Abstract
Twenty-one non-peptide substituted desloratadine class compounds were synthesized as novel arginine vasopressin receptor antagonists from desloratadine via successive acylation, reduction and acylation reactions. Their structures were characterized by 1H-NMR and HRMS, their biological activity was evaluated by in vitro and in vivo studies. The in vitro binding assay and cAMP accumulation assay indicated that these compounds are potent selective V2 receptor antagonists. Among them compounds 1n, 1t and 1v exhibited both high affinity and promising selectivity for V2 receptors. The in vivo diuretic assay demonstrated that 1t presented remarkable diuretic activity. In conclusion, 1t is a potent novel AVP V2 receptor antagonist candidate.
Keywords: substituted desloratadine, synthesis, arginine vasopressin receptor antagonists, biological activity
1. Introduction
Arginine vasopressin (AVP), a neurohypophysial peptide hormone that is secreted mainly from the posterior pituitary gland in response to low blood volume or high serum osmolality exerts its biological action through three major G-protein-coupled receptors, V1a, V1b and V2 [1,2,3]. The V2 receptors, which are localized predominately in the kidney collecting tubules, are responsible for controlling water reabsorption and salt (NaCl) balance [4]. The receptor stimulates adenylate cyclase, which results in the production of cyclic AMP [5]. Thus, there is potential to develop a vasopressin V2 receptor antagonist for the treatment of disorders such as congestive heart failure [6,7,8,9], hypertension [10,11], renal disease [12,13], edema [14,15], liver cirrhosis [16,17], hyponatremia [18,19,20,21,22], inappropriate antidiuretic hormone secretion (SIADH) syndrome [23] and any state involving excessive retention of water.
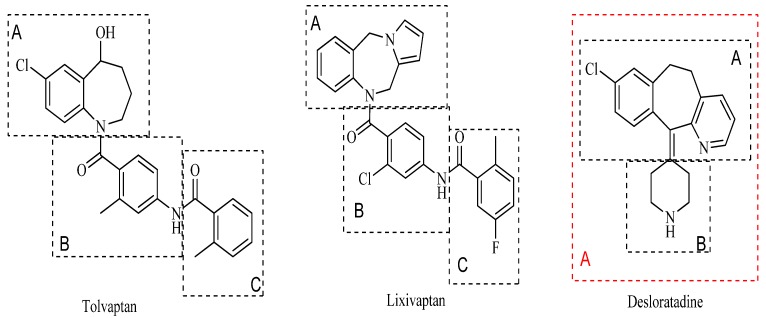
Numerous AVP receptor antagonists were developed and evaluated in recent decades [24,25,26,27,28,29,30]. A few of them have undergone sufficient clinical trials to be on the market, such as the dual V1a/V2 receptor antagonist conivaptan and the selective V2 receptor antagonist tolvaptan approved for the treatment of hyponatremia in the USA. Another promising selective AVP V2 receptor antagonist, lixivaptan, is still undergoing phase 3 clinical trials at this moment. The structures of most extant AVP receptor antagonists include a benzene-fused seven membered ring system (ring A) and two aromatic rings (ring B and ring C) linked through amide bonds. Recently, we reported some amide and sulfamide derivatives of desloratadine, which are potent AVP V2 receptor antagonists [31]. Desloratadine is a selective, H1-receptor antagonist, and also has anti-inflammatory activity [32]. In the previous study, ring A and ring B of classic V2 receptor antagonists were replaced by desloratadine (Figure 1). In a continuous study, we synthesized several compounds centered on a desloratadine scaffold as ring A (compounds 1a, 1b, 1h and 1i, see Table 1) and found that they exhibited potent diuretic activity [33]. Therefore, additional compounds with a desloratadine scaffold as ring A were designed, synthesized and evaluated. Herein, we report the synthesis and biological evaluation of this series of substituted desloratadine designed as potent AVP V2 receptor agonists.
Figure 1.
Chemical structures of tolvaptan, lixivaptan and desloratadine (black: previous study; red: reported here in this article).
Table 1.
The structures of the target compounds and their biological activity evaluation.
