Abstract
Snakebite is a neglected disease and serious health problem in Brazil, with most bites being caused by snakes of the genus Bothrops. Although serum therapy is the primary treatment for systemic envenomation, it is generally ineffective in neutralizing the local effects of these venoms. In this work, we examined the ability of 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM), an isoflavone from Dipteryx alata, to neutralize the neurotoxicity (in mouse phrenic nerve-diaphragm preparations) and myotoxicity (assessed by light microscopy) of Bothrops jararacussu snake venom in vitro. The toxicity of TM was assessed using the Salmonella microsome assay (Ames test). Incubation with TM alone (200 μg/mL) did not alter the muscle twitch tension whereas incubation with venom (40 μg/mL) caused irreversible paralysis. Preincubation of TM (200 μg/mL) with venom attenuated the venom-induced neuromuscular blockade by 84% ± 5% (mean ± SEM; n = 4). The neuromuscular blockade caused by bothropstoxin-I (BthTX-I), the major myotoxic PLA2 of this venom, was also attenuated by TM. Histological analysis of diaphragm muscle incubated with TM showed that most fibers were preserved (only 9.2% ± 1.7% were damaged; n = 4) compared to venom alone (50.3% ± 5.4% of fibers damaged; n = 3), and preincubation of TM with venom significantly attenuated the venom-induced damage (only 17% ± 3.4% of fibers damaged; n = 3; p < 0.05 compared to venom alone). TM showed no mutagenicity in the Ames test using Salmonella strains TA98 and TA97a with (+S9) and without (−S9) metabolic activation. These findings indicate that TM is a potentially useful compound for antagonizing the neuromuscular effects (neurotoxicity and myotoxicity) of B. jararacussu venom.
Keywords: ames test; bothropstoxin-I; 7,8,3'-trihydroxy-4'-methoxyisoflavone; neuromuscular junction; Salmonella mutagenicity; snake venoms
1. Introduction
Envenomation by Bothrops snakes is characterized by local (pain, edema, inflammation, blistering, hemorrhage and necrosis) and systemic (coagulopathy, systemic hemorrhage, acute kidney injury and circulatory shock) manifestations [1]. Bothrops jararacussu is a large pit viper found in southeastern Brazil and northern Argentina [2]. Envenomation by this species shares many of the foregoing features with other Bothrops species [3], with most of the clinical manifestations of envenoming being mediated predominantly by snake venom metalloproteases (SVMPs), serine proteases, phospholipases (PLA2) and C-type lectins. Transcriptomic analysis has confirmed that these are indeed the major protein classes in this venom, with PLA2 being particularly abundant: Lys49-PLA2 homologs accounted for 83.2% of PLA2 transcripts, acidic Asp49-for 0.6% and basic Asp49-PLA2 for 0.1% [4].
In addition to the features indicated above, clinical studies in the early 1900s suggested that bites by B. jararacussu also involved systemic manifestations reminiscent of envenoming by the South American rattlesnake, Crotalus durissus terrificus, viz., neurotoxicity, blindness, blurred vision, difficulty in swallowing and paralysis [5], in addition to other unspecified signs of neurotoxicity [6,7,8]. In agreement with this, various studies using isolated vertebrate nerve-muscle preparations in vitro have shown that B. jararacussu venom [9,10] and PLA2 from this venom [11,12,13,14,15,16] can cause neuromuscular blockade by pre- and post-synaptic mechanisms.
Serum therapy, the treatment of choice for snakebites, efficiently neutralizes the systemic manifestations but is generally ineffective against the local effects (edema, inflammation, hemorrhage and necrosis) of venoms [17]. Extensive local tissue damage leading to tissue loss after Bothrops bites can result in permanent disability and amputations [18,19]. Hence, there is a clinical and therapeutic impetus to develop alternatives for treating these local manifestations, with plant-derived bioactive products providing important candidate or lead molecules [20,21].
Several studies have shown that the neurotoxic and myotoxic effects of B. jararacussu venom and its PLA2 myotoxins can be neutralized by some plant extracts and their isolated compounds [21,22,23,24,25,26,27,28,29,30]. In particular, a methanolic extract of bark from Dipteryx alata Vogel, a plant species native to the Brazilian cerrado, fully protects against the neuromuscular blockade caused by B. jararacussu venom, whereas a dichloromethane extract provides partial protection against the blockade caused by B. jararacussu and C. d. terrificus venoms [31]. Lupane triterpenoids (lupeol, lupenone, betulin and 28-OH-lupenone) from D. alata prevent the paralysis induced by B. jararacussu venom [32], whereas betulin and lupenone only partially neutralize the paralysis induced by C. d. terrificus venom [33].
To increase our understanding of the components involved in the protective action of D. alata extracts, in this study we examined the ability of 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM), an isoflavone from D. alata [32], to attenuate the neurotoxicity and myotoxicity of B. jararacussu venom and its main myotoxin, bothropstoxin-I (BthTX-I), in mouse phrenic nerve-diaphragm preparations in vitro. The mutagenicity (toxicity) of TM, as an indicator of it potential use as a clinical agent, was assessed in the Ames test using Salmonella strains TA 98 and TA 97a.
2. Results and Discussion
This work was based on three premises: (1) an understanding of the principal clinical manifestations of B. jararacussu envenomation [1]; (2) the low efficacy of antivenom against the local effects of Bothrops venoms [18,19]; and (3) the availability of a molecule, 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM) from D. alata, with potentially interesting activity against B. jararacussu snake venom.
2.1. Molecule
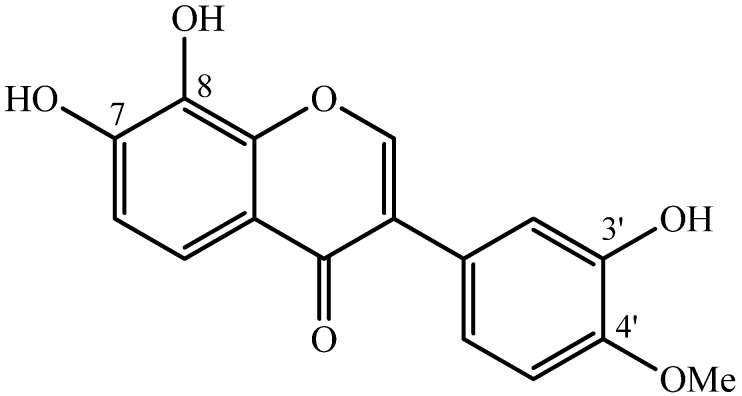
Figure 1 shows the chemical structure of TM from D. alata [32], a compound also found in Xanthocercis zambesiaca (Baker) plant extract [34].
