Abstract
A mild and efficient method catalyzed by α-chymotrypsin was developed for the synthesis of bis(indolyl)methanes through a cascade process between indole and aromatic aldehydes. In the ethanol aqueous solution, a green medium, a wide range of aromatic aldehydes could react with indole to afford the desired products with moderate to good yields (from 68% to 95%) using a little α-chymotrypsin as catalyst.
Keywords: bis(indolyl)methanes, tandem protocol, biocatalysis, promiscuity, α-chymotrypsin
1. Introduction
Bis(indolyl)methane and their derivatives are members of an important class of heterocyclic compounds that display diverse biological properties, and can act as a selective colorimetric sensor for F− (or HSO4−) and also as a highly selective fluorescent molecular sensor for Cu2+ [1,2]. They are of immense interest because of their wide spectrum of pharmacological properties, such as antibacterial activity [3], antiangiogenic activity [4], acting as cytotoxic agents [4] and tumor growth inhibitors [5]. In the past years, various methods were mentioned for the synthesis of bis(indolyl)methanes, generally, these compounds could be obtained by the cascade reaction between indole and aromatic (or aliphatic) aldehydes in the present of protic or Lewis acids, such as I2 [6], Ionic liquids [7], Fe(DS)3 [8], CeCl3·7H2O [9], AuCl [10], SBA-15/SO3H [11], TPPMS/CBr4 [12], PEG-supported dichlorophosphate [13], H3PW12O40 [14], zeolites [15]. In fact, the bis(indolyl)methanes could also be prepared from indole and benzyl alcohols [16,17]. Although bis(indolyl)methanes could be effectively synthesized via most of the above attempts in excellent yields, there are some drawbacks in the vast majority of the described methods including the use of expensive and toxic heavy metals, requirement of a stoichiometric amount of catalysts, complicated post-treatment process, and so on.
As an efficient, high selectivity and eco-friendly catalyst for the organic synthesis, enzymes have attracted much attention in the field of synthetic chemistry [18,19,20]. Especially, in recent years, some hydrolases have demonstrated high activity for unnatural substrates and alternative chemical transformations, namely, biocatalytic promiscuity, which provides a new tool for organic synthesis and largely extends the application of enzymes [21,22]. Enzyme promiscuity has been widely used in multiple types of organic reactions, such as C-C [23], C-N [24] and C-S [25] bond-formation reactions. Recently, Lin and coworkers [26] reported the synthesis of bis(indolyl)methanes catalyzed by PPL (Lipase from porcine pancreas), but the results are waiting to be lifted. Therefore, effective, eco-friendly and sustainable biocatalytic methods are still to be explored.
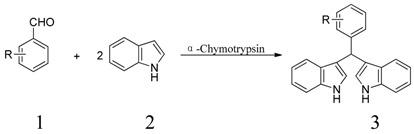
Thus, as a part of our continuing interest in green chemistry and enzyme promiscuity, we wish to report the protease-catalyzed cascade reaction between indole and aromatic aldehydes (Scheme 1). Here, α-chymotrypsin was selected as the biocatalyst, and a series of aromatic aldehydes could react with indole to afford the corresponding bis(indolyl)methanes in ethanol aqueous solution, which was obviously a green synthesis method.
Scheme 1.
α-Chymotrypsin-catalyzed tandem reaction of indole and aldehydes.
2. Results and Discussion
Based on our previous research, initial efforts were performed in 40% ethanol solution using 4-nitrobenzaldehyde and indole as a model reaction and some hydrolases were investigated to screen the optimal catalyst. As shown in Table 1, α-chymotrypsin displayed the best catalytic activity and gave the corresponding product in 77% yield (Table 1, entry 8), pepsin also provided 51% yield (Table 1, entry 7). However, the other tested hydrolase, such as Neutral protease, Alkaline protease, Papain, Acylase I from Aspergillus melleus (Acylase I), PPL and Amano Lipase M from Mucor javanicus (Amano Lipase M) only provided fewer products (Table 1, entries 1–6). At the same time, non-enzyme protein BSA (Bovine serum albumin) (Table 1, entry 9) and denatured α-chymotrypsin (Table 1, entry 10) were also used as catalysts, while both showed inferior catalytic ability, and gained similar results with the blank control reaction (Table 1, entry 11). These results confirmed that the catalytic activity of α-chymotrypsin for the tandem reaction did not arise from unspecific amino acids, and its tertiary structure played a great role in this enzyme-catalyzed reaction.