| Compound | Structures of Target Compound | Bingding Assy (IC50; nmol/mL) | cAMP Assay (V2, IC50; nmol/mL) | Volume of Urine (mL, 0–20 h) | ||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | Position Of -NH2 | R | V2 | V1a | |||
| 1a | CO | CO | p-NH2 | -CH2CH3 | 6.3 | 130 | 26 | 27.5±5.7 |
| 1b | CO | CO | p-NH2 | -CH2CH2CH3 | 47 | >1000 | ||
| 1c | CO | CO | p-NH2 | -CH2CH2CH2Cl | 25 | >1000 | ||
| 1d | CO | SO2 | p-NH2 | -CH2CH3 | 11 | 92 | 160 | 21.4±4.1 |
| 1e | SO2 | CO | m-NH2 | -CH2CH2CH3 | 26 | 176 | ||
| 1f | SO2 | CO | p-NH2 | -CH2CH2CH3 | 40 | 480 | ||
| 1g | CO | CO | p-NH2 | H | 19 | 330 | ||
| 1h | CO | CO | p-NH2 | 2-Me | 23 | 210 | ||
| 1i | CO | CO | p-NH2 | 4-Me | 18 | 220 | ||
| 1j | CO | CO | p-NH2 | 3-Me | 15 | 370 | 53 | 19.3 ± 5.5 |
| 1k | CO | CO | p-NH2 | 2-Cl | 27 | 490 | ||
| 1l | CO | CO | p-NH2 | 3-Cl | 16 | 560 | ||
| 1m | CO | CO | p-NH2 | 2-F | 20 | 170 | ||
| 1n | CO | CO | p-NH2 | 3-OMe | 8.5 | 390 | 380 | 19.9 ± 6.7 |
| 1o | CO | CO | p-NH2 | 3-NO2 | 18 | 550 | ||
| 1p | CO | CO | p-NH2 | 4-NO2 | 52 | >1000 | ||
| 1q | CO | SO2 | p-NH2 | 4-Me | 24 | 720 | ||
| 1r | CO | SO2 | p-NH2 | 2-Cl | 11 | 830 | 220 | 16.1 ± 3.2 |
| 1s | CO | SO2 | p-NH2 | 2,5-DiCl | 9.2 | 320 | 37 | 18.3 ± 4.3 |
| 1t | SO2 | CO | m-NH2 | H | 7.7 | >1000 | 98 | 28.1 ± 5.0 |
| 1u | SO2 | CO | m-NH2 | 3-Cl | 19 | 840 | ||
| 1v | SO2 | CO | m-NH2 | 3- NO2 | 5.5 | 630 | 110 | 11.9 ± 2.7 |
| 1w | SO2 | SO2 | m-NH2 | 4-Me | 30 | 860 | ||
| 1x | SO2 | CO | p-NH2 | 3-Cl | 310 | >1000 | ||
| 1y | SO2 | SO2 | p-NH2 | 4-Me | 170 | 920 | ||
| Control | 6.5 ± 0.5 | |||||||
| tolvaptan | 28.0 ± 6.5 | |||||||
2. Results and Discussion
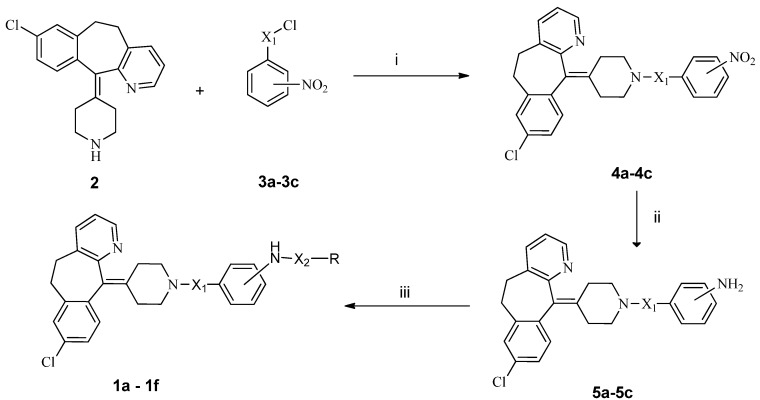
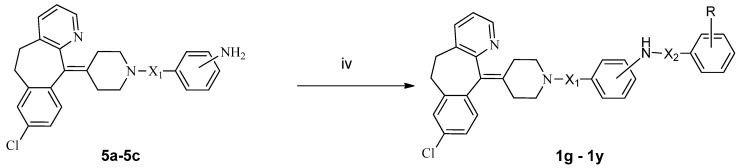
The synthetic routes used in this study are illustrated in Scheme 1 and Scheme 2, respectively. As shown in Scheme 1, the acylation of 2 with a p-nitrobenzoyl chloride, p-nitrobenzene sulfonyl chloride or m-nitrobenzene sulfonyl chloride provided 4a–c, which were subsequently reduced with SnCl2 to provide the corresponding anilines 5a–c in satisfied yields. Acylation of 5a–c with alkyl chloride or alkylsulfonyl chloride yielded the target compounds 1a–f. Similarly, as shown in Scheme 2, acylation of 5a–c with substituted benzoyl chlorides or benzenesulfonyl chlorides gave target product 1g–y.
Scheme 1.
Synthetic route to 1a–f.
Reagents and Conditions: i: DCM, TEA, 0 °C. ii: SnCl2 and concentrated hydrochloric acid in refluxing ethanol; iii: Alkyl chloride or alkylsulfonyl chloride; DCM, TEA, 0 °C.
