Abstract
An eco-benign synthesis of pyrimidine derivatives 2a,b containing different functional groups with different electronic character starting from nitroalkenes 1a and 2b has been described. The structures for 1a and 2a,b have been characterized by single crystal X-ray diffraction analysis. The thermal data of the molecules pointed towards important structural aspects of their stability. The mechanism of their thermal decomposition is discussed. The thermodynamic parameters of the dissociation steps were evaluated and discussed. DFT calculations reveal that the compound 1a possesses a high calculated dipole moment value (8.28 D) which indicates its high reactivity towards its surrounding molecules.
Keywords: pyrimidine, X-ray, DFT, TGA, FT-IR
1. Introduction
Pyrimidine and its derivatives, which are important N-heterocyclic molecules, have received the consideration of researchers due to their significant biological and pharmaceutical properties. Pyrimidine and its derivatives have immense importance as antibiotics, and as crucial parts of many vitamins, and coenzymes. Indeed, some of them are the basic constituents of DNA and RNA, and play an important role in the constitutional properties of nucleic acids. Pyrimidine-derived biomolecules have received much attention from spectroscopists, drug, clinical, and industrial researchers because of their therapeutic importance [1,2,3,4,5,6,7,8,9]. Barbiturates (barbituric acid derivatives) are a class of central nervous system depressants, [10] utilized as sedatives, sleeping agents, hypnotics, anxiolytics, anticonvulsants, and anesthetics [7]. In addition, they have additional pharmacological activities as antioxidant anxiolitics, analeptics, anti-AIDA, immunomodulatory, anticancer agents and in other psychiatric disorders, and possess effects on motor and sensory functions [11,12,13,14,15] For example, phenobarbital, a 5-alkylated barbituric acid, was reported to exhibit sedative and hypnotic properties, and most importantly is an anticonvulsant [16].
In continuation of our research program [17,18,19,20,21,22,23], in the present work, the structures of some pyrimidine derivatives were characterized for the first time by single-crystal crystallography and TGA studies. DFT calculations were undertaken to study the optimized molecular structural parameters, vibrational frequencies, thermodynamic parameters, total dipole moment and HOMO-LUMO energy gap for the synthesized molecules using B3LYP/6-311G(d,p) basis set. The findings of these spectroscopic and theoretical studies are reported herein.
2. Results and Discussion
2.1. Synthesis of the Pyrimidine Derivatives
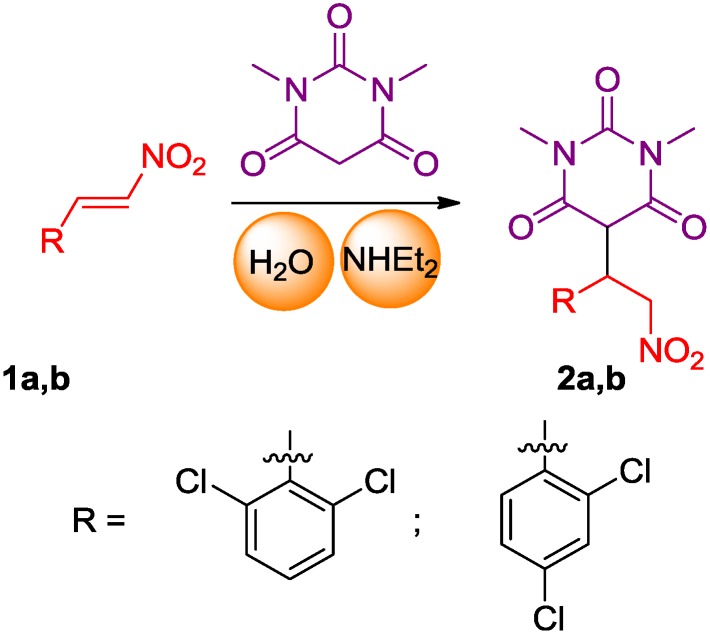
Green chemistry is being increasingly exploited as a powerful tool for the generation of privileged medicinal scaffolds and fine chemicals due to its numerous advantages, such as providing high enantio- and regioselectivity and more environmentally friendliness.
Recently, research groups have become involved in using green chemistry as a synthetic tool for the generation of valuable scaffolds to achieve new biological knowledge. The chemistry used in this paper involves an aqueous diethylamine catalyzed Michael addition of 1,3-dimethylbarbituric acid to nitro-olefins at room temperature for less than 1 h [16] (Scheme 1).
Scheme 1.
Synthesis of pyrimidine derivatives 2a and 2b.
2.2. X-ray Crystal Structures
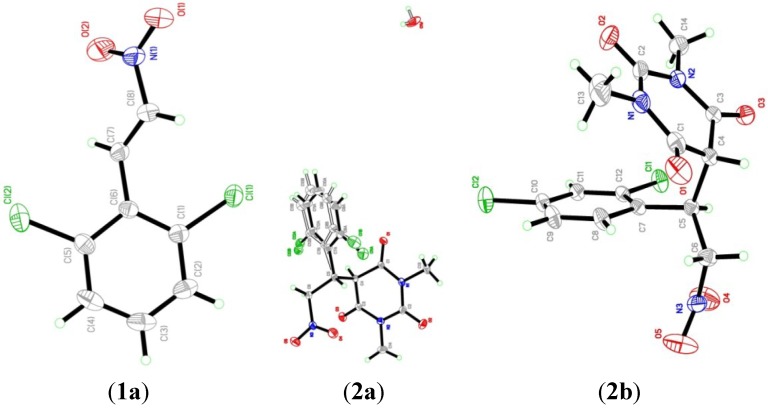
The structures of 1a and 2a,b were confirmed by single crystal X-ray analysis (Figure 1). Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 display the crystal data and main geometrical parameters of the compounds.