Figure 1.
Chemical structure of 7,8,3'-trihydroxy-4'-methoxyisoflavone isolated from D. alata Vogel [32].
In preliminary screening for anti-snake venom activity, fraction F7 of a dichloromethane extract of D. alata bark [32] showed the best activity out of 11 fractions when tested against the neuromuscular activity of B. jararacussu venom [31]. Isoflavonoids are a large and very distinctive subclass of flavonoids, but only a few plants, including D. alata, have been reported to contain isoflavonoids [35]. Isoflavones are biologically active plant-food constituents that are common in the human diet and food industry, and are widely used as preservatives in pharmaceutical products [36]. This range of activities and uses suggested the possibility that isoflavones could be potentially useful as an adjuvant treatment for snakebites alongside standard serum therapy, particularly since antiserum shows little or no neutralizing activity towards the local effects of Bothrops venoms [18,19].
2.2. Pharmacological Assays
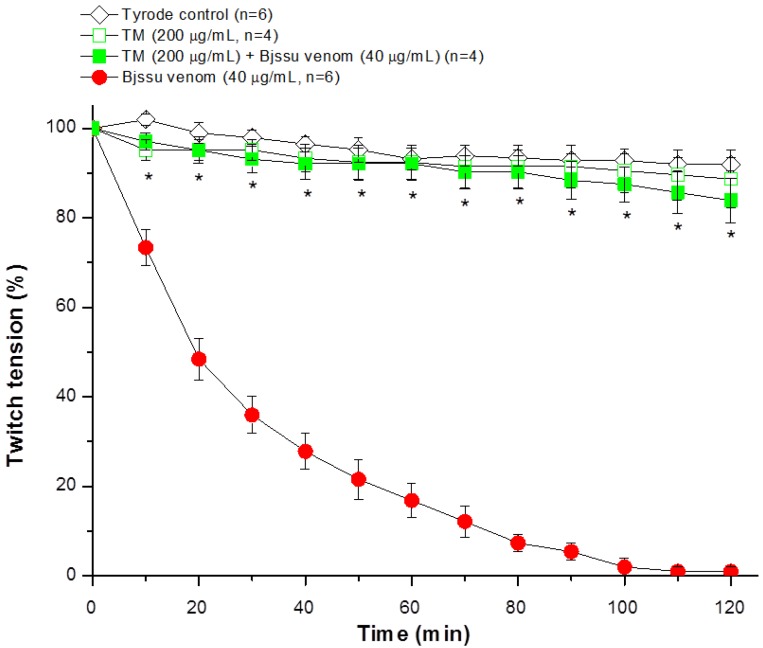
The ability of TM to inhibit the neuromuscular blockade caused by B. jararacussu venom was investigated in mouse phrenic nerve-diaphragm (PND) preparations. Figure 2 shows the characteristic irreversible neuromuscular blockade induced by B. jararacussu venom in vitro (Bjssu, 40 µg/mL, n = 6, * p < 0.05 compared to control), in agreement with that originally reported by Rodrigues-Simioni et al. [9]. Incubation with TM alone (200 µg/mL, n = 4) did not change the muscle twitch-tension responses, which were similar to those of preparations incubated with Tyrode solution (negative control; n = 6). Preincubation of TM (200 µg/mL) with venom (40 µg/mL) for 30 min prior to testing totally abolished the venom-induced neuromuscular blockade (n = 4, * p < 0.05 compared to venom alone).
Figure 2.
Twitch tension responses of indirectly stimulated PND incubated with 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM, 200 μg/mL), B. jararacussu (Bjssu) venom (40 μg/mL) or a mixture of TM (200 µg/mL) and venom (40 μg/mL). The TM + venom mixture was preincubated at 37 °C for 30 min prior to testing. The points are the mean ± SEM of the number of experiments indicated in the figure. * p < 0.05 for TM + venom compared to venom alone.
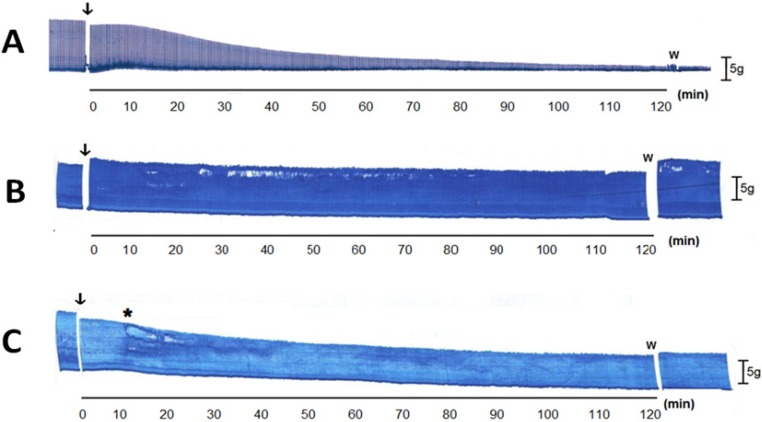
Bothrops jararacussu venom is rich in PLA2, most of which are Lys49-PLA2, with considerably fewer basic and acidic Asp49-PLA2 [4]. Bothropstoxin-I (BthTX-I) is the major Lys49-PLA2 myotoxin and totally reproduces the in vitro neurotoxicity and myotoxicity of B. jararacussu venom [11]. This toxin acts presynaptically before causing membrane depolarization [15,37]. As shown in Figure 3A, incubation of PND preparations with BthTX-I (20 µg/mL) mimicked the characteristic progressive, irreversible decrease in muscle twitch-tension caused by the venom. As with the venom, preincubation of BthTX-I (20 µg/mL) with TM (200 µg/mL) fully protected the PND against toxin-induced neuromuscular blockade (Figure 3B). When TM was added 10 min after BthTX-I there was still attenuation of the neuromuscular blockade, although this was much less marked than with the preincubation protocol (Figure 3C). These findings suggest that the protective action of TM against venom-induced neuromuscular blockade most likely resulted from the inhibition of BthTX-I, presumably by attenuating the presynaptic activity of the toxin [15,37].