Scheme 2.
Synthetic route to 1g–y.
Reagents and Conditions: iv. Substituted benzoyl chloride or benzenesulfonyl chloride, DCM, TEA, 0 °C.
Twenty-one compounds 1c–g, 1j–y were synthesized and characterized by 1H-NMR and HRMS. In order to provide a comprehensive understanding of the structure-activity relationships, compounds 1a, 1b, 1h and 1i were included in this research as well. The structures of the target compounds 1a–y and evaluation of the biological features were summarized in Table 1. The binding affinity was determined by a radioligand binding assay and cAMP assay on V1a and V2 over-expressing cells. These compounds had specific affinity to human AVP receptors. Furthermore, they showed high selectivity to V2 receptors. When ring C was replaced by an alkyl group with straight chain, their binding constants to V2 receptor were reduced significantly along the length increase of the carbon chain. Halogen-substituted alkyl group slightly increased their binding affinity to V2 receptors. When ring C was a substituted benzene ring, the different substituted positions of methyl or halogen did not significantly affect the binding affinity to V2 receptors or V1a/V2 selectivity of the compounds.
Moreover, meta-substituted compounds had a relatively higher binding affinity to V2 receptors than ortho-substituted or para-substituted compounds. Compounds 1x and 1y showed poor binding activity to both V1a and V2 receptors. Compounds 1a, 1n, 1t and 1v presented encouraging binding affinity, with both remarkable binding affinity and selectivity for the V2 receptor.
Several compounds with satisfactory binding affinity were selected to conduct the in vivo diuretic assay, with 1a as the reference compound [33]. As shown in Table 1, it is evident that the selected compounds have significant diuretic activity, as they strongly increased urine volume compared with the control group. Compound 1t exhibited an excellent diuretic activity which was equivalent to tolvaptan. It is very difficult to declare the relationship between diuretic activity and binding affinity. Because of specific differences in the vasopressin receptors, it may be difficult to draw a direct comparison between the diuretic assay in rats and the binding assay in cells expressing the human receptor.
3. Experimental
3.1. General Information
Desloratadine was purchased from Beijing Datian Fengtuo Chemistry Co., Ltd (Beijing, China). Other reagents and solvents were obtained from commercial suppliers. The human recombinant vasopressin V1a (Cat. ES-361-C) and V2 (ES-363-C) receptors in 1321N1 host cell were obtained from Perkin Elmer Inc (Waltham, MA, USA). The Sprague-Dawley rats were purchased from Tianjin Shanchuanhong Experimental Animals Co., Ltd (Tianjin, China). All reactions were monitored by thin layer chromatography. Silica gel chromatography was conducted on a Teledyne Isco COMBIFLASH Rf200 Purification System (Teledyne Isco, Inc., Lincoln, NE, USA) (petroleum ether and ethyl acetate, gradient elution). HPLC data was obtained with an Agilent 1260 (Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a Grace C18 column (5 μm, 250 mm × 4.6 mm, Lot No. 55/182). 1H-NMR spectra were recorded on a Bruker AV400 NMR (Bruker, Billerica, MA, USA) and HRMS were measured on a VG ZAB-HS instrument (VG Instruments, London, UK). Melting points (uncorrected) were determined on a YRT-3 Melting Point Tester (Precision Instrument of Tianjin University, Tianjin, China).
3.2. Synthesis
(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)(4-nitrophenyl) methanone (4a) [33]. To a solution of desloratadine (100 g, 322 mmol) in CH2Cl2 (500 mL), Et3N (48 g, 480 mmol) was added and the mixture was stirred at 0 °C for 10 min. Then 2a (59.8 g, 322 mmol) dissolved in CH2Cl2(200 mL) was added dropwise into the mixture and stirring was continued for another 2 h. The reaction misxture was washed successively with 1 mol/L hydrochloric acid and water. The organic layer was dried over anhydrous magnesium sulfate and evaporated to give the crude product as a yellow powder, which was recrystallized from ethanol affording 4a as a white powder. Yield: 95%; m.p.: 188.2–189.0 °C; 1H-NMR (400 MHz, DMSO-d6): δ 2.18–2.32 (m, 2H), 2.41 (br s, 1H), 2.79–2.84 (m, 2H), 3.17–3.36 (m, 6H), 3.97 (br s, 1H), 7.05–7.32 (m, 4H), 7.54–7.57 (m, 1H), 7.68 (d, J = 8.4 Hz, 2H), 8.25–8.36 (m, 3H).
