Abstract
Five new taraxerene-type triterpenes, 2-nor-D-friedoolean-14-en-28-ol (1), 2-nor-d-friedoolean-14-en-3α,28-diol (2), 6α-hydroxy-2-nor-D-friedoolean-14-en-3,21-dione (3), 6α,11α,29-trihydroxy-D-friedoolean-14-en-3,16,21-trione (4), and 6α,23,29-trihydroxy-D-friedoolean-14-en-3,16,21-trione (5), were isolated from the MeOH extract of the branch barks of Davidia involucrata, together with five known compounds. Their structures were elucidated by means of various spectroscopic analyses. Five of the identified compounds showed moderate cytotoxicities against the cell proliferation of SGC-7901, MCF-7, and BEL-7404.
Keywords: Davidia involucrata, Nyssaceae, taraxerene, triterpenoid
1. Introduction
Davidia involucrata Baill., an ornamental tree known as the Chinese dove tree or handkerchief tree, is the only species in genus Davidia. D. involucrata is a relic deciduous tree species of the Tertiary period with important ecological, scientific and horticultural values [1,2,3]. An initial and the only report of study on chemical components of D. involucrata besides our program appeared in 1989, which revealed the presence of sterols, tannins and triterpenes [4]. The branch barks of D. involucrata have been intensively studied in our previous work and were found containing diverse constituents including flavonoids, alkaloids, lignans, and phenols, among which were three alkaloids including vicosamide, strictosidinic acid and puimiloside, which were proposed to be the intermediate precursors between strictosamide and camptothecin [5,6,7,8,9,10,11,12]. Moreover, we have recently reported the identification of two novel 2-nor-ursane triterpenes having a unique five-membered A-ring from the water insoluble fraction of the branch barks of D. involucrata [13].
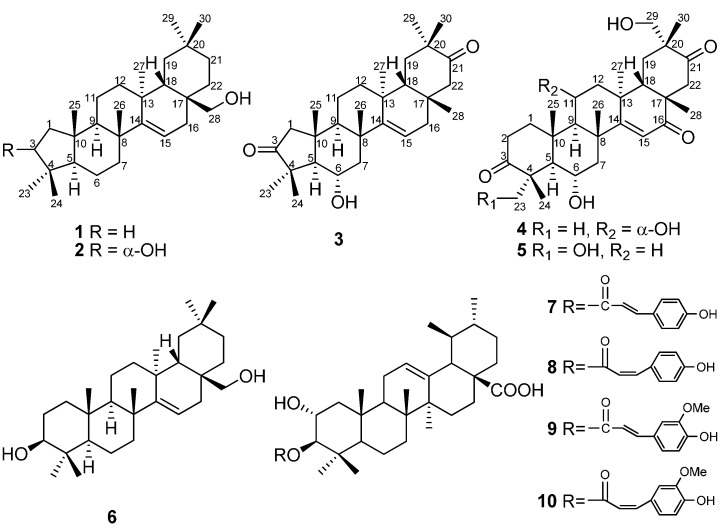
Our continuing work led to the isolation of another five novel triterpenoids, namely davinvolunols A-B (1–2) and davinvolunones A-C (3–5), together with a known taraxerene triterpene, myricadiol (6) [14,15,16], and four known triterpene esters, 3β-O-trans-p-coumaroyl-2α-hydroxy-urs-12-en-28-oic acid (7) [17,18,19], 3β-O-cis-p-coumaroyl-2α-hydroxy-urs-12-en-28-oic acid (8), 3β-O-trans-p-feruloyl-2α-hydroxy-urs-12-en-28-oic acid (9), 3β-O-cis-p-feruloyl-2α-hydroxy-urs-12-en-28-oic acid (10) [19] (Figure 1). Compounds 1–3 feature a contracted five-membered A-ring, whereas 4–5 are highly oxidized. The novel structures were unequivocally determined by extensive spectroscopic analysis and comparison with literature data. We herein report in this paper the structure elucidation of the new triterpenes, as well as the in vitro cytotoxic activities against three tumor cell lines (SGC-7901, MCF-7 and BEL-7404) of the isolated compounds.
Figure 1.
Chemical structures of compounds isolated from the branch barks of Davidia involucrata.