Figure 1.
ORTEP diagrams of the structures of 1a and 2a,b.
Table 1.
Crystal and experimental data of compounds 1a, 2a and 2b.
| Parameters | Compound 1a | Compound 2a | Compound 2b |
|---|---|---|---|
| Empirical formula | C8H5Cl2NO2 | C14H13Cl2N3O5·H2O | C14H13Cl2N3O5 |
| Formula weight | 218.03 | 391.02 | 374.17 |
| Temperature | 293(2) K | 293(2) K | 293(2) K |
| Wavelength (Mo Kα radiation, λ) | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system | monoclinic | trigonal | Monoclinic |
| Space group | P 21/c | R-3 | Cc |
| Unit cell dimensions | a = 3.8395 (1) Å, α = 90.00° b = 19.8653 (7) Å β = 90.6258 (10)° c = 11.9619 (4) Å, γ = 90.00° | a = 18.0177(7) Å, α = 90.00° b = 18.0177(7) Å, β = 90.00° c = 26.6558(9) Å, γ = 120.00° | a = 12.9432 (4) Å, α = 90.00° b = 9.3084 (4) Å, β = 101.3572 (16)° c = 13.4650 (5) Å, γ = 90.00° |
| Volume | 912.31 (5)Å3 | 7494.1(6) Å3 | 1590.51 (11) Å3 |
| Z | 4 | 6 | 4 |
| Density (calculated) | 1.587 Mg/m3 | 1.535 Mg/m3 | 1.563 Mg/m3 |
| Absorption coefficient | 0.67 mm−1 | 0.424 mm−1 | 0.439 mm−1 |
| F(000) | 440 | 3564 | 768 |
| Crystal size | 0.58 × 0.35 × 0.27 mm | 0.33 × 0.21 × 0.09 mm | 0.57 × 0.35 × 0.26 mm |
| Theta range for data collection | 2.7 to 30.5°. | 2.3 to 30.6°. | 2.7 to 30.6°. |
| Index ranges | −5 ≤ h ≤ 5, −28 ≤ k ≤ 28, −17 ≤ l ≤ 17 | −25 ≤h ≤ 25, −25 ≤ k ≤ 25, −38 ≤ l ≤ 38 | −18 ≤ h ≤ 18, −13 ≤ k ≤ 13, −19 ≤ l ≤ 19 |
| Reflections collected/ unique | 41303/2404 [R(int) = 0.042] | 107278/5130 [R(int) = 0.059] | 35961/4754 [R(int) = 0.024] |
| Completeness to theta = 30.57° | 99.8% | 99.6% | 99.6% |
| Absorption correction | multi-scan | multi-scan | multi-scan |
| Refinement | method Full-matrix least-squares on F2 | method Full-matrix least-squares on F2 | method Full-matrix least-squares on F2 |
| Goodness-of-fit on F2 | 1.04 | 1.03 | 1.27 |
| Largest diff. peak and hole | 0.28 and −0.22 e.Å−3 | 0.72 and −0.26 e.Å−3 | 0.22 and −0.25 e.Å−3 |
Table 2.
Selected geometric parameters (Å, °) of compound 1a.
| Bond | Experimental | Calculated |
| Cl1-C1 | 1.7320 (13) | 1.7314 |
| Cl2-C5 | 1.7295 (14) | 1.7405 |
| O1-N1 | 1.222 (2) | 1.2370 |
| O2-N1 | 1.211 (2) | 1.2372 |
| N1-C8 | 1.4563 (18) | 1.4511 |
| Atom Angle | Experimental | Calculated |
| O1-N1-O2 | 124.37 (14) | 125.1111 |
| O1-N1-C8 | 115.73 (14) | 115.6492 |
| O2-N1-C8 | 119.90 (14) | 119.2397 |
| Cl1-C1-C2 | 117.62 (11) | 124.7209 |
| Cl1-C1-C6 | 120.34 (9) | 113.6358 |
| Cl2-C5-C4 | 118.18 (11) | 114.3525 |
| Cl2-C5-C6 | 119.58 (10) | 123.4094 |
| N1-C8-C7 | 120.57 (13) | 119.8515 |
Table 3.
Hydrogen-bond geometry (Å, °) of compound 1a.
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| C7-H7A···O2 | 0.9300 | 2.4000 | 2.7323 (18) | 101.00 |
Table 4.
