Abstract
Ribavirin has been used as an antiviral agent for several decades. Although it has activity against numerous viruses, its major use clinically has been in the treatment of respiratory syncytial virus in paediatric patients and chronic HCV infection in both children and adults. This review highlights the clinical application and mechanism of action of ribavirin and discusses the future role of ribavirin in treatment of HCV where there are intense research efforts to improve therapy.
Introduction
Ribavirin (1-b-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide) was synthesized in 1970 by researchers at ICN pharmaceuticals (now Valeant International Pharmaceuticals). Initial studies demonstrated that ribavirin possessed antiviral activity against both DNA and RNA viruses in various animal models of infection [1]. Following this discovery, efforts were undertaken to establish a clinical use for the compound. Subsequent investigation demonstrated its efficacy for treatment of paediatric respiratory syncytial virus (RSV) infection in 1986, currently, one of its major indications [2]. Over a decade later, ribavirin was approved for treatment of HCV in combination with interferon-α [3]. Since then, ribavirin has continued to be used primarily for these indications. Interestingly, despite its proven effectiveness against a variety of viral infections, its mechanism of action has not been firmly established [4]. Although there have been several proposed mechanisms of action, each with some experimental evidence, there has been no consensus as to which one explains the major antiviral activity of ribavirin [5]. Regardless of this scientific quandary, ribavirin continues to be a necessary agent to treat many viral infections, including its use in the current standard of care for HCV therapy.
Chemistry and pharmacokinetics
Ribavirin is a water-soluble, guanosine nucleoside analogue that mimics other purines including inosine and adenosine [6]. Its chemical structure is shown in Figure 1. Notable aspects of its structure include a heterocyclic base with only one ring, as opposed to the two rings found in guanine. As such, ribavirin’s base moiety more closely resembles the monocyclic base found in nicotinamide as well as 5-aminoimidazole-4-carboxyamide ribonucleoside, both naturally occur-ring metabolites [7]. In addition, the 4′ carbon contains a methanol group found naturally on ribose and it is the hydroxyl group on the 5′ carbon that is phosphorylated to the triphosphate form, as occurs for other nucleosides. In addition, its sugar moiety, present as ribose with a hydroxyl group at the 2′-carbon position instead of a deoxyribose, allows ribavirin to be preferentially active in RNA-related metabolism [6].
Figure 1.
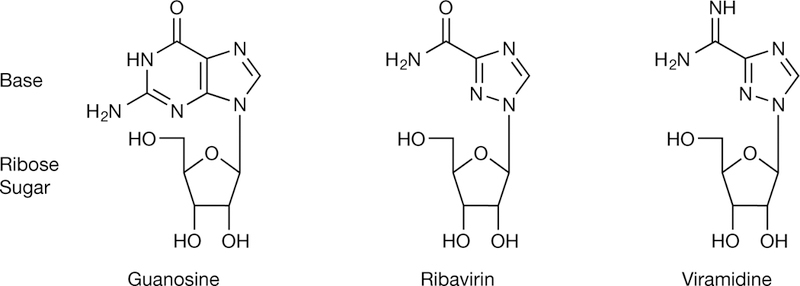
Structure of ribavirin and related molecules
Absorption and distribution
Ribavirin may be administered orally, intravenously and through inhalation. Each method of delivery offers advantages and disadvantages and can be utilized to target ribavirin against a specific viral pathogen. Ribavirin delivered by inhalation is the preferred method for treatment of RSV in infants and only a small amount of ribavirin enters the systemic circulation by this route [8]. For cases of haemorrhagic fever (for example, Lassa fever virus and hantavirus) and influenza infection, intravenous ribavirin has been used experimentally for short-term treatment following infection with these, sometimes life-threatening, pathogens [9,10]. For other indications, including HCV, ribavirin is administered orally, can be administered for as long as 1 year and has been utilized with continual dosing for much longer periods of time [11].
Oral ribavirin is distributed systemically following intestinal absorption facilitated by nucleoside transporters on the luminal epithelial cells. Bioavailability of the oral formulation is approximately 50%; coad-ministration with a fatty meal increases bioavailability to approximately 75%. Once it enters the plasma, ribavirin is widely distributed and accumulates in erythrocytes and also in the cerebrospinal fluid and brain. It has a multiple dose half-life of 12 days in the serum, and it may persist in non-plasma compartments for as long as 6 months. Several studies have indicated that it takes approximately 4 weeks of oral dosing to achieve steady-state plasma levels with the possibility that levels continue to increase slightly after 4 weeks [12,13].
The mechanism by which ribavirin enters eukaryotic cells has been an area of intense study. Two distinct sets of nucleoside transporters have been implicated in ribavirin uptake and these include the concentrative nucleotide transporters (CNTs) and equilibrative nucleotide transporters (ENTs) [14]. A major difference between these two transporters is their sodium dependence. CNTs are characterized as sodium-dependent where import of nucleosides is coupled to sodium regardless of nucleoside concentration, while the ENTs facilitate bidirectional, sodium-independent transport. In addition, the CNTs also demonstrate high affinity for nucleosides, whereas the ENTs tend to be lower affinity transporters [15]. Previous studies have specifically determined that, in humans, ENT1 is the major transporter for the majority of ribavirin, while CNTs may contribute minimally to uptake into the intracellular compartment [16,17]. Interestingly, single nucleotide polymorphisms (SNPs) in these transporters may dictate the effectiveness of ribavirin in antiviral therapy for HCV and also its toxicity [18,19].
Metabolism and elimination
Once transported into the intracellular compartment, ribavirin is actively phosphorylated to the triphosphate nucleotide by cellular kinases, with adenosine kinase being the major enzyme [20]. Kinetic analysis of the phosphorylation events have established that the generation of the monophosphate species of ribavirin is the rate-limiting step during conversions of ribavirin to the triphosphate form [21]. Ultimately, it is the triphosphate form that is thought to confer most of the antiviral activity of ribavirin although the monophosphate form (approximately 5–10% of total intracellular levels) may also contribute to the antiviral activity [22–24]. In nucleated cells, ribavirin triphosphate is actively dephosphorylated by cellular phosphatases allowing ribavirin to be exported back into the extracellular compartment. How-ever, enucleated cells, such as erythrocytes, lack these phosphatases, resulting in the accumulation of intracellular ribavirin triphosphate [25]. Ribavirin is cleared from plasma by the kidney with reduced clearance being observed in patients with renal impairment [26,27]. About one-third of absorbed ribavirin is excreted into the urine unchanged, and the rest is excreted into urine as the deribosylated base 1,2,4-triazole 3-carboxamide, and its hydrolysis product, 1,2,4-triazole 3-carboxylic acid [28,29].
Current role in HCV antiviral therapy
Although ribavirin has been investigated as therapy for numerous viral infections, its principal use has been for the treatment of chronic HCV infection, having gained FDA approval for this indication in 1998 [30]. The number of individuals with chronic HCV infection is estimated to be >150 million globally [31]. Chronic hepatitis C (CHC) is a major cause of cirrhosis, decompensated liver disease and hepatocellular carcinoma worldwide and in the US [32]. A recent US study suggested that the numbers of cases of cirrhosis and hepatocellular carcinoma in individuals with CHC are expected to peak in 2020, underscoring the need for better therapies to prevent further morbidity and mortality from CHC [33]. Ribavirin has been included in the therapeutic regimen targeting HCV for over a decade [34].