8-Chloro-11-(1-((3-nitrophenyl)sulfonyl)piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta-[1,2-b]pyridine (4b). Compound 4b was prepared using a similar method as for 4a. Yield: 97%, m.p.: 221.7–222.8 °C; 1H-NMR (CDCl3): δ 2.40–2.41 (m, 2H), 2.50–2.57 (m, 1H), 2.62–2.68 (m, 1H), 2.71–2.86 (m, 2H), 3.09–3.15 (m, 2H), 3.22–3.36 (m, 4H), 7.01 (d, J = 8.0 Hz, 2H), 7.11–7.15 (m, 3H), 7.45 (d, J = 7.6 Hz, 1H), 7.76 (t, J = 8.0 Hz, 1H), 8.07–8.10 (m, 1H), 8.37 (d, J = 6.4 Hz, 1H), 8.44–8.59 (m, 1H), 8.59 (d, J = 2.0 Hz, 1H).
8-Chloro-11-(1-((4-nitrophenyl)sulfonyl)piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta-[1,2-b]pyridine (4c). Compound 4c was prepared using a similar method as for 4a. Yield: 88%, m.p.: >230 °C; 1H-NMR (CDCl3): δ 2.34–2.39 (m, 2H), 2.46–2.49 (m, 1H), 2.60–2.62 (m, 1H), 2.72–2.81 (m, 2H), 2.99–3.03 (m, 2H), 3.20–3.33 (m, 4H), 6.98 (d, J = 8.4 Hz, 1H), 7.05–7.13 (m, 3H), 7.39 (d, J = 7.6 Hz, 1H), 7.91–7.94 (m, 2H), 8.33–8.37 (m, 3H).
(4-Aminophenyl)(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)-methanone (5a) [33]. To a mixture of 4a (100 g, 217 mmol) dissolved in ethanol (600 mL) and concentrated hydrochloric acid (150 mL), SnCl2 (171 g, 760 mmol) dissolved in ethanol (400 mL) was added. After the addition was completed, the mixture was heated to reflux and stirred for 4–6 h. Then the mixture was poured into ice water and extracted with dichloromethane. The organic layer was collected and concentrated under reduced pressure to give the crude product as a brown powder. Compound 5a was obtained by recrystallization from ethyl acetate. Yield: 82%, 1H-NMR (CDCl3): δ 2.38–2.56 (m, 4H), 2.75–2.87 (m, 2H), 3.20–3.42 (m, 4H), 3.82 (s, 2H), 3.94 (br s, 2H), 6.59–6.62 (m, 2H), 7.06–7.15 (m, 4H), 7.22–7.26 (m, 2H), 7.40–7.43 (dd, J1 = 1.2 Hz, J2 = 7.6 Hz, 1H), 8.37 (d, J = 3.6 Hz, 1H).
3-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) aniline (5b). Compound 5b was prepared using a similar method as used for 5a. Yield: 78%, m.p.: 227.9–228.8 °C; 1H-NMR (CDCl3): δ 2.34–2.37 (m, 2H), 2.44–2.50 (m, 1H), 2.56–2.63 (m, 1H), 2.68–2.81 (m, 2H), 2.88–2.94 (m, 2H), 3.20–3.39 (m, 4H), 3.89 (s, 1H), 6.83 (dd, J1 = 2.0 Hz, J2 = 8.0 Hz, 1H), 6.99–7.01 (m, 2H), 7.04–7.12 (m, 4H), 7.23–7.27 (m, 1H), 7.38 (dd, J1 = 1.2 Hz, J2 = 7.6 Hz, 1H), 8.34 (dd, J1 = 2.0 Hz, J2 = 4.8 Hz, 1H).
4-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) aniline (5c). Compound 5c was prepared using a similar method as used for 5a. Yield: 88%, m.p.: 110.1–111.6 °C; 1H-NMR (CDCl3): δ 2.30–2.36 (m, 2H), 2.43–2.46 (m, 1H), 2.56–2.60 (m, 1H), 2.70–2.90 (m, 4H), 3.17–3.30 (m, 4H), 6.65–6.67 (m, 2H), 6.99–7.11 (m, 4H), 7.38 (dd, J1 = 1.6 Hz, J2 = 8.0 Hz, 1H), 7.50 (dd, J1 = 1.8 Hz, J2 = 6.6 Hz, 2H), 8.35 (dd, J1 = 1.6 Hz, J2 = 4.8 Hz, 1H).