Selected geometric parameters (Å, °) of compound 2a.
| Bond | Experimental | Calculated |
| Cl1A-C8A | 1.738 (4) | 1.7277 |
| Cl1B-C8B | 1.721 (14) | NC |
| Cl2A-C12A | 1.751 (6) | 1.7365 |
| Cl2B-C12B | 1.715 (14) | NC |
| O1-C1 | 1.204 (2) | 1.2278 |
| O2-C2 | 1.205 (2) | 1.2325 |
| O3-C3 | 1.212 (2) | 1.2276 |
| O4-N3 | 1.196 (3) | 1.2362 |
| O5-N3 | 1.215 (3) | 1.2378 |
| O1W-O1W i | 0.969 (19) | NC |
| O1W-O1W ii | 0.97 (2) | NC |
| N1-C1 | 1.378 (2) | 1.3855 |
| N1-C13 | 1.473 (3) | 1.4513 |
| N1-C2 | 1.382 (2) | 1.3724 |
| N2-C2 | 1.387 (3) | 1.3925 |
| N2-C14 | 1.463 (3) | 1.4515 |
| N2-C3 | 1.370 (2) | 1.3881 |
| N3-C6 | 1.500 (2) | 1.4962 |
| Atom Angle | Experimental | Calculated |
| O1W ii-O1W-O1W i | 60.0 (18) | NC |
| C1-N1-C2 | 124.36 (15) | 122.9525 |
| C2-N1-C13 | 117.61 (15) | 117.6313 |
| C1-N1-C13 | 117.39 (15) | 117.0595 |
| C2-N2-C3 | 124.74 (15) | 123.0628 |
| C3-N2-C14 | 117.62 (17) | 116.9594 |
| C2-N2-C14 | 117.50 (16) | 117.4505 |
| O4-N3-C6 | 121.03 (18) | 117.2445 |
| O4-N3-O5 | 123.45 (19) | 125.7819 |
| O5-N3-C6 | 115.53 (16) | 116.9697 |
| O1-C1-N1 | 121.69 (16) | 123.7575 |
| O1-C1-C4 | 121.91 (15) | 122.9936 |
| N1-C1-C4 | 116.24 (15) | 113.2488 |
| O2-C2-N2 | 120.68 (18) | 123.3871 |
| N1-C2-N2 | 117.42 (14) | 116.2983 |
| O2-C2-N1 | 121.9 (2) | 121.7454 |
| O3-C3-C4 | 121.55 (15) | 123.7560 |
| O3-C3-N2 | 121.13 (15) | 121.7802 |
| N2-C3-C4 | 117.24 (14) | 112.8565 |
| N3-C6-C5 | 112.43 (14) | 111.9825 |
| Cl1A-C8A-C7A | 119.7 (3) | 124.0885 |
| Cl1A-C8A-C9A | 117.4 (3) | 114.4806 |
| Cl1B-C8B-C9B | 116.7 (10) | NC |
| Cl1B-C8B-C7B | 121.7 (9) | NC |
| Cl2A-C12A-C7A | 120.8 (3) | 122.7008 |
| Cl2A-C12A-C11A | 114.7 (4) | 115.2511 |
| Cl2B-C12B-C7B | 122.5 (8) | NC |
| Cl2B-C12B-C11B | 114.4 (10) | NC |
Symmetry codes: (i) –x + y + 1, −x + 1, z; (ii) –y + 1, x − y, z. NC: Not calculated.
Table 5.
Hydrogen-bond geometry (Å, °) of compound 2a.
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| O1W-H2W1···O1W iii | 0.9100 | 2.1000 | 2.859 (6) | 140.00 |
| O1W-H2W1···O1W iv | 0.9100 | 1.8500 | 2.689 (6) | 152.00 |
| O1W-H2W1···O1W v | 0.9100 | 2.0500 | 2.689 (7) | 125.00 |
| C5-H5A···O4 | 0.9800 | 2.2700 | 2.741 (3) | 108.00 |
| C6-H6A···O1 vi | 0.9700 | 2.5300 | 3.337 (3) | 141.00 |
| C6-H6B···Cl2A | 0.9700 | 2.6800 | 3.125 (4) | 109.00 |
| C6-H6B···O3 | 0.9700 | 2.5800 | 2.979 (2) | 105.00 |
| C13-H13A···O2 | 0.9600 | 2.2900 | 2.716 (3) | 106.00 |
| C13-H13C···O5 vii | 0.9600 | 2.5100 | 3.425 (3) | 159.00 |
| C14-H14B···O2 | 0.9600 | 2.2400 | 2.690 (4) | 108.00 |
Symmetry codes: (iii) −x + 4/3, −y + 2/3, −z + 2/3; (iv) y + 1/3, −x + y + 2/3, −z + 2/3; (v) x − y + 1/3, x − 1/3, −z + 2/3; (vi) y − 1/3, −x + y + 1/3, −z + 4/3; (vii) −x + y, −x, z.
Table 6.
Selected geometric parameters (Å, °) of compound 2b.