Use of ribavirin monotherapy for HCV
In the early 1990s, attempts at using ribavirin mono-therapy to treat HCV were initiated [3,35,36]. Because ribavirin was demonstrated to be a broad-spectrum antiviral agent with activity against RNA viruses (for example, RSV and hantavirus) and HCV is related to flavivirus with a positive sense RNA genome, pilot studies of monotherapy were undertaken. The first study utilized weight-based ribavirin at doses of 1,000–1,200 mg per day for 12 weeks in 10 patients [35]. A second study was conducted in 13 patients utilizing incremental increases in dose for 6 months with 1,200 mg per day being the largest dose administered [36]. Overall, patients treated with ribavirin monotherapy demonstrated improvements in serum alanine aminotransferase levels with some indication that patients infected with HCV genotype 2/3 may be more responsive [37]. However, ribavirin monotherapy had little effect on HCV RNA levels (<2 log reduction) with no patients achieving viral clearance [38].
Combination therapy: use of ribavirin and interferon-α for HCV
Interferon-α was the first therapy approved for HCV, gaining FDA licensure in 1991, but had a dismal cure rate of only 6% [39,40]. There were no other significant advancements in HCV therapy until 1998 when the combination of ribavirin and interferon-α gained approval. The disappointing trials with ribavirin monotherapy demonstrated a measurable therapeutic effect as evidenced by improvements in liver function tests [3]. Small pilot studies of the combination of interferon-α and ribavirin demonstrated a synergistic effect between the two antiviral agents [41]. Subsequent randomized controlled trials demonstrated an almost sixfold improvement in sustained virological response (SVR) rates from 6–15% with standard interferon monotherapy for 6 months to 36% with combination therapy [42,43]. The next major advancement was the development of pegylated forms of interferon-α used in conjunction with ribavirin, which further increased SVR rates to approximately 55% [44]. This regimen was FDA-approved in 2002.
Clinically, ribavirin has a small, additive antiviral effect when combined with interferon, but its main effect appears to be prevention of virological relapse, an effect that seems to be dose-dependent [44]. Mathematical analysis of the rate of viral decline following therapy with interferon described a biphasic kinetic model: a rapid first phase (24–48 h) attributed to a inhibition of viral replication by interferon and a slower second phase (days to weeks) due to immune-mediated clearance of virally infected hepatocytes. Mathematical modelling of viral decay from the combination of interferon and ribavirin showed no effect of ribavirin on the rapid first phase decline in HCV RNA level. However, the second slower phase of viral decline was enhanced by ribavirin in some patients supporting a weak antiviral or perhaps immunomodulatory effect [45]. Nonetheless, as mentioned above, a great improvement in the long-term response rates over interferon monotherapy resulted in the approval of ribavirin in combination with interferon-α.
Between 2002 and 2010, there were no significant improvements in antiviral therapy for HCV. Many clinical trials utilizing multiple permutations of the standard regimen including higher doses of both pegylated interferon-α and ribavirin, longer durations of treatment as well as a ribavirin lead-in phase resulted in no or minor improvements in response rates [46–48]. A provocative pilot-study that utilized high dose ribavirin in combination with pegylated interferon-α with the goal of achieving ribavirin levels >15 mmol/l reported a 90% SVR rate in genotype 1 patients [49]. Whether ribavirin imparts greater antiviral activity at this level is unknown. How-ever, side effects seriously limit the clinical applicability of such a strategy as profound anaemia can result. Simi-lar studies have not been replicated to date.
In order to optimize ribavirin’s use for therapy of HCV, several groups have correlated serum concentrations of ribavirin during therapy with subsequent out-come [50,51]. It is believed that patient variability can lead to large variations in effective serum concentrations of ribavirin. This suggests that individual dosing based on serum concentration may be a more effective method to administer ribavirin. Although clinical trials have demonstrated that low serum concentrations of ribavirin result in decreased antiviral responses and that higher levels improve response rates [52], the utility of doing this is questionable due to the technical sophistication needed to accurately measure ribavirin levels. The expensive and complicated instrumentation (liquid chromatography/tandem mass spectrometry) limits routine use of this method to determine adequate serum ribavirin concentrations [53,54]. As a surrogate marker of ribavirin concentration, the degree of anaemia achieved with ribavirin seems to be predictive of SVR [55].
Triple therapy: use with telaprevir and boceprevir
In May 2011, direct-acting antiviral (DAA) agents specifically targeting the NS3/4A protease of HCV gained FDA approval for use as triple therapy in patients with HCV genotype 1 infection. This triple-therapy regimen is associated with SVR rates of >70% in treatment-naive patients, but has additional serious side effects [56,57]. Initially, it was hoped that these protease inhibitors would abolish the need for interferon and ribavirin; however, studies with these small molecule inhibitors demonstrated rapid selection of resistant viral strains when used as monotherapy [58,59]. Ribavirin appears to be crucial to the success of triple therapy regimens as was demonstrated in the PROVE 2 trial [60], which evaluated the safety and efficacy of telaprevir with or without ribavirin, as compared to pegylated interferon-α 2a and ribavirin alone, in previously untreated patients infected with HCV genotype 1. This effect of ribavirin on improving SVR rate with DAAs appeared to be on the prevention of relapse and the emergence of both low-level and high-level protease inhibitor resistance [60].
Adverse events and contraindications
The main toxicity observed with ribavirin is haemolytic anaemia [61]. Ribavirin is actively transported into erythrocytes and subsequently phosphorylated to its triphosphate form by intracellular kinases. In the case of the erythrocyte, these cells lack the phosphatase that converts ribavirin back to its unphosphorylated form. Consequently, high levels of ribavirin triphosphate accumulate in the erythrocyte due to the fact that the triphosphate form is not actively exported from the cell [25]. Intracellular erythrocyte concentrations can reach approximately 100× the concentration found in the serum [62]. Consequently, extremely high intracellular concentrations of the nucleotide deplete intracellular ATP concentrations and lead to oxidative stress, ultimately resulting in damage to the cell membrane and lyses of the erythrocyte [61]. Most patients treated with oral ribavirin experience some degree of anaemia. In the registration trials of pegylated interferon and ribavirin, anaemia defined as a haemoglobin concentration <10 mg/dl, was observed in approximately one-third of patients, reaching a nadir within 4 to 8 weeks of starting therapy. Dose modification for anaemia was required in 9–15% of subjects in the two Phase III registration trials of pegylated interferon and ribavirin [44,63]. Use of erythrocyte stimulating agents, such as erythropoietin and darbepoietin, have been used to counter the anaemia associated with pegylated interferon and ribavirin [64] Although the use of growth factors improves a patient’s sense of well-being and reduces the requirement for ribavirin dose reduction, it has not been consistently shown to improve SVR rates [65,66]. These agents are not approved for use in patients with chronic hepatitis C.
The degree of anaemia observed during treatment was greater with the triple therapy regimens. Haemoglobin decreases <10 g/dl occurred in 49% of patients who received a boceprevir regimen compared to 29% of those who received a pegylated interferon plus ribavirin regimen, while 9% had a haemoglobin decline of <8.5 g/dl [56,67]. Among patients treated with the approved telaprevir triple therapy regimen, haemoglobin levels of <10 g/dl were observed in 36% of patients compared to 14% of patients who received pegylated interferon plus ribavirin regimen, and 9% had haemoglobin decreases to <8.5 g/dl [57,60].