4-Chloro-N-(4-(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl)phenyl)butanamide (1c). The syntheses of 1a, 1b, 1h and 1i have been reported in our previous study [33]. Other target compounds were prepared using similar methods. Taking 1c as an example, to a solution of 5a (5.0 g, 12 mmol) in CH2Cl2 (40 mL), Et3N (1.8 g, 18 mmol)was added and the resulting mixture was then stirred at 0 °C for 10 min. Then 4-chlorobutanoyl chloride (1.7 g, 12 mmol) dissolved in CH2Cl2 (20 mL) was added dropwise into the mixture that was stirred for another 3 h. The reaction solution was washed successively with 1 mol/L hydrochloric acid and water. The organic layer was dried over anhydrous magnesium sulfate and concentrated to give the crude product as a yellow solid that was purified by silica gel chromatography to give compound 1c. Yield: 85%, m.p.: 204.8–207.6 °C; 1H-NMR (CDCl3): δ 2.15–2.20 (m, 2H), 2.29–2.64 (m, 6H), 2.77–2.90 (m, 2H), 3.25–3.44 (m, 4H), 3.61–3.69 (m, 3H), 4.15 (br s, 1H), 7.10–7.17 (m, 4H), 7.33 (d, J = 8.4 Hz, 2H), 7.43–7.49 (m, 3H), 7.95 (s, 1H), 8.39 (d, J = 2.8 Hz, 1H); HRMS (ESI): calcd for C30H29Cl2N3O2 [M + H]+ m/z: 534.1710, found: 534.1705.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)ethanesulfonamide (1d). Yield: 44%, m.p.: 210.0–211.1 °C; 1H-NMR (CDCl3): δ 2.32–2.46 (m, 4H), 2.75–2.89 (m, 2H), 3.21–3.40 (m, 4H), 3.57 (br s, 1H), 4.13 (br s, 1H), 6.11–6.14 (m, 2H), 6.24–6.28 (d, J = 16.8 Hz, 2H), 6.98–7.16 (m, 5H), 7.24–7.28 (m, 2H), 7.41–7.45 (m, 3H), 8.37 (s, 1H); HRMS (ESI): calcd for C28H28ClN3O3S [M + H]+ m/z: 522.1613, found: 522.1609.
N-(3-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)butyramide (1e). Yield: 50%, m.p.: 174.1–174.9 °C; 1H-NMR (CDCl3): δ 1.00 (t, J = 7.4 Hz, 3H), 1.75 (d, J = 7.6 Hz, 2H), 2.32–2.37 (m, 4H), 2.44–2.50 (m, 1H), 2.57–2.62 (m, 1H), 2.70–2.81 (m, 2H), 2.90 (t, J = 3.8 Hz, 2H), 3.22–3.31 (m, 4H), 7.00 (d, J = 8.0 Hz, 1H), 7.08–7.12 (m, 3H), 7.40–7.47 (m, 3h), 7.78 (s, 1H), 7.92 (d, J = 4.0 Hz, 1H), 8.35 (dd, J1 = 1.2 Hz, J2 = 4.8 Hz, 1H); HRMS (ESI): calcd for C29H30ClN3O3S [M + H]+ m/z: 536.1769, found: 536.1770.
N-(4-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)butyramide (1f). Yield: 60%, mp: 210.0–211.1 °C; 1H-NMR (CDCl3): δ 1.01 (t, J = 7.4 Hz, 3H), 1.77 (q, J = 7.5 Hz, 1H), 2.31–2.39 (m, 4H), 2.44–2.47 (m, 1H), 2.57–2.59 (m, 1H), 2.70–2.89 (m, 4H), 3.23–3.30 (m, 4H), 7.00 (d, J = 8.0 Hz, 1H), 7.08–7.12 (m, 3H), 7.33 (s, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.67 (s, 4H), 8.35 (dd, J1 = 1.6 Hz, J2 = 4.8 Hz, 1H); HRMS (ESI): calcd for C29H30ClN3O3S [M + H]+ m/z: 536.1769, found: 536.1771.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)benzamide (1g). Yield: 85%, m.p.: 128.5–131.6 °C; 1H-NMR (CDCl3): δ 2.41–2.53 (m, 4H), 2.78–2.88 (m, 2H), 3.31–3.42 (m, 4H), 3.68 (s, 1H), 4.14 (s, 1H), 7.12–7.17 (m, 4H), 7.36 (d, J = 8.8Hz, 2H), 7.43–7.55 (m, 4H), 7.64 (d, J = 8.8 Hz, 2H), 7.88 (dd, J1 = 1.2 Hz, J2 = 7.2 Hz, 2H), 8.34 (s, 1H), 8.40 (s, 1H); HRMS (ESI): calcd for C33H28ClN3O2 [M + H]+ m/z: 534.1943, found: 534.1939.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-3-methylbenzamide (1j). Yield: 79%; m.p.: 154.9–156.0 °C; 1H-NMR (CDCl3): δ 2.40–2.56 (m, 7H), 2.76–2.89 (m, 2H), 3.28–3.43 (m, 4H), 3.68–3.71 (m, 1H), 4.14 (s, 1H), 7.09–7.17 (m, 4H), 7.31–7.45 (m, 5H), 7.63–7.70 (m, 4H), 8.28 (s, 1H), 8.34 (s, 1H); HRMS (ESI): calcd for C34H30ClN3O2 [M + H]+ m/z: 548.2099, found: 548.2108.