| Bond | Experimental | Calculated |
| Cl1-C12 | 1.7310 (11) | 1.7247 |
| Cl2-C10 | 1.7338 (13) | 1.7195 |
| O1-C1 | 1.2101 (19) | 1.2276 |
| O2-C2 | 1.219 (2) | 1.2325 |
| O3-C3 | 1.2148 (16) | 1.2278 |
| O4-N3 | 1.212 (2) | 1.2362 |
| O5-N3 | 1.204 (3) | 1.2378 |
| N1-C1 | 1.376 (2) | 1.3881 |
| N1-C2 | 1.386 (2) | 1.3925 |
| N1-C13 | 1.469 (2) | 1.4515 |
| N2-C2 | 1.3947 (19) | 1.4071 |
| N2-C3 | 1.3661 (16) | 1.3855 |
| N2-C14 | 1.471 (2) | 1.4513 |
| N3-C6 | 1.496 (2) | 1.4962 |
| Atom Angle | Experimental | Calculated |
| C1-N1-C2 | 124.73 (12) | 122.7743 |
| C1-N1-C13 | 117.37 (15) | 117.4505 |
| C2-N1-C13 | 117.89 (15) | 117.0006 |
| C2-N2-C3 | 124.33 (11) | 122.9525 |
| C2-N2-C14 | 117.89 (12) | 117.0593 |
| C3-N2-C14 | 117.64 (12) | 117.6313 |
| O4-N3-O5 | 122.4 (2) | 125.7819 |
| O4-N3-C6 | 118.79 (15) | 116.9697 |
| O5-N3-C6 | 118.86 (17) | 117.2445 |
| O1-C1-N1 | 122.31 (14) | 123.3871 |
| O1-C1-C4 | 120.81 (13) | 123.7560 |
| N1-C1-C4 | 116.80 (12) | 112.8565 |
| O2-C2-N1 | 121.70 (15) | 121.7802 |
| O2-C2-N2 | 120.54 (14) | 121.7454 |
| N1-C2-N2 | 117.74 (12) | 116.2983 |
| O3-C3-N2 | 121.68 (12) | 123.7575 |
| O3-C3-C4 | 121.06 (11) | 122.9936 |
| N2-C3-C4 | 117.18 (10) | 113.2488 |
| N3-C6-C5 | 109.74 (11) | 111.9825 |
| Cl2-C10-C9 | 118.98 (10) | 119.8634 |
| Cl2-C10-C11 | 119.27 (9) | 119.9545 |
| Cl1-C12-C7 | 121.01 (8) | 123.6597 |
| Cl1-C12-C11 | 116.44 (9) | 115.4596 |
Table 7.
Hydrogen-bond geometry (Å, °) of compound 2b.
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| C6-H6A···O2 i | 0.9700 | 2.3900 | 3.222 (2) | 144.00 |
| C6-H6B···O1 | 0.9700 | 2.5500 | 3.118 (2) | 117.00 |
| C9-H9A···O3 ii | 0.9300 | 2.5100 | 3.2290 (18) | 134.00 |
| C13-H13B···O2 | 0.9600 | 2.3100 | 2.727 (3) | 105.00 |
| C14-H14C···O2 | 0.9600 | 2.2700 | 2.715 (2) | 107.00 |
Symmetry codes: (i) x, −y + 1, z+1/2; (ii) x − 1/2, y + 1/2, z.
X-ray analysis was performed using a Bruker Apex II D8 Venture diffractometer (Bruker AXS GmbH, Karlsruhe, Germany). The data were processed with SAINT and corrected for absorption using SADABS [24]. The structure was solved by direct method using the program SHELXTL [25] and were refined by full-matrix least squares technique on F2 using anisotropic displacement parameters. The non-hydrogen atoms were refined anisotropically. In these compounds, all the H atoms were calculated geometrically with isotropic displacement parameters set to 1.2 times the equivalent isotropic U values of the parent carbon atoms.
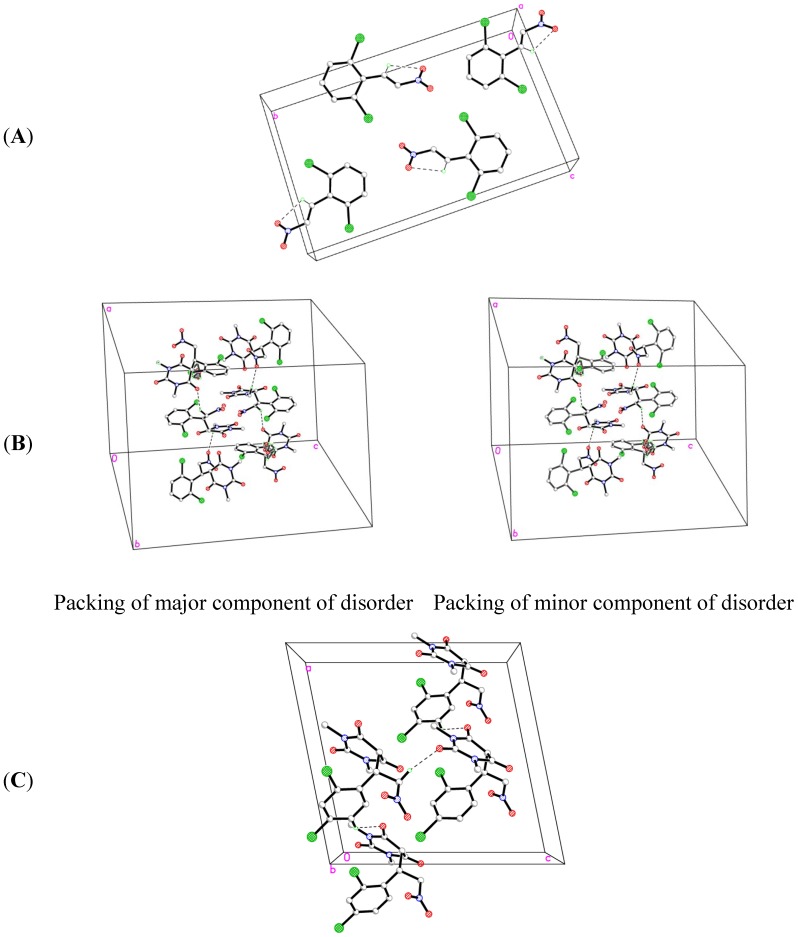
The title compound 1a, C8H5Cl2NO2, which crystallizes in the monoclinic space group P 21/c comprises one crystallographically independent molecule in its asymmetric unit, as depicted in Figure 1. In the crystal structure (Figure 2A), there is an intramolecular C7-H7A···O2 hydrogen bond. The crystal is essentially consolidated by Van Der Waals interactions. The crystal data and parameters for structure refinement of the title compound are given in Table 1. Selected geometric parameters are given in Table 2. H-bonding interactions are listed in Table 3.