Since the major dose-limiting toxicity of ribavirin is haemolytic anaemia, it can result in worsening of cardiac disease and can lead to fatal and non-fatal myocardial infarctions. Therefore, patients with a history of significant or unstable cardiac disease should not be treated. Additionally, in patients with other base-line risks of severe anaemia (for example, spherocytosis and history of gastrointestinal bleeding), ribavirin should not be used [64]. Since ribavirin is cleared by the kidney, the drug should be used with extreme caution in patients with chronic kidney disease [68]. Other side effects associated with ribavirin include mild lymphopaenia, hyperuricaemia, itching, rash, cough and nasal stuffiness. Furthermore, significant teratogenic and/or embryocidal effects have been observed in all animal species exposed to ribavirin except for baboons [67] Consequently, ribavirin is contraindicated in both males and females who are attempting conception with a recommended 6-month washout period before trying to get pregnant [70,71].
Inosine triphosphatase polymorphism
Recently, a landmark discovery was made with respect to ribavirin’s marked ability to cause anaemia in patients undergoing combination antiviral treatment [72]. A genome-wide association study demonstrated that SNPs were present in the human genome that correlate with both the development and severity of ribavirin-induced anaemia. Initial analysis demonstrated that polymorphisms near the inosine triphosphatase (ITPA) gene locus were predictive of the development of anaemia secondary to ribavirin but subsequent analysis demonstrated that two SNPs located directly in the ITPA gene were the functional SNPs and accounted for the observed phenotype. Heterozygotes were less susceptible to ribavirin-induced anaemia than homozygotes with the risk allele [73]. Subsequent in vitro experiments demonstrated how these polymorphisms resulted in anaemia with a dose-dependent effect. Specifically, the two SNPs in the ITPA gene result in the reduced activity of the enzyme, leading to an increase in basal inosine triphosphate levels in the erythrocyte. Inosine triphosphate subsequently confers protection against ribavirin-induced ATP reduction by substituting for erythrocyte GTP, which is depleted by ribavirin, in the biosynthesis of ATP [74]. Although the finding is scientifically significant, the clinical utility of the polymorphism has been limited as the frequency of the protective allele is low in the human population. Additionally, it is unknown whether or not the polymorphism plays a role in the antiviral activity of ribavirin against HCV.
Mechanism of action of ribavirin HCV antiviral therapy
There are multiple mechanisms of action reported for ribavirin, each with some experimental evidence; how-ever, the major antiviral mechanism responsible for ribavirin activity has been enigmatic (Figure 2) [4]. Many factors contribute to this area of scientific controversy and include the fact that ribavirin displays antiviral activity against numerous viruses including those with RNA and DNA genomes [1]. It is unlikely that one mechanism can be responsible for these observations. In addition, ribavirin’s use in HCV therapy is dependent on administration with interferon. Since patients are not cured of HCV with ribavirin monotherapy, interactions with the multifaceted antiviral activity of interferon-a, the potent antiviral agent in the regimen, complicates analysis of ribavirin’s contribution in combination therapy [45]. In order to understand each of the proposed mechanisms of action of ribavirin against HCV, the ability of ribavirin to exert its antiviral effect either by a direct mechanism targeting the virus or by an indirect mechanism targeting host cells is discussed below.
Figure 2.
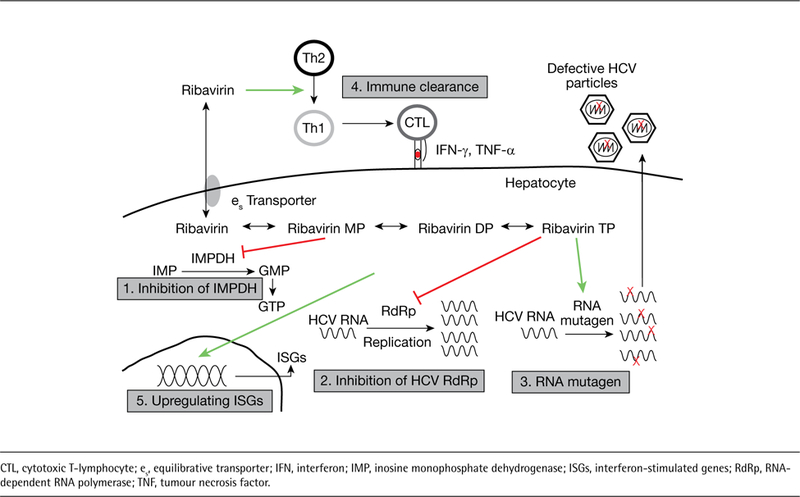
Proposed antiviral mechanisms of action of ribavirin
HCV polymerase inhibition
Since ribavirin is a nucleoside analogue of guanosine, the most straightforward possible mechanism of action would be that ribavirin acts as an inhibitor of the viral polymerase. Since viral polymerases utilize intracellular nucleotides to replicate their genome, ribavirin triphosphate would potentially be recognized by the viral polymerase and either inhibit subsequent elongation due to chain termination or prevent the binding of other endogenous nucleotides necessary for the completion of genome replication [24,75]. This is an attractive mechanism of action because of the conservation in the nucleotide binding site between viral genotypes. As a result, nucleoside/nucleotide polymerase inhibitors should confer pan-genotypic antiviral activity [76,77]. One of the strongest arguments against this mechanism of action is the fact that ribavirin is a very weak inhibitor of HCV polymerase in vitro and retains antiviral activity against most HCV strains that harbour resistance mutations in the polymerase region that arise following treatment with other nucleoside/nucleotide inhibitors [78,79]. However, potential mutations that may confer resistance to ribavirin in the polymerase gene have been identified in patients undergoing treatment with ribavirin [80]. Furthermore, there is limited data showing that ribavirin is a chain terminator, as are many other nucleoside/nucleotide analogues [76]. Proponents of this mechanism of action argue that ribavirin has weak activity against the polymerase and thus there is not enough selective pressure to drive the preferential expansion of resistant strains. Claims have also been made that this weak antiviral activity would also be of importance with coad-ministration of interferon-a, where the interferon will drive down virus levels allowing ribavirin’s weak antiviral effect to be of more importance when the viral levels and quasispecies diversity are much lower. Thus, this weak and constant antiviral activity can help eradicate low-level virus that usually relapses following cessation of interferon-α monotherapy.
RNA mutagenesis (error catastrophe)
A second mechanism of action is inferred from data that demonstrated ribavirin triphosphate can be incorporated in the HCV viral genome. Once incorporated, since ribavirin can base pair with both cytosine and uracil, it would subsequently drive an increasing number of mutations in the HCV genome, ultimately resulting in the production of less fit viruses: a process termed ‘error catastrophe’ [6,81]. Evidence to support this mechanism is also very controversial as several laboratories have published conflicting studies demonstrating that there ‘is’ or ‘is not’ an observable increase in the number of mutations being generated during exposure to ribavirin [38,80,82,83]. This mechanism of action, although directly affecting the virus, would not select for a particular resistant strain since mutations would occur randomly throughout the genome. Two independent studies have reported a small but significant increase in the error rate following mutational analysis of the viral polymerase [80,82]. Another study reported no increase in mutation frequency but the analysis was performed examining only the NS5A and NS3 sequences [84].
A controversial area of ribavirin research has been whether or not ribavirin selects for resistant viral strains. In tissue culture models, it has been clearly demonstrated that resistance to ribavirin develops from adaptations occurring in host factors mainly in the equilibrative nucleotide transporters [85]. However, viral mutants have also been observed in the replicon cell lines treated with ribavirin [86]. Specific mutants have been isolated in both the NS5A and NS5B genes and these mutants emerged in patients undergoing ribavirin monotherapy [80]. However these mutations have not been shown to confer ribavirin resistance in vitro. In these cell culture models (for example, replicon and JFH1), a clear antiviral effect for ribavirin has been reported and the effect appears to be lasting without appearance of viral resistance. This in vitro antiviral effect of ribavirin is also synergistic with the addition of interferon [87,88]. Interestingly, in vivo studies in patients undergoing monotherapy have yielded additional insight. The impact on HCV RNA levels as reported by Pawlotsky et al. [38], demonstrated a transient decline in viral level that returned to baseline levels after approximately 4 days. Longer ribavirin mono-therapy trials have found a weak antiviral effect that increases over time [89]. Therefore, a two-tier mechanism may be operational, in which the initial dose of ribavirin monotherapy has a transient effect that is subsequently followed by a more lasting effect. By contrast, there is very clear evidence that the combination of pegylated interferon-α and ribavirin does not select for viral resistance in vivo [89].