2. Results and Discussion
Compound 1, a white amorphous powder, had a molecular formula of C29H48O as determined by HR-TOF-MS at m/z = 435.3596 [M + Na]+ (calcd for C29H48ONa, 435.3603). The IR spectrum exhibited absorption bands due to hydroxyl (3560 cm−1) and olefinic (1640 cm−1) groups. Observed in the 1H-NMR (500 MHz, CDCl3 and CD3OD) spectrum were signals for seven tertiary methyl groups at δH 0.87, 0.94, 0.94, 1.05, 1.07, 1.08 and 1.22 (s, each 3H), while resonances at δH 3.10 and 3.23 (d, J = 10.9 Hz, each 1H) were attributed to proton signals attached to an oxygenated methylene carbon. In addition, one olefinic proton at δH 5.51 (dd, J = 8.2, 3.1 Hz, 1H) of a trisubstituted double bond, coupled with the protons of a methylene at δH 1.68 (dd, J = 15.2, 3.1 Hz, 1H) and 2.13 (dd, J = 15.2, 8.2 Hz, 1H) as deduced from their coupling constants, was recognized in the 1H-NMR spectrum. The 13C-NMR (125 MHz, CDCl3 and CD3OD) spectrum of 1 showed 29 carbon signals, which were classified from DEPT and HSQC data as seven methyls, ten methylenes, three methines, six quaternary carbons, one oxygen-bearing secondary carbon and a trisubstituted double bond. One of the six degrees of unsaturation came from a trisubstituted double bond at δC 116.0 and 158.5, and the remaining five degrees of unsaturation were therefore indicative of a pentacyclic skeleton. The characteristics of NMR data of compound 1 were comparable to those of myricadiol (6) [13]. Comparison of the MS, 1D- and 2D-NMR data of 1 with those of 6 revealed that they both shared the same B/C/D/E rings, indicating that 1 might bear a contracted five-membered A-ring. This inference was confirmed by the HMBC and ROESY experiments. The key HMBC correlations (Figure 2) from H3-23 (δH 1.22, s, 3H) and H3-24 (δH 0.94, s, 3H) to C-3 (δC 29.8), C-4 (δC 47.5) and C-5 (δC 55.7) enabled the establishment of an unusual five-membered A-ring. The observed ROESY cross-peaks (Figure 3) of H3-24/H3-25, H3-25/H-26, and H-18/H-28 indicated that they were on the same side of the molecule, and were arbitrarily assigned as β-oriented. As a consequence, the ROESY correlations of H3-23/H-5, H-5/H-9, and H-9/H3-27 revealed that they were α-configured. Hence, the structure of compound 1 was determined to be 2-nor-d-friedoolean-14-en-28-ol, and this substance has been accorded the trivial name davinvolunol A.
Figure 2.
Key HMBC (H→C) correlations of compounds 1–5.
Figure 3.
Key ROESY (H↔C) correlations of compounds 1–5.
Compound 2 was isolated as a white amorphous solid. It was assigned to have a molecular formula of C29H48O2 by HR-TOF-MS at m/z = 451.3546 [M + Na]+ (calcd for C29H48O2Na, 451.3552). The 1H and 13C-NMR spectra of 2 were highly similar to those of 1 except for the absence of methylene proton signals at δH 2.01 and 2.25 (m, each 1H) with the presence of an additional oxymethine proton signal at δH 3.18 (dd, J = 9.8, 6.8 Hz, 1H) instead, suggesting that a hydroxyl group was attached to C-3. This was confirmed by the key HMBC correlations (Figure 2) from H3-23 (δH 1.05, s, 3H) and H3-24 (δH 0.91, s, 3H) to C-3 (δC 77.3). The ROESY correlations (Figure 3) of H-3/H3-24, H3-25 implied that H-3 was α-orientated, and thus 3-OH was in β-orientation. Other observed ROESY effects indicated the relative configuration of the remaining part of the molecule of 2 was identical with that of 1. Thus, the structure of compound 2 (davinvolunol B) was elucidated as 2-nor-d-friedoolean-14-en-3α,28-diol.
Compound 3, a white amorphous solid, presented a molecular formula of C29H44O3, as determined by HR-TOF-MS at m/z = 463.3180 [M + Na]+ (calcd for C29H44O3Na, 463.3188) with eight double-bond equivalents. The IR spectrum revealed the presence of hydroxyl (3437 cm−1), carbonyl (1726 cm−1) and olefinic (1635 cm−1) groups. The 1H-NMR (400 MHz, CDCl3) spectrum showed eight tertiary methyls at δH 0.78, 0.82, 1.02, 1.05, 1.06, 1.11, 1.29 and 1.32 (s, each 3H), and a characteristic olefinic proton signal at δH 5.60 (dd, J = 8.1, 2.9 Hz, 1H) coupled with the protons of a methylene at δH 1.79 (dd, J = 14.5, 8.1 Hz, 1H) and 2.05 (dd, J = 14.5, 2.9 Hz, 1H), as deduced from their coupling constants, indicative of the same D-friedoolean-14-en skeleton of 3 as compounds 1 and 2. In addition, an oxygenated methylene at δH 3.91 (ddd, J = 15.0, 10.9, 4.1 Hz, 1H) coupled with the protons of a methylene at δH 1.38 (t, J = 10.9 Hz, 1H) and 2.28 (dd, J = 10.9, 4.1 Hz, 1H) was observed in the 1H-NMR spectrum of 3. The 13C-NMR (100 MHz, CDCl3) spectrum, along with HSQC and DEPT data, resolved 29 carbons that came from eight methyls, seven methylenes, three methines, six quaternary carbons, an oxygenated secondary carbon, two keto carbonyls and a trisubstituted double bond. The HMBC correlations (Figure 2) from H3-23 (δH 1.29, s, 3H) and H3-24 (δH 1.32, s, 3H) to C-3 (δC 219.7) rationalized the existence of a keto group at C-3, while another keto group was assigned to C-21 as deduced from the HMBC correlations from H3-29 (δH 1.06, s, 3H) and H3-30 (δH 1.05, s, 3H) to C-21 (δC 219.7). An OH group was attached at C-6 as deduced from the key HMBC correlations of H-6 (δH 3.91, ddd, J = 15.0, 10.9, 4.1 Hz, 1H) to C-5 (δC 58.8) and C-7 (δC 50.4), as well as the observation of an AMX spin system of H-5 (δH 1.61, d, J = 10.9 Hz, 1H), H-6 (δH 3.91, ddd, J = 15.0, 10.9, 4.1 Hz, 1H), H-7a (δH 1.38, t, J = 10.9 Hz, 1H) and H-7b (δH 2.28, dd, J = 10.9, 4.1 Hz, 1H) in the 1H-NMR spectrum. A Δ14 double bond was determined by the HMBC correlations from H3-26 (δH 1.11, s, 3H) to C-14 (δC 157.3), and from H-16a (δH 1.79, dd, J = 14.5, 8.1 Hz) and H-16b (δH 2.05, dd, J = 14.5, 2.9 Hz, 1H) to C-14 (δC 157.3) and C-15 (δC 116.5). The relative stereochemistry of 3 was established by the ROESY spectrum. The ROESY correlations (Figure 3) of H-6/H3-24, H3-25, H3-26 and H-18/H3-28, H3-30 implied that H-6, H3-24, H3-25, H3-26, H3-28, H3-30 were cofacial and placed in β-orientation, and thus indicated the α-orientation of the hydroxyl group at C-6. The structure of compound 3 was therefore determined as 6α-hydroxy-2-nor-d-friedoolean-14-en-3,21-dione and named davinvolunone A.
