Abstract
In this study, we describe the synthesis of 1,4-disustituted-1,2,3-triazolo-quinazoline ribonucleosides or acyclonucleosides by means of 1,3-dipolar cycloaddition between various O or N-alkylated propargyl-quinazoline and 1'-azido-2',3',5'-tri-O-benzoylribose or activated alkylating agents under microwave conditions. None of the compounds selected showed significant anti-HCV activity in vitro.
Keywords: quinazoline ribonucleosides; 1,2,3-triazole-acyclonucleosides; Huisgen cycloaddition; quinazolinone alkylation; HCV
1. Introduction
An estimated 150 million people worldwide are chronically infected with hepatitis C virus (HCV) and have an increased risk of eventually developing liver cirrhosis or liver cancer [1]. We believe that a successful approach to cure HCV in most patients will likely require treatment with a combination of drugs that attacks different mechanisms necessary for replication and survival of HCV. Currently, patients undergo treatment with a combination of pegylated interferon alpha and ribavirin or a virus-specific protease inhibitor like telaprevir or boceprevir [2,3,4].
Heterocyclic structures are the basic elements of many pharmaceuticals, agrochemicals and veterinary products. Quinazolinone derivatives are an important class of these heterocyclic compounds that has been shown to display a broad-range of biological activities, for example, anticancer, diuretic, anti-inflammatory, anti-convulsant and antihypertensive activities [5,6,7].
In addition, 1,2,3-triazole nucleosides and carbanucleosides are N-Heterocyclic compounds which have been the subject of considerable research, mainly due to their value in synthetic organic chemistry [8,9,10,11,12,13,14] based on the Sharpless-Meldal modified Huisgen reaction. The classical 1,3-dipolar cycloaddition of azides and alkynes discovered by Huisgen [15] often gives mixtures of regioisomers (1,4- and 1,5-disubstituted triazoles). “Click Chemistry” is a term that was developed by Sharpless and independently by Meldal to illustrate a regioselective 1,3-dipolar cycloaddition using Cu(I) salts as catalyst. The catalyst can be added directly in the form of Cu(I) or Cu(II) salts using reducing agents to form active Cu(I) in-situ [16,17]. Cu(I) salts require at least an amine base to form the Cu-acetylide complexes. Many studies have shown that the presence of base under the process conditions provides stability for Cu(I) salts against oxidation. It has been used especially in anhydrous media and also under catalytic conditions [18].
In addition, the combination of two different and independently linked hybrid compounds can display synergy and result in a pharmacological potency greater than the sum of each individual moiety’s potencies. For instance, nucleoside analogues incorporating triazole units are a valuable area of therapeutic research, and some triazole-containing compounds have shown activities against hepatitis and HIV-1 [19,20].
We previously reported the preparation of various 1,2,3-triazole acyclonucleosides from propargylated nucleobases by high temperature and long duration copper-free Huisgen 1,3-dipolar cycloadditiona and the evaluation of the resulting compounds for their HIV activity [21]. Recently, we also reported the preparation of several triazolo-acyclic nucleoside phosphonates using copper(I)-catalyzed Huisgen 1,3-dipolar cycloadditions between azido alkylphosphonates and propargylated nucleobases and the evaluation of the resulting compounds for their HIV and HCV activity [22,23,24,25,26,27].
The poor treatment response, combined with often-severe side effects induced by therapy, highlights the need for improved antiviral drugs with better efficacy and safety profiles. Furthermore, in continuation of our research program centered on click chemistry [28,29], the aim of the present work was to synthesize some new hybrid compounds combining the two heterocycles: quinazolinone and 1,2,3-triazole. The new compounds were also assessed for their anti-HCV activities.
2. Results and Discussion
2.1. Synthesis of Protected Nucleosides and Acyclonucleosides 6a–i
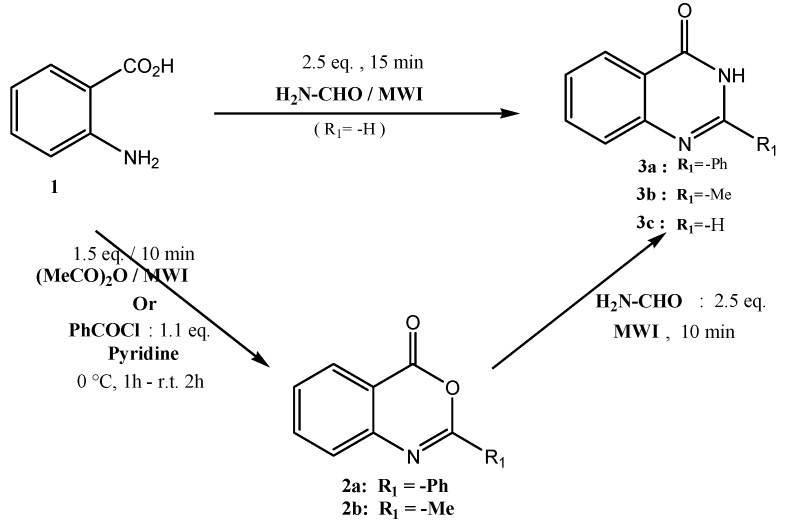
Typically, quinazolinone structures were constructed using anthranilic acid or its derivatives via a sequence of acylation and condensation, which requires strong acidic or basic reaction conditions [30,31,32]. The quinazoline derivatives were prepared from anthranilic acid (1) in three steps. Initially, the acid was reacted with benzoyl chloride in anhydrous pyridine at 0–5 °C for one hour. Afterwards the reaction mixture was stirred (two hours) at room temperature until 2-phenylbenzoxazinone (2a) was formed [33,34]. Alternatively, 2-methylbenzoxazinone (2b) was obtained by reaction of anthranilic acid with acetic anhydride using microwave irradiation [35,36]. The benzoxazinones were further treated with formamide under microwave irradiation to obtain the quinazolinones 3a–b. On the other hand, the synthesis of quinazolin-4-one 3c was achieved by condensing anthranilic acid with 2.5 equivalents of formamide under microwave irradiation [37] (Scheme 1).
Scheme 1.
Synthesis of benzoxazinones 2a,b.
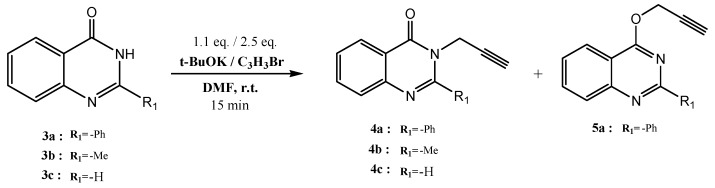
The alkylation of quinazol-4-ones which are substituted in position 2 sometimes leads to two isomers resulting from competing N-alkylation and O-alkylation and the ratio of these isomers depends on the substituent at position 2. An earlier study confirmed that the substituents and the reaction conditions play a significant role in influencing the ratio of O-alkylation vs. N-alkylation [38]. In this investigation, the quinazolinones 3a‒c were treated with propargyl bromide in the presence of potassium t-butoxide. The reaction was carried out using DMF as the solvent [39,40,41] (Scheme 2). The alkylation of 2-methylquinazolin-4-ones 2b and 2c leads exclusively to the formation of N-propargylated quinazolines, and O-propargylated isomers were not detected. In the case of 2-phenylquinazolin-4-one (2a), alkylation preferentially results in O-propargylation, with O and N-alkylated products 5a and 4a being obtained in a ratio of 58:23.