Inosine monophosphate dehydrogenase inhibition
One mechanism that has received significant attention from pharmaceutical companies stems from the fact that ribavirin is a competitive inhibitor of the inosine monophosphate dehydrogenase (IMPDH) enzyme [91,92]. IMPDH is the necessary enzyme for the con-version of inosine-5-monophosphate to xanthine-5-monophosphate and this is the rate-limiting step in guanosine nucleotide biosynthesis through the de novo synthesis pathway. Ribavirin specifically binds to the substrate-binding site of the IMPDH enzyme limiting access of the enzyme to its endogenous substrate (inosine-5-monophosphate) ultimately leading to the decreased synthesis and lower levels of GTP. Since viruses need guanosine nucleotides to rapidly replicate their genomes, it is thought that depletion of GTP by ribavirin’s inhibition of IMPDH may result in significant antiviral activity [93]. Based on this mechanism of action, pharmaceutical companies have attempted to use more potent IMPDH inhibitors (for example, VX-497 and mychophenolate mofetil) to treat HCV but have reported minimal success [94,95]. As a result, this mechanism has lost favour as the dominant mechanism of action of ribavirin. However, proponents argue that IMPDH inhibition could also contribute to anti-viral activity by ultimately lowering GTP pools facilitating the activity of ribavirin triphosphate in error catastrophe or as a polymerase inhibitor. Thus, it is a combination of IMPDH inhibition and a direct mechanism that may explain ribavirin’s efficacy and also why other non-nucleoside IMPDH inhibitors fail to show a significant effect during HCV therapeutic trials.
Immunomodulation
Additional in vivo and in vitro experiments from mouse models and small cohorts of HCV-infected patients have generated evidence that ribavirin can skew the activity of helper T-cells suppressing Th2 responses while inducing Th1 responses, a more cytotoxic pattern facilitating the clearance of infected cells [96–98]. Studies have demonstrated the importance of T-cells in clearance of acute HCV infection [99]. However, it is unclear if T-cells play an important role in antiviral clearance during combination therapy with pegylated interferon-α and ribavirin [100] and therefore this mechanism is probably less likely to have a significant contribution.
Potentiation of induction of interferon-stimulated genes
The mechanism of interferon action has largely been attributed to the induction of multiple genes that support an antiviral state [101]. Since ribavirin is particularly effective when given with interferon, it was evident that ribavirin may have some interferon potentiating activity. In order to study this further, gene expression analyses were performed on liver biopsies from patients on treatment with interferon with or without ribavirin. It was observed that the levels of some interferon-stimulated genes (ISGs) were higher in patients treated with both interferon and ribavirin and it was hypothesized that ribavirin may contribute to ISG induction [102]. Several additional studies, both in vitro and in vivo, have now demonstrated that ribavirin can directly induce ISGs, albeit at a much lower level and with a much smaller number of affected genes, when compared to the effect of interferon [103–107]. In addition, this effect of ribavirin was seen in cells from various mammalian species including cells from humans, mice and rats [88,108]. This is not an entirely unexpected phenomenon as it has been reported that guanine nucleoside analogues can be recognized by toll-like receptors resulting in the induction of antiviral genes [109].
The mechanism by which ribavirin up-regulates antiviral genes can explain ribavirin’s antiviral activity against both DNA and RNA viruses. In addition, it can explain how ribavirin synergistically acts with interferon. This mechanism of action would also limit development of resistant viral strains. The exact mechanism by which ribavirin stimulates this response is an area needing further study. It has been proposed that ribavirin may inhibit a transcriptional repressor allowing up-regulation of antiviral genes that are normally kept at low basal levels [88]. This novel mechanism of action could be utilized to develop broad-spectrum antivirals by enhancing the potent innate immune response in humans. Toxicity would be limited as these agents would mainly be active in cells infected with virus and may protect uninfected cells from future infection. Several strategies to develop small molecule agonists to potentiate the interferon response have been attempted and showed some promise [110,111].
Other nucleosides/nucleotides and HCV therapy
Although ribavirin is the first and only approved nucleoside analogue for the treatment of HCV, there is currently a significant amount of excitement about the future use of this class of compounds in antiviral treatment regimens for HCV [112]. Initial attempts at utilizing taribavirin/viramidine (Figure 1), a liver-targeted ribavirin analogue that elicits fewer side effects, ultimately failed to demonstrate non-inferiority in Phase III clinical trials [113]. Unfortunately, a weight-based dosing regimen was not used for taribavirin/ viramidine in these studies as is utilized for ribavirin. Therefore, future studies with taribavirin/viramidine may demonstrate added therapeutic efficacy if a similar treatment regimen is used between the two agents in order to facilitate its further development. However, new nucleoside and nucleotide analogues have been developed that have received significant attention due to their potent and pangenotypic antiviral activity with high barrier to viral resistance. These include the nucleoside analogue mericitabine (RG7128-cytidine nucleoside analogue), GS-7977 (uracil nucleotide analogue), GS-938 (guanosine nucleotide analogue), INX-189 (guanosine nucleotide analogue) and IDX-184 (guano-sine nucleotide analogue). Since the generation of the monophosphorylated form is the rate-limiting step toward generation of the active triphosphate form of these compounds, there has been an increasing focus on developing nucleotides as these compounds appear to have the greatest potency in this class. It is interesting that the combination of a nucleoside and a nucleotide analogue (for example, ribavirin and GS-7977) showed synergistic activity in treatment of hepatitis C [114].
Future role for ribavirin in HCV therapy
Quadruple treatment regimens and the role of ribavirin in regimens utilizing novel classes of antivirals
The current standard of care for HCV genotype 1 gained approval in 2011 and it consists of a triple-therapy regimen including pegylated interferon-α, ribavirin and a protease inhibitor (either telaprevir or boceprevir) [56,57,60,67]. The next likely improvement in HCV treatment would be the inclusion of second-generation protease inhibitors with fewer side effects and simpler dosing schedules given with ribavirin and pegylated interferon-α [115,116]. Although this would make triple therapy easier to tolerate for the patient, it is anticipated that there would be minimal improvement in cure rates that will remain at approximately 70–75%. Following approval of these second-generation protease inhibitors, the next most likely treatment regimen that will gain approval would utilize four antiviral agents. This would again include a ribavirin and pegylated interferon-a backbone and a protease inhibitor with either an NS5A inhibitor or a non-nucleoside polymerase inhibitor. Preliminary data from these trials are reporting cure rates approaching 100% [117]. This would be a significant step forward in HCV therapy and these quad-regimens are a much needed treatment strategy as they would offer the best alternative for any patients that harbor resistant strains following treatment failure in a clinical trial that was conducted without interferon. One recent study specifically demonstrated the combination of two DAAs with pegylated interferon and ribavirin for 24 weeks resulted in 100% cure rate in 10 patients with genotype 1 who had failed to respond to pegylated interferon and ribavirin alone [117]. These patients are thought to be the hardest to cure; consequently, the high cure rate in this population is very encouraging. In addition to NS5A and non-nucleoside polymerase inhibitors, other classes of antivirals are being combined with ribavirin and pegylated interferon-α. One class, including the family of cyclophilin inhibitors, specifically targets components of the cellular machinery and are designated host targeting antivirals [118]. Recent Phase I and II studies utilizing triple regimens of ribavirin, pegylated interferon-α and the cyclophilin inhibitor alisporivir have suggested an improvement in efficacy over dual therapy [119] although recent discovery of toxicity may hamper subsequent development.