2-Chloro-N-(4-(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl)phenyl)benzamide (1k). Yield: 96%, mp: 120.4–123.1 °C; 1H-NMR (CDCl3): δ 2.42–2.58 (m, 4H), 2.79–2.89 (m, 2H), 3.29–3.43 (m, 4H), 3.70 (br s, 1H), 4.14 (br s, 1H), 7.10–7.17 (m, 4H), 7.35–7.46 (m, 7H), 7.65–7.75 (m, 3H), 8.14 (s, 1H), 8.41 (s, 1H); HRMS (ESI): calcd for C33H27Cl2N3O2 [M + H]+ m/z: 568.1553, found: 568.1547.
3-Chloro-N-(4-(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl)phenyl)benzamide (1l). Yield: 70%, m.p.: 241.8–243.7 °C; 1H-NMR (CDCl3): δ 2.43–2.54 (m, 4H), 2.77–2.90 (m, 2H), 3.31–3.44 (m, 4H), 3.69–3.73 (m, 1H), 4.11–4.14 (m, 1H), 7.01–7.18 (m, 4H), 7.39–7.58 (m, 7H), 7.79 (d, J = 8.0 Hz, 1H), 7.92 (t, J = 2.0 Hz, 1H), 8.39 (s, 1H); HRMS (ESI): calcd for C33H27Cl2N3O2 [M + H]+ m/z: 568.1553, found: 568.1556.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-2-fluorobenzamide (1m). Yield: 94%, m.p.: 166.9–167.8 °C; 1H-NMR (CDCl3): δ 2.41–2.56 (m, 4H), 2.77–2.90 (m, 2H), 3.32–3.44 (m, 4H), 3.71 (br s, 1H), 4.16 (br s, 1H), 7.09–7.23 (m, 5H), 7.30–7.32 (m, 1H), 7.44–7.46 (m, 3H), 7.51–7.57 (m, 1H), 7.70 (d, J = 8.4 Hz, 2H), 8.14–8.19 (m, 1H), 8.40 (s, 1H), 8.54 (d, J = 15.6 Hz, 1H); HRMS (ESI): calcd for C33H27ClFN3O2 [M + H]+ m/z: 552.1849, found: 552.1841.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-3-methoxybenzamide (1n). Yield: 52%, m.p.: 148.3–151.0 °C; 1H-NMR (CDCl3): δ 2.39–2.59 (m, 4H), 2.77–2.89 (m, 2H), 3.28–3.43 (m, 4H), 3.71 (br s,1H), 3.84 (s, 3H), 4.17 (br s, 1H), 7.05–7.20 (m, 5H), 7.33–7.45 (m, 6H), 7.63 (d, J = 8.8 Hz, 2H), 8.31 (s, 1H), 8.39 (s, 1H); HRMS (ESI): calcd for C34H30ClN3O3 [M + H]+ m/z: 564.2048, found: 564.2049.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-3-nitrobenzamide (1o). Yield: 53%, m.p.: >250 °C; 1H-NMR (CDCl3): δ 2.32–2.45 (m, 4H), 2.75–2.89 (m, 2H), 3.25–3.42 (m, 4H), 3.62 (br s, 1H), 4.16 (br s, 1H), 7.09–7.24 (m, 6H), 7.43 (d, J = 8.4 Hz, 3H), 7.61–7.65 (m, 1H), 8.32–8.41 (m, 3H), 8.86 (t, J = 2.0 Hz,1H), 9.37 (s, 1H); HRMS (ESI): calcd for C33H27ClN4O4 [M + H]+ m/z: 579.1794, found: 579.1786.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-4-nitrobenzamide (1p). Yield: 68%, m.p.: >250 °C; 1H-NMR (CDCl3): δ 2.27–2.56 (m, 4H), 2.79–2.91 (m, 2H), 3.32–3.43 (m, 4H), 3.66 (br s, 1H), 4.16 (br s, 1H), 7.12–7.18 (m, 4H), 7.37–7.46 (m, 3H), 7.60 (d, J = 8.0 Hz, 2H), 8.10 (d, J = 8.4 Hz, 2H), 8.32–8.37 (m, 4H); HRMS (ESI): calcd for C33H27ClN4O4 [M + H]+ m/z: 579.1794, found: 579.1792.
N-(4-(4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl) phenyl)-4-methylbenzenesulfonamide (1q). Yield: 92%, m.p.: 161.5–162.9 °C; 1H-NMR (CDCl3): δ 2.33–2.50 (m, 7H), 2.78–2.86 (m, 2H), 3.28–3.39 (m, 4H), 3.58 (br s, 1H), 4.13 (br s, 1H), 7.04–7.25 (m, 10 H), 7.42 (d, J = 6.4 Hz, 1H), 7.59–7.65 (m, 3H), 8.36 (s, 1H); HRMS (ESI): calcd for C33H30ClN3O3S [M + H]+ m/z: 584.1769, found: 584.1777.