Compound 4 was obtained as a white amorphous powder. Its molecular formula was deduced to be C30H44O6 by the HR-TOF-MS at m/z 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036). The IR spectrum exhibited the presence of hydroxyl (3500 cm−1), carbonyl (1700 cm−1) and conjugated carbonyl (1610 cm−1) functional groups. The 1H-NMR spectrum of compound 4 showed the presence of proton signals for seven tertiary methyls at δH 0.98, 1.06, 1.07, 1.22, 1.26, 1.35 and 1.36 (s, each 3H), and resonances at δH 3.98 (ddd, J = 15.2, 11.1, 4.2 Hz, 1H) and δH 4.28 (t, J = 7.0 Hz, 1H) attributed to proton attached to two oxygenated methine carbons, as well as signals of two protons at δH 3.26 and 3.71 (d, J = 10.3 Hz, each 1H) attached to an oxygenated methylene carbon. In addition, an olefinic proton signal at δH 5.96 (br. s, 1H) was observed in the 1H-NMR spectrum. The 13C-NMR (400 MHz, CD3OD) including DEPT experiments showed that four of the nine degrees of unsaturation came from one trisubstituted double bond at δC 118.8 and 177.9, and three keto carbonyls at δC 206.3, 219.9 and 222.5. The remaining five degrees of unsaturation were therefore indicative of a pentacyclic skeleton. A Δ14 double bond was determined by the key HMBC correlations (Figure 2) from H3-26 (δH 1.26, s, 3H) and H3-27 (δH 1.22, s, 3H) to C-14 (δC 177.9). Key HMBC correlations from the olefinic proton at δH 5.96 (br. s, 1H) to a carbonyl at δC 206.3 indicated that one keto group was placed at C-16. Another two keto groups were assigned to C-3 and C-21, which was confirmed by the observed key HMBC correlations from H3-23 (δH 1.35, s, 3H) and H3-24 (δH 1.36, s, 3H) to C-3 (δC 222.5), and correlations from H3-30 (δH 1.06, s, 3H) to C-21 (δC 219.9). Moreover, three hydroxyls were attached to C-6, C-11 and C-29 respectively, which was supported by the key HMBC correlations observed from H-5 (δH 1.78, d, J = 11.1 Hz, 1H), H-7a (δH 1.49, t, J = 11.1 Hz, 1H) and H-7b (δH 2.30, dd, J = 11.1, 4.2 Hz, 1H) to C-6 (δC 67.5), from H-9 (δH 1.60, d, J = 7.0 Hz, 1H) and H-12a (δH 1.98, d, J = 14.5 Hz, 1H) to C-11 (δC 65.9), and from H3-30 (δH 1.06, s, 3H) to C-29 (δC 71.2). ROESY experiment was undertaken to establish the relative configuration of 4. The significant ROESY correlations (Figure 3) of H-11/H3-25, H-6/H3-24, H3-25 and H3-26, H-18/ H3-28 and H3-30 indicated that they were on the same side of the molecule, and were assigned as β-oriented. As a consequence, the ROESY correlations of H3-23/H-5, H-5/H-9 and H-9/H3-27 revealed that they were α-configured. Thus, the structure of compound 4 (davinvolunone B) was determined as 6α,11α,29-trihydroxy-d-friedoolean-14-en-3,16,21-trione.
Compound 5, obtained as a white amorphous powder, was assigned to have a molecular formula of C30H44O6 by HR-TOF-MS at m/z = 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036), which was an isomer of 4. The NMR data of 5 were comparable to those of 4. Observed in the 1H-NMR spectra was the absence of proton signal of an oxymethine at H-11 (δH 4.28, t, J = 7.0 Hz, 1H), with the appearance of an additional oxygenated methylene proton resonances at δH 3.58 (d, J = 10.2 Hz, 1H) and 3.80 (d, J =10.2 Hz, 1H) in the 1H-NMR spectra of 5. Correspondingly, an oxymethine carbon at δC 65.9 (C-11) was absent with the presence of an oxygenated secondary carbon at δC 72.5 in the 13C-NMR spectra of 5. The HMBC correlations from H-23a (δH 3.58, d, J = 10.2 Hz, 1H) and H-23b (δH 3.80, d, J = 10.2 Hz, 1H) to C-3 (δC 220.9), C-4 (δC 54.3) and C-5 (δC 54.2) indicated that a hydroxyl group was attached to C-23, which was also confirmed by the key ROESY correlations (Figure 3) of H-23/H-5, as well as the downfield shifted carbon resonance of C-4, as compared with those of compound 4. Analysis of the ROESY spectrum suggested the relative configuration of the remainder of the molecule of 5 was identical with that of 4. Compound 5 (davinvolunone C) was therefore determined as 6α,23,29-trihydroxy-d-friedoolean-14-en-3,16,21-trione.
