounds before substantial time and money are invested in testing [24,25]. The pharmacokinetic and toxic profiles of the tested compounds were predicted by the ADMET descriptors protocol in ACD/Labs. The parameters with higher prediction accuracy and/or Reliability Index values (RI; a number ranging from 0 to 1 (0: Unreliable prediction, 1: Ideal, fully reliable prediction), is used to estimate reliability of prediction.) containing absorption, Log BB, Log PS, PPB, Vd and LD50 as ADMET descriptors, were selected and predicted using ACD/Percepta Platform available online. In order to further understand the above-mentioned properties, pKa and Log p also were predicted.
4. Conclusions
In summary, we have synthesized seven new pleuromutilin derivatives containing thiadiazole moieties. In evaluation of the in vivo antibacterial activity of the synthesized compounds, all the synthesized compounds showed moderate to good inhibitory characteristics. Compound 6d, bearing a l(−)phenylglycinamide group on the C-14 glycolic acid side chain, was the most active antibacterial agents against MRSA (MIC = 0.5 μg/mL), MRSE (MIC = 4 μg/mL), S. aureus (MIC = 0.5 μg/mL), S. epidermidis (MIC = 2 μg/mL) and B. cereus (MIC = 0.25 μg/mL). Docking studies revealed that the binding free energies (ΔGb) were in the range of −10.42 to −15.09 kcal/mol, with an RMSD range of 1.00 to 1.23 Å. Compound 6d with the most antibacterial activity displayed the highest binding affinity (ΔGb = −15.09). All of the compounds were further predicted their ADMET properties including absorption, Log BB, Log PS, PPB, Vd and LD50, as well as pKa and Log p. The results indicate that the seven compounds might have moderate to good ADMET properties and the compound 6d which shows the highest antibacterial activity among them may be a drug-like compound.
Acknowledgments
This work was financed by Basic Scientific Research Funds in Central Agricultural Scientific Research Institutions (No. 1610322014003), The Agricultural Science and Technology Innovation Program (ASTIP) and the “Five-Year” plan of national science and technology projects in rural areas (No. 2011AA10A214).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/19050/s1.
Author Contributions
RFS, JPL, and YL designed research; RFS, GHW, XMX, SJL, CZ and YPY performed research and analyzed the data; RFS and YL wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 2–4, 5a–c and 6a–d are available from the corresponding author.
References
- 1.Kavanagh F., Hervey A., Robbins W.J. Antibiotic Substances From Basidiomycetes: VIII. Pleurotus Multilus (Fr.) Sacc. and Pleurotus Passeckerianus Pilat. Proc. Natl. Acad. Sci. USA. 1951;37:570–574. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arigoni D. Structure of a new type of terpene. Gazz. Chem. Ital. 1962;92:884–901. [Google Scholar]
- 3.Birch A.J., Holzapfel C.W., Richards R.W. Diterpenoid nature of pleuromutilin. Chem. Ind. 1963;14:374–375. [Google Scholar]
- 4.Shang R.F., Liu Y., Xin Z.J., Guo W.Z., Guo Z.T., Hao B.C., Liang J.P. Synthesis and antibacterial evaluation of novel pleuromutilin derivatives. Eur. J. Med. Chem. 2013;63:231–238. doi: 10.1016/j.ejmech.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y.Z., Liu Y.H., Chen J.X. Pleuromutilin and its derivatives—The lead compounds for novel antibiotics. Mini Rev. Med. Chem. 2012;12:53–61. doi: 10.2174/138955712798868968. [DOI] [PubMed] [Google Scholar]
- 6.Moody M.N., Morrison L.K., Tyring S.K. Retapamulin: What is the role of this topical antimicrobial in the treatment of bacterial infections in atopic dermatitis? Skin Ther. Lett. 2010;15:1–4. [PubMed] [Google Scholar]
- 7.Sader H.S., Paukner S., Ivezic-Schoenfeld Z., Biedenbach D.J., Schmitz F.J., Jones R.N. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs) J. Antimicrob. Chemother. 2012;67:1170–1175. doi: 10.1093/jac/dks001. [DOI] [PubMed] [Google Scholar]
- 8.Shang R.F., Wang J.T., Guo W.Z., Liang J.P. Efficient antibacterial agents: A review of the synthesis, biological evaluation and mechanism of pleuromutilin derivatives. Curr. Top. Med. Chem. 2013;13:3013–3025. doi: 10.2174/15680266113136660217. [DOI] [PubMed] [Google Scholar]