Table 1.
Catalytic activities of different enzymes a.

| Entry | Enzyme | Yield b (%) |
|---|---|---|
| 1 | Neutral Protease | 9 |
| 2 | Alkaline protease | 15 |
| 3 | Papain | 22 |
| 4 | Acylase I | 10 |
| 5 | PPL | 29 |
| 6 | Amano Lipase M | 6 |
| 7 | Pepsin | 51 |
| 8 | α-Chymotrypsin | 77 |
| 9 | BSA | 5 |
| 10 | Denatured α-chymotrypsin c | 5 |
| 11 | No enzyme | 4 |
a Conditions: 4-nitrobenzaldehyde 75.6 mg (0.5 mmol), indole 117.1 mg (1.0 mmol), enzyme 10 mg, ethanol 2 mL and deionized water 3 mL, at 40 °C for 24 h; b Isolated yield after column chromatography; c Pretreated with urea solution (8 mol/L).
α-Chymotrypsin was selected as the best catalyst, the influence of the ethanol content was then investigated, because the reaction medium has been recognized as a significant factor for enzymatic reaction. The results are shown in Figure 1, it can be seen that the yield was significantly influenced by ethanol content, and 30% was selected as the optimum concentration. A possible reason may be that ethanol is conducive to the dissolution of the substrates; nevertheless, over much ethanol can lead α-chymotrypsin to lose activity. As a result, the yield was rising greatly by increasing the concentration of ethanol from 0% to 30% and reached the maximum at 30%, and then the yield decreased significantly with the rise of the concentration.
Figure 1.
Influence of the ethanol content on the cascade reaction a.
a Conditions: 4-nitrobenzaldehyde 75.6 mg (0.5 mmol), indole 117.1 mg (1.0 mmol), α-chymotrypsin 10 mg, solvent 5 mL, at 40 °C for 24 h; b Isolated yield after column chromatography.
Temperature is another key influencing factor on the biocatalytic reaction, because of its effect on the biological activity of enzyme and the rate of the reaction. As can be seen from Figure 2, a yield of 88% was gained at 50 °C and 90% was gained at 60 °C. Though a slightly higher yield was obtained at 60 °C, 50 °C was chosen as the optimal temperature considering of the energy consumption and the inactivation of enzyme at high temperature for a long time.
Figure 2.
The influence of temperature on the cascade reaction a.
a Conditions: 4-nitrobenzaldehyde 75.6 mg (0.5 mmol), indole 117.1 mg (1.0 mmol), α-chymotrypsin 10 mg, ethanol 1.5 mL and deionized water 3.5 mL, at a certain temperature for 24 h; b Isolated yield after column chromatography.
To further optimize the reaction conditions, the effect of enzyme loading on the α-chymotrypsin-catalyzed tandem reaction was investigated. As shown in Figure 3, only a little product was detected in the absence of enzyme, however, the yield was improved sharply when 2 mg α-chymotrypsin was loaded. After that, only a slight rising trend was appeared with the increase of catalyst dosage (2–10 mg), at last 8 mg was chosen as the best enzyme loading. However, it is particularly worth mentioning that the enzyme dosage is much lesser than most of the other reports about enzyme promiscuity, for example, 200 mg enzyme was used in a protease-catalyzed aldol reaction with 1.15 mL reaction medium [27].
Figure 3.
Influence of the enzyme loading on the cascade reaction a.
a Conditions: 4-nitrobenzaldehyde 75.6 mg (0.5 mmol), indole 117.1 mg (1.0 mmol), α-chymotrypsin 0–10 mg, ethanol 1.5 mL and deionized water 3.5 mL, at 50 °C for 24 h; b Isolated yield after column chromatography.