The novel compounds, namely davinvolunols A–B (1–2) and davinvolunones A–C (3–5), are all taraxerene-type triterpenoids with a d-friedoolean-14-en skeleton. To the best of our knowledge, compounds 1–3, as well as previously reported two ursane triterpenes, davinvolunic acid A and B from the same plant [13], are the first reported 2-nor pentacyclic triterpenoids with 29 carbons and a five-membered A-ring. The results provided important, evolutionary and chemotaxonomic knowledge of monotypic genus Davidia.
The isolated triterpenoids were evaluated for cytotoxic activities against SGC-7901 human gastric cancer cells, MCF-7 human breast cancer cells and BEL-7404 human hepatoma cells. The cytotoxicities were summarized in Table 1. Among the pure triterpenoids isolated from Davidia involucrata, compounds 3–5, 7 and 9 showed moderate cytotoxic activities against all three cell lines. Moreover, davinvolunone B (4) exhibited the most potent cytotoxicities towards SGC-7901, MCF-7, and BEL-7404 cancer cells with IC50 value of 30.57 ± 1.63, 41.34 ± 1.24, and 37.29 ± 1.64 μM, respectively.
Table 1.
Cytotoxicities of compounds 1–7 and 9 against three human tumor cell lines (IC50, μM).
| Compounds | Cell Lines | ||
|---|---|---|---|
| SGC-7901 | MCF-7 | BEL-7404 | |
| 1 | >100 | >100 | >100 |
| 2 | >100 | >100 | >100 |
| 3 | 58.23 ± 2.36 | 65.35 ± 3.12 | 70.26 ± 4.21 |
| 4 | 30.57 ± 1.63 | 41.34 ± 1.24 | 37.29 ± 1.64 |
| 5 | 32.22 ± 1.22 | 42.54 ± 1.67 | 39.26 ± 1.14 |
| 6 | >100 | >100 | >100 |
| 7 | 35.47 ± 1.55 | 55.24 ± 2.28 | 53.82 ± 2.11 |
| 9 | 37.65 ± 1.85 | 58.93 ± 2.58 | 54.77 ± 2.05 |
| Doxorubicin | 0.19 ± 0.032 | 0.08 ± 0.007 | 0.12 ± 0.011 |
3. Experimental Section
3.1. General
Optical rotations were measured with a Jasco DIP-180 digital polarimeter. IR spectra were recorded with a Perkin-Elmer 1750 FT-IR spectrometer in KBr discs. High-resolution mass spectra were recorded on an IonSpec 4.7 Tesla FTMS instrument. The NMR spectra were obtained by using a Bruker AV-400 or a DRX-500 spectrometer. Semipreparative HPLC was performed with an Elite P230 pump equipped with a Schambeck SFD GmbH RI2000 detector and a YMC-Pack SIL column (250 × 10 mm, 5 µm). Sephadex LH-20 (25–100 µm, Pharmacia Fine Chemicals, Uppsala, Sweden), Silica gel (200–300 mesh) and Silica gel H (Qingdao Oceanic Chemical Co., Qingdao, China) were used for column chromatography. Thin-layer chromatography was performed on TLC plates (H, Qingdao Oceanic Chemical Co.), with compounds visualized by spraying with 5% (v/v) H2SO4 in alcohol solution, followed by heating.
3.2. Plant Material
The branch barks of D. involucrata were collected from Shennongjia Forest Region of Hubei province, P. R. China in 2002. The plant was authenticated by Prof. Y. P. Yang. A voucher specimen (No. 12245) is deposited in the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China.
3.3. Extraction and Isolation
The air-dried branch barks of the plant (10 kg) were extracted with MeOH (2 × 10 L) at room temperature to give 600 g of crude extract, which was solubilized in water (1 L) and then filtered. The water-insoluble fraction (175 g) was separated on a silica gel column (200–300 mesh, 80 × 5 cm, i.d.) that was eluted with a gradient of Petroleum ether/Acetone (from 10:1 to 10:5, v/v) to afford seven fractions 1–7.
Fraction 5 (6 g) was subjected to Sephadex LH-20 column (120 × 3 cm, i.d.) eluting with CHCl3/MeOH (1:1, v/v) to afford five fractions 5-1–5-5. Fraction 5-2 was subjected to chromatography over a silica gel column (silica gel H, 25 × 2.5 cm, i.d.) with a gradient of Petroleum ether/CHCl3/EtOAc (10:6:2, v/v) to yield 1 (11.0 mg). Fraction 5-3 was purified by chromatography over a silica gel column (silica gel H, 25 × 2.5 cm, i.d.) eluting with a gradient of Petroleum ether/Acetone/MeOH (10/20/2, v/v) to afford 2 (15.2 mg), 3 (16.6 mg) and 6 (21.4 mg).