Interferon-free treatment – the holy grail
Ultimately, the goal is to develop an all-oral treatment regimen that excludes interferon. Unfortunately, several factors make HCV a particularly difficult pathogen to cure including its rapid replication rate, existence as a quasispecies and the that fact that its genome encodes a low fidelity, error-prone polymerase that allows for pre-existing drug-resistant mutations and the rapid generation of mutant strains that can evade the antiviral activity of some classes of targeted small molecules [120] However, the considerable side effect profile of interferon and the need for the drug to be given as a subcutaneous injection makes it impractical for wide-spread use. As discussed above, ribavirin itself is contraindicated in several patient populations. However, there is considerable debate as to the necessity of ribavirin in an all-oral treatment regimen [121]. Regard-less of its contraindications, ribavirin has proven to be useful in interferon sparing all-oral treatment regimens with several classes of small molecule inhibitors. One of the first studies to demonstrate the utility of ribavirin in interferon-free treatment regimens was a study with a protease inhibitor and a non-nucleoside polymerase inhibitor with and without ribavirin [122]. All patients without ribavirin developed viral breakthrough during therapy. By contrast, the addition of ribavirin enhanced antiviral activity, delayed the emergence/selection of resistance, and resulted in a greater proportion of patients achieving a rapid virological response (patient is HCV-RNA-negative after 4 weeks of therapy). Additional interferon-free treatment regimens have been pursued but most are using ribavirin in ≥1 of the treatment arms (Table 1). Another recently published study demonstrated no viral breakthrough in patients on triple combinations including ribavirin [123]. Interestingly, this study did not include a ribavirin-free treatment arm, highlighting its probable necessity in all-oral combination. In addition, there was minimal anaemia in this patient cohort.
Table 1.
Use of ribavirin in interferon-sparing regimens for the treatment of HCVa
| Small molecule antivirals | Company |
|---|---|
| ABT-450/r +ABT-333 +ABT-267 ±RBV | Abbott |
| BI 207127 +BI 201335 ±RBV | Boehringer Ingelheim |
| BMS-790052 +BMS-650032 ±RBV | Bristol–Myers Squibb |
| GS-5885 +GS-9451 +GS-9190 ±RBV | Gilead Sciences |
| GS-7977 +RBV | Gilead Sciences |
| GS-7977 +TMC435 ±RBV | Tibotec |
| RO5190591/r +RO5024048 ±RBV | Hoffmann–La Roche |
| DEB025 ±RBV | Novartis Pharmaceuticals |
Information obtained from ClinicalTrials.gov. RBV, ribavirin; /r, ritonavir-boosted.
An additional study, designated Electron, treated HCV genotype-2/3-infected patients in an interferon-free regimen utilizing another DAA that is a nucleotide analogue (PSI-7977). A study of 10 patients with genotype 2/3 HCV infection that combined ribavirin and PSI-7977 for 12 weeks resulted in end-of-treatment viral clearance in all 10 patients, alluding to the possibility that the two small molecule combination might be enough to cure the genotype 2/3 HCV viruses [114].
Finally, the aforementioned study [117] also demonstrated that the combination of the two DAAs alone yielded a 36% SVR rate in 11 additional difficult-to-cure patients with genotype 1a and 1b. This was the first published demonstration of cure of HCV in an interferon-free regimen. In addition, the dual combination regimen was able to cure this difficult-to-treat patient population without ribavirin. This study sup-ports a possible future when ribavirin may not be necessary. However, had ribavirin been included in this regimen, one might predict higher cure rates.
Prospects for the future use of ribavirin in HCV therapy
If a combination of DAAs is developed that is effective and safe with minimal side effects, the contraindications of ribavirin may limit its future use in antiviral therapy for HCV. However, based on previous studies, ribavirin may have to be included in treatment regimens for HCV that include interferon [44,60]. In interferon-sparing all-oral treatment regimens, there are multiple reasons to include ribavirin in the small molecule cock-tail. First, improvements in alanine aminotransferase by ribavirin may mask any slight toxicity of other DAAs in the combination [11]. This could subsequently prove to be beneficial for rapid FDA approval of an all-oral treatment regimen. However, this effect is not seen in all patients treated with ribavirin monotherapy so markers are needed to facilitate identification of patients who will most benefit from ribavirin being included in their all-oral treatment regimen. In addition, ribavirin should be utilized if its use can accelerate approval of DAA-only combinations by increasing SVR (>50%) or in order to shorten the treatment regimen (8/12 weeks on treatment followed by sustained virological response where the patient is HCV-RNA-negative three months after stopping therapy [SVR 12]).
In interferon-sparing regimens, ribavirin may also be particularly effective since its use without interferon, with its effects on bone marrow (for example, neutropaenia) [124], may cause less anaemia. Further-more, it may be utilized at lower doses in these regimens to minimize side effects, as has been done with HCV genotype 2/3 (800 mg) [125] but a dose <600 mg has been demonstrated to be insufficient in certain settings [126]. In addition, ribavirin can be used with other nucleoside/nucleotide analogues, that may be considered to be in the same class of compounds, as has been done in previously reported studies [112] or with other classes that are very different, such as the host-targeting antivirals [119]. One interesting caveat would be to determine if taribavirin/viramidine should be utilized in interferon-free regimens since it has a better side effect profile and because it may be able to contribute significantly to viral eradication. Phase III trials with taribavirin and pegylated interferon failed to demonstrate lack of inferiority [113]; however, this would not preclude the use of taribavirin in interferon-sparing treatment regimens [127]. Ultimately, the future of HCV therapy should be free of interferon. However, much work needs to be done to determine if the DAAs currently in development can cure HCV of all genotypes quickly and safely without ribavirin in the regimen.