With the optimized conditions in hand, more kinds of aromatic aldehydes were used to show the generality and scope of this enzymatic cascade reaction. The results are summarized in Table 2, it can be seen that a wide range of aromatic aldehydes can effectively react with indole to give the corresponding products, and the best yield of 95% has been obtained. However, the substituent in aromatic aldehyde has marked impact on the yield. Generally, aromatic aldehydes bearing an electron-withdrawing substituent, such as a nitro group, gave better results (Table 2, entries 1–7). However, some aldehydes with electron-donating groups, e.g., 4-hydroxybenzaldehyde and 4-methylbenzaldehyde showed lower activity, and moderate yields were gotten. However, beyond that, we also examined the reaction of aliphatic aldehydes with indole, however, the results were far from satisfactory. Caproaldehyde, one of the tested aliphatic aldehydes, only gave the best yield of 20% (data not given). Meanwhile, the tandem reactions about other heterocyclic compounds are proceeding.
Table 2.
Investigation of the reactant scope of the α-chymotrypsin-catalyzed cascade reaction a.
| Entry | R | Product | Yield b (%) |
|---|---|---|---|
| 1 | 4-NO2 | 3a | 95 |
| 2 | 3-NO2 | 3b | 92 |
| 3 | 2-NO2 | 3c | 90 |
| 4 | 4-Cl | 3d | 95 |
| 5 | 2-Cl | 3e | 94 |
| 6 | 3-Br | 3f | 89 |
| 7 | 2-Br | 3g | 86 |
| 8 | 4-OH | 3h | 70 |
| 9 | 2-OH | 3i | 68 |
| 10 | 4-CH3 | 3j | 71 |
| 11 | 4-OCH3 | 3k | 75 |
| 12 | 4-OH, 3-OCH3 | 3l | 79 |
a Conditions: aromatic aldehyde 0.5 mmol, indole 1.0 mmol, α-chymotrypsin 8 mg, ethanol 1.5 mL and deionized water 3.5 mL, at 50 °C for 32 h; b Isolated yield after column chromatography.
Finally, we attempt to propose the mechanism for the α-chymotrypsin-catalyzed cascade reaction. As one of the family of serine proteases, α-chymotrypsin is comprised of 245 amino acids and the catalytic triad is formed by His57, Asp102, and Ser195 [28,29,30]. According to this information, a mechanism of α-chymotrypsin-catalyzing such reaction was invisaged. As shown in Scheme 2, the aldehyde carbonyl bind to the oxyanion hole and be effectively activated [31,32]. His 57 may abstract the proton from the C-3 position of indole, allowing the indole as an available nucleophile to bind to aromatic aldehyde, Ser195, and Gly193 could stabilize the formation of an oxyanion by hydrogen-bonding with the carbonyl oxygen of the aromatic aldehyde. Subsequently, the released indolyl methanol and another molecule of indole are joined together in a similar fashion, which could lead to the formation of the product and liberated α-chymotrypsin.
Scheme 2.
Proposed mechanism for α-chymotrypsin-catalyzed cascade reaction.
3. Experimental Section
3.1. General Information
All chemicals were purchased from commercial suppliers and the solvents were not redistilled before used. Amano Lipase M from Mucor javanicus (Amano Lipase M), Acylase I from Aspergillus melleus (Acylase I) and α-chymotrypsin were obtained from Sigma-Aldrich. Lipase from porcine pancreas (PPL), Bovine serum albumin (BSA), Alkaline protease, Neutral protease, Pepsin from bovine serum and Papain were obtained from Aladdin.
1H and 13C-NMR spectra were recorded on a Bruker AV-400 spectrometer in CDCl3 or DMSO-d6. High-resolution mass measurements (HRMS) were recorded on a Thermo Fisher Scientific LTQ Orbitrap-XL mass Spectrometer. Chemical shifts were reported in ppm(δ). IR spectra were recorded on a Nicolet 380 FT-IR spectrophotometer. Melting points were measured using a WRS-1B Digital Melting Point Apparatus.