Figure 3.
Neuromuscular responses of indirectly stimulated PND incubated with BthTX-I (20 µg/mL) in the absence (A) and presence (B,C) of 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM, 200 µg/mL). In (B), TM (200 µg/mL) was preincubated with BthTX-I (20 µg/mL) at 37 °C for 30 min before addition to the bath, whereas in (C) TM (200 µg/mL) was added separately (at *) 10 min after the addition of BthTX-I (20 µg/mL). Arrows show the moment of sample addition to the bath. Bar = tension of 5 g/cm; W = wash.
2.3. Quantitative Histological Analysis
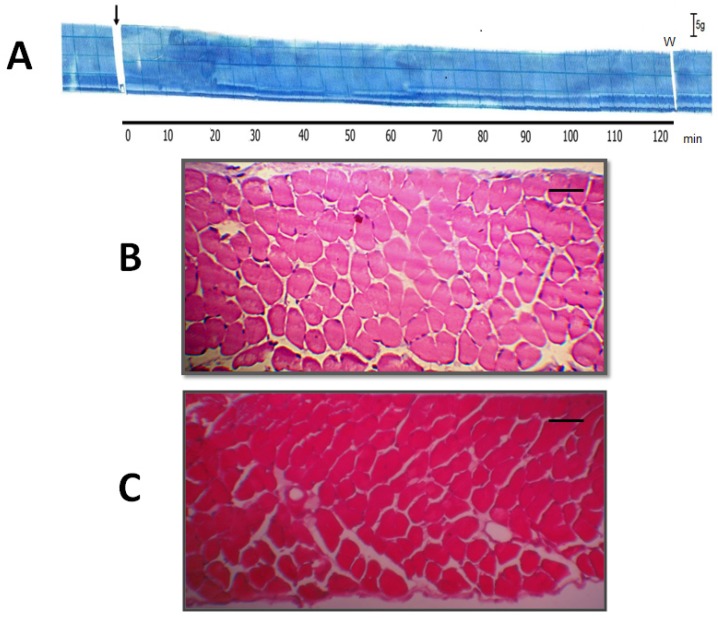
To assess the anti-myotoxic activity of TM, diaphragm muscles exposed to different treatments (TM, venom or TM + venom) were analyzed by light microscopy, as described by Queiroz et al. [38] for mouse tibialis anterior muscle. Figure 4A shows a characteristic myographic recording of PND contractile activity during incubation with TM (200 µg/mL) for 120 min; similar responses were observed in preparations incubated with Tyrode solution alone (results not shown). The percentage of damage cells in these two protocols was <10% [9.7% ± 1.6% (n = 6) for Tyrode solution and 9.2% ± 1.7% (n = 4) for TM-treated muscles].
Figure 4.
Histological analysis of muscle damage in PND incubated with or without 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM). Panel (A) shows a representative trace of PND incubated with TM (n = 4). The arrow indicates the addition of TM. Bar = tension of 5 g/cm. W = wash. Panels B and C show cross-sections of diaphragm muscle incubated with Tyrode solution alone (negative control; n = 6) (B) or TM alone (n = 4) (C). Note the normal appearance of the fibers (polygonal aspect and peripheral nuclei) in both panels. Bar = 50 μm in B and C.
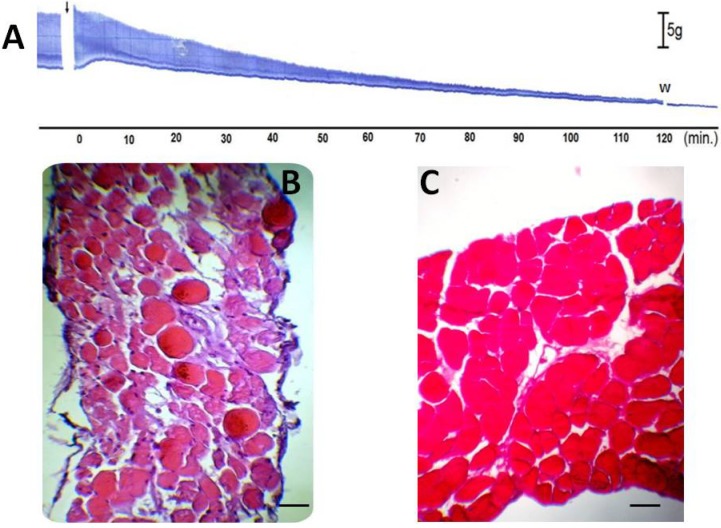
In indirectly stimulated PND, B. jararacussu venom (40 μg/mL, n = 6) induced a transient contracture that was followed by progressive neuromuscular blockade of muscle twitches during the following 120 min (Figure 5A). In contrast to the foregoing controls, there was a marked increase in the percentage of damaged fibers (to 50.3% ± 5.4%; p < 0.05 compared to the Tyrode control) in diaphragms incubated with venom alone (40 μg/mL, n = 3) for 120 min (Figure 5B). In diaphragm muscle incubated with a mixture of TM (200 μg/mL) + venom (40 μg/mL) (Figure 5C, n = 3) there was a significant decrease in the percentage of damaged fibers (17% ± 3.4%; p < 0.05 compared to venom alone). Although the venom produced complete neuromuscular blockade, quantitative analysis of the fibers showed that only 50% were damaged. This discrepancy may reflect the fact that muscle cross-sections do not allow observation of the entire fiber length. Although diaphragm muscle has a high resistance to fatigue and a safety margin of 10% [39], the venom nevertheless had a striking effect on the physiological excitation-contraction coupling mechanism. As shown here, the preincubation of venom with TM was effective in protecting the muscle against venom-induced myotoxicity.
Figure 5.
Neuromuscular responses of indirectly stimulated PND incubated with B. jararacussu venom (40 μg/mL) for 120 min (A). Notice the irreversible blockade. Venom was added at the arrow. Bar = tension of 5 g/cm. W = wash. Panel (B)—Cross-section of diaphragm muscle incubated with venom alone (40 μg/mL, n = 6). Note the edema and intense myonecrosis (muscle fiber atrophy, hyaline aspect, sarcolemmal disruption and myofibril lysis). Panel (C)—Cross-section of diaphragm muscle incubated with a mixture of TM (200 μg/mL) + venom (40 μg/mL). Note the normal appearance of the fibers (polygonal aspect and peripheral nuclei), but with occasional edema. Bar = 50 μm for B and C.