Scheme 2.
Synthesis of alkylated quinazolines 4a–c and 5a.
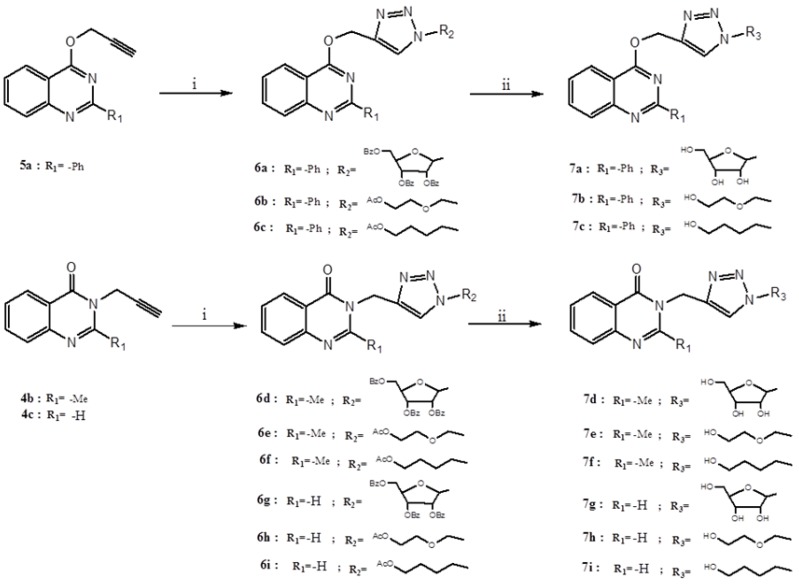
The propargylated quinazolines 4 and 5 were used in the 1,3-dipolar cycloaddition reaction to link 1,2,3-triazole with the quinazoline nucleus to yield compounds 6. Reaction of the triple bonds of propargylated quinazoline and the azide of sugar and pseudo-sugar was performed under microwave irradiation using Cul as catalyst without solvent (Scheme 3).
Scheme 3.
Syntheses of 1,2,3-triazoles-quinazoline 6a–i and 7a–i.
Reaction conditions: (i) 5a/4b–c (1 mmol), alkylazide (2.5 mmol), Et3N (1.1 mmol), CuI (0.1 eq), MWI (400W, 2 min); (ii) 6a,d,g (1 mmol), NaOMe (1 eq), MeOH, r.t (30 min) or 6b–c,e,f,h,i (1 mmol), K2CO3 (1 eq), MeOH, r.t (15 min).
This protocol (click chemistry) for the formation of the triazole rings is efficient, easy and convenient and typically gives almost quantitative reaction yields [42]. The cycloaddition reaction is drawn in Scheme 3 and the obtained products are tabulated in Table 1.
Table 1.
Synthesized 1,2,3-triazoloquinazolines 6a–i and 7a–i.
| Product | Yielda (%) | Product | Yield a (%) |
|---|---|---|---|
| 6a | 95 | 7a | 98 |
| 6b | 88 | 7b | 86 |
| 6c | 93 | 7c | 88 |
| 6d | 93 | 7d | 97 |
| 6e | 87 | 7e | 95 |
| 6f | 86 | 7f | 90 |
| 6g | 84 | 7g | 98 |
| 6h | 90 | 7h | 93 |
| 6i | 89 | 7i | 94 |
a Yields of isolated products.
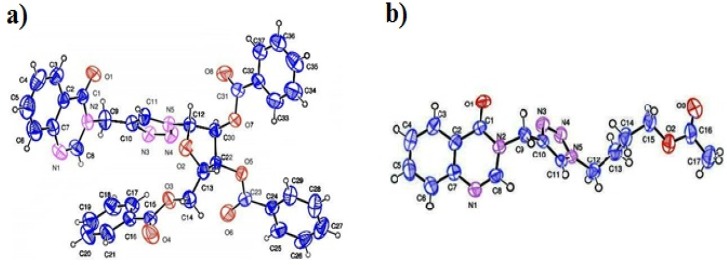
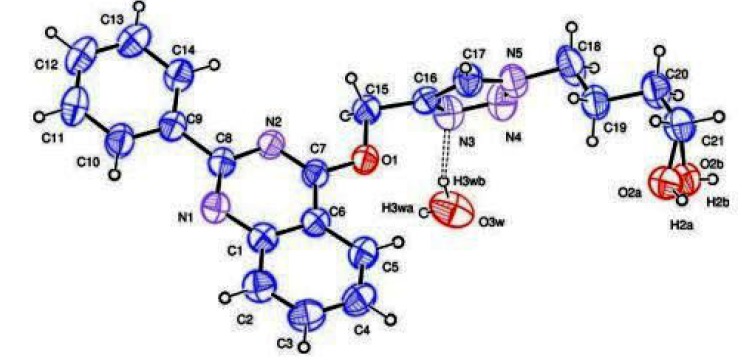
We characterized the structures of all products by 1H-NMR, 13C-NMR and mass spectrometry. In addition, the structures of 6g and 6i were confirmed by X-ray crystallographic analysis. The structure of crystal (a) illustrates that the anomeric configuration at the C1'-stereocentre has not been affected during the click reaction, the steric effect of benzoyl group in the position 2' and 3' (ribose) directs the reaction towards the β-configuration. The crystal structures of these compounds are shown in Figure 1 [43,44].
Figure 1.
X-ray crystal structures of compounds (a) 6g and (b) 6i with the atom numbering used in the crystallographic analysis.
2.2. Deprotection of Nucleosides and Acyclonucleosides: Preparation of 7a–i
For deprotection base catalyzed methods were employed. Sodium methoxide (NaOMe) in methanol was used for the deprotection of the benzoyl group of compounds 6a,d,g [45]. On the other hand, the deprotection of the acetyl group of compounds 6b,c,e,f,h,i was carried out using potassium carbonate (K2CO3) in methanol (Scheme 3 and Table 1). The structure of 7c was confirmed by X-ray crystallographic analysis. The crystal structure of this compound is shown in Figure 2 [46].
Figure 2.
X-ray molecular structure of compound 7c.
Finally, we were also interested in studying the biological activity of 1,2,3-triazole ribonucleosides 7a‒i. These derivatives were tested in vitro to evaluate their anti- HCV activity. None of the new compounds were found to inhibit HCV replication in vitro (Table 2).
Table 2.
Anti-HCV activity of compounds 7a–i.
| Compound | CC50 (µM) a | IC50 (µM) b | SI c |
|---|---|---|---|
| 7a | ≥50 µM | >10 | 5 |
| 7b | ≥100 µM | >10 | 10 |
| 7c | ≥50 µM | >10 | 5 |
| 7d | ≥100 µM | >10 | 10 |
| 7e | ≥50 µM | >10 | 5 |
| 7f | ≥50 µM | >10 | 5 |
| 7g | ≥100 µM | >10 | 10 |
| 7h | ≥50 µM | >10 | 5 |
| 7i | ≥50 µM | >10 | 5 |
| 2CmeCyt | >300 | 1.5 | 200 |
| aIFNB2 | >10,000 d | 1.5 d | 6667 |
a CC50 Concentrations of compound required for 50% extinction of Huh 5.2 cells; b IC50 Concentrations of compound achieving 50% inhibition of the replicon system; c SI selectivity index = CC50/IC50; d interferon reported as IU/mL.