3.2. General Procedure for the Synthesis of Bis(indolyl)methane
A mixture of 4-nitrobenzaldehyde (0.0756 g, 0.5 mmol, 1 equiv.), indole (0.1171 g, 1 mmol, 2 equivalent) and α-chymotrypsin (10 mg) in mixed solvents (3 mL water and 2 mL ethanol) was incubated at 50 °C and 260 r.p.m. for 24 h. After completion of the reaction (TLC), the products were extraction with 3 × 5 mL ethylacetate. Then, the combined organic layer was concentrated under reduced pressure to afford the crude product and purified by column chromatography on silica gel (PE:EtOAc = 9:1) to give the pure product.
3,3'-((4-Nitrophenyl)methylene)bis(1H-indole) (3a)

Red solid, mp: 219–220 °C; IR ν (cm−1) (KBr): 3455, 1637, 1506, 1456, 1414, 1341, 1101, 744; 1H-NMR (400 MHz, DMSO): δ 10.95 (s, 2H), 8.15 (d, J = 8.7 Hz, 2H), 7.61 (d, J = 8.7 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 7.9 Hz, 2H), 7.06 (t, J = 7.5 Hz, 2H), 6.96–6.78 (m, 4H), 6.04 (s, 1H); 13C-NMR (100 MHz, DMSO): δ 153.61, 146.26, 137.10, 129.93, 124.35, 123.87, 121.58, 119.39, 118.91, 112.07, 40.66, 40.45, 40.24, 40.03, 39.83, 39.62, 39.41; HRMS (EI) m/z calcd for C23H16O2N3 [M−H]− 366.12480, found: 366.12896.
3,3'-((3-Nitrophenyl)methylene)bis(1H-indole) (3b)

Red solid, mp: 86–87 °C; IR ν (cm−1) (KBr): 3411, 1637, 1508, 1456, 1417, 1339, 1094, 744; 1H-NMR (400 MHz, DMSO-d6): δ 10.94 (s, 2H), 8.17 (s, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 8.4, 16.0, 7.6 Hz, 1H), 7.37 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8 Hz, 2H), 7.06 (t, J = 7.2, 14.8, 7.6 Hz, 2H), 6.88 (m, 4H), 6.07 (s, 1H).
3,3'-((2-Nitrophenyl)methylene)bis(1H-indole) (3c)

Red solid, mp: 140–142 °C; IR ν (cm−1) (KBr): 3419, 1636, 1560, 1523, 1454, 1417, 1352, 1096, 744; 1H NMR (400 MHz, DMSO): δ 10.93 (s, 2H), 7.88 (d, J = 7.9 Hz, 1H), 7.56 (t, J = 7.5 Hz, 1H), 7.46 (t, J = 7.3 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.36 (d, J = 8.1 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.06 (t, J = 7.5 Hz, 2H), 6.89 (t, J = 7.4 Hz, 2H), 6.78 (s, 2H), 6.40 (s, 1H).
3,3'-((4-Chlorophenyl)methylene)bis(1H-indole) (3d)

Red solid, mp: 75–76 °C; IR ν (cm−1) (KBr): 3417, 1637, 1486, 1455, 1416, 1338, 1092, 743; 1H-NMR (400 MHz, DMSO): δ 10.86 (s, 2H), 7.39–7.33 (m, 4H), 7.31 (d, J = 8.5 Hz, 2H), 7.27 (d, J = 7.9 Hz, 2H), 7.04 (t, J = 7.5 Hz, 2H), 6.87 (t, J = 7.5 Hz, 2H), 6.83 (s, 2H), 5.87 (s, 1H).