In this work, we focused on the ability of TM to antagonize the neuromuscular action (neurotoxicity and myotoxicity) of B. jararacussu venom and its main PLA2 myotoxin, BthTX-I. However, B. jararacussu venom has a variety of other biological activities (edema-formation, hemorrhage and lethality) against which it would be interesting to assess the neutralizing capacity of TM. Nevertheless, with the isolation procedure used in this study, the amount of isoflavone obtained was low, which severely limited the number of protocols and activities that could be tested. This limitation (low yield/availability of purified TM) is common to other phytochemical compounds with biological activity [40]. Nonetheless, as shown here, TM faithfully mimicked the protection against the venom-induced neuromuscular blockade seen with a crude extract of D. alata [31].
Several mechanisms have been proposed to explain the anti-snake venom activity of phytochemical compounds [21], with the specific interactions depending on the plant compound (tannins, coumarins, flavonoids, etc.) and the snake genus involved. In the case of TM, we suggest that its interaction with B. jararacussu venom and BthTX-I involves the hydroxyl groups at positions C-8 and C-7 of the isoflavone (see Figure 1). Since the neighboring hydroxyls are susceptible to radical formation [35], this chemical interaction would ultimately lead to a very reactive 7,8-quinone moiety that could modify specific sites in enzymes responsible for the local and systemic effects of the venom.
2.4. Salmonella Mutagenicity Assay
The toxicology of TM was assessed in the Salmonella mutagenicity assay, using test strains TA97a and TA98 that can be reverted by frameshift mutagens [41,42]. Although the Organisation for Economic Cooperation and Development (OECD) [41] recommends the use of at least five Salmonella strains to meet regulatory demands, the low yield of isoflavonoid limited the use of five strains in this work. On the other hand, satisfactory toxicological results have been obtained using only TA97a and TA98 [43] or TA97a and TA100 [44], and were considered as valid mutagenic screening. In our experimental conditions a GC frameshift was evaluated. The mutagenicity assays showed that TM did not increase the number of revertant colonies relative to the negative control, indicating no mutagenicity for this compound (Table 1).
Table 1.
Assessment of the mutagenicity of 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM) against S. typhimurium. The table shows the revertants per plate, the standard deviation and the mutagenicity index (in brackets) for strains TA98 and TA97a after treatment with TM.
| TM | ||||
|---|---|---|---|---|
| Treatment | TA 98 | TA 97a | ||
| mg/plate | −S9 | +S9 | −S9 | +S9 |
| DMSO | 20 ± 2 | 30 ± 2 | 151 ± 8 | 143 ± 8 |
| 0.19 | 20 ± 4 (1.0) | 34 ± 3 (1.1) | 194 ± 4 (1.3) | 172 ± 4 (1.2) |
| Control+ | 1319 ± 41 a | 1696 ± 41 b | 1875 ± 62 a | 1623 ± 48 b |
The values are the mean ± SEM of two determinations. DMSO: dimethyl sulfoxide (50 μL/plate; negative control); Control+: positive control; a 4-nitro-o-phenylenediamine (10 μg/plate); b 2-anthramine (1.25 μg/plate).
The genes affected in Salmonella strains TA98 and TA97a were hisD6610 and hisD3052, respectively, although these strains also contained the mutations ∆urvB, rfa and pKM101. The former was designed to enhance the mutagenicity of compounds, presumably through the nucleotide excision repair system [42] that extended into a gene for biotin synthesis and required the addition of biotin to the culture medium because of the deletion in this region. The rfa mutation changes the properties of the bacterial cell wall and results in partial loss of the lipopolysaccharide (LPS) barrier, thereby increasing the permeability of cells to certain chemicals; this mutation is generally detected based on the altered sensitivity to crystal violet. The R factor plasmid (pKM101) makes the strains more responsive to a variety of mutagens [45].
3. Experimental
3.1. Plant Material and Extraction
Bark samples were collected from an adult D. alata Vogel tree in Pedro Afonso (Tocantins, Brazil) in December 2007 and identified by Dr. Roseli B. Torres (Institute of Agronomy of Campinas—IAC). A voucher specimen was deposited in the herbarium of the IAC (IAC 50629). The bark (1.269 kg) was dried at 37 °C for 48 h and then powdered, ground in a mill, macerated for 5 days (200 g in 2 L of 70% ethanol) and the suspension then percolated (protected from light) at 20 drops/min, resulting in a 20% (m/v) hydroalcoholic extract. The extract was concentrated under reduced pressure and lyophilized to yield a final residue of 170 g that corresponded to an extraction efficiency of 85% [46].
3.1.1. Isolation of 7,8,3'-trihydroxy-4'-methoxyisoflavone
For isolation of the isoflavone, lyophilized extract (50 g) was dissolved in a methanol/water mixture (80:20, v/v) and partitioned successively with the corresponding solvents to yield, after concentration, hexane (1.5 g), CH2Cl2 (18 g), EtOAc (3.7 g) and MeOH (21 g) residues. The CH2Cl2 fraction was subjected to silica gel flash column chromatography and eluted with hexane/EtOAc (9:1). This procedure yielded 12 subfractions that were further successively flash-chromatographed on silica gel and purified by Sephadex LH-20 column chromatography and eluted with hexane/CH2Cl2/MeOH (2:2:1) to yield 18 compounds, including 19 mg of 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM), characterized [32] as a yellow amorphous solid. IR (KBr) cm−1: 3429, 1619, 1604, 1290, 1124. 1H-NMR (CD3OD) δ: 3.80 (s, 3H), 6.93 (s, 1H), 6.94 (d, J = 8.7 Hz, 1H), 6.96 (s, 1H), 7.03 (s, 1H), 7.57 (d, J = 8.7 Hz, 1H), 8.17 (s, 1H). 13C-NMR (CD3OD) δ: 56.4 (CH3), 112.6 (CH), 115.4 (CH), 117.3 (CH), 117.4 (CH), 118.8 (C), 121.6 (CH), 125.3 (C), 126.3 (C), 134.1 (C), 147.4 (C), 147.8 (C), 149.2 (C), 171.0 (C), 154.6 (CH), 178.5 (C).