Figure 2.
Crystal packings of (A) 1a, (B) 2a and (C) 2b.
The title compound 2a, C14H13Cl2N3O5·H2O, which crystallizes in the trigonal space group R-3 comprises one crystallographically independent molecule with disorder in the phenyl ring and a water molecule in its asymmetric unitas shown in Figure 1. Figure 2B shows the crystal packing for the major and minor components of 2a respectively with occupancy ratio 0.755:0.245. There are two intermolecular C6-H6A···O2 and C9-H9A···O3 hydrogen bonds (Table 5). The crystal structure is further consolidated by Van Der Waals interactions. The crystal data and parameters for structure refinement of the title compound are given in Table 1. Selected geometric parameters are given in Table 4. H-bonding interactions are listed in Table 5.
The title compound 2b, C14H13Cl2N3O5, which crystallizes in the monoclinic space group Cc comprises one crystallographically independent molecule in its asymmetric as shown in Figure 1. In the crystal structure (Figure 2C), there are two intermolecular C6-H6A···O1 and C13-H13C···O5 hydrogen bonds (Table 7). The crystal is further consolidated by Van Der Waals interactions. The crystal data and parameters for structure refinement of the title compound are given in Table 1. Selected geometric parameters are given in Table 6. H-bonding interactions are listed in Table 7.
2.3. Optimized Molecular Geometry
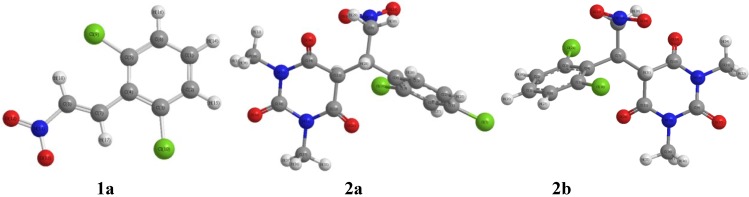
From the XRD data, it is clear that the compounds 1a and 2b possess monoclinic, whereas, 2a has trigonal crystal structures. The cell dimensions and other data are tabulated in Table 1. Selected values of experimental and DFT calculated geometric parameters for the compounds are listed in Table 2, Table 4, Table 6 and Table 8. Figure 3 shows the optimized structures for 1a and 2a,b.
Table 8.
Optimized calculations of various parameters for 1a and 2a,b using B3LYP/6-311G basis set.
| Parameter | 1a | 2a | 2b |
|---|---|---|---|
| Heat of Formation (kcal/mol) | 22.075 | −100.903 | −99.472 |
| Total Energy (kcal/mol) | −896424.216 | −1244859.443 | −102495.469 |
| Dipole (Debye) | 8.281 | 4.116 | 3.848 |
| HOMO energy (eV) | −10.019 | −10.575 | −10.813 |
| LUMO energy (eV) | −4.320 | −2.278 | −2.383 |
| HOMO–LUMO energy gap (eV) | 5.699 | 8.297 | 8.430 |
Figure 3.
The optimized structures for 1a and 2a,b.
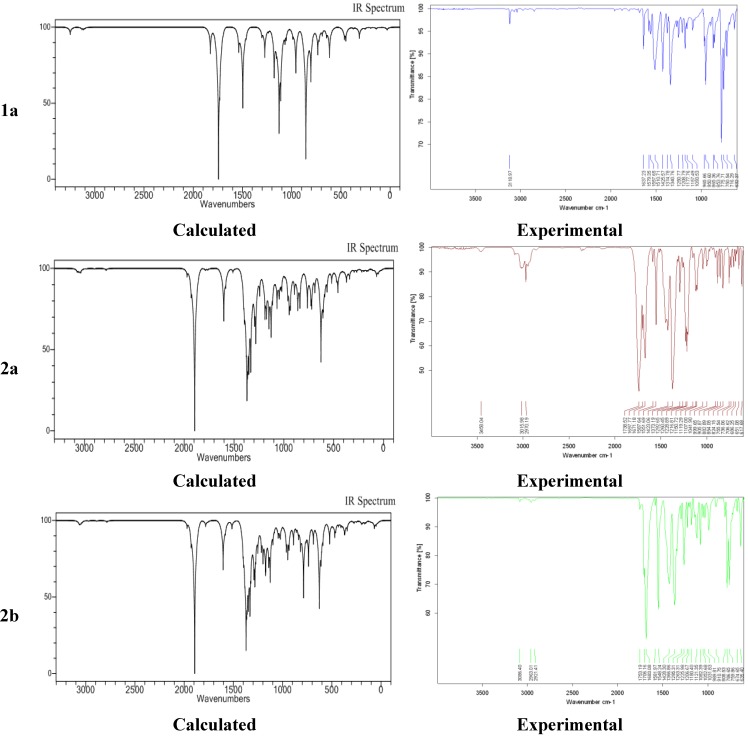
Figure 4 shows the experimental and calculated (B3LYP/6-311G) IR spectrum for 1a and 2a,b. A comparison of predicted (B3LYP/6-311G) with experimental IR reveals that B3LYP/6-311G basis set gives reasonable deviations from the experimental values. The discrepancies observed between the calculated and the experimental vibrational frequencies may be attributable to the fact that the calculations have been actually performed on a single molecule in the gaseous state contrary to the experimental values recorded in the solid state in the presence of intermolecular interactions.