3,3'-((2-Chlorophenyl)methylene)bis(1H-indole) (3e)

Red solid, mp: 71–72 °C; IR ν (cm−1) (KBr): 3414, 1627, 1458, 1417, 1338, 1093, 744; 1H-NMR (400 MHz, CDCl3): δ 7.93 (s, 2H), 7.64 (d, J = 7.8 Hz, 1H), 7.44 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 7.23 (s, 1H), 7.21 (s, 1H), 7.17 (s, 2H), 7.14–7.10 (m, 1H), 7.06 (d, J = 15.0 Hz, 2H), 6.62 (s, 2H), 6.34 (s, 1H).
3,3'-((3-Bromophenyl)methylene)bis(1H-indole) (3f)

Red solid, mp: 104–106 °C; IR ν (cm−1) (KBr): 3440, 1636, 1454, 1416, 1342, 1096, 742; 1H-NMR (400 MHz, CDCl3): δ 7.97 (s, 2H), 7.49 (s, 1H), 7.41–7.34 (m, 5H), 7.31 (d, J = 21.5 Hz, 1H), 7.22–7.11 (m, 3H), 7.02 (t, J = 7.5 Hz, 2H), 6.65 (s, 2H), 5.85 (s, 1H).
3,3'-((2-Bromophenyl)methylene)bis(1H-indole) (3g)

Red solid, mp: 76–77 °C; IR ν (cm−1) (KBr): 3416, 1637, 1457, 1416, 1339, 1094, 1017, 743; 1H-NMR (400 MHz, CDCl3): δ 7.88 (s, 2H), 7.46–7.27 (m, 5H), 7.19 (s, 1H), 7.15 (d, J = 5.8 Hz, 1H), 7.13–7.06 (m, 2H), 7.05–7.00 (m, 1H), 6.95 (t, J = 7.5 Hz, 2H), 6.56 (s, 2H), 6.27 (s, 1H); HRMS (EI) m/z calcd for C23H16BrN2 [M−H]− 399.05023, found: 399.05086.
3,3'-((4-Hydroxylphenyl)methylene)bis(1H-indole) (3h)

Red solid, mp: 195–196 °C; IR ν (cm−1) (KBr): 3416, 1617, 1509, 1454, 1416, 1338, 1218, 1166, 1095, 744; 1H-NMR (400 MHz, DMSO): δ 10.77 (s, 2H), 9.14 (s, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 7.9 Hz, 2H), 7.13 (d, J = 8.3 Hz, 2H), 7.02 (t, J = 7.5 Hz, 2H), 6.85 (t, J = 7.4 Hz, 2H), 6.77 (s, 2H), 6.65 (d, J = 8.4 Hz, 2H), 5.70 (s, 1H).
3,3'-((2-Hydroxylphenyl)methylene)bis(1H-indole) (3i)

Red solid, mp: 255–256 °C; IR ν (cm−1) (KBr): 3419, 2924, 1620, 1516, 1483, 1455, 1420, 1195, 1096, 745; 1H-NMR (400 MHz, CDCl3): δ 7.96 (s, 2H), 7.34 (s, 1H), 7.31 (d, J = 3.4 Hz, 2H), 7.29 (s, 1H), 7.19 (s, 2H), 7.13 (d, J = 8.1 Hz, 2H), 7.09 (d, J = 7.0 Hz, 2H), 6.95 (t, J = 7.5 Hz, 2H), 6.80 (d, J = 3.8 Hz, 1H), 6.78 (d, J = 6.2 Hz, 1H), 6.70 (s, 2H), 5.93 (s, 1H).
3,3'-((4-Methylphenyl)methylene)bis(1H-indole) (3j)

Red solid, mp: 99–101 °C; IR ν (cm−1) (KBr): 3421, 1635, 1512, 1454, 1416, 1339, 1095, 742; 1H-NMR (400 MHz, CDCl3): δ 7.91 (s, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.35 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 7.9 Hz, 2H), 7.16 (t, J = 7.5 Hz, 2H), 7.08 (d, J = 7.7 Hz, 2H), 7.00 (t, J = 7.5 Hz, 2H), 6.66 (s, 2H), 5.85 (s, 1H), 2.31 (s, 3H).