3.1.2. Isoflavone Solubilization
For the pharmacological assays, fresh solutions of TM were prepared daily by dissolving in 30 µL of DMSO (CAQ – Casa da Química Ind. E Com. Ltd, Diadema, SP, Brazil); this volume of solvent did not alter the basal response of the neuromuscular preparation [47,48].
3.2. Pharmacological Assays
3.2.1. Venom and Purification of BthTX-I
Bothrops jararacussu venom was collected from two adult specimens kept in the Serpentário do Centro de Estudos da Natureza (CEN). The venom was lyophilized and certified by Dr. José Carlos Cogo (Vale do Paraiba University—UNIVAP, São José dos Campos, SP, Brazil). BthTX-I was purified and its identity confirmed as described by Homsi-Brandeburgo et al. [11].
3.2.2. Animals
Male Swiss white mice (26–32 g) were supplied by Anilab (Animais de Laboratório, Paulínia, SP, Brazil). The animals were housed at 25 ± 3 °C on a 12 h light/dark cycle and had access to food and water ad libitum. This project was approved by the institutional Committee for Ethics in Animal Research of Vale do Paraiba University (protocol no. A013/CEUA/2011) and the experiments were done in accordance with the general guidelines of the Brazilian Society for Laboratory Animal Science (SBCAL).
3.2.3. Mouse Phrenic Nerve-Diaphragm Muscle (PND) Preparation
The PND preparation [49] was obtained from mice anesthetized with halothane (Cristália, Itapira, SP, Brazil) and killed by exsanguination. The diaphragm was removed and mounted under a tension of 5 g/cm in a 5 mL organ bath containing aerated Tyrode solution (control) of the following composition (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 0.49, NaH2PO4 0.42, NaHCO3 11.9 and glucose 11.1. After equilibration with 95% O2/5% CO2 (v/v), the pH of this solution was 7.0. The preparations were indirectly stimulated with supramaximal stimuli (4× threshold, 0.06 Hz, 0.2 ms) delivered from a stimulator (model ESF-15D, Ribeirão Preto, SP, Brazil) to the nerve by bipolar electrodes. Isometric twitch tension was recorded with a force displacement transducer (cat. no. 7003, Ugo Basile, Varese, Italy) coupled to a 2-channel Gemini physiograph recorder (cat. no. 7070, Ugo Basile) via a basic preamplifier (cat. no. 7080, Ugo Basile) [33]. The preparations were allowed to stabilize for at least 20 min before initiating the treatments described below.
Control preparations were incubated with Tyrode solution alone (n = 6), whereas other preparations were incubated with TM (200 µg/mL, n = 4), venom (40 µg/mL, n = 6) or a mixture of TM + venom preincubated for 30 min at 37 °C prior addition to the organ bath (n = 4) [33]. BthTX-I (20 µg/mL) was assayed in two protocols: in one, PND preparations were incubated with toxin followed by the addition of TM (200 µg/mL) while in the other the toxin was preincubated with TM (200 µg/mL) prior to testing in PND.
3.2.4. Quantitative Histological Analysis
At the end of the incubations (120 min), the preparations from the various protocols (control, TM, venom and venom + TM) were processed for histological analysis. The preparations (n = 3 for each treatment) were fixed in Bouin solution and processed by routine morphological techniques. Cross-sections (5 µm thick) of diaphragm muscle were stained with 0.5% (w/v) hematoxylin-eosin for examination by light microscopy. Tissue damage (edema, intense myonecrosis characterized by muscle fiber atrophy, a hyaline aspect, sarcolemmal disruption and myofibril lysis) was expressed as a myotoxicity index (MI), defined as (the number of damaged muscle cells/the total number of cells) × 100 in three non-overlapping, non-adjacent areas of each preparation [28].
3.2.5. In vitro Mutagenicity Assay
Mutagenic activity was evaluated by the Salmonella microsome assay, using the Salmonella typhimurium test strains TA98 and TA97a, kindly provided by Dr. B.N. Ames (Berkeley, CA, USA), with (+S9) and without (−S9) metabolization, by the preincubation method [50]. As discussed in the Results section, the OECD [41] recommends the use of five strains (S. typhimurium 1535, TA97a, TA98, TA100, TA102), but this was not feasible in this study because of the low yield of isoflavonoid during extraction. The strains were grown from frozen cultures overnight for 12–14 h in Oxoid Nutrient Broth No. 2. The metabolic activation mixture (S9 fraction), prepared from livers of Sprague–Dawley rats treated with the polychlorinated biphenyl mixture Aroclor 1254 (500 mg/kg), was purchased from Molecular Toxicology Inc. (Boone, NC, USA) and freshly prepared before each test. The metabolic activation system consisted of 4% S9 fraction, 1% 0.4 M MgCl2, 1% 1.65 M KCl, 0.5% 1 M D-glucose-6-phosphate disodium, 4% 0.1 M NADP (nicotinamide adenine dinucleotide phosphate), 50% 0.2 M phosphate buffer and 39.5% sterile distilled water. The isoflavone was dissolved in DMSO (Sigma Chemical Co., St. Louis, MO, USA) to provide a non-toxic concentration of 0.19 mg/plate. This concentration was added to 0.5 mL of 0.2 M phosphate buffer, or to 0.5 mL of 4% S9 mixture, with 0.1 mL of bacterial culture and then incubated at 37 °C for 20–30 min. Subsequently, 2 mL of top agar [0.6% agar, histidine and biotin (0.5 mM each) and 0.5% NaCl] was added and the mixture poured onto a plate containing minimal agar. The plates were incubated at 37 °C for 48 h and the His+ revertant colonies were counted manually. All experiments were done only in duplicate because of the low amount of isoflavone available. The use of duplicate plating is acceptable when scientifically justified [41]. The results were analyzed with the statistical software package Salanal 1.0 (U.S. Environmental Protection Agency, Monitoring Systems Laboratory, Las Vegas, NV, from Research Triangle Institute, Research Triangle Park, NC, USA), using the model of Bernstein et al. [51]. The mutagenic index (MI), defined as the average number of revertants per plate with the test compound divided by the average number of revertants per plate with the negative (solvent) control, was also calculated. A sample was considered positive when the MI was equal to or greater than two for at least one of the concentrations, and if it had a reproducible dose-response curve [52]. The standard mutagen used as a positive control in experiments without the S9 mix was 4-nitro-o-phenylenediamine (NOPD, 10 μg/plate). In experiments with S9 activation, 2-anthramine (2-AA, 1.25 μg/plate) was used. DMSO (50 μL/plate) served as the negative (solvent) control.