Antiviral activity was assessed in a 3-day cell culture assay using the HCV-replicon-containing cell line, AVA5 (genotype 1b, CON1) (provided to GUMC by Apath, Inc., Brooklyn, NY, USA) as previously described [47].
3. Experimental
3.1. General
1H- and 13C-NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker 300 MHz instrument using SiMe4 as internal standard. Chemical shifts are given in ppm and coupling constants (J) in MHz (br, broad; m, multiplet; t, triplet; d, doublet; and s, singlet). Mass spectra were obtained using ESI/MS and MALDI-TOF-MS. Reactions were carried out in a microwave oven Model AVM510/WP/WH. The reactions were controlled by thin layer chromatography (TLC) on precoated silica gel 60 F254 (Merck, Darmstadt, Germany); UV light was used for visualization of the spots. All products were purified by column chromatography on silica gel (100–200 mesh) Merck.
3.2. Synthesis of 2-Substituted Quinazolinones
3.2.1. Preparation of 2-Phenylquinazolin-4-one (3a) [36]
Initially, anthranilic acid (1, 2 g, 14.5 mmol) was dissolved in dry pyridine (20 mL). Then benzoyl chloride (1.1 equiv) was added dropwise at 0 °C. The reaction mixture was maintained at 0 °C for 1 h and then allowed to stir at room temperature for 2 h, during which time a solid product precipitated out. The mixture was neutralized using a saturated solution of sodium bicarbonate. The pale yellow solid was filtered and washed with water. The product, 2-phenylbenzo[d][1,3]-oxazin-4-one (2a) thus obtained was reacted with formamide under microwave irradiation to obtain 3a. The crude compound obtained was crystallized in ethanol; Mp 225–226 °C; Yield 83%; Rf 0.5; eluent CH2Cl2/MeOH = 99/1, v/v; 1H-NMR (CDCl3): δ 7.61 (m, 4H, H-Aromatic), 7.83 (m, 1H, H-Aromatic), 8.01 (m, 1H, H-Aromatic), 8.24 (m, 3H, H-Aromatic), 11.9 (s, 1H, N-H). 13C-NMR (CDCl3): δ 120.81 (C-Aromatic), 126.67, 127.46, 127.85, 128.01, 129.90, 130.11, 131.66, 132.81, 134.93 (CH-Aromatic), 129.04, 149.54, 151.81 (C-Aromatic), 164.04 (CO).
3.2.2. Preparation of 2-Methylquinazolin-4-one (3b) [38]
A mixture of anthranilic acid (1, 500 mg, 3.64 mmol) and acetic anhydride (1.5 eq) was reacted under microwave irradiation at 400W until total conversion (10 min). After this first step, 2-methylquinazolin-4-one (3b) was obtained by addition of formamide (2.5 equiv) to the reaction mixture and irradiated by microwave for 10 min. After cooling to room temperature, the solid obtained was recrystallized from ethanol; Mp 238–240 °C; Yield 94%; Rf 0.65; eluent CH2Cl2/MeOH = 99/1, v/v; 1H-NMR (CDCl3) δ 2.34 (s, 3H, CH3-Aromatic), 7.34–8.02 (m, 4H, H- Aromatic), 12.12 (br s, 1H, N-H). 13C-NMR (CDCl3) δ 39.16 (-CH3), 123.31 (C-Aromatic), 125.73, 126.64, 127.15, 134.63 (CH-Aromatic), 156.02, 154.16 (C-Aromatic), 161.63 (CO).
3.2.3. Preparation of Quinazolin-4-one (3c) [38]
Anthranilic acid (1, 500 mg, 3.64 mmol) and formamide (2.5 equiv) were mixed and irradiated at 400 W for 15 min, the reaction was monitored by TLC. The reaction mixture was cooled to give the crude compound 3c which was crystallized from ethanol; Mp 217–219 °C; Yield 95%; Rf 0.63 (CH2Cl2); eluent CH2Cl2; 1H-NMR (CDCl3) δ (ppm): 7.49 (m, 1H, H-Aromatic), 7.64 (d, 1H, J = 8.2 Hz, H-Aromatic), 7.78 (m, 1H, H-Aromatic), 8.07 (s, 1H, H-Aromatic), 8.10 (d, 1H, J = 8.2 Hz, H-Aromatic), 12.29 (br s, 1H, NH). 13C-NMR (CDCl3) δ(ppm): 121.72 (C-Aromatic), 125.46, 126.88, 127.39, 133.98, 145.66 (CH-Aromatic), 147.31 (C-Aromatic), 161.63 (CO).
3.3. Synthesis of Propargylated Quinazolines
The appropriate quinazolin-4-one 3a–c (2 mmol) was dissolved in dry DMF (2.5 mL); KOt-Bu (1.1 equiv) was added. The mixture was stirred for 15 min at room temperature. Afterwards, propargyl bromide (2.5 mmol) was added dropwise to the mixture. The reaction was performed for 15 min at room temperature. The reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (2 × 20 mL); the organic phase was dried over Na2SO4 and evaporated under vacuum. The crude products were purified by column chromatography using CH2Cl2/MeOH = 99:1, v/v as eluent.
2-Phenyl-4-(prop-2-ynyloxy)quinazoline (5a). Yield 58%; Rf 0.80; eluent CH2Cl2; 1H-NMR (CDCl3) δ 2.09 (s, 1H, CCH), 5.28 (s, 2H, -CH2-N), 7.49–8.60 (m, 9H, H-quinazoline, H-Aromatic). 13C-NMR (CDCl3) δ 29.93 (-CH2-), 53.26 (CH-alkyne), 75.59 (C-alkyne), 121.17 (C-Aromatic) 126.17–135.07 (CH-Aromatic), 128.94, 148.86, 152.19 (C-Aromatic), 162.13 (CO).
2-Methyl-3-(prop-2-ynyl)quinazolin-4-one (4b). Yield 76%; Rf 0.75; eluent CH2Cl2; 1H-NMR (CDCl3) δ 2.32 (s, 1H, CCH), 2.76 (s, 3H, CH3-Aromatic), 4.94 (s, 2H, -CH2-N), 7.48–8.27 (m, 9H, H-quinazoline). 13C-NMR (CDCl3) δ 22.81 (-CH3), 33.13 (-CH2-), 72.66 (CH-alkyne), 77.06 (C-alkyne), 120.21(C-Aromatic), 126.71, 134.63, 143.14, 145.06 (CH-Aromatic), 147.10, 153.72 (C-Aromatic), 161.32 (CO).
3-(Prop-2-ynyl)quinazolin-4-one (4c). Yield 80%; Rf 0.40; eluent CH2Cl2; 1H-NMR (CDCl3) δ 2.50 (s, 1H, CCH), 4.83 (s, 2H, -CH2-N), 7.47–8.30 (m, 9H, H-quinazoline). 13C-NMR (CDCl3) δ (ppm): 29.08 (-CH2-), 71.58 (CH-alkyne), 77.33 (C-alkyne), 120.18 (C-Aromatic), 126.43, 134.26, 142.98, 145.32 (CH-Aromatic), 146.89, 151.62 (C-Aromatic), 162.06 (CO).