2-Chloro-N-(4-(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl)phenyl)benzenesulfonamide (1r). Yield: 80%, m.p.: 153.9–155.5 °C; 1H-NMR (CDCl3): δ 2.39–2.46 (m, 4H), 2.73–2.87 (m, 2H), 3.17–3.39 (m, 4H), 3.57 (br s, 1H), 4.09 (br s, 1H), 7.06–7.14 (m, 6H), 7.23–7.34 (m, 3H), 7.40–7.45 (m, 3H), 7.630 (s, 1H), 8.01 (d, J =8.4 Hz, 1H), 8.36 (s, 1H); HRMS (ESI): calcd for C32H27Cl2N3O3S [M + H]+ m/z: 604.1223, found: 604.1224.
2,5-Dichloro-N-(4-(4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidine-1-carbonyl)phenyl)benzenesulfonamide (1s). Yield: 96%, m.p.: 147.8–149.5 °C; 1H-NMR (CDCl3): δ 2.15–2.40 (m, 4H), 2.74–2.87 (m, 2H), 3.20–3.52 (m, 5H), 4.11 (br s, 1H), 7.08–7.11 (m, 6H), 7.24–7.28 (m, 2H), 7.34–7.43 (m, 3H), 7.51–7.53 (m, 1H), 7.98 (d, J =2.0 Hz, 1H), 8.38 (s, 1H); HRMS (ESI): calcd for C32H26Cl3N3O3S [M + H]+ m/z: 640.0833, found: 640.0824.
N-(3-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)benzamide (1t). Yield: 78%, m.p.: 119.8–122.4 °C; 1H-NMR (CDCl3): δ 2.34–2.37 (m, 2H), 2.44–2.50 (m, 1H), 2.56–2.63 (m, 1H), 2.68–2.81 (m, 2H), 2.88–2.94 (m, 2H), 3.20–3.39 (m, 4H), 3.89 (s, 1H), 6.83 (dd, J1 = 2.0 Hz, J2 = 8.0 Hz, 1H), 6.99–7.01 (m, 2H), 7.04–7.12 (m, 4H), 7.23–7.27 (m, 1H), 7.38 (dd, J1 = 1.2 Hz, J2 = 7.6 Hz, 1H), 8.34 (dd, J1 = 2.0 Hz, J2 = 4.8 Hz, 1H); HRMS (ESI): calcd for C32H28ClN3O3S [M + H]+ m/z: 570.1613, found: 570.1612.
3-Chloro-N-(3-((4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)-sulfonyl)phenyl)benzamide (1u). Yield: 70%, m.p.: 131.2–133.5 °C; 1H-NMR (CDCl3): δ 2.31–2.36 (m, 2H), 2.42–2.49 (m, 1H), 2.55–2.62 (m, 1H), 2.67–2.81 (m, 2H), 2.85–2.91 (m, 2H), 3.19–3.38 (m, 4H), 6.98 (d, J = 8.0 Hz, 1H), 7.04–7.11 (m, 3H), 7.37–7.54 (m, 5H), 7.77 (d, J = 7.6 Hz, 1H), 7.90 (dd, J1 = 1.6 Hz, J2 = 10.0 Hz, 1H), 8.10 (dd, J1 = 2.0 Hz, J2 = 7.2 Hz, 1H), 8.19 (s, 1H), 8.33 (d, J = 3.6 Hz, 1H); HRMS (ESI): calcd for C32H27Cl2N3O3S [M + H]+ m/z: 604.1223, found: 604.1218.
N-(3-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)-3-nitrobenzamide (1v). Yield: 87%, m.p.: 206.1–208.2 °C; 1H-NMR (CDCl3): δ 2.34–2.43 (m, 2H), 2.56–2.62 (m, 1H), 2.80–2.84 (m, 2H), 2.94–3.07 (m, 2H), 3.29–3.53 (m, 4H), 4.19 (s, 1H), 7.16–7.23 (m, 3H), 7.54–7.57 (m, 2H), 7.66–7.70 (m, 2H), 8.07 (d, J = 7.6 Hz, 1H), 8.20 (s, 1H), 8.35 (dd, J1 = 2.0 Hz, J2 = 8.0 Hz, 1H), 8.51–8.65 (m, 3H), 9.17 (s, 1H); HRMS (ESI): calcd for C32H27ClN4O5S [M + H]+ m/z: 615.1464, found: 615.1461.