Figure 4.
Experimental and calculated IR spectra for 1a and 2a,b.
2.4. Experimental and Calculated IR Vibrations
The calculated (B3LYP/6-311G) and experimental C-H, C-Cl, N=O, C=O, and C=C vibration values for all the molecules have good agreement, except for C=O for 2a. For 2a, the computed C=O stretching vibrations at 1894 cm−1 deviated substantially from the experimental result. A plausible reason for this could be the intermolecular interactions present in solid state whereas during DFT calculations the molecule is assumed to be in isolated gaseous state.
2.5. Geometric Parameters
The solid state X-ray structure analysis revealed disorder in the phenyl moiety for 2a (see Figure 2). This incongruity noted between the calculated and the experimental vibrational frequencies may be due to the fact that the calculations have been actually performed on a single molecule in the gaseous state contrary to the experimental values recorded in the solid state. In DFT calculations, bond lengths and angles have been reported only for the major component of the phenyl moiety of 2a (designated by the suffix “A”). The selected calculated and experimental geometric parameters for 1a and 2a,b have been tabulated in Table 2, Table 4 and Table 6.
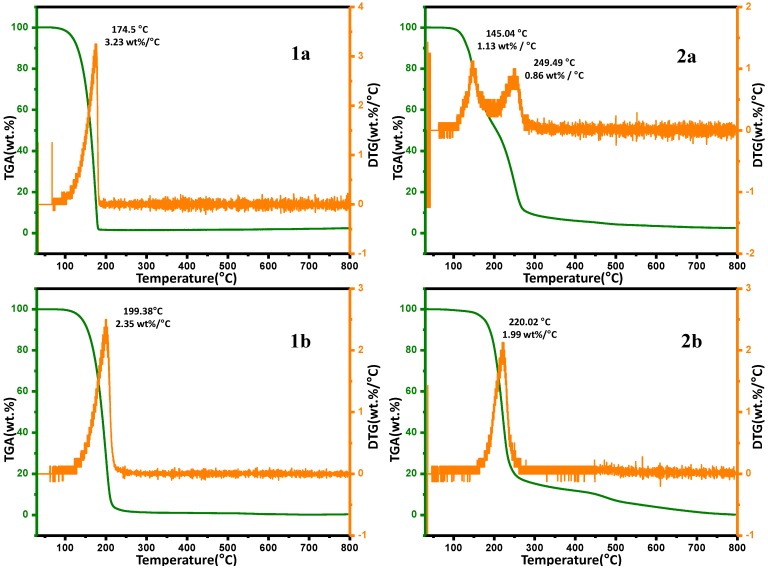
2.6. Thermal Gravimetric Analysis (TGA)
The synthesized novel compounds were subjected to thermogravimetric analysis (TGA) to evaluate their thermal stability and degradation patterns (Table 9) and the TGA and DTG patterns are shown in Figure 5. All samples were subjected to analysis in a nitrogen atmosphere in the temperature range from 30 °C to 800 °C with a heating ramp rate of 10 °C per min. It was observed that the different nitrostyrene derivatives displayed different thermal stabilities and degradation patterns. The pyrolysis processes of the materials are characterized by single-stage degradation, with an exception of 2a, which displays two-stage degradation. Interestingly, it was detected that upon the incorporation of the barbituric acid ring into the styrene, the resulting molecule was slightly more thermally stable than its precursor. The weight loss pattern of all the compounds was found to be different at different intervals with 1a,b displaying a weight loss of 70% and 98% respectively at 200 °C, but at the same temperature the weight loss percentage for the barbituric acid derivatives 2a,b were found to be 48% and 18 % respectively. When a comparison of thermal degradation pattern between 1a and 2a was made it was found that 1a undergoes single stage degradation with decomposition range of 100–190 °C (90 °C), while 2a displays two stage degradation with decomposition taking place in the range of 105–195 °C (90 °C) and 200–275 °C (75 °C). The degradation temperature range of 1b and 2b was found to take place at a 162–262 °C (100 °C) and 125–230 °C (105 °C), respectively. The peak temperature of 2b (220.02 °C) was higher than that of 1b (199.38 °C), while the decomposition intensity of 1b was found to be high with 2.35 wt %/°C compared to 1.99 wt %/°C 2b. This behaviour can be attributed to the presence of barbituric acid. However, when the temperature was increased further all the compounds followed similar weight loss patterns with varying percentages of residue at the final temperature of 800 °C. From the residual weight values obtained at ~800 °C it can be concluded that there is no significant thermal stability among any of the four compounds tested, however it can be said that thermal stability of the barbituric acid derivative of the styrene molecule is slightly improved compared to that of its precursor. The weight loss percentage at different temperatures is mentioned in the table below and a comparative graphical representation of the compounds is given in Figure 5. A detailed study into the thermal behaviour of the synthesized compounds shall be carried out the thermal kinetics will be reported separately.
Table 9.