3.4. Synthesis of 1,2,3-Triazol-4-yl substituted O-, N-Quinazolines
Propargylated quinazolines 5a/4b–c (1 mmol), alkyl azide (sugar or pseudosugar, 2.5 mmol) and Et3N (1.1 mmol) were mixed with CuI (0.1 equiv). For homogenization, the reaction mixture was dissolved in dry MeCN and stirred for 5 min. Then, the solvent was removed under vacuum. The mixture was subjected to microwave irradiation at the power level 400 W for 2 min. The residue was purified on silica gel using CH2Cl2 as eluent.
4-((1-(2,3,5-Tri-O-benzoyl-β-d-ribofuranos-1-yl)1H-1,2,3-triazol-4-yl)methoxy)-2-phenylquinazoline (6a). Yield 95%; Rf 0.63; eluent CH2Cl2; 1H-NMR (CD Cl3) δ 4.48 (m, 1H, H5'), 4.64 (m, 1H,H5'), 4.75 (m, 1H,H4'), 5.70 (s, 2H, -CH2-), 6.05 (dd, 1H, J = 8.2 Hz, H3'), 6.21 (dd, 1H, J = 8.2 Hz, H2'), 6.32 (d, 1H, H1'), 7.14–7.44 (m, 10H, H-Aromatic) 7.65–7,81 (m, 12H, H-Aromatic), 7.62 (s, 1H, H-Triazole), 8.83–8.88 (m, 2H, H-Aromatic). 13C-NMR (CDCl3) δ 57.21, 61.07 (CH2), 69.13(C5'), 72.81(C2'), 78.72(C3'), 87.72 (C4'), 112.58(C1'), 123.30, 126.59, 127.91, 128.44–133.88 (CH-Aromatic), 135.68–158.34 (C-Aromatic), 163.12, 164.45 (CO). ESI-MS(M+H)+, m/z calcd for C43H33N5O8 748.75, found 748.90.
2-((4-((2-Phenylquinazolin-4-yloxy)methyl)-1,2,3-triazol-1-yl)methoxy)ethylacetate (6b). Yield 88; Rf 0.62; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.80 (s, 3H, -CO-CH3), 3.63 (t, 2H, -CH2-O), 4.35 (t, 2H, -CH2-O-CO), 4.51 (s, 2H, N-CH2-Triazole), 5.88 (s, 2H, O-CH2-Triazole), 7.45 (m, 4H, H-Aromatic), 7.73 (m, 2H, H-Aromatic), 7.84 (s, 1H, H-Triazole), 8.11 (dd, 1H, H-Aromatic), 8.54(dd, 2H, H-Aromatic). 13C-NMR (CDCl3) δ 20.42(CH3), 49.13 (CH2-quinazoline), 59.78, 62.62 (-OCH2CH2O-), 67.69 (-O-CH2-N<), 123.51–133.80 (-CH-Aromatic), 142.13,146.46, 152.22 (C-Aromatic), 162.43, (CO-Aromatic),172.12 (CO-Ester). ESI-MS(M+H)+, m/z calcd for C22H21N5O4 420.43, found 420.40.
4-(4-((2-Phenylquinazolin-4-yloxy)methyl)-1,2,3-triazol-1-yl)butylacetate (6c). Yield 83%; Rf 0.68; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.56 (s, 3H, -CO-CH3), 1.92 (m, 4H, -CH2-CH2-), 3.97 (t, 2H, J = 6.3 Hz,-CH2-N(Triazole)), 4.29 (t, 2H, J = 7.2 Hz, -CH2-O-CO), 5.85 (s, 2H, O-CH2-C(Triazole)), 7.46 (m, 4H, H-Aromatic), 7.72 (m, 2H, H-Aromatic), 7.93 (s, 1H, H-Triazole), 8.10 (dd, 1H, H-Aromatic), 8.56 (dd, 2H, H-Aromatic).13C-NMR (CDCl3) δ 18.61(CH3), 23.38, 24.71 (-CH2-CH2-) 47.62(-CH2-quinazoline), 57.67 (CH2-N(Triazole)), 61.06 (CH2-O), 123.59–133.77 (CH-Aromatic), 142.13, 146.46, 152.22 (C-Aromatic),163.86, (CO-Aromatic), 168.70 (CO-Ester). ESI-MS (M+H)+, m/z calcd for C23H23N5O3 418.46, found 418.60.
3-((1-(2,3,5-Tri-O-benzoyl-β-D-ribofuranos-1-yl)-1,2,3-triazol-4-yl)methyl)-2-methylquinazolin-4-one (6d). Yield 83%; Rf 0.60; eluent CH2Cl2; 1H-NMR (CDCl3) δ 2.76 (s, 3H, -CH3), 4.54 (m, 1H, H5'), 4.65 (m, 1H, H5'), 4.81 (m, 1H, H4'), 5.23 (s, 2H, -CH2-), 6.10 (dd, 1H, J = 8.6 Hz, H3'), 6.19 (dd, 1H, J = 8.6 Hz, H2'), 6.31(d, 1H, H1'), 7.23–7,40(m, 13H, H-Aromatic), 7.57(s, 1H, H-Triazole), 8.62–7.86 (m, 5H, H-Aromatic), 7.88 (m, 1H, H-Aromatic).13C-NMR (CDCl3) δ 23.67(CH3), 39.45, 63.75(CH2), 71.70 (C5'), 75.22 (C2'), 81.13 (C3'), 90.34 (C4'), 117.84 (C1'), 124.07–134.35 (CH-Aromatic), 140.73–159.53 (C-Aromatic), 162.51, 163.58 (CO). ESI-MS(M+H)+, m/z calcd for C38H31N5O8 686,68, found 686.50.
2-((4-((2-Methyl-4-oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl) methoxy) ethylacetate (6e). Yield 87%; Rf 0.59; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.9(s, 3H, -CO-CH3), 2.84 (s, 3H, Aromatic-CH3), 3.64 (t, 2H, -CH2-O), 4.35 (t, 2H, -CH2-O-CO), 4.52 (s, 2H, N-CH2-Triazole), 5.33 (s, 2H, O-CH2-Triazole), 7.34–7.55 (m, 3H, H-Aromatic), 7.78 (s, 1H, H-Triazole), 8.13 (m, 1H, H-Aromatic). 13C-NMR (CDCl3) δ 20.23, 23.86 (CH3), 39.59 (CH2-quinazoline), 49.04, 62.16 (OCH2CH2O), 78.77 (O-CH2-N), 120.24–134.34 (CH-Aromatic), 143.00, 147.35, 154.36 (C-Aromatic), 162.01 (CO-Aromatic), 170.25 (CO-Ester). ESI-MS(M+H)+, m/z calcd for C17H19N5O4 358.36, found 358.40.
4-(4-((2-Methyl-4-oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)butylacetate (6f). Yield 86%; Rf 0.60; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.55 (s, 3H, -CO-CH3), 1.90 (m, 4H,-CH2-CH2-), 2.83 (s, 3H, Aromatic-CH3), 3.99 (t, 2H, J = 6.2Hz, -CH2-N(Triazole)), 4.27 (t, 2H, J = 7.2Hz, -CH2-O-CO), 5.29 (s, 2H, O-CH2-C(Triazole)), 7.20–7,52 (m, 3H, H-Aromatic), 7.63 (s, 1H, H-Triazole), 8.17 (m, 1H, H-Aromatic). 13C-NMR (CDCl3) δ (ppm): 20.88, 23.74 (CH3), 25.67, 26.87 (CH2-CH2) 39.72 (CH2-quinazoline), 49.91 (CH2-N(Triazole)), 63.32 (CH2-O), 123.89–134.46(CH-Aromatic), 142.93, 147.42, 154.44 (C-Aromatic), 162.71, (CO-Aromatic), 168.70 (CO-Ester). ESI-MS(M+H)+, m/z calcd for C18H21N5O3 356.39, found 356.40.