And fraction 7 (8 g) was subjected to Sephadex LH-20 column (120 × 3 cm, i.d.) chromatography eluting with MeOH to afford four fractions 7-1–7-4. Fraction 7-4 was then purified by semi-preparative HPLC with a gradient of CHCl3–MeOH (100:4, v/v) to give 4 (7.2 mg) and 5 (6.3 mg).
3.4. Spectral Data
Davinvolunol A (1): white amorphous powder;  = +99.0 (c 0.15, CHCl3); IR (KBr) νmax: 3560, 2950, 1640, 1470 cm−1; HR-TOF-MS m/z = 435.3596 [M + Na]+ (calcd for C29H48ONa, 435.3603); 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (125 MHz, CDCl3 and CD3OD) data (Table 2 and Table 3).
= +99.0 (c 0.15, CHCl3); IR (KBr) νmax: 3560, 2950, 1640, 1470 cm−1; HR-TOF-MS m/z = 435.3596 [M + Na]+ (calcd for C29H48ONa, 435.3603); 1H-NMR (500 MHz, CDCl3 and CD3OD) and 13C-NMR (125 MHz, CDCl3 and CD3OD) data (Table 2 and Table 3).
Table 2.
1H-NMR Data of compounds 1–5.
| Position | 1a [δH (J in Hz)] | 2b [δH (J in Hz)] | 3c [δH (J in Hz)] | 4d [δH (J in Hz)] | 5d [δH (J in Hz)] |
|---|---|---|---|---|---|
| 1 | 0.80, m | 0.84, m | 1.60, d (14.3) | 2.05, m | 1.80, m |
| 1.53, m | 1.51, m | 1.79, d (14.3) | 2.12, m | 1.85, m | |
| 2 | – | – | – | 2.30, m | 2.32, m |
| 2.75, ddd (16.3, 11.2, 4.9) | 2.59, ddd (16.3, 11.2, 6.4) | ||||
| 3 | 2.01, m | 3.18, dd (9.8, 6.8) | – | – | – |
| 2.25, m | |||||
| 4 | – | – | – | – | – |
| 5 | 1.00, m | 0.98, m | 1.61, d (10.9) | 1.78, d (11.1) | 2.03, d (11.0) |
| 6 | 1.23, m | 1.21, m | 3.91, ddd (15.0, 10.9, 4.1) | 3.98, ddd (15.2, 11.1, 4.2) | 3.98, ddd (15.2, 11.2, 4.0) |
| 1.83, d (13.0) | 1.80, d (13.0) | ||||
| 7 | 1.35, m | 1.27, m | 1.38, t (10.9) | 1.49, t (11.1) | 1.50, t (11.2) |
| 2.03 dt-like (13.0) | 2.01 dt-like (13.0) | 2.28, dd (10.9, 4.1) | 2.30, dd (11.1, 4.2) | 2.28, dd (11.2, 4.0) | |
| 8 | – | – | – | – | – |
| 9 | 1.49, m | 1.51, m | 1.52, m | 1.60, d (7.0) | 1.70, m |
| 10 | – | – | – | – | – |
| 11 | 1.51, m | 1.50, m | 1.60, m | 4.28, t (7.0) | 1.85, m |
| 1.65 m | 1.60, m | 1.72, m | 1.92, m | ||
| 12 | 1.48, m | 1.46, m | 1.60, m | 1.98, d (14.5) | 1.80, m |
| 1.60, m | 1.53, m | 1.75, m | 2.12, d (14.5) | 1.92, m | |
| 13 | – | – | – | – | – |
| 14 | – | – | – | – | – |
| 15 | 5.51, dd (8.2, 3.1) | 5.47, dd (8.0, 3.1) | 5.60, dd (8.1, 2.9) | 5.96, br. s | 5.94, br. s |
| 16 | 1.68, dd (15.2, 3.1) | 1.66, dd (15.2, 3.1) | 1.79, dd (14.5, 8.1) | – | – |
| 2.13, dd (15.2, 8.2) | 2.09, dd (15.2, 8.0) | 2.05, dd (14.5, 2.9) | |||
| 17 | – | – | – | – | – |
| 18 | 0.57, dd (13.5, 3.8) | 0.60, dd (13.5, 3.8) | 1.22, dd (13.5, 5.1) | 1.88, dd (13.5, 5.2) | 1.92, dd (13.5, 5.1) |
| 19 | 0.91, dd (13.5, 3.8) | 0.96, dd (13.5, 3.8) | 1.44, dd (13.5, 5.1) | 1.63, dd (13.5, 5.2) | 1.65, dd (13.5, 5.1) |
| 1.37, t (13.5) | 1.32, t (13.5) | 1.88, t (13.5) | 2.54, t (13.5) | 2.49, t (13.5) | |
| 20 | – | – | – | – | – |
| 21 | 1.53, m | 1.46, m | – | – | – |
| 1.65, m | 1.60, m | ||||
| 22 | 1.24, m | 1.20, m | 1.87, d (12.8) | 2.51, d (13.8) | 2.49, d (13.7) |
| 2.05, m | 2.03, m | 2.63, d (12.8) | 2.62, d (13.8) | 2.61, d (13.7) | |
| 23 | 1.22, s | 1.05, s | 1.29, s | 1.35, s | 3.58, d (10.2) |
| 3.80, d (10.2) | |||||
| 24 | 0.94, s | 0.91, s | 1.32, s | 1.36, s | 1.27, s |
| 25 | 0.87, s | 0.84, s | 0.82, s | 0.98, s | 1.13, s |
| 26 | 1.08, s | 1.02, s | 1.11, s | 1.26, s | 1.30, s |
| 27 | 1.07, s | 1.01, s | 1.02, s | 1.22, s | 0.96, s |
| 28 | 3.10 (d, 10.9) | 3.06 (d, 10.9) | 0.78, s | 1.07, s | 1.06, s |
| 3.23, (d, 10.9) | 3.18, (d, 10.9) | ||||
| 29 | 1.05, s | 1.02, s | 1.06, s | 3.26, d (10.3) | 3.24, d (10.4) |
| 3.71, d (10.3) | 3.69, d (10.4) | ||||
| 30 | 0.94, s | 0.90, s | 1.05, s | 1.06, s | 1.05, s |
a Recorded in CDCl3 and CD3OD (10:1) in 500 MHz; b Recorded in CDCl3 and CD3OD (10:1) in 400 MHz; c Recorded in CDCl3 in 400 MH; d Recorded in CD3OD in 400 MHz.