3,3'-((4-Methoxylphenyl)methylene)bis(1H-indole) (3k)

Pink solid, mp: 189–191 °C; IR ν (cm−1) (KBr): 3412, 1636, 1564, 1509, 1455, 1417, 1340, 1250, 1175, 1095, 1027, 744; 1H-NMR (400 MHz, CDCl3): δ 7.90 (s, 2H), 7.38 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 8.1 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 7.16 (t, J = 7.4 Hz, 2H), 7.00 (t, J = 7.3 Hz, 2H), 6.81 (d, J = 8.6 Hz, 2H), 6.63 (s, 2H), 5.83 (s, 1H), 3.77 (s, 3H).
4-(Di(1H-indol-3-yl)methyl)-2-methoxyphenol (3l)

Red solid, mp 123–125 °C; IR ν (cm−1) (KBr): 3428, 1635, 1511, 1456, 1418, 1128, 744; 1H-NMR (400 MHz, CDCl3): δ 7.85 (s, 2H), 7.38 (d, J = 7.9 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H), 7.15 (t, J = 7.6 Hz, 2H), 6.99 (t, J = 7.5 Hz, 2H), 6.87 (s, 1H), 6.83–6.74 (m, 2H), 6.58 (s, 2H), 5.79 (s, 1H), 3.72 (s, 3H).
4. Conclusions
In conclusion, an effective, eco-friendly and convenient method for synthesis of bis(indolyl)alkanes was developed, which is the first example of such a protease-catalyzed reaction. α-Chymotrypsin, a promiscuous hydrolase, showed excellent catalytic activity for a series of substrates. As a new example of enzyme promiscuity, it is beneficial for expanding the application of biocatalysis in non-natural reactions.
Acknowledgments
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21262002, 21462001, 21465002), Natural Science Foundation of Jiangxi (No. 20122BAB203006, 20142BAB203008), the Science and Technology Foundation of the Jiangxi Education Department (KJLD12006, GJJ13468, GJJ14466, KJLD14050).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/12/19665/s1.
Author Contributions
Zhang-Gao Le and Zong-Bo Xie designed research; Da-Zhao Sun and Guo-Fang Jiang performed research and analyzed the data; Zong-Bo Xie and Da-Zhao Sun wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.He X.-M., Hu S.-Z., Liu K., Guo Y., Xu J., Shao S. Oxidized Bis(indolyl)methane: A Simple and Efficient Chromogenic-Sensing Molecule Based on the Proton Transfer Signaling Mode. Org. Lett. 2006;8:333–336. doi: 10.1021/ol052770r. [DOI] [PubMed] [Google Scholar]
- 2.Martínez R., Espinosa A., Tárraga A., Molina P. Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu2+ cations. Tetrahedron. 2008;64:2184–2191. doi: 10.1016/j.tet.2007.12.025. [DOI] [Google Scholar]
- 3.Lounasmaa M., Tolvanen A. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit (July 1997 to December 1998) Nat. Prod. Rep. 2000;17:175–191. doi: 10.1039/a809402k. [DOI] [PubMed] [Google Scholar]
- 4.Shiri M., Zolfigol M.A., Kruger H.G., Tanbakouchian Z. Bis- and Trisindolylmethanes (BIMs and TIMs) Chem. Rev. 2010;110:2250–2293. doi: 10.1021/cr900195a. [DOI] [PubMed] [Google Scholar]
- 5.Safe S., Papineni S., Chintharlapalli S. Cancer chemotherapy with indole-3-carbinol, bis(3'-indolyl)methane and synthetic analogs. Cancer Lett. 2008;269:326–338. doi: 10.1016/j.canlet.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji S.-J., Wang S.-Y., Zhang Y., Loh T.-P. Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions. Tetrahedron. 2004;60:2051–2055. doi: 10.1016/j.tet.2003.12.060. [DOI] [Google Scholar]