3-((1-(2,3,5-Tri-O-benzoyl-β-d-ribofuranos-1-yl)-1,2,3-triazol-4-yl)methyl)quinazolin-4-one (6g). Yield 84%; Rf 0.60; eluent CH2Cl2; 1H-NMR (CDCl3) δ (ppm): 4.68 (m, 1H, H5'), 4.75 (m, 1H, H5'), 4.91 (m, 1H, H4'), 5.30 (s, 2H, -CH2-), 6.16 (dd, 1H, J = 7.8 Hz, H3'), 6.26 (dd, 1H, J = 7.8 Hz, H2'), 6.38(d, 1H, H1'), 7.27–7.40 (m, 13H, H-Aromatic), 7.69 (s, 1H, H-Triazole), 7.87–8.08 (m, 5H, H-Aromatic), 7.30 (m, 1H, H-Aromatic). 13C-NMR (CDCl3) δ (ppm): 41.46, 63.68 (CH2), 71.65 (C5'), 75.20 (C2'), 81.24 (C3'), 90.42 (C4'), 122 (C1'), 123.56–134.36 (CH-Aromatic), 142.46–160.90 (C-Aromatic), 164.98, 166.05 (CO). ESI-MS(M+H)+, m/z calcd for C37H29N5O8 672.65, found 672.80.
2-((4-((4-Oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)methoxy)ethylacetate (6h). Yield 90%; Rf 0.57; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.9 (s, 3H, -CO-CH3), 3.64 (t, 2H,-CH2-O), 4.35 (t, 2H, -CH2-O-CO), 4.52 (s, 2H, N-CH2-Triazole), 5.33 (s, 2H, O-CH2-Triazole), 7.21-7.42 (m, 3H, H-Aromatic), 7.77 (s, 1H, H-Triazole), 8.18–8.30 (m, 2H, H-Aromatic). 13C-NMR (CDCl3) δ 20.63 (CH3), 41.63 (CH2-quinazoline), 49.15, 62.19 (-OCH2CH2O-), 79.11 (-O-CH2-N<), 121.94–134.44 (CH-Aromatic), 142.43, 146.30, 148.14 (C-Aromatic), 160.99 (CO-Aromatic), 170.34 (CO-Ester). ESI-MS (M+H)+, m/z calcd for C16H17N5O4 344.34, found 344.10.
4-(4-((4-Oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)butylacetate (6i). Yield 89%; Rf 0.60; eluent CH2Cl2; 1H-NMR (CDCl3) δ 1.55 (s, 3H, -CO-CH3), 1.90 (m, 4H,-CH2-CH2-), 3.40 (t, 2H, J = 6.3 Hz, -CH2-N(Triazole)), 4.28 (t, 2H, J = 7.2 Hz, -CH2-O-CO), 5.20 (s, 2H, O-CH2-C(Triazole)), 7.20 (m, 1H, H-Aromatic), 7.65 (s, H, H-Aromatic, H-Triazole), 8.19–8.28 (m, 2H, H-Aromatic). 13C-NMR (CDCl3) δ 20.88 (CH3), 25.66, 26.87 (CH2-CH2) 39.72(CH2-quinazoline), 49.91 (CH2-N(Triazole)), 63.32 (CH2-O), 123.89–134.46 (CH-Aromatic), 142.93, 147.42, 154.44 (C-Aromatic), 162.71, (CO-Aromatic), 168.70 (CO-Ester). ESI-MS(M+H)+, m/z calcd for C17H19N5O3 342.36, found 342.33.
3.4.1. Benzoyl Group Deprotection
The compound (6a,d,g, 1 mmol) was dissolved in dry methanol (2.5 mL). NaOMe (1 eq) was added to the solution with stirring for 30 min at room temperature. The neutralization was performed with AmberliteIR120 hydrogen form. After, filtration and evaporation the residue was purified by silica gel flash column chromatography.
3.4.2. Acetyl Group Deprotection
A solution of (6b,c,e,f,h,i, 1 mmol) in dry methanol (2.5 mL) was treated with 1 eq of K2CO3. The reaction mixture was stirred at room temperature for 15 min. The deprotected compound was purified by silica gel flash column chromatography.
4-((1-(β-D-Ribofuranos-1-yl)-1,2,3-triazol-4-yl)methoxy)-2-phenylquinazoline (7a). Yield 98%; Rf 0.56; eluent CH2Cl2/MeOH = 95/5: v/v; 1H-NMR (DMSO-d6) δ 2.50 (d, 2H, -CH2-), 3.35–3.65 (m, 3H, -OH), 4.18 (m, 1H, H5'), 4.42 (m, 1H,H5'), 4.91 (m, 1H,H4'), 5.88 (s, 2H, -CH2-), 5.28 (dd, 1H, J = 8.2 Hz, H3'), 5.71 (dd, 1H, J = 8.2 Hz, H2'), 6.01 (d, 1H,H1'),. 7.55 (m, 4H, H-Aromatic) 7.97 (m, 2H, H-Aromatic), 8.25 (s, 1H, H-Triazole), 8.58 (m, 3H, H-Aromatic). 13C-NMR (DMSO-d6) δ 59.81 (CH2), 61.31 (C5'), 70.36 (C2'), 75.08 (C3'), 85.89 (C4'), 92.10 (C1'), 114.38–130.83 (CH-Aromatic), 134.36–158.82 (C-Aromatic), 165.74 (CO). ESI-MS(M+H)+, m/z calcd for C22H21N5O5 436.43, found 436.30.
2-((4-((2-Phenylquinazolin-4-yloxy)methyl)-1,2,3-triazol-1-yl)methoxy)etanol (7b). Yield 86%; Rf 0.43; eluent CH2Cl2/MeOH, 95/5: v/v; 1H-NMR (DMSO-d6) δ 3.63 (t, 4H,-CH2-O), 4.24 (s, 2H, N-CH2-Triazole), 4.65 (t, 1H, -OH), 5.28 (s, 2H, O-CH2-Triazole), 7.67 (m, 4H, H-Aromatic), 7.67 (m, 2H, H-Aromatic), 7.96 (s, 1H, H-Triazole), 8.54 (dd, 3H, H-Aromatic). 13C-NMR (DMSO-d6) δ 20.32 (CH3), 49.13 (CH2-quinazoline), 59.78 (CH2OH), 62.62 (OCH2), 66.87 (O-CH2-N), 124.50–134.20 (CH-Aromatic), 141.7–153.60 (C-Aromatic), 161.32, (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C20H19N5O3 378.40, found 378.30.
4-(4-((2-Phenylquinazolin-4-yloxy)methyl)-1,2,3-triazol-1-yl)butan-1-ol (7c). Yield 88%; Rf 0.44; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (DMSO-d6) δ 1.45–1.86 (m, 4H,-CH2-CH2-), 2.50 (t, 1H, -OH), 4.38 (s, 2H, -CH2OH), 4.39 (t, 2H, J = 6.3Hz, -CH2-N(Triazole)), 5.85 (s, 2H, N-CH2-Triazole), 7.54 (m, 4H, H-Aromatic), 7.87 (m, 3H, H-Aromatic), 8.32 (s, 1H, H-Triazole), 8.57 (m, 2H, H-Aromatic). 13C-NMR (DMSO-d6) δ 26.56–29.19 (CH2-CH2) 47.62 (CH2-quinazoline), 57.67 (CH2-N(Triazole)), 61.06 (CH2-O), 114.41–130.80 (CH-Aromatic), 134.30–158.80 (C-Aromatic), 165.47 (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C21H23N5O2 376.44, found 376.50.