Table 3.
13C-NMR Data of compounds 1–5.
| Position | 1a (δc mult.) | 2b (δc mult.) | 3c (δc mult.) | 4d (δc mult.) | 5e (δc mult.) |
|---|---|---|---|---|---|
| 1 | 38.2 (CH2) | 38.1 (CH2) | 37.3 (CH2) | 39.3 (CH2) | 36.4 (CH2) |
| 2 | – | – | – | 34.1 (CH2) | 31.6 (CH2) |
| 3 | 29.8 (CH2) | 77.3 (CH) | 219.7 (C) | 222.5 (C) | 220.9 (C) |
| 4 | 47.5 (C) | 47.7 (C) | 47.0 (C) | 49.2 (C) | 54.3 (C) |
| 5 | 55.7 (CH) | 55.7 (CH) | 58.8 (CH) | 59.9 (CH) | 54.2 (CH) |
| 6 | 19.9 (CH2) | 19.8 (CH2) | 67.3 (CH) | 67.5 (CH) | 67.2 (CH) |
| 7 | 40.6 (CH2) | 40.5 (CH2) | 50.4 (CH2) | 50.2 (CH2) | 50.3 (CH2) |
| 8 | 38.9 (C) | 38.9 (C) | 39.7 (C) | 41.2 (C) | 42.5 (C) |
| 9 | 48.5 (CH) | 48.5 (CH) | 47.0 (CH) | 55.9 (CH) | 47.2 (CH) |
| 10 | 40.3 (C) | 40.3 (C) | 38.5 (C) | 40.7 (C) | 40.0 (C) |
| 11 | 17.3 (CH2) | 17.2 (CH2) | 16.8 (CH2) | 65.9 (CH) | 17.7 (CH2) |
| 12 | 30.6 (CH2) | 30.5 (CH2) | 33.2 (CH2) | 43.3 (CH2) | 37.4 (CH2) |
| 13 | 37.6 (C) | 37.6 (C) | 37.3 (C) | 38.9 (C) | 39.3 (C) |
| 14 | 158.5 (C) | 158.3 (C) | 157.3 (C) | 177.9 (C) | 178.3 (C) |
| 15 | 116.0 (CH) | 116.1 (CH) | 116.5 (CH) | 118.8 (CH) | 119.4 (CH) |
| 16 | 32.6 (CH2) | 32.5 (CH2) | 37.5 (CH2) | 206.3 (C) | 206.3 (C) |
| 17 | 37.9 (C) | 37.9 (C) | 37.3 (C) | 47.1 (C) | 47.2 (C) |
| 18 | 44.8 (CH) | 44.7 (CH) | 48.8 (CH | 46.4 (CH) | 46.7 (CH) |
| 19 | 35.7 (CH2) | 35.7 (CH2) | 37.3 (CH2 | 31.4 (CH2) | 31.8 (CH2) |
| 20 | 28.9 (C) | 28.8 (C) | 43.1 (C) | 48.7 (C) | 48.5 (C) |
| 21 | 33.3 (CH2) | 33.2 (CH2) | 219.7 (C) | 219.9 (C) | 220.0 (C) |
| 22 | 27.8 (CH2) | 27.6 (CH2) | 51.5 (CH2) | 48.4 (CH2) | 48.2 (CH2) |
| 23 | 25.6 (CH3) | 25.9 (CH3) | 31.5 (CH3) | 32.2 (CH3) | 72.5 (CH2) |
| 24 | 21.3 (CH3) | 21.3 (CH3) | 19.7 (CH3) | 20.6 (CH3) | 16.4 (CH3) |
| 25 | 14.7 (CH3) | 14.7 (CH3) | 16.0 (CH3) | 17.9 (CH3) | 17.1 (CH3) |
| 26 | 25.7 (CH3) | 25.7 (CH3) | 25.6 (CH3) | 25.5 (CH3) | 24.6 (CH3) |
| 27 | 21.6 (CH3) | 21.5 (CH3) | 20.2 (CH3) | 29.1 (CH3) | 26.9 (CH3) |
| 28 | 65.1 (CH2) | 64.7 (CH2) | 32.5 (CH3) | 33.8 (CH3) | 33.9 (CH3) |
| 29 | 33.4 (CH3) | 33.3 (CH3) | 28.1 (CH3) | 71.2 (CH2) | 71.2 (CH2) |
| 30 | 29.8 (CH3) | 29.7 (CH3) | 23.0 (CH3) | 20.2 (CH3) | 20.2 (CH3) |
a Recorded in CDCl3 and CD3OD (10:1) in 125 MHz; b Recorded in CDCl3 and CD3OD (10:1) in 100 MHz; c Recorded in CDCl3 in 100 MHz; d Recorded in CD3OD in 100 MH; e Recorded in CD3OD in 125 MHz.