3.2.6. Statistical Analysis
Each pharmacological protocol was repeated at least four times and the results are shown as the mean ± SEM. The number of experiments (n) is indicated in the corresponding figure legend. Student’s t-test was used for statistical comparison of the data and the confidence level was set as 5% (α = 0.05).
4. Conclusions
In conclusion, the bioactive isoflavone, 7,8,3'-trihydroxy-4'-methoxyisoflavone (TM) from D. alata Vogel efficiently counteracted the myotoxicity and neuromuscular activity of B. jararacussu venom and its major myotoxin, BthTX-I, in vitro. TM was not mutagenic in S. typhimurium strains TA 97a and TA 98, indicating its safety. The results of this study reinforce the potential of TM for therapeutic use in the treatment of venomous snakebites.
Acknowledgments
The authors thank Roseli B. Torres for plant identification. This study was supported by FAPESP (grant nos. 04/09705-8, 07/53883-6, 08/50669-6, 08/52643-4 and 08/11005-5), CAPES/Prosup, PROBIC/Uniso and USAL:18KAC9/463AC01.
Author Contributions
MCF and EHY did the experimental work. RVST was responsible for fractionation of the lyophilized extract. JCC provided and certified the snake venom. ACOC purified the myotoxin and confirmed its identity. MGS and CADB were responsible for collection of plant samples in Tocantins. FAF and EAV interpreted the Salmonella microsome assay. SH contributed to the writing and editing of the manuscript. LMF, PP, ASF and YOF were responsible for isolation and identification of the isoflavonoid from D. alata. All authors read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Warrell D.A. Snakebites in central and south america: Epidemiology, clinical features and clinical management. In: Campbell J.A., Lamar W.W., editors. The Venomous Reptiles of the Western Hemisphere. Volume 2. Comstock Publishing Associates/Cornell University Press; Ithaca, NY, USA: 2004. pp. 709–761. [Google Scholar]
- 2.Campbell J.A., Lamar W.W. The Venomous Reptiles of the Western Hemisphere. Comstock Publishing Associates/Cornell University Press; Ithaca, NY, USA: 2004. p. 870. [Google Scholar]
- 3.Milani Junior R., Jorge M.T., de Campos F.P., Martins F.P., Bousso A., Cardoso J.L.C., Ribeiro L.A., Fan H.W., França F.O.S., Sano-Martins I.S., et al. Snake bites by the jararacuçu (Bothrops jararacussu): Clinicopathological studies of 29 proven cases in São Paulo State, Brazil. Q. J. Med. 1997;90:323–334. doi: 10.1093/qjmed/90.5.323. [DOI] [PubMed] [Google Scholar]
- 4.Kashima S., Robert P.G., Soares A.M., Astolfi-Filho S., Pereira J.O., Giuliati S., Faria Junior M., Xavier M.A.S., Fontes M.R.M., Giglio J.R., et al. Analysis of Bothrops jararacussu venomous gland transcriptome focusing on structural and functional aspects: I—gene expression profile of highly expressed phospholipases A2. Biochimie. 2004;86:211–219. doi: 10.1016/j.biochi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Brazil V. Do envenamento ophidico e seu tratamento. Collet. Trab. Inst. Butantan. 1901;1:31–55. [Google Scholar]
- 6. Vellard J.A. Serpentes venenosas Terapêutica Clínica Cardini C., Beretervide J.J. Libreria y Editorial “El Ateneo”; Buenos Aires, Argentina: 1945. ' Volume 4 265 273 [Google Scholar]
- 7.Alves E. Medicina de Urgência. 3rd ed. Livraria Atheneu; Rio de Janeiro, Brazil: 1956. p. 562. [Google Scholar]
- 8.Teixeira R. Forma grave do acidente por ofídios da sub família Crotalinae. Ann. Acad. Med. Bahia. 1979;2:109–135. [Google Scholar]
- 9.Rodrigues-Simioni L., Borgese N., Ceccarelli B. The effects of Bothrops jararacussu venom and its components on frog nerve-muscle preparation. Neuroscience. 1983;10:475–489. doi: 10.1016/0306-4522(83)90147-1. [DOI] [PubMed] [Google Scholar]
- 10.Zamunér S.R., Cruz-Höfling M.A., Corrado A.P., Hyslop S., Rodrigues-Simioni L. Comparison of the neurotoxic and myotoxic effects of Brazilian Bothrops venoms and their neutralization by commercial antivenom. Toxicon. 2004;44:259–271. doi: 10.1016/j.toxicon.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Homsi-Brandeburgo M.I., Queiroz L.S., Santo-Neto H., Rodrigues-Simioni L., Giglio J.R. Fractionation of Bothrops jararacussu snake venom: Partial chemical characterization and biological activity of bothropstoxin. Toxicon. 1988;26:615–627. doi: 10.1016/0041-0101(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 12.Heluany N.F., Homsi-Brandeburgo M.I., Giglio J.R., Prado-Franceschi J., Rodrigues-Simioni L. Effects induced by bothropstoxin, a component from Bothrops jararacussu snake venom, on mouse and chick muscle preparations. Toxicon. 1992;30:1203–1210. doi: 10.1016/0041-0101(92)90436-9. [DOI] [PubMed] [Google Scholar]
- 13.Andrião-Escarso S.H., Soares A.M., Rodrigues V.M., Ângulo Y., Díaz C., Lomonte B., Gutiérrez J.M., Giglio J.R. Myotoxic phospholipases A2 in Bothrops snake venoms: Effects of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie. 2000;82:755–763. doi: 10.1016/S0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- 14.Bonfin V.L., Toyama M.H., Novello J.C., Hyslop S., Oliveira C.R., Rodrigues-Simioni L., Marangoni S. Isolation and enzymatic characterization of a basic phospholipase A2 from Bothrops jararacussu snake venom. J. Protein Chem. 2001;20:239–245. doi: 10.1023/A:1010956126585. [DOI] [PubMed] [Google Scholar]