Conclusions
Since its initial use as monotherapy over 20 years ago to treat HCV infection, ribavirin has continued to be a critical antiviral agent in treating this virus. Although strategies to eliminate its use have been attempted and subsequently failed, there is no doubt that ribavirin greatly enhances the antiviral effect of interferon-a. With new therapies being developed for chronic HCV infection and the impending reality that interferon- α will likely be of decreasing use for this indication, interest in ribavirin has increased. It is very likely that future studies assessing the effect of DAAs with and without ribavirin will yield a tremendous amount of insight on the antiviral activity of this small molecule and its mechanism of action. Additional data on mechanism of action of ribavirin may re-energize efforts to develop broadly-acting small molecule antivirals with ribavirin-like activity for many other viruses. How-ever, regardless of whether or not the scientific com-munity ever embraces one major mechanism of action to explain ribavirin’s utility in antiviral therapy for HCV, its impact on the treatment of this virus will never be questioned.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972; 177:705–706. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Walsh EE, Hruska JF, Betts RF, Hall WJ. Ribavirin treatment of experimental respiratory syncytial viral infection. A controlled double-blind study in young adults. JAMA 1983; 249:2666–2670. [PubMed] [Google Scholar]
- 3.Buti M, Esteban R, Rodriguez-Frias F, Jardi R, Guardia J. Ribavirin therapy for chronic type C hepatitis. J Hepatol 1991; 13 Suppl 2:S103. [DOI] [PubMed] [Google Scholar]
- 4.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005; 436:967–972. [DOI] [PubMed] [Google Scholar]
- 5.Dixit NM, Perelson AS. The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci 2006; 63:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty S, Maag D, Arnold JJ, et al. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med 2000; 6:1375–1379. [DOI] [PubMed] [Google Scholar]
- 7.Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res 2005; 107:165–171. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez WJ, Parrott RH. Ribavirin aerosol treatment of serious respiratory syncytial virus infection in infants. Infect Dis Clin North Am 1987; 1:425–439. [PubMed] [Google Scholar]
- 9.McCormick JB, King IJ, Webb PA, et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med 1986; 314:20–26. [DOI] [PubMed] [Google Scholar]
- 10.Hayden FG, Sable CA, Connor JD, Lane J. Intravenous ribavirin by constant infusion for serious influenza and parainfluenzavirus infection. Antivir Ther 1996; 1:51–56. [PubMed] [Google Scholar]
- 11.Hoofnagle JH, Ghany MG, Kleiner DE, et al. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology 2003; 38:66–74. [DOI] [PubMed] [Google Scholar]
- 12.Glue P The clinical pharmacology of ribavirin. Semin Liver Dis 1999; 19 Suppl 1:17–24. [PubMed] [Google Scholar]
- 13.Preston SL, Drusano GL, Glue P, Nash J, Gupta SK, McNamara P. Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother 1999; 43:2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab 2004; 5:63–84. [DOI] [PubMed] [Google Scholar]
- 15.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci 2006; 27:416–425. [DOI] [PubMed] [Google Scholar]
- 16.Fukuchi Y, Furihata T, Hashizume M, Iikura M, Chiba K. Characterization of ribavirin uptake systems in human hepatocytes. J Hepatol 2010; 52:486–492. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Kuniki K, Takekuma Y, Hirano T, Iseki K, Sugawara M. Ribavirin uptake by cultured human choriocarcinoma (BeWo) cells and Xenopus laevis oocytes expressing recombinant plasma membrane human nucleoside transporters. Eur J Pharmacol 2007; 557:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Morello J, Cuenca L, Soriano V, et al. Influence of a single nucleotide polymorphism at the main ribavirin transporter gene on the rapid virological response to pegylated interferon-ribavirin therapy in patients with chronic hepatitis C virus infection. J Infect Dis 2010; 202:1185–1191. [DOI] [PubMed] [Google Scholar]
- 19.Doehring A, Hofmann WP, Schlecker C, et al. Role of nucleoside transporters SLC28A2/3 and SLC29A1/2 genetics in ribavirin therapy: protection against anemia in patients with chronic hepatitis C. Pharmacogenet Genomics 2011; 21:289–296. [DOI] [PubMed] [Google Scholar]
- 20.Willis RC, Carson DA, Seegmiller JE. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A 1978; 75:3042–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumarapperuma SC, Sun Y, Jeselnik M, et al. Structural effects on the phosphorylation of 3-substituted 1-beta-D-ribofuranosyl-1,2,4-triazoles by human adenosine kinase. Bioorg Med Chem Lett 2007; 17:3203–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streeter DG, Witkowski JT, Khare GP, et al. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A 1973; 70:1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vo NV, Young KC, Lai MM. Mutagenic and inhibitory effects of ribavirin on hepatitis C virus RNA polymerase. Biochemistry 2003; 42:10462–10471. [DOI] [PubMed] [Google Scholar]
- 24.Bougie I, Bisaillon M. Initial binding of the broad spectrum antiviral nucleoside ribavirin to the hepatitis C virus RNA polymerase. J Biol Chem 2003; 278:52471–52478. [DOI] [PubMed] [Google Scholar]
- 25.Page T, Connor JD. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem 1990; 22:379–383. [DOI] [PubMed] [Google Scholar]
- 26.Bruchfeld A, Lindahl K, Schvarcz R, Stahle L. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit 2002; 24:701–708. [DOI] [PubMed] [Google Scholar]
- 27.Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit 2000; 22:555–565. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara EA, Oishi JS, Wannemacher RW Jr., Stephen EL. Plasma disappearance, urine excretion, and tissue distribution of ribavirin in rats and rhesus monkeys. Antimicrob Agents Chemother 1981; 19:1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paroni R, Del Puppo M, Borghi C, Sirtori CR, Galli Kienle M. Pharmacokinetics of ribavirin and urinary excretion of the major metabolite 1,2,4-triazole-3-carboxamide in normal volunteers. Int J Clin Pharmacol Ther Toxicol 1989; 27:302–307. [PubMed] [Google Scholar]
- 30.Picardi A, Gentilucci UV, Zardi EM, D’Avola D, Amoroso A, Afeltra A. The role of ribavirin in the combination therapy of hepatitis C virus infection. Curr Pharm Des 2004; 10:2081–2092. [DOI] [PubMed] [Google Scholar]
- 31.Lavanchy D Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect 2011; 17:107–115. [DOI] [PubMed] [Google Scholar]
- 32.Alter HJ. HCV natural history: the retrospective and prospective in perspective. J Hepatol 2005; 43:550–552. [DOI] [PubMed] [Google Scholar]
- 33.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010; 138:513–521. [DOI] [PubMed] [Google Scholar]
- 34.Jain MK, Zoellner C. Role of ribavirin in HCV treatment response: now and in the future. Expert Opin Pharmacother 2010; 11:673–683. [DOI] [PubMed] [Google Scholar]
- 35.Reichard O, Andersson J, Schvarcz R, Weiland O. Ribavirin treatment for chronic hepatitis C. Lancet 1991; 337:1058–1061. [DOI] [PubMed] [Google Scholar]
- 36.di Bisceglie AM, Shindo M, Fong TL, et al. A pilot study of ribavirin therapy for chronic hepatitis C. Hepatology 1992; 16:649–654. [DOI] [PubMed] [Google Scholar]
- 37.Brok J, Gluud LL, Gluud C. Ribavirin monotherapy for chronic hepatitis C infection: a Cochrane Hepato-Biliary Group systematic review and meta-analysis of randomized trials. Am J Gastroenterol 2006; 101:842–847. [DOI] [PubMed] [Google Scholar]
- 38.Pawlotsky JM, Dahari H, Neumann AU, et al. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology 2004; 126:703–714. [DOI] [PubMed] [Google Scholar]
- 39.Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med 1986; 315:1575–1578. [DOI] [PubMed] [Google Scholar]