Davinvolunol B (2): white amorphous powder;  = +104.5 (c 0.17, CHCl3); IR (KBr) νmax: 3500, 2920, 1640, 1470 cm−1; HR-TOF-MS m/z = 451.3546 [M + Na]+ (calcd for C29H48O2Na, 451.3552); 1H-NMR (400 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (Table 2 and Table 3).
= +104.5 (c 0.17, CHCl3); IR (KBr) νmax: 3500, 2920, 1640, 1470 cm−1; HR-TOF-MS m/z = 451.3546 [M + Na]+ (calcd for C29H48O2Na, 451.3552); 1H-NMR (400 MHz, CDCl3 and CD3OD) and 13C-NMR (100 MHz, CDCl3 and CD3OD) data (Table 2 and Table 3).
Davinvolunone A (3): white amorphous powder;  = +116.8 (c 0.16, CHCl3); IR (KBr) νmax: 3437, 2930, 1726, 1635, 1456 cm−1; HR-TOF-MS m/z 463.3180 [M + Na]+ (calcd for C29H44O3Na, 463.3188); 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) data (Table 2 and Table 3).
= +116.8 (c 0.16, CHCl3); IR (KBr) νmax: 3437, 2930, 1726, 1635, 1456 cm−1; HR-TOF-MS m/z 463.3180 [M + Na]+ (calcd for C29H44O3Na, 463.3188); 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) data (Table 2 and Table 3).
Davinvolunone B (4): white amorphous powder;  = +126.0 (c 0.12, CHCl3); IR (KBr) νmax: 3500, 2930, 1700, 1610, 1456 cm−1; HR-TOF-MS m/z 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036); 1H-NMR (400 MHz, CD3OD) and 13C-NMR (100 MHz, CD3OD) data (Table 2 and Table 3).
= +126.0 (c 0.12, CHCl3); IR (KBr) νmax: 3500, 2930, 1700, 1610, 1456 cm−1; HR-TOF-MS m/z 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036); 1H-NMR (400 MHz, CD3OD) and 13C-NMR (100 MHz, CD3OD) data (Table 2 and Table 3).
Davinvolunone C (5): white amorphous powder;  = +118.2 (c 0.20, CHCl3); IR (KBr) νmax: 3489, 2920, 1700, 1610, 1453 cm−1; HR-TOF-MS m/z 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036); 1H-NMR (400 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD) data (Table 2 and Table 3).
= +118.2 (c 0.20, CHCl3); IR (KBr) νmax: 3489, 2920, 1700, 1610, 1453 cm−1; HR-TOF-MS m/z 523.3030 [M + Na]+ (calcd for C30H44O6Na, 523.3036); 1H-NMR (400 MHz, CD3OD) and 13C-NMR (125 MHz, CD3OD) data (Table 2 and Table 3).
3.5. Cytotoxicity Assay
The in vitro cytotoxic activity was determined by the MTT colorimetric method as described previously in our paper [13]. Three tumor cell lines, SGC-7901 cells (human gastric adenocarcinoma), MCF-7 cells (human breast cancer) and BEL-7404 (human hepatocellular carcinoma), provided by Department of Hepatobiliary Surgery, Affiliated Union Hospital, Fujian Medical University, were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 100 IU·mL−1 of penicillin-streptomycin at 37 °C in humidified atmosphere with 5% CO2. For the cytotoxicity tests, cells in exponential growth stage were harvested from culture by trypsin digestion and centrifuging at 180× g for 3 min, and then resuspended in fresh medium at a cell density of 5 × 104 per mL. The cell suspension was dispensed into a 96-well microplate at 100 μL and incubated for 24 h, and then treated with compounds at various concentrations. After 48 h of treatment, 50 μL solution of 1 mg·mL−1 MTT was added to each well, and the cells were further incubated for 4 h. Finally, supernatants were removed and the formazan crystals were dissolved by adding 100 μL DMSO and the optical density was recorded at 570 nm. All drug doses were tested with doxorubicin as positive control in triplicate.
4. Conclusions
The phytochemical study of the methanol extract from the branch barks of Davidia involucrata has led to the isolation of five new taraxerene-type triterpenes, namely 2-nor-d-friedoolean-14-en-28-ol (1), 2-nor-d-friedoolean-14-en-3α,28-diol (2), 6α-hydroxy-2-nor-d-friedoolean-14-en-3,21-dione (3), 6α,11α,29-trihydroxy-d-friedoolean-14-en-3,16,21-trione (4), and 6α,23,29-trihydroxy-d-friedoolean-14-en-3,16,21-trione (5), in addition to five known compounds. This is the first report of the isolation of taraxerene-type triterpenes from the plant, and compounds 1–3 are rarely reported 2-nor pentacyclic triterpenoids with 29 carbons and a five-membered A-ring. Our studies suggested that pure triterpenoids isolated from the relic deciduous plant demonstrated moderate cytotoxic activities against the proliferation of SGC-7901, MCF-7, and BEL-7404 cell lines.