Weight loss percentage at different temperatures for 1a,b and 2a,b.
| Temperature | Weight Loss (%) | |||
|---|---|---|---|---|
| 1a | 2a | 1b | 2b | |
| 100 | 0.33 | 0.63 | 1.42 | 0.44 |
| 200 | 70.85 | 48.06 | 98.37 | 18.45 |
| 300 | 98.72 | 91.07 | 98.46 | 84.73 |
| 400 | 98.98 | 93.95 | 98.40 | 88.19 |
| 500 | 99.11 | 95.67 | 98.29 | 92.93 |
| 600 | 99.56 | 96.47 | 98.08 | 96.10 |
| 700 | 99.77 | 97.14 | 97.87 | 98.52 |
| 800 | 99.66 | 97.47 | 97.55 | 99.81 |
Figure 5.
TGA and DTG curves of the synthesized compounds.
3. Experimental Section
3.1. General
All the chemicals were purchased from Aldrich (Riedstraße, Germany), Sigma-Aldrich (St. Louis, MO, USA), Fluka (Buchs, Switzerland), and used without further purification, unless otherwise stated. The structure of 1a was confirmed by X-ray crystal structure analysis (Bruker AXS GmbH, Karlsruhe, Germany). The crystallographic data for 1a (CCDC-992700), 2a (CCDC-992327) and 2b (CCDC-992701) have been submitted to the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk/data_request/cif). Compound 1a was prepared from 2,6-dicholorobenzaldehyde and nitromethane as white crystals according to the reported procedure [16]. Colorless block-shaped crystals of the compound suitable for X-ray analysis were formed in isopropanol/heptane at room temperature after 3 days.
3.2. 5-(1-(2,6-Dichlorophenyl)-2-nitroethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (2a)
According to the reported procedure [16], 2a was prepared from 1,3-dimethylbarbituric acid (1a) and (E)-2,6-dichloro-1-(2-nitrovinyl)benzene (1a) as white crystals. Colorless block-shaped crystals of the compound suitable for X-ray analysis were formed in CHCl3/Et2O at room temperature after 2 days.
3.3. 5-(1-(2,4-Dichlorophenyl)-2-nitroethyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (2b)
According to the reported procedure [16], 2b was prepared from 1,3-dimethylbarbituric acid and (E)-2,4-dichloro-1-(2-nitrovinyl)benzene (1a) as yellow crystals. Colorless block-shaped crystals of the compound suitable for X-ray analysis were formed in DCM/Pet. ether at room temperature after 1 day.
3.4. DFT Calculations
The DFT calculations were performed using the GAMESS package. The input geometry of the synthesized molecules were optimized without imposing any external constraint on the potential energy surfaces generated by the B3LYP/6-311G(d,p) basis set for C, O, N and H atoms. The resulting optimized geometry was used as an input for vibrational frequencies calculations, with inclusion of polarization functions to handle the polar bonds such as N=O, C=O, etc.
4. Conclusions
In a summary, pyrimidine derivatives 2a,b were prepared starting from nitroalkenes 1a,b using an eco-benign method. The structures for 1a and 2a,b have been characterized by their single crystal X-ray diffraction analysis. DFT/TGA/IR analyses were performed and discussed.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project Number RGP-VPP-257.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/11/17187/s1.
Supplementary Files
Author Contributions
Hany J. Al-Najjar and Mohamed Ali carried out the experimental part; Syed F. Adil carried out thermo-analysis; Vijay H. Masand carried out computational studies; Hazem A Ghabbour and Hoong-Kun Fun carried out X-ray part; Abdullah M. Al-Majid helped in the results and discussion and writing the manuscript; Assem Barakat: proposed the subject and designed the study. All the authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1a,b and 2a,b are available from the authors.
References
- 1.Rastogi V.K., Mittal H.P., Sharma Y.C., Sharma S.N. In: Spectroscopy of Biological Molecules. Hester R.E., Girling R.B., editors. Royal Society of Chemistry; London, UK: 1991. pp. 403–404. [Google Scholar]
- 2.Bandekar J., Zundel G. Normal coordinate analysis treatment on uracil in solid state. Spectrochim. Acta A. 1983;39:343–355. [Google Scholar]
- 3.Aruna S., Shanmugam G. Vibrational assignments of six-membered heterocyclic compounds: Normal vibrations of 6-amino uracil and 6-amino 2-thio uracil. Spectrochim. Acta A. 1985;41:531–536. doi: 10.1016/0584-8539(85)80040-4. [DOI] [Google Scholar]
- 4.Portalone G., Ballirano P., Maras A. The crystal structure of 3-methyluracil from X-ray powder diffraction data. J. Mol. Struct. 2002;608:35–39. doi: 10.1016/S0022-2860(01)00929-2. [DOI] [Google Scholar]
- 5.Grosmaire L., Delabre J.L. Vibrational spectra of 6-methyluracil, 6-methyl-2-thiouracil and their deuterated analogues. J. Mol. Struct. 2012;1011:42–49. doi: 10.1016/j.molstruc.2011.12.007. [DOI] [Google Scholar]
- 6.Yaniv M., Folk W.R. The nucleotide sequences of the two glutamine transfer ribonucleic acids from Escherichia coli. J. Biol. Chem. 1975;250:3243–3253. [PubMed] [Google Scholar]