- 40.di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interferon alfa therapy for chronic hepatitis C A randomized, double-blind, placebo-controlled trial. N Engl J Med 1989; 321:1506–1510. [DOI] [PubMed] [Google Scholar]
- 41.Chemello L, Cavalletto L, Bernardinello E, Guido M, Pontisso P, Alberti A. The effect of interferon alfa and ribavirin combination therapy in naive patients with chronic hepatitis C. J Hepatol 1995; 23 Suppl 2:8–12. [PubMed] [Google Scholar]
- 42.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998; 339:1485–1492. [DOI] [PubMed] [Google Scholar]
- 43.Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998; 352:1426–1432. [DOI] [PubMed] [Google Scholar]
- 44.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347:975–982. [DOI] [PubMed] [Google Scholar]
- 45.Dixit NM, Layden-Almer JE, Layden TJ, Perelson AS. Modelling how ribavirin improves interferon response rates in hepatitis C virus infection. Nature 2004; 432:922–924. [DOI] [PubMed] [Google Scholar]
- 46.Reddy KR, Shiffman ML, Rodriguez-Torres M, et al. Induction pegylated interferon alfa-2a and high dose ribavirin do not increase SVR in heavy patients with HCV genotype 1 and high viral loads. Gastroenterology 2010; 139:1972–1983. [DOI] [PubMed] [Google Scholar]
- 47.Thomas E, Fried MW. Hepatitis C: current options for nonresponders to peginterferon and ribavirin. Curr Gastroenterol Rep 2008; 10:53–59. [DOI] [PubMed] [Google Scholar]
- 48.Sánchez-Tapias JM, Diago M, Escartin P, et al. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 2006; 131:451–460. [DOI] [PubMed] [Google Scholar]
- 49.Lindahl K, Stahle L, Bruchfeld A, Schvarcz R. High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C. Hepatology 2005; 41:275–279. [DOI] [PubMed] [Google Scholar]
- 50.Nicot F, Legrand-Abravanel F, Lafont T, et al. Serum concentrations of ribavirin and pegylated interferon and viral responses in patients infected with HIV and HCV. J Med Virol 2008; 80:1523–1529. [DOI] [PubMed] [Google Scholar]
- 51.Dumortier J, Ducos E, Scoazec JY, Chevallier P, Boillot O, Gagnieu MC. Plasma ribavirin concentrations during treatment of recurrent hepatitis C with peginterferon alpha-2b and ribavirin combination after liver transplantation. J Viral Hepat 2006; 13:538–543. [DOI] [PubMed] [Google Scholar]
- 52.Arase Y, Ikeda K, Tsubota A, et al. Significance of serum ribavirin concentration in combination therapy of interferon and ribavirin for chronic hepatitis C. Intervirology 2005; 48:138–144. [DOI] [PubMed] [Google Scholar]
- 53.Larrat S, Stanke-Labesque F, Plages A, Zarski JP, Bessard G, Souvignet C. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother 2003; 47:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svensson JO, Bruchfeld A, Schvarcz R, Stahle L. Determination of ribavirin in serum using highly selective solid-phase extraction and high-performance liquid chromatography. Ther Drug Monit 2000; 22:215–218. [DOI] [PubMed] [Google Scholar]
- 55.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361:580–593. [DOI] [PubMed] [Google Scholar]
- 56.Poordad F, McCone J Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009; 360:1827–1838. [DOI] [PubMed] [Google Scholar]
- 58.Susser S, Welsch C, Wang Y, et al. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 2009; 50:1709–1718. [DOI] [PubMed] [Google Scholar]
- 59.Sarrazin C, Kieffer TL, Bartels D, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 2007; 132:1767–1777. [DOI] [PubMed] [Google Scholar]
- 60.Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360:1839–1850. [DOI] [PubMed] [Google Scholar]
- 61.Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem 2006; 13:3351–3357. [DOI] [PubMed] [Google Scholar]
- 62.Baiocchi L, De Leonardis F, Delle Monache M, et al. Plasma/erythrocyte ribavirin ×100 ratio as an indicator of sustained virological response in HCV genotype 1 patients with early virological response. Antivir Ther 2010; 15:633–639. [DOI] [PubMed] [Google Scholar]
- 63.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–965. [DOI] [PubMed] [Google Scholar]
- 64.McHutchison JG, Manns MP, Brown RS Jr., Reddy KR, Shiffman ML, Wong JB. Strategies for managing anemia in hepatitis C patients undergoing antiviral therapy. Am J Gastroenterol 2007; 102:880–889. [DOI] [PubMed] [Google Scholar]
- 65.Stickel F, Helbling B, Heim M, et al. Critical review of the use of erythropoietin in the treatment of anaemia during therapy for chronic hepatitis C. J Viral Hepat 2012; 19:77–87. [DOI] [PubMed] [Google Scholar]
- 66.Alavian SM, Tabatabaei SV, Behnava B. Impact of erythropoietin on sustained virological response to peginterferon and ribavirin therapy for HCV infection: a systematic review and meta-analysis. J Viral Hepat 2012; 19:88–93. [DOI] [PubMed] [Google Scholar]
- 67.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Freah MA, Zeino Z, Heneghan MA. Management of hepatitis C in patients with chronic kidney disease. Curr Gastroenterol Rep 2012; 14:78–86. [DOI] [PubMed] [Google Scholar]
- 69.Copegus (ribavirin). Package insert 2011 Genentech, San Francisco, CA, USA. [Google Scholar]
- 70.Roberts SS, Miller RK, Jones JK, et al. The Ribavirin Pregnancy Registry: findings after 5 years of enrollment, 2003– 2009. Birth Defects Res A Clin Mol Teratol 2010; 88:551–559. [DOI] [PubMed] [Google Scholar]
- 71.Hofer H, Donnerer J, Sator K, et al. Seminal fluid ribavirin level and functional semen parameters in patients with chronic hepatitis C on antiviral combination therapy. J Hepatol 2010; 52:812–816. [DOI] [PubMed] [Google Scholar]
- 72.Fellay J, Thompson AJ, Ge D, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature 2010; 464:405–408. [DOI] [PubMed] [Google Scholar]
- 73.Thompson AJ, Fellay J, Patel K, et al. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia and decrease the need for ribavirin dose reduction. Gastroenterology 2010; 139:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hitomi Y, Cirulli ET, Fellay J, et al. Inosine triphosphate protects against ribavirin-induced adenosine triphosphate loss by adenylosuccinate synthase function. Gastroenterology 2011; 140:1314–1321. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Larsson R, O’Connell K, Koumans E, Patterson JL. Molecular analysis of the inhibitory effect of phosphorylated ribavirin on the vesicular stomatitis virus in vitro polymerase reaction. Antimicrob Agents Chemother 1989; 33:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maag D, Castro C, Hong Z, Cameron CE. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of the antiviral activity of ribavirin. J Biol Chem 2001; 276:46094–46098. [DOI] [PubMed] [Google Scholar]
- 77.Young KC, Lindsay KL, Lee KJ, et al. Identification of a ribavirin-resistant NS5B mutation of hepatitis C virus during ribavirin monotherapy. Hepatology 2003; 38:869–878. [DOI] [PubMed] [Google Scholar]
- 78.Howe AY, Cheng H, Johann S, et al. Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796. Antimicrob Agents Chemother 2008; 52:3327–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura M, Saito H, Ikeda M, et al. Possible molecular mechanism of the relationship between NS5B polymorphisms and early clearance of hepatitis C virus during interferon plus ribavirin treatment. J Med Virol 2008; 80:632–639. [DOI] [PubMed] [Google Scholar]
- 80.Lutchman G, Danehower S, Song BC, et al. Mutation rate of the hepatitis C virus NS5B in patients undergoing treatment with ribavirin monotherapy. Gastroenterology 2007; 132:1757–1766. [DOI] [PubMed] [Google Scholar]
- 81.Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A 2001; 98:6895–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofmann WP, Polta A, Herrmann E, et al. Mutagenic effect of ribavirin on hepatitis C nonstructural 5B quasispecies in vitro and during antiviral therapy. Gastroenterology 2007; 132:921–930. [DOI] [PubMed] [Google Scholar]