Acknowledgments
Financial support from National Science and Technology Major Project (Grant No. 2011AA10A203, and 2012BAD19B03), National Natural Science Foundation of China (Grant No. 31371987), Chinese Ministry of Education (Grant No. 20103515110007), Fujian Provincial Department of Science and Technology Major Project (Grant No. 2012N4001), Natural Science Foundation of Fujian Province (Grant No. 2013J05046), Fujian Provincial Department of Education (Grant No. JA12125) is gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/17619/s1.
Supplementary Files
Author Contributions
Qing-Wei Tan took charge of the isolation, purification and structure elucidation of the isolated compounds. Ming-An Ouyang participated in the structure elucidation of the isolated compounds. Qi-Jian Chen and Zu-Jian Wu were responsible for the cytotoxicity assay.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–7 are available from the authors.
References
- 1.Li Y.X., Chen L., Lin J., Li Y.F., Chen F. Suppression subtractive hybridization cloning of cDNAs of differentially expressed genes in dovetree (Davidia involucrata) bracts. Plant Mol. Biol. Rep. 2002;20:231–238. [Google Scholar]
- 2.Sun J.F., Huang S.Q. White bracts of the Dove Tree (Davidia involucrata): Umbrella and pollinator lure? Mag. Arnold Arboretum. 2011;68:2–10. [Google Scholar]
- 3.Sun J.F., Gong Y.B., Renner S.S., Huang S.Q. Multifunctional bracts in the Dove Tree Davidia involucrata (Nyssaceae: Cornales): Rain protection and pollinator attraction. Am. Nat. 2008;171:119–124. doi: 10.1086/523953. [DOI] [PubMed] [Google Scholar]
- 4.Xiang G.Q., Lu F.S. Study on chemical components of Davidia involucrata Baill native to China. Acta Bot. Sin. 1989;31:540–543. [Google Scholar]
- 5.Ouyang M.A., Zhou J.N. Flavonoid glycosides from the leaves of Davidia involucrata. Guihaia. 2003;23:568–570. [Google Scholar]
- 6.Huang J., Liu R., Wang C.Z., Ouyang M.A. Progress in study on chemical constituents of Nyssaceae family. Subtrop. Plant Sci. 2005;34:70–75. [Google Scholar]
- 7.Liu R., Ouyang M.A. Structural identification of pumiloside, a quinolone alkaloid glycoside isolated from leaves of Davidia involucrata. Subtrop. Plant Sci. 2006;35:35–38. [Google Scholar]
- 8.Liu R., Wang C.Z., Ouyang M.A. Studies on the alkaloid glycoside constituents from branch-bark of Davidia involucrata. Guihaia. 2007;27:277–280. [Google Scholar]
- 9.Ouyang M.A., Huang J., Tan Q.W. Neolignan and lignan glycosides from branch bark of Davidia involucrata. J. Asian Nat. Prod. Res. 2007;9:487–492. doi: 10.1080/10286020600604146. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang M.A., Zhou J.N., Wang S.B. New caffeoyl derivatives from the leaves of Davidia involucrata. Nat. Prod. Res. 2008;22:471–476. doi: 10.1080/14786410600898730. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z.J., Ouyang M.A., Wang S.B. Two new phenolic water-soluble constituents from branch bark of Davidia involucrata. Nat. Prod. Res. 2008;22:483–488. doi: 10.1080/14786410600906426. [DOI] [PubMed] [Google Scholar]
- 12.Sirikantaramas S., Asano T., Sudo H., Yamazaki M., Saito K. Camptothecin: Therapeutic potential and biotechnology. Curr. Pharm. Biotechnol. 2007;8:196–202. doi: 10.2174/138920107781387447. [DOI] [PubMed] [Google Scholar]
- 13.Tan Q.W., Ouyang M.A., Gao B. Three new ring-A modified ursane triterpenes from Davidia involucrata. Molecules. 2014;19:4897–4906. doi: 10.3390/molecules19044897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr P.G., Longmore R.B., Betts T.J. Myricadiol and other taraxerenes from Scaevola spinescens. Planta Med. 1996;62:519–522. doi: 10.1055/s-2006-957961. [DOI] [PubMed] [Google Scholar]
- 15.Merfort I., Buddrus J., Nawwar M.A.M., Lambert J. A triterpene from the bark of Tamarix aphylla. Phytochemistry. 1992;31:4031–4032. [Google Scholar]
- 16.Sakurai N., Yaguchi Y., Inoue T. Triterpenoids from Myrica rubra. Phytochemistry. 1986;26:217–219. [Google Scholar]
- 17.Ogura M., Cordell G.A., Farnsworth N.R. Jacoumaric acid, a new triterpene ester from Jacaranda caucana. Phytochemistry. 1977;16:286–287. [Google Scholar]
- 18.Wang Q., Ju P., Wang Y.F., Luo S.D. Triterpenoids from Saurauia napaulensis (Saurauiaceae) Acta Bot. Yunnanica. 2008;30:121–124. [Google Scholar]
- 19.Häberlein H., Tschiersch K.P. Triterpenoids and flavonoids from Leptospermum scoparium. Phytochemistry. 1994;35:765–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.