- 7.Amir M., Javed S.A., Kumar H. Pyrimidine as antiinflammatory agent: A review. Indian J. Pharm. Sci. 2007;69:337–343. doi: 10.4103/0250-474X.34540. [DOI] [Google Scholar]
- 8.Çırak Ç., Sert Y., Ucun F. Experimental and computational study on molecular structure and vibrational analysis of a modified biomolecule: 5-Bromo-2'-deoxyuridine. Spectrochim. Acta A. 2012;92:406–414. doi: 10.1016/j.saa.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Demirbaş N., Ug̵urluog̵lu R., Demirbaş A. Synthesis of 3-alkyl(aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg. Med. Chem. 2002;10:3717–3723. doi: 10.1016/s0968-0896(02)00420-0. [DOI] [PubMed] [Google Scholar]
- 10.Elinson M.N., Vereshchagin A.N., Stepanov N.O., Zaimovskaya T.A., Merkulova V.M., Nikishin G.I. The first example of the cascade assembly of a spirocyclopropane structure: Direct transformation of benzylidenemalononitriles and N,N-dialkylbarbituric acids into substituted 2-aryl-4,6,8-trioxo-5,7-diazaspiro[2.5]octane-1,1-dicarbonitriles. Tetrahedron Lett. 2010;51:428–431. doi: 10.1016/j.tetlet.2009.11.065. [DOI] [Google Scholar]
- 11.Barakat A., Al-Majid A.M., Al-Najjar H.J., Mabkhot Y.N., Javaid S., Yousuf S., Choudhary M.I. Zwitterionic pyrimidinium adducts as antioxidants with therapeutic potential as nitric oxide scavenger. Eur. J. Med. Chem. 2014;84:146–154. doi: 10.1016/j.ejmech.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Ray R., Krishna S.H., Sharma J.D., Limaye S.N. Variations in the Physico-Chemical Parameters of Some 5a and 5b Substituted Barbiturate Derivatives. Asian J. Chem. 2007;19:2497–2501. [Google Scholar]
- 13.Brunton L.L., Lazo J.S., Lazo P., Keith L. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. McGraw-Hill; New York, NY, USA: 2006. [Google Scholar]
- 14.Fillaut J.L., de Los Rios I., Masi D., Romerosa A., Zanobini F., Peruzzini M. Synthesis and Structural Characterization of (Carbene)ruthenium Complexes Binding Nucleobases. Eur. J. Inorg. Chem. 2002:935–942. [Google Scholar]
- 15.Temiz-Arpaci O., Ozdemir A., Yalcin I., Yildiz I., Aki-Sener E., Altanlar N. Synthesis and antimicrobial activity of some 5-[2-(morpholin-4-yl)acetamido] and/or 5-[2-(4-substituted piperazin-1-yl)acetamido]-2-(p-substituted phenyl)benzoxazoles. Arch. Pharm. 2005;338:105–111. doi: 10.1002/ardp.200400923. [DOI] [PubMed] [Google Scholar]
- 16.Kwan P., Brodie M.J. Phenobarbital for the treatment of epilepsy in the 21st century: A critical review. Epilepsia. 2004;45:1141–1149. doi: 10.1111/j.0013-9580.2004.12704.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Najjar H.J., Barakat A., Al-Majid A.M., Mabkhot Y.N., Weber M., Ghabbour H.A., Fun H.-K. A greener and efficient approach to Michael addition of barbituric acid to nitroalkene in aqueous diethylamine mediu. Molecules. 2014;19:1150–1162. doi: 10.3390/molecules19011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Majid A.M., Barakat A., Al-Najjar H.J., Mabkhot Y.N., Ghabbour H.A., Fun H-K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to bis-pyrimidine derivatives. Int. J. Mol. Sci. 2013;14:23762–23773. doi: 10.3390/ijms141223762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barakat A., Al-Majid A.M., Al-Ghamdi A.M., Mabkhot Y.N., Siddiqui M.R., Ghabbour H.A., Fun H-K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to dimedone-barbituric acid derivatives. Chem. Cent. J. 2014;8 doi: 10.1186/1752-153X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat A., Al-Majid A.M., Al-Najjar H.J., Mabkhot Y.N., Ghabbour H.A., Fun H-K. An efficient and green procedure for synthesis of rhodanine derivatives by Aldol-thia-Michael protocol using aqueous diethylamine. RSC Adv. 2014;4:4909–4916. doi: 10.1039/c3ra46551a. [DOI] [Google Scholar]
- 21.Barakat A., Al Majid A.M.A., Islam M.S., Al-Othman Z.A. Highly enantioselective Friedel-Crafts alkylations of indoles with α,β-unsaturated ketones under Cu(II)-simple oxazoline-imidazoline catalysts. Tetrahedron. 2013;69:5185–5192. doi: 10.1016/j.tet.2013.04.063. [DOI] [Google Scholar]
- 22.Al-Majid A.M., Islam M.S. Barakat, Elkahatany N., Yousuf S.S., Choudhary M.I. Tandem Knoevenagel-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to bis-Dimedone derivatives. Arab. J. Chem. 2014 doi: 10.1016/j.arabjc.2014.04.008. [DOI] [Google Scholar]
- 23.Islam M.S., Al-Majid A.M.A., Al-Othman Z.A., Barakat A. Highly Enantioselective Friedel-Crafts Alkylation of Indole with electron deficient trans-β-Nitroalkenes under simple Zn(II)-Oxazoline-Imidazoline Catalysts. Tetrahedron: Asymmetry. 2014;25:245–251. doi: 10.1016/j.tetasy.2013.11.018. [DOI] [Google Scholar]
- 24.Bruker, APEX2, SAINT and SADABS. Bruker AXS Inc.; Madison, WI, USA: 2009. [Google Scholar]
- 25.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.