3-((1-(β-d-Ribofuranos-1-yl)-1,2,3-triazol-4-yl)methyl)-2-methylquinazolin-4-one (7d). Yield 97%; Rf 0.60; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (DMSO-d6) δ 2.74 (s, 3H, -CH3), 3.56–4.22 (m, 3H, -OH), 4.67 (m, 1H, H5'), 4.98 (m, 1H, H5'), 5.22 (m, 1H, H4'), 5.41 (s, 2H, -CH2-), 6.10 (dd, 1H, J = 8.6 Hz, H3'), 5.62 (dd, 1H, J = 8.6 Hz, H2'), 6.01 (d, 1H, H1'), 7.46–7.60 (m, 2H, H-Aromatic), 7.83 (m, 1H, H-Aromatic), 8.11 (s, 1H, H-Triazole), 8.32 (m, 1H, H-Aromatic). 13C-NMR (DMSO-d6) δ(ppm): 23.67 (CH3), 58.81 (CH2), 61.23 (C5'), 70.28 (C2'), 74.94 (C3'), 85.83 (C4'), 92.03 (C1'), 119.80–126.52 (CH-Aromatic), 134.45–155.00 (C-Aromatic), 160.90 (CO). ESI-MS (M+H)+, m/z calcd for C17H19N5O5 374.36, found 374.30.
2-((4-((2-Methyl-4-oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)methoxy)ethanol (7e). Yield 95%; Rf 0.40; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (DMSO-d6) δ 2.74 (s, 3H, -CH3), 3.39 (t, 2H, -CH2-O), 3.64 (t, 2H, -CH2-O), 4.50 (s, 2H, N-CH2-Triazole), 4.63 (t, 1H, -OH), 5.21 (s, 2H, O-CH2-Triazole), 7.46–7,65 (m, 2H, H-Aromatic), 7.82 (s, 1H, H-Triazole), 8.07 (m, 2H, H-Aromatic). 13C-NMR (DMSO-d6) δ 22.98 (CH3), 40.31 (CH2-quinazoline), 59.78 (CH2OH), 62.62 (OCH2), 80.27 (O-CH2-N), 119.83–134.42 (CH-Aromatic), 142.04, 147.03, 155.03 (C-Aromatic), 160.92 (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C15H17N5O3 316.33, found 316.40.
4-(4-((2-Methyl-4-oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)butan-1-ol (7f). Yield 90%; Rf 0.39; eluent CH2Cl2/MeO = 95/5, v/v; 1H-NMR (DMSO-d6) δ (ppm): 1.43–1.88 (m, 4H, -CH2-CH2-), 2.61 (s,3H, -CH3), 2.72 (t, 1H, -OH), 4.37 (s, 2H, -CH2OH), 4.40 (t, 2H, J = 6.3 Hz, -CH2-N(Triazole)), 5.56 (s, 2H, N-CH2-Triazole), 7.58–7.69 (m, 2H, H-Aromatic), 7.87 (s, 1H, H-Triazole), 8.17(m, 2H, H-Aromatic). 13C-NMR (DMSO-d6) δ 22.96 (CH3), 26.48, 29.19 (CH2-CH2) 40.30 (CH2-quinazoline), 49.31 (CH2-N(Triazole)), 59.94 (CH2-O), 119.82–134.41 (CH-Aromatic), 142.23, 147.03, 155.01 (C-Aromatic), 160.91 (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C16H19N5O2 314.35, found 314.30.
3-((1-(β-D-Ribofuranos-1-yl)-1,2,3-triazol-4-yl)methyl)quinazolin-4-one (7g). Yield 98%; Rf: 0.62; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (DMSO-d6) δ 3.52–4.33 (m,3H,-OH), 4.59 (m, 1H, H5'), 4.70 (m, 1H, H5'), 4.88 (m, 1H, H4'), 5.28 (s, 2H, -CH2-), 5.66 (dd, 1H, J = 7.8 Hz, H3'), 5.73 (dd, 1H, J = 7.8 Hz, H2'), 6.20 (d, 1H, H1'),. 7.44–7.58 (m, 3H, H-Aromatic), 7.63 (s, 1H, H-Triazole), 7.92 (m, 2H, H-Aromatic). 13C-NMR (75 MHz, DMSO-d6) δ 40.86 (CH2), 61.28 (C5'), 70.31 (C2'), 74.97 (C3'), 85.85 (C4'), 92.02 (C1'), 121.53–132.23 (CH-Aromatic), 134.44, 142.60, 147.83 (C-Aromatic), 159.84 (CO). ESI-MS(M+H)+, m/z calcd for C16H17N5O5 360.34, found 360.42.
2-((4-((4-Oxoquinazolin-3-yl)methyl)-1,2,3-triazol-1-yl)methoxy)ethanol (7h). Yield 93%; Rf: 0.39; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (DMSO-d6) δ 3.64 (m, 4H, -CH2-O), 4.23 (t, 1H, -OH), 4.56 (s, 2H, N-CH2-Triazole), 5.40 (s, 2H, O-CH2-Triazole), 7.62–7.79 (m, 3H, H-Aromatic), 7.80 (m, 2H, H-Triazole, H-Aromatic), 8.33 (s, 1H, H-Aromatic). 13C-NMR (DMSO-d6) δ 52.20 (CH2-quinazoline), 59.70 (CH2OH), 70.97 (OCH2), 78.21 (O-CH2-N), 121.45–134.41 (CH-Aromatic), 142.00, 147.82, 155.12 (C-Aromatic), 159.84 (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C14H15N5O3 302.30, found 302.30.
4-(4-((4-Oxoquinazolin-3-yl)methyl)-1H-1,2,3-triazol-1-yl)butan-1-ol (7i). Yield 94%; Rf: 0.43; eluent CH2Cl2/MeOH = 95/5, v/v; 1H-NMR (CDCl3) δ 1.39–1.87 (m, 4H,-CH2-CH2-), 2.56 (t, 1H, -OH), 4.36 (s, 2H, -CH2OH), 4.43 (t, 2H, J = 6.3 Hz, -CH2-N(Triazole)), 5.32 (s, 2H, N-CH2-Triazole), 7.58–7.69 (m, 2H, H-Aromatic), 7.87 (s, 1H, H-Triazole), 8.17 (m, 2H, H-Aromatic). 13C-NMR (DMSO-d6) δ 26.49, 29.18 (CH2-CH2) 40.94 (CH2-quinazoline), 49.30 (CH2-N(Triazole)), 59.94 (CH2-O), 121.54–127.19 (CH-Aromatic), 142.20, 147.81, 147.815 (C-Aromatic), 159.84, (CO-Aromatic). ESI-MS(M+H)+, m/z calcd for C15H17N5O2 300.33, found 300.30.
4. Conclusions
In summary, the synthesis of a series of 1,2,3-triazole-4-yl-quinazoline derivatives starting from anthranilic acid was performed efficiently using click chemistry under microwave irradiation. None of the compounds selected showed significant anti-HCV activity.