- 7.Chakraborti A.K., Roy S.R., Kumar D., Chopra P. Catalytic application of room temperature ionic liquids: [bmim][MeSO4] as a recyclable catalyst for synthesis of bis(indolyl)methanes. Ion-fishing by MALDI-TOF-TOF MS and MS/MS studies to probe the proposed mechanistic model of catalysis. Green Chem. 2008;10:1111–1118. doi: 10.1039/b807572g. [DOI] [Google Scholar]
- 8.Wang S.Y., Ji S.J. Facile Synthesis of Bis(indolyl)methanes catalyzed by Ferric Dodecyl Sulfonate [Fe(DS)3] in Water at Room Temperature. Synth. Commun. 2008;38:1291–1298. doi: 10.1080/00397910701873318. [DOI] [Google Scholar]
- 9.Silveira C.C., Mendes S.R., Líbero F.M., Lenardão E.J., Perin G. Glycerin and CeCl3·7H2O: A new and efficient recyclable medium for the synthesis of bis(indolyl)methanes. Tetrahedron Lett. 2009;50:6060–6063. doi: 10.1016/j.tetlet.2009.08.062. [DOI] [Google Scholar]
- 10.Praveen C., Wilson Sagayaraj Y., Perumal P.T. Gold(I)-catalyzed sequential cycloisomerization/bis-addition of o-ethynylanilines: An efficient access to bis(indolyl)methanes and di(indolyl)indolin-2-ones. Tetrahedron Lett. 2009;50:644–647. doi: 10.1016/j.tetlet.2008.11.086. [DOI] [Google Scholar]
- 11.Naik M.A., Sachdev D., Dubey A. Sulfonic acid functionalized mesoporous SBA-15 for one-pot synthesis of substituted aryl-14H-dibenzo xanthenes and bis(indolyl) methanes. Catal. Commun. 2010;11:1148–1153. doi: 10.1016/j.catcom.2010.06.004. [DOI] [Google Scholar]
- 12.Huo C., Sun C., Wang C., Jia X., Chang W. Triphenylphosphine-m-sulfonate/Carbon Tetrabromide as an Efficient and Easily Recoverable Catalyst System for Friedel–Crafts Alkylation of Indoles with Carbonyl Compounds or Acetals. ACS Sustain. Chem. Eng. 2013;1:549–553. doi: 10.1021/sc400033t. [DOI] [Google Scholar]
- 13.Naidu K.R., Khalivulla S.I., Rasheed S., Fakurazi S., Arulselvan P., Lasekan O., Abas F. Synthesis of bisindolylmethanes and their cytotoxicity properties. Int. J. Mol. Sci. 2013;14:1843–1853. doi: 10.3390/ijms14011843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azizi N., Torkian L., Saidi M.R. Highly efficient synthesis of bis(indolyl)methanes in water. J. Mol. Catal. A Chem. 2007;275:109–112. doi: 10.1016/j.molcata.2007.05.024. [DOI] [Google Scholar]
- 15.Karthik M., Tripathi A., Gupta N., Palanichamy M., Murugesan V. Zeolite catalyzed electrophilic substitution reaction of indoles with aldehydes: Synthesis of bis(indolyl)methanes. Catal. Commun. 2004;5:371–375. doi: 10.1016/j.catcom.2004.04.007. [DOI] [Google Scholar]
- 16.Hikawa H., Suzuki H., Yokoyama Y., Azumaya I. Mechanistic Studies for Synthesis of Bis(indolyl)methanes: Pd-Catalyzed C–H Activation of Indole–Carboxylic Acids with Benzyl Alcohols in Water. Catalysts. 2013;3:486–500. doi: 10.3390/catal3020486. [DOI] [Google Scholar]
- 17.Hikawa H., Yokoyama Y. Pd-catalyzed C–H activation in water: Synthesis of bis(indolyl)methanes from indoles and benzyl alcohols. RSC Adv. 2013;3:1061–1064. doi: 10.1039/c2ra21887a. [DOI] [Google Scholar]
- 18.Cerqueira Pereira S., Bussamara R., Marin G., Lima Camargo Giordano R., Dupont J., de Campos Giordano R. Enzymatic synthesis of amoxicillin by penicillin G acylase in the presence of ionic liquids. Green Chem. 2012;14:3146–3156. [Google Scholar]