- 9.Novak R., Shlaes D.M. The pleuromutilin antibiotics: A new class for human use. Curr. Opin. Invest. Drugs. 2010;11:182–191. [PubMed] [Google Scholar]
- 10.Schlunzen F., Pyetan E., Fucini P., Yonath A., Harms J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004;54:1287–1294. doi: 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- 11.Davidovich C., Bashan A., Auerbach-Nevo T., Yaggie R.D., Gontarek R.R., Yonath A. Induced-fit tightens pleuromutilins, binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA. 2007;104:4291–4296. doi: 10.1073/pnas.0700041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long K.S., Hansen L.H., Jakobsen L., Vester B. Interaction of pleuromutilin derivatives with the ribosomal peptidyl transferase center. Antimicrob. Agent. Chemother. 2006;50:1458–1462. doi: 10.1128/AAC.50.4.1458-1462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popiolek L., Kosikowska U., Mazur L., Dobosz M., Malm A. Synthesis and antimicrobial evaluation of some novel 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Med. Chem. Res. 2013;22:3134–3147. doi: 10.1007/s00044-012-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliabadi A., Eghbalian E., Kiani A. Synthesis and evaluation of the cytotoxicity of a series of 1,3,4-thiadiazole based compounds as anticancer agents. Iran J. Basic Med. Sci. 2013;16:1133–1138. [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrakantha B., Isloor A.M., Shetty P., Fun H.K., Hegde G. Synthesis and biological evaluation of novel substituted 1,3,4-thiadiazole and 2,6-di aryl substituted imidazo [2,1-b] [1,3,4] thiadiazole derivatives. Eur. J. Med. Chem. 2014;71:316–323. doi: 10.1016/j.ejmech.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 16.Shang R.F., Pu X.Y., Xu X.M., Xin Z.J., Zhang C., Guo W.Z., Liu Y., Liang J.P. Synthesis and Biological Activities of Novel Pleuromutilin Derivatives with a Substituted Thiadiazole Moiety as Potent Drug-Resistant Bacteria Inhibitors. J. Med. Chem. 2014;57:5664–5678. doi: 10.1021/jm500374c. [DOI] [PubMed] [Google Scholar]
- 17.Marialke J., Tietze S., Apostolakis J. Similarity based docking. J. Chem. Inf. Model. 2008;48:186–196. doi: 10.1021/ci700124r. [DOI] [PubMed] [Google Scholar]
- 18.Vyas V.K., Gupta N., Ghate M., Patel S. Design, synthesis, pharmacological evaluation and in silico ADMET prediction of novel substituted benzimidazole derivatives as angiotensin II–AT1 receptor antagonists based on predictive 3D QSAR models. SAR QSAR Environ. Res. 2014;25:117–146. doi: 10.1080/1062936X.2013.868825. [DOI] [PubMed] [Google Scholar]
- 19.Lanevskij K., Japertasa P., Didziapetrisa R., Petrauskasa A. Ionization-specific QSAR models of blood-brain penetration of drugs. Chem. Biodivers. 2009;6:2050–2054. doi: 10.1002/cbdv.200900079. [DOI] [PubMed] [Google Scholar]
- 20.Waterbeemd H., Gifford E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- 21.Marcus D.H., Donald E.C., David C.L., Tim V., Eva Z., Geoffrey R.H. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stierand K., Maaß P., Rarey M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics. 2006;22:1710–1716. doi: 10.1093/bioinformatics/btl150. [DOI] [PubMed] [Google Scholar]
- 23.Delano W.L., Ultsch M.H., De Vos A.M., Wells J.A. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 24.Hou T.J., Xu X.J. ADME evaluation in drug discovery. 3. Modeling blood-brain barrier partitioning using simple molecular descriptors. J. Chem. Inf. Comput. Sci. 2003;43:2137–2152. doi: 10.1021/ci034134i. [DOI] [PubMed] [Google Scholar]
- 25.Stankovic N., Mladenovic M., Mihailovic M., Arambasic J., Uskokovic A., Stankovic V., Mihailovic V., Katanic J., Matic S., Solujic S., et al. Synthesis and toxicological studies of in vivo anticoagulant activity of novel 3-(1-aminoethylidene)chroman-2,4-diones and 4-hydroxy-3-(1-iminoethyl)-2H-chromen-2-ones combined with a structure-based 3-D pharmacophore model. Eur. J. Pharm. Sci. 2014;55:20–35. doi: 10.1016/j.ejps.2014.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.