Acknowledgments
This project was supported by the CNRST Research program RS 2011/01. We gratefully acknowledge Ananthan Sam, Southern Research Institute Birmingham, Alabama, AL, USA, for helpful discussion.
Author Contributions
J. W. Engels, H. B. Lazrek and M. Taourirte supervised and designed the study. A. Ouahrouch performed the experiments, analysed the data and wrote the paper. S. Benjelloun performed the antiviral activity.
Conflictts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 7a–i are available from the authors.
References
- 1.Seeff L.B. Natural history of hepatitis C. Hepathology. 1997;26(Suppl. 1):21S–28S. doi: 10.1002/hep.510260704. [DOI] [PubMed] [Google Scholar]
- 2.Zeuzem S. Interferon-based therapy for chronic hepatitis C: Current and future perspectives. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008;5:610–622. doi: 10.1038/ncpgasthep1274. [DOI] [PubMed] [Google Scholar]
- 3.Feld J.J., Hoofnagle J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 4.Butt A.A., Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin. Infect. Dis. 2012;54:96–104. doi: 10.1093/cid/cir774. [DOI] [PubMed] [Google Scholar]
- 5.Murgan V., Thomas C.C., Rama Sarma G.V.S., Kumar E.P. Synthesis of 2-substituted quinazolin-4(3H)-ones as a new class of anticancer agents. Indian J. Pharm. Sci. 2003;65:386–389. [Google Scholar]
- 6.Chan J.H., Hong J.S., Kuyper L.F., Jones M.L., Baccanari D.P., Tansik R.L., Boytos C.M., Rudolph S.K., Brown A.D. Synthesis of 1,3-diamino-7,8,9,10-tetrahydropyrido[3,2-f]-quinazolines. Inhibitors of Candida albicans dihydrofolate reductase as potential antifungal agents. J. Heterocycl. Chem. 1997;34:145–151. doi: 10.1002/jhet.5570340123. [DOI] [Google Scholar]
- 7.Dempcy R.O., Skibo E.B. Rational design of quinazoline-based irreversible inhibitors of human erythrocyte purine nucleoside phosphorylase. Biochemistry. 1991;30:8480–8487. doi: 10.1021/bi00098a028. [DOI] [PubMed] [Google Scholar]
- 8.Broggi J., Joubert N., Aucagne V., Berteina-Raboin S., Diez-Gonzales S., Nolan S.P., Topalis D., Deville-Bonne D., Balzarini J., Neyts J., et al. Alkyne-azide click chemistry mediated carbanucleosides synthesis. Nucleos. Nucleot. Nucl. 2007;26:1391–1394. doi: 10.1080/15257770701534139. [DOI] [PubMed] [Google Scholar]
- 9.El Akri K., Bougrin K., Balzarini J., Faraj A., Benhida R. Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides. Bioorg. Med. Chem. Lett. 2007;17:6656–6659. doi: 10.1016/j.bmcl.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 10.Broggi J., Diez-Gonzalez S., Petersen J., Berteina-Raboin S., Nolan S.P., Agrofoglio L.A. Study of copper (I) catalysts for the synthesis of carbanucleosides via azide-alkyne 1,3-Dipolar Cycloaddition. Synthesis. 2008;1:141–144. [Google Scholar]
- 11.Broggi J., Joubert N., Díez-González S., Berteina-Raboin S., Zevaco T., Nolan S.P., Agrofoglio L.A. Synthesis of (±)-1,2,3-triazolo-3'-deoxy-4'-hydroxymethyl carbanucleosides via ‘click’ cycloaddition. Tetrahedron. 2009;65:1162–1170. doi: 10.1016/j.tet.2008.11.065. [DOI] [Google Scholar]
- 12.Broggi J., Kumamoto H., Berteina-Raboin S., Nolan S.P., Agrofoglio L.A. Click azide-alkyne cycloaddition for the synthesis of D-(–)-1,4-disubstituted triazolo-Carbanucleosides. Eur. J. Org. Chem. 2009;10:1880–1888. [Google Scholar]
- 13.Guezguez R., Bougrin K., El Akri K., Benhida R. A highly efficient microwave-assisted solvent-free synthesis of α- and β-2'-deoxy-1,2,3-triazolyl-nucleosides. Tetrahedron Lett. 2006;47:4807–4811. doi: 10.1016/j.tetlet.2006.05.050. [DOI] [Google Scholar]
- 14.Malnuit V., Duca M., Manout A., Bougrin K., Benhida R. Tandem azide-alkyne 1,3-dipolar cycloaddition/electrophilic addition: A concise three-component route to 4,5-disubstituted triazolyl-nucleosides. Synlett. 2009;13:2123–2128. [Google Scholar]
- 15.Huisgen R. Kinetics and Mechanism of 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 1963;2:633–696. doi: 10.1002/anie.196306331. [DOI] [Google Scholar]
- 16.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Tornøe C.W., Christensen C., Meldal M.J. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 18.Meldal M., Tornøe C.W. Cu-Catalyzed Azide Alkyne Cycloaddition. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 19.Koev G., Dekhtyar T., Han L., Ng T.I., Lin C.T., Mo H., Molla A. Antiviral interactions of an HCV polymerase inhibitor with an HCV protease inhibitor or interferon in vitro. Antivir. Res. 2007;73:78–83. doi: 10.1016/j.antiviral.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20.De Clercq E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez R., Velazquez S., San-Felix A., Aquaro S., de Clercq E., Perno C.F., Karlsson A., Balzarini J., Camarasa M.J. 1,2,3-Triazole-[2,5-Bis-O-(tert-butyldimethylsilyl)-.beta.-D-ribofuranosyl]-3'-spiro-5''-(4''-amino-1'',2''-oxathiole 2'',2''-dioxide) (TSAO) analogs: Synthesis and Anti-HIV-1 Activity. J. Med. Chem. 1994;1994:4185–4194. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 22.Kabbaj Y., Lazrek H.B., Barascut J.L., Imbach J.L. Synthesis and biological activity of some unsaturated 6-azauracil acyclonucleosides. Nucleos. Nucleot. Nucl. 2005;24:161–172. doi: 10.1081/NCN-55695. [DOI] [PubMed] [Google Scholar]
- 23.Redwane N., Lazrek H.B., Barascut J.L., Imbach J.L., Balzarini J., Witvrouw M., de Clerq E. Synthesis and biological activities of (Z) and (E) α-ethenyl acyclonucleosides. Nucleos. Nucleot. Nucl. 2001;20:1439–1447. doi: 10.1081/NCN-100105239. [DOI] [PubMed] [Google Scholar]
- 24.Lazrek H.B., Rochdi A., Khaider H., Barascut J.L., Imbach J.L., Balzarini J., Witvrouw M., Pannecouque C., de Clercq E. Synthesis of (Z) and (E) α-alkenyl phosphonic acid derivatives of purines and pyrimidines. Tetrahedron. 1998;54:3807–3816. doi: 10.1016/S0040-4020(98)00107-0. [DOI] [Google Scholar]