- 19.Kloosterman W.M.J., Roest S., Priatna S.R., Stavila E., Loos K. Chemo-enzymatic synthesis route to poly(glucosyl-acrylates) using glucosidase from almonds. Green Chem. 2014;16:1837–1846. doi: 10.1039/c3gc41115j. [DOI] [Google Scholar]
- 20.Paggiola G., Hunt A.J., McElroy C.R., Sherwood J., Clark J.H. Biocatalysis in bio-derived solvents: an improved approach for medium optimisation. Green Chem. 2014;16:2107–2110. doi: 10.1039/c3gc42526f. [DOI] [Google Scholar]
- 21.Humble M.S., Berglund P. Biocatalytic Promiscuity. Eur. J. Org. Chem. 2011;19:3391–3401. doi: 10.1002/ejoc.201001664. [DOI] [Google Scholar]
- 22.Busto E., Gotor-Fernandez V., Gotor V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010;39:4504–4523. doi: 10.1039/c003811c. [DOI] [PubMed] [Google Scholar]
- 23.Xu J.-M., Zhang F., Liu B.-K., Wu Q., Lin X.-F. Promiscuous zinc-dependent acylase-mediated carbon-carbon bond formation in organic media. Chem. Commun. 2007:2078–2080. doi: 10.1039/b700327g. [DOI] [PubMed] [Google Scholar]
- 24.Wu W.-B., Xu J.-M., Wu Q., Lv D.-S., Lin X.-F. Promiscuous Acylases-Catalyzed Markovnikov Addition of N-Heterocycles to Vinyl Esters in Organic Media. Adv. Synth. Catal. 2006;348:487–492. doi: 10.1002/adsc.200505342. [DOI] [Google Scholar]
- 25.Lou F.-W., Liu B.-K., Wu Q., Lv D.-S., Lin X.-F. Candida antarcticaLipase B (CAL-B)-Catalyzed Carbon-Sulfur Bond Addition and Controllable Selectivity in Organic Media. Adv. Synth. Catal. 2008;350:1959–1962. doi: 10.1002/adsc.200800207. [DOI] [Google Scholar]
- 26.Xiang Z., Liu Z., Chen X., Wu Q., Lin X. Biocatalysts for cascade reaction: Porcine pancreas lipase (PPL)-catalyzed synthesis of bis(indolyl)alkanes. Amino Acids. 2013;45:937–945. doi: 10.1007/s00726-013-1547-4. [DOI] [PubMed] [Google Scholar]
- 27.Li H.-H., He Y.-H., Guan Z. Protease-catalyzed direct aldol reaction. Catal. Commun. 2011;12:580–582. doi: 10.1016/j.catcom.2010.12.003. [DOI] [Google Scholar]
- 28.Kumar A., Venkatesu P. Overview of the Stability of α-Chymotrypsin in Different Solvent Media. Chem. Rev. 2012;112:4283–4307. doi: 10.1021/cr2003773. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Liu R. The interaction of α-chymotrypsin with one persistent organic pollutant (dicofol): Spectroscope and molecular modeling identification. Food Chem. Toxicol. 2012;50:3298–3305. doi: 10.1016/j.fct.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Blow D.M., Birktoft J.J., Hartley B.S. Role of a Buried Acid Group in the Mechanism of Action of Chymotrypsin. Nature. 1969;221:337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- 31.Martichonok V., Jones J.B. Probing the Specificity of the Serine Proteases Subtilisin Carlsberg and α-Chymotrypsin with Enantiomeric 1-Acetamido Boronic Acids. An Unexpected Reversal of the Normal “l”-Stereoselectivity Preference. J. Am. Chem. Soc. 1996;118:950–958. doi: 10.1021/ja952816j. [DOI] [Google Scholar]
- 32.Svedendahl M., Hult K., Berglund P. Fast Carbon-Carbon Bond Formation by a Promiscuous Lipase. J. Am. Chem. Soc. 2005;127:17988–17989. doi: 10.1021/ja056660r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