N-(3-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)-4-methylbenzenesulfonamide (1w). Yield: 98.4%; m.p.: 192.7–194.9 °C; 1H-NMR (CDCl3): δ 2.26–2.28 (m, 2H), 2.31 (s, 3H), 2.50–2.54 (m, 1H), 2.77–2.83 (m, 2H), 2.94–3.03 (m, 2H), 3.10 (s, 1H), 3.22–3.25 (m, 1H), 3.32–3.36 (m, 1H), 3.46–3.50 (m, 1H), 3.88 (s, 1H), 7.14–7.22 (m, 4H), 7.33–7.43 (m, 2H), 7.55 (d, J = 8.0 Hz, 1H), 7.61 (s, 1H), 7.66 (t, J = 6.4 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 8.04 (d, J = 8.0 Hz, 1H), 8.61 (d, J = 4.8 Hz, 1H), 9.14 (s, 1H); HRMS (ESI): calcd for C32H30ClN3O4S2 [M + H]+ m/z: 620.1439, found: 620.1439.
3-Chloro-N-(4-((4-(8-chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)-sulfonyl)phenyl)benzamide (1x). Yield: 96%; m.p.: 130.5–133.0 °C; 1H-NMR (CDCl3): δ 2.32–2.38 (m, 2H), 2.45–2.47 (m, 1H), 2.58–2.59 (m, 1H), 2.71–2.89 (m, 4H), 3.22–3.33 (m, 4H), 6.99 (d, J = 8.4 Hz, 1H), 7.06–7.11 (m, 3H), 7.39–7.45 (m, 2H), 7.52–7.55 (m, 1H), 7.70–7.87 (m, 6H), 8.13 (s, 1H), 8.34 (dd, J1 = 1.6 Hz, J2 = 4.8 Hz, 1H); HRMS (ESI): calcd for C32H27Cl2N3O3S [M + H]+ m/z: 604.1223, found: 604.1219.
N-(4-((4-(8-Chloro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6H)-ylidene)piperidin-1-yl)sulfonyl) phenyl)-4-methylbenzenesulfonamide (1y). Yield: 78%; m.p.: 126.1–129.1 °C; 1H-NMR (CDCl3): δ 2.29–2.35 (m, 2H), 2.38 (s, 3H), 2.43–2.46 (m, 1H), 2.57–2.59 (m, 1H), 2.70–2.82 (m, 2H), 2.89–2.93 (m, 2H), 3.14–3.30 (m, 4H), 6.98 (d, J = 8.0 Hz, 1H), 7.07–7.12 (m, 3H), 7.16–7.19 (m, 2H), 7.26–7.30 (m, 3H), 7.42 (d, J = 6.4 Hz, 1H), 7.57–7.60 (m, 2H), 7.73 (d, J = 8.4 Hz, 2H), 8.35 (d, J = 4.0 Hz, 1H); HRMS (ESI): calcd for C32H30ClN3O4S2 [M + H]+ m/z: 620.1439, found: 620.1437.
3.3. Biological Evaluation
The in vitro evaluation was done by a slightly modified method we reported previously [31]. An in vitro radioligand binding assay was performed to determine the binding affinity of the candidates to human V2 and V1a receptors. The functional activity was then subsequently determined by measuring the activation or inhibition of vasopressin induced cAMP accumulation in V2 receptor expressing cells. We investigated some potent derivatives for in vivo diuretic activity in conscious hydrated male Sprague-Dawley rats at 8 weeks of age (body weight: (260 ± 20) g). Urine volume was measured 20 h after oral administration of the test compounds.
4. Conclusions
Twenty-one derivatives of desloratadine designed as AVP V2 receptor antagonists were synthesized and characterized by 1H-NMR, HRMS and HPLC. Their biological activity was evaluated by in vitro radioligand binding assay, cAMP assay and in vivo diuretic assay. Compounds 1n, 1t and 1v exhibited both high affinity and promising selectivity for V2 receptors. The selected compounds showed promising diuretic results in rats, especially compound 1t, which produced a total urine volume equivalent to tolvaptan during the experimental period. Through the present studies, compound 1t, which has good efficacy both in vitro and in vivo, could be a novel AVP V2 receptor antagonist candidate. Further preclinical studies are however still required.
Acknowledgments
This project was supported by the National Basic Research Program of China (973 Program, granted Nos. 2010CB735602 and 2012CB724002) and National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No. 2013ZX09102014).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1a–y are available from the authors.
References
- 1.Bowman B.T., Rosner M.H. Lixivaptan-an evidence-based review of its clinical potential in the treatment of hyponatremia. Core Evid. 2013;8:47–56. doi: 10.2147/CE.S36744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiatkowska A., Lewandowska M., Borovickova L., Slaninova J., Lammek B., Prahl A. Design, synthesis and structure-activity relationship of new arginine vasopressin analogues containing proline derivatives in position 2. Chem. Biol. Drug Des. 2013;81:420–428. doi: 10.1111/cbdd.12093. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowska A., Sobolewski D., Prahl A