- 15.Oshima-Franco Y., Leite G.B., Dal Belo C.A., Hyslop S., Prado-Franceschi J., Cintra A.C.O., Giglio J.R., Cruz-Höfling M.A., Rodrigues-Simioni L. The presynaptic activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom. Basic Clin. Pharmacol. Toxicol. 2004;95:175–182. doi: 10.1111/j.1742-7843.2004.pto_950405.x. [DOI] [PubMed] [Google Scholar]
- 16.Ponce-Soto L.A., Bonfim V.L., Rodrigues-Simioni L., Novello J.C., Marangoni S. Determination of primary structure of two isoforms 6–1 and 6–2 PLA2 D49 from Bothrops jararacussu snake venom and neurotoxic characterization using in vitro neuromuscular preparation. Protein J. 2006;25:147–155. doi: 10.1007/s10930-006-0006-4. [DOI] [PubMed] [Google Scholar]
- 17.Gutiérrez J.M., Lomonte B., León G., Rucavado A., Chaves F., Angulo Y. Trends in snakebite envenomation therapy: Scientific, technological and public health considerations. Curr. Pharm. Des. 2007;13:2935–2950. doi: 10.2174/138161207782023784. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva N.M.V., Arruda E.Z., Murakami Y.L.B., Moraes R.A.M., El-Kik C.Z., Tomaz M.A., Fernandes F.F.A., Oliveira C.Z., Soares A.M., Gigliio J.R., et al. Evaluation of three Brazilian antivenom ability to antagonize myonecrosis and hemorrhage induced by Bothrops snake venom in mouse model. Toxicon. 2007;50:196–205. doi: 10.1016/j.toxicon.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez J.M., Lomonte B., León G., Alape-Girón A., Flores-Díaz M., Sanz L., Angulo Y., Calvete J.J. Snake venomics and antivenomics: Proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J. Proteomics. 2009;72:165–182. doi: 10.1016/j.jprot.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Mors W.B., Nascimento M.C., Pereira B.M., Pereira N.A. Plant natural products active against snake bite—The molecular approach. Phytochemistry. 2000;55:627–642. doi: 10.1016/S0031-9422(00)00229-6. [DOI] [PubMed] [Google Scholar]
- 21.Melo R.F., Farrapo N.M., Rocha Junior D.S., Silva M.G., Cogo J.C., Dal Belo C.A., Rodrigues Simioni L., Groppo F.C., Oshima-Franco Y. Antiophidian mechanisms of medicinal plants. In: Keller R.B., editor. Flavonoids: Biosynthesis, Biological Effects and Dietary Sources. Nova Science; New York, NY, USA: 2009. pp. 249–262. [Google Scholar]
- 22.Melo P.A., Nascimento M.C., Mors W.B., Suarez-Kurtz G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon. 1994;32:595–603. doi: 10.1016/0041-0101(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 23.Oshima-Franco Y., Alves C.M.V., Andréo Filho N., Gerenutti M., Cintra A.C.O., Leite G.B., Rodrigues-Simioni L., Silva M.G. Neutralization of the neuromuscular activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom, by a hydroalcoholic extract of Casearia sylvestris sw (guaçatonga) J. Venom. Anim. Toxins Incl. Trop. Dis. 2005;11:465–478. [Google Scholar]
- 24.Cavalcante W.L., Campos T.O., Dal Pai-Silva M., Pereira P.S., Oliveira C.Z., Soares A.M., Gallacci M. Neutralization of snake venom phospholipase A2 toxins by aqueous extract of Casearia sylvestris (Flacourtiaceae) in mouse neuromuscular preparation. J. Ethnopharmacol. 2007;112:490–497. doi: 10.1016/j.jep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Cintra-Francischinelli M., Silva M.G., Andréo-Filho N., Gerenutti M., Cintra A.C.O., Giglio J.R., Leite G.B., Cruz-Höfling M.A., Rodrigues-Simioni L., Oshima-Franco Y. Antibothropic action of Casearia sylvestris Sw. (Flacourtiaceae) extracts. Phytother. Res. 2008;22:784–790. doi: 10.1002/ptr.2365. [DOI] [PubMed] [Google Scholar]
- 26.Camargo T.M., Nazato V.S., Silva M.G., Cogo J.C., Groppo F.C., Oshima-Franco Y. Bothrops jararacussu venom-induced neuromuscular blockade inhibited by Casearia gossypiosperma Briquet hydroalcoholic extract. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010;16:432–441. [Google Scholar]
- 27.Farrapo N.M., Silva G.A.A., Costa K.N., Silva M.G., Cogo J.C., Dal Belo C.A., Santos M.G., Groppo F.C., Oshima-Franco Y. Inhibition of Bothrops jararacussu venom activities by Plathymenia reticulata Benth extracts. J. Venom Res. 2010;2:52–58. [PMC free article] [PubMed] [Google Scholar]
- 28.Oshima-Franco Y., Rosa L.J.R., Silva G.A.A., Amaral Filho J., Silva M.G., Lopes P.S., Cogo J.C., Cintra A.C.O., da Cruz-Höfling M.A. Antibothropic action of Camellia sinensis extract against the neuromuscular blockade by Bothrops jararacussu snake venom and its main toxin, bothropstoxin-I. In: Gallelli L., editor. Pharmacology. Intechopen; Croatia, Croatia: 2012. pp. 469–489. [Google Scholar]
- 29.Tribuiani N., da Silva A.M., Ferraz M.C., Silva M.G., Bentes A.P.G., Graziano T.S., dos Santos M.G., Cogo J.C., Varanda E.A., Groppo F.C., et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement. Altern. Med. 2014;14:48. doi: 10.1186/1472-6882-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veronese E.L., Esmeraldino L.E., Trombone A.P., Santana A.E., Bechara G.H., Kettelhut I., Cintra A.C., Giglio J.R., Sampaio S.V. Inhibition of the myotoxic activity of Bothrops jararacussu venom and its two major myotoxins, BthTX-I and BthTX-II, by the aqueous extract of Tabernaemontana catharinensis A. DC. (Apocynaceae) Phytomedicine. 2005;12:123–130. doi: 10.1016/j.phymed.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Nazato V.S., Rubem-Mauro L., Vieira N.A.G., Rocha-Junior D.S., Silva M.G., Lopes P.S., Dal-Belo C.A., Cogo J.C., Santos M.G., Cruz-Höfling M.A., et al. In vitro antiophidian properties of Dipteryx alata Vogel bark extracts. Molecules. 2010;15:5956–5970. doi: 10.3390/molecules15095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puebla P., Oshima-Franco Y., Franco L.M., dos Santos M.G., da Silva R.V., Rubem-Mauro L., San Feliciano A. Chemical constituents of the bark of Dipteryx alata Vogel, an active species against Bothrops jararacussu venom. Molecules. 2010;15:8193–8204. doi: 10.3390/molecules15118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferraz M.C., Parrilha L.A.C., Moraes M.S.D., Amaral Filho J., Cogo J.C., dos Santos M.G., Franco L.M., Groppo F.C., Puebla P., San Feliciano A., et al. The effect of lupane triterpenoids (Dipteryx alata Vogel) in the in vitro neuromuscular blockade and myotoxicity of two snake venoms. Curr. Org. Chem. 2012;16:2717–2723. doi: 10.2174/138527212804004481. [DOI] [Google Scholar]