- 83.Lanford RE, Guerra B, Lee H, et al. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(i)-poly(c), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J Virol 2003; 77:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schinkel J, de Jong MD, Bruning B, van Hoek B, Spaan WJ, Kroes AC. The potentiating effect of ribavirin on interferon in the treatment of hepatitis C: lack of evidence for ribavirin-induced viral mutagenesis. Antivir Ther 2003; 8:535–540. [PubMed] [Google Scholar]
- 85.Pfeiffer JK, Kirkegaard K. Ribavirin resistance in hepatitis C virus replicon-containing cell lines conferred by changes in the cell line or mutations in the replicon RNA. J Virol 2005; 79:2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feigelstock DA, Mihalik KB, Feinstone SM. Selection of hepatitis C virus resistant to ribavirin. Virol J 2011; 8:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SS, Peng LF, Lin W, et al. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology 2007; 132:311–320. [DOI] [PubMed] [Google Scholar]
- 88.Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology 2011; 53:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rotman Y, Noureddin M, Feld JJ, et al. Ribavirin exerts a weak interferon-like effect on gene expression in the liver of patients with chronic hepatitis C. Hepatology 2011; 54 Suppl 1:1327A–1328A. [Google Scholar]
- 90.Feld JJ, Modi AA, El-Diwany R, et al. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology 2011; 140:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malinoski F, Stollar V. Inhibitors of IMP dehydrogenase prevent sindbis virus replication and reduce GTP levels in Aedes albopictus cells. Virology 1981; 110:281–291. [DOI] [PubMed] [Google Scholar]
- 92.Zhou S, Liu R, Baroudy BM, Malcolm BA, Reyes GR. The effect of ribavirin and IMPDH inhibitors on hepatitis C virus subgenomic replicon RNA. Virology 2003; 310:333–342. [DOI] [PubMed] [Google Scholar]
- 93.Franchetti P, Grifantini M. Nucleoside and non-nucleoside IMP dehydrogenase inhibitors as antitumor and antiviral agents. Curr Med Chem 1999; 6:599–614. [PubMed] [Google Scholar]
- 94.Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother 2000; 44:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McHutchison JG, Shiffman ML, Cheung RC, et al. A randomized, double-blind, placebo-controlled dose-escalation trial of merimepodib (VX-497) and interferon-alpha in previously untreated patients with chronic hepatitis C. Antivir Ther 2005; 10:635–643. [PubMed] [Google Scholar]
- 96.Tam RC, Pai B, Bard J, et al. Ribavirin polarizes human T cell responses towards a Type 1 cytokine profile. J Hepatol 1999; 30:376–382. [DOI] [PubMed] [Google Scholar]
- 97.Ning Q, Brown D, Parodo J, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 1998; 160:3487–3493. [PubMed] [Google Scholar]
- 98.Hultgren C, Milich DR, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol 1998; 79:2381–2391. [DOI] [PubMed] [Google Scholar]
- 99.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5:215–229. [DOI] [PubMed] [Google Scholar]
- 100.Rahman F, Heller T, Sobao Y, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology 2004; 40:87–97. [DOI] [PubMed] [Google Scholar]
- 101.Borden EC, Sen GC, Uze G, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 2007; 6:975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feld JJ, Nanda S, Huang Y, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: identifying molecular pathways for treatment response. Hepatology 2007; 46:1548–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feld JJ, Lutchman GA, Heller T, et al. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology, 2010; 139:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Jamaluddin M, Wang S, et al. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol 2003; 77:5933–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu WL, Su WC, Cheng CW, et al. Ribavirin up-regulates the activity of double-stranded RNA-activated protein kinase and enhances the action of interferon-alpha against hepatitis C virus. J Infect Dis 2007; 196:425–434. [DOI] [PubMed] [Google Scholar]
- 106.Su WC, Liu WL, Cheng CW, et al. Ribavirin enhances interferon signaling via stimulation of mTOR and p53 activities. FEBS Lett 2009; 583:2793–2798. [DOI] [PubMed] [Google Scholar]
- 107.Tokumoto Y, Hiasa Y, Uesugi K, et al. Ribavirin regulates hepatitis C virus replication through enhancing interferon-stimulated genes and interleukin 8. J Infect Dis 2012; 205:1121–1130. [DOI] [PubMed] [Google Scholar]
- 108.Ye J, Maniatis T. Negative regulation of interferon-beta gene expression during acute and persistent virus infections. PLoS ONE 2011; 6:e20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee J, Chuang TH, Redecke V, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A 2003; 100:6646–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boonstra A, Liu BS, Groothuismink ZM, et al. Potent immune activation in chronic hepatitis C patients upon administration of an oral inducer of endogenous interferons that acts via Toll-like receptor 7. Antivir Ther 2012; 17:657–667. [DOI] [PubMed] [Google Scholar]
- 111.Konishi H, Okamoto K, Ohmori Y, et al. An orally available, small-molecule interferon inhibits viral replication. Sci Rep 2012; 2:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferenci P Treatment of chronic hepatitis C – are interferons really necessary? Liver Int 2012; 32 Suppl 1:108–112. [DOI] [PubMed] [Google Scholar]
- 113.Marcellin P, Gish RG, Gitlin N, et al. Safety and efficacy of viramidine versus ribavirin in ViSER2: randomized, double-blind study in therapy-naive hepatitis C patients. J Hepatol 2010; 52:32–38. [DOI] [PubMed] [Google Scholar]
- 114.Gane EJ, Stedman CA, Hyland RH, et al. Once daily PSI-7977 plus RBV: pegylated interferon alfa not required for complete rapid viral response in treatment-naive patients with HCV GT2 or GT3. Hepatology 2011; 54 Suppl 1:377A.21710476 [Google Scholar]
- 115.Ciesek S, von Hahn T, Manns MP. Second-wave protease inhibitors: choosing an heir. Clin Liver Dis 2011; 15:597–609. [DOI] [PubMed] [Google Scholar]
- 116.Manns M, Reesink H, Berg T, et al. Rapid viral response of once-daily TMC435 plus pegylated interferon/ribavirin in hepatitis C genotype-1 patients: a randomized trial. Antivir Ther 2011; 16:1021–1033. [DOI] [PubMed] [Google Scholar]
- 117.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 2012; 366:216–224. [DOI] [PubMed] [Google Scholar]
- 118.Gallay PA. Cyclophilin inhibitors: a novel class of promising host-targeting anti-HCV agents. Immunol Res 2012; 52:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Flisiak R, Jaroszewicz J, Flisiak I, Lapinski T. Update on alisporivir in treatment of viral hepatitis C. Expert Opin Investig Drugs 2012; 21:375–382. [DOI] [PubMed] [Google Scholar]
- 120.Domingo E, Gomez J. Quasispecies and its impact on viral hepatitis. Virus Res 2007; 127:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clark V, Nelson DR. The role of ribavirin in direct acting antiviral drug regimens for chronic hepatitis C. Liver Int 2012; 32 Suppl 1:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeuzem S, Buggisch P, Agarwal K, et al. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology 2012; 55:749–758. [DOI] [PubMed] [Google Scholar]
- 123.Zeuzem S, Asselah T, Angus P et al. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology 2011; 141:2047–2055. [DOI] [PubMed] [Google Scholar]
- 124.Renou C, Harafa A, Cummins C, et al. Threshold for neutropenia in the adjustment of interferon treatment in HCV infection. Hepatology 2003; 37:949–950. [DOI] [PubMed] [Google Scholar]
- 125.Rizzetto M Treatment of hepatitis C virus genotype 2 and 3 with pegylated interferon plus ribavirin. J Hepatol 2005; 42:275–276. [DOI] [PubMed] [Google Scholar]
- 126.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology 2007; 132:103–112. [DOI] [PubMed] [Google Scholar]
- 127.Kwo PY, Vinayek R. The next step for taribavirin. Hepatology 2010; 52:1185–1188. [DOI] [PubMed] [Google Scholar]