- 25.Lazrek H.B., Taourirte M., Oulih T., Barascut J.L., Imbach J.L., Pannecouque C., Witrouw M., de Clercq E. Synthesis and anti-HIV activity of new modified 1,2,3-triazole acyclonucleosides. Nucleos. Nucleot. Nucl. 2001;20:1949–1960. doi: 10.1081/NCN-100108325. [DOI] [PubMed] [Google Scholar]
- 26.Moukha-chafiq O., Taha M.L., Lazrek H.B., Vasseur J.J., Pannecouque C., Witvrouw M., de Clercq E. Synthesis and biological activity of some 4-substituted 1-[1-(2,3-dihydroxy-1-propoxy)methyl-1,2,3-triazol-(4&5)-ylmethyl]-1H-pyrazolo[3,4-d]pyrimidines. Farmaco. 2002;57:27–32. doi: 10.1016/S0014-827X(01)01152-1. [DOI] [PubMed] [Google Scholar]
- 27.Moukha-Chafiq O., Taha M.L., Lazrek H.B., Vasseur J.J., Pannecouque C., Witvrouw M., de Clercq E. Synthesis and biological evaluation of some 4-substituted 1-[1-(4-hydroxybutyl)-1,2,3-triazol-(4 & 5)-ylmethyl]-1H-pyrazolo-[3,4-d]pyrimidines. Nucleos. Nucleot. Nucl. 2001;20:1811–1821. doi: 10.1081/NCN-100107192. [DOI] [PubMed] [Google Scholar]
- 28.Elayadi H., Smietana M., Pannecouque C., Leyssen P., Neyts J., Vasseur J.J., Lazrek H.B. Straightforward synthesis of triazoloacylonucleotide phosphonates as potential HCV inhibitors. Bioorg. Med. Chem. Lett. 2010;20:7365–7368. doi: 10.1016/j.bmcl.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Krim J., Taourirte M., Gruenewald C., Krstic I., Engels J.W. Microwave assisted click chemistry for nucleoside functionalization: Useful derivatives for analytical and biological applications. Synthesis. 2013;45:396–405. doi: 10.1055/s-0032-1317964. [DOI] [Google Scholar]
- 30.Elayadi H., Mesnaoui M., Korba B.E., Smietana M., Vasseur J.J., Secrist A.J., Lazrek H.B. Preparation of 1,4-disubstituted-1,2,3-triazolo ribonucleosides by Na2CuP2O7 catalyzed azide-alkyne 1,3-dipolar cycloaddition. ARKIVOC. 2012;8:76–89. [Google Scholar]
- 31.Elayadi H., Ait Ali M., Mehdi A., Lazrek H.B. Nanoscrystalline CuO: Synthesis and application as an efficient catalyst for the preparation of 1,2,3-triazole acyclic nucleosides via 1,3-dipolar cycloaddition. Catalysis. Comm. 2012;26:155–158. doi: 10.1016/j.catcom.2012.05.016. [DOI] [Google Scholar]
- 32.Elayadi H., Smietana M., Vasseur J.J., Balzarini J., Lazrek H.B. Synthesis of 1,2,3-triazolyl nucleoside analogs as potential anti-influenza A (H3N2 subtype) virus agents. Arch. Pharm. (Weinheim) 2014;347:134–141. doi: 10.1002/ardp.201300260. [DOI] [PubMed] [Google Scholar]
- 33.Chandrika P.M., Yakaiah T., Narsaiah B., Sridhar V., Venugopal G., Venkateshwara R.J., Kumar K.P., Murthy U.S.N., Rao A.R.R. Synthesis leading to novel 2,4,6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell lines. Indian J. Chem. 2009;48B:840–847. [Google Scholar]
- 34.Patil D.A., Patil P.O., Patil G.B., Saurana S.J. Synthesis of 2, 3-Disubstituted-Quinazolin-4-(3H)-ones. Mini Rev. Med. Chem. 2011;11:633–641. doi: 10.2174/138955711796268778. [DOI] [PubMed] [Google Scholar]
- 35.Rocco S.A., Barbarini J.E., Rittner R. Syntheses of some 4-anilinoquinazoline derivatives. Synthesis. 2004;3:429–435. [Google Scholar]
- 36.Gao X., Cai X., Yan K., Song B., Gao L., Chen Z. Synthesis and Antiviral Bioactivities of 2-Aryl- or 2-Methyl-3-(substituted- Benzalamino)-4(3H)-quinazolinone Derivatives. Molecules. 2007;12:2621–2642. doi: 10.3390/12122621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexandre F.R., Berecibara A., Besson T. Microwave-assisted Niementowski reaction. Back to the roots. Tetrahedron Lett. 2002;43:3911–3913. doi: 10.1016/S0040-4039(02)00619-6. [DOI] [Google Scholar]
- 38.Nouira I., Kostakis I.K., Dubouilh C., Chosson E., Iannelli M., Besson T. Decomposition of formamide assisted by microwaves, a tool for synthesis of nitrogen-containing heterocycles. Tetrahedron Lett. 2008;49:7033–7036. doi: 10.1016/j.tetlet.2008.09.135. [DOI] [Google Scholar]
- 39.Bogentoft C., Krongberg L., Danielsson B. Studies on the medicinal chemistry of oxoquinazolines. IV. N- and O-alkylation of some 2-substituted 3,4-dihydro-4-oxoquinazolines. Acta Pharm. Suec. 1969;6:489–500. [PubMed] [Google Scholar]
- 40.Hori M., Ohtaka H. Effects of a 2-substituent on the ratio of N- and O-alkylation of 4(3H)quinazolinones. Chem. Pharm. Bull. 1993;41:1114–1117. doi: 10.1248/cpb.41.1114. [DOI] [Google Scholar]
- 41.Usifoh C.O., Scriba G.K.E. Synthesis and Anticonvulsant activity of Acetylenic Quinazolinone derivatives. Arch. Pharm. 2000;333:261–266. doi: 10.1002/1521-4184(20008)333:8<261::AID-ARDP261>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Chandrika P.M., Yakaiah T., Gayatri G., Kumar K.P., Narsaiah B., Murthy U.S.N., Rao R.A.R. Click chemistry: Studies on the synthesis of novel fluorous tagged triazol-4-yl substituted quinazoline derivatives and their biological evaluation—Theoretical and experimental validation. Eur. J. Med. Chem. 2010;45:78–84. doi: 10.1016/j.ejmech.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Krim J., Sillahi B., Taourirte M., Rakib E.M., Engels J.W. Microwave-assisted click chemistry: Synthesis of mono and bis-1,2,3-triazole acyclonucleoside analogues of ACV via copper(I)-catalyzed cycloaddition. ARKIVOC. 2009;8:142–152. [Google Scholar]
- 44.Ouahrouch A., Taourirte M., Lazrek H.B., El Azhari M., Saadi M., El Ammari L. 4-{4-[(4-Oxoquinazolin-3-yl)methyl]-1H-1,2,3-triazol-1-yl}butyl acetate. Acta Crystallogr. 2011;E67:o3191. doi: 10.1107/S1600536811045600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouahrouch A., Taourirte M., El Azhari M., Saadi M., El Ammari L. 3-{[1-(2,3,5-Tri-O-benzoyl-β-d-ribofuranos-1-yl)-1H-1,2,3-triazol-4-yl]methyl}quinazolin-4(3H)-one. Acta Crystallogr. 2012;E68:o3166. doi: 10.1107/S1600536812042778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfadler W., Pfleiderer W. Syntheses and properties of pterin ribonucleosides. ARKIVOC. 2009;3:95–114. [Google Scholar]
- 47.Korba B.E., Montero A.B., Farrar K., Gaye K., Mukerjee S., Ayers M., Rossignol J.F. Nitazoxanide, tizoxanide, and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antivir. Res. 2008;77:56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]