- 34.Bezuidenhout S.C., Bezuidenhout B.C.B., Ferreira D. α-Hydroxydihydrochalcones and related 1,3-diarylpropan-2-ones from Xanthocercis zambesiaca. Phytochemistry. 1988;27:2329–2334. doi: 10.1016/0031-9422(88)80154-7. [DOI] [Google Scholar]
- 35.Dewick P.M. Isoflavonoids. In: Harborne J.B., editor. The Flavonoids Advances in Research Since 1986. Chapman & Hall/CRC; London, UK: 1994. pp. 117–386. [Google Scholar]
- 36.Lampe J.W. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J. Nutr. 2003;133(Suppl.):956s–964s. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 37.Correia-de-Sá P., Noronha-Matos J.B., Ferreirinha F., Marques P., Soares A.M., Carvalho C., Cavalcante W.L., Gallacci M. Bothropstoxin-Ireduces evoked acetylcholine release from rat motor nerve terminals: Radiochemical and real-time video-microscopy studies. Toxicon. 2013;61:16–25. doi: 10.1016/j.toxicon.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Queiroz L.S., Santo Neto H., Rodrigues-Simioni L., Prado-Franceschi J. Muscle necrosis and regeneration after envenomation by Bothrops jararacussu snake venom. Toxicon. 1984;22:339–346. doi: 10.1016/0041-0101(84)90077-1. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhry I.A., Nitahara K., Nagashima H., Vizi E.S. Neurochemical evidence that [Ca2+]o antagonizes the effect of neomycin on acetylcholine release from mouse hemidiaphragm preparation: An attempt to assess the margin to safety. Acta Anaesthesiol. Scand. 1995;39:494–497. doi: 10.1111/j.1399-6576.1995.tb04106.x. [DOI] [PubMed] [Google Scholar]
- 40.Harborne J.B. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 3rd ed. Chapman & Hall; London, UK: 1998. [Google Scholar]
- 41.OECD Guideline for Testing of Chemicals Bacterial Reverse Mutation Test. Adopted: 21st July 1997. [(accessed on 27 January 2014)]. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf.
- 42.Kaur K., Mathur N., Bhatnagar P. Comparative study of usage of microbial strains for monitoring waste water treatment plants. Univers. J. Environ. Res. Technol. 2012;2:26–37. [Google Scholar]
- 43.Vedmaurthy R.B., Padmanabhan S., Vijayan M., Jamal Z.A., Kunjumman J., Narayanan M.L. Compatibility of different solvents with Salmonella typhimurium mutant strains in bacterial reverse mutation assay. Int. J. Pharm. Pharm. Sci. 2012;4:283–284. [Google Scholar]
- 44.Swartz C., Parks N., Schaaper R.M., Demarini M. General enhancement of mutagenic potency of various mutagens due to deleted genes in the uvrB strains TA 98 and TA 100 of Salmonella compared with strains containing only a point mutation in uvr B. U.S. Environmental Protection Agency; San Francisco, CA, USA. 3–8 September 2005; [(accessed on 28 January 2014)]. Presented at The 9th International Conference on Environmental Mutagens, and 36th Annual Meeting of the International Conference on Environmental Mutagen Society. Available online: http://www.sciencedirect.com/science/journal/00275107/577/supp/S. [Google Scholar]
- 45.Mortelmans K., Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2009;455:29–36. doi: 10.1016/S0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 46.Esteves-Pedro N.M., Borim T., Nazato V.S., Silva M.G., Lopes P.S., dos Santos M.G., Dal Belo C.A., Cardoso C.R.P., Varanda E.A., Groppo F.C., et al. In vitro and in vivo safety evaluation of Dipteryx alata Vogel extract. BMC Complement. Altern. Med. 2012;12:9. doi: 10.1186/1472-6882-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cintra-Francischinelli M., Silva M.G., Andreo-Filho N., Cintra A.C.O., Leite G.B., Cruz-Höfling M.A., Rodrigues-Simioni L., Oshima-Franco Y. Effects of commonly used solubilizing agents on a model nerve-muscle synapse. Lat. Am. J. Pharm. 2008;27:721–726. [Google Scholar]
- 48.Oshima M., Leite G.B., Rostelato-Ferreira S., Cruz-Höfling M.A., Rodrigues-Simioni L., Oshima-Franco Y. Insights of the effects of polyethylene glycol 400 on mammalian and avian nerve terminals. Muscle Nerve. 2010;41:540–546. doi: 10.1002/mus.21531. [DOI] [PubMed] [Google Scholar]
- 49.Bülbring E. Observation on the isolated phrenic nerve diaphragm preparation of the rat. Br. J. Pharmacol. 1946;1:38–61. doi: 10.1111/j.1476-5381.1946.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein L., Kaldor J., McCann J., Pike M.C. An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test. Mutat. Res. 1982;97:267–281. doi: 10.1016/0165-1161(82)90026-7. [DOI] [PubMed] [Google Scholar]
- 52.Varella S.D., Pozetti G.L., Vilegas W., Varanda E.A. Mutagenic activity of sweepings and pigments from a household-wax factory assayed with Salmonella typhimurium. Food Chem. Toxicol. 2004;42:2029–2035. doi: 10.1016/j.fct.2004.07.019. [DOI] [PubMed] [Google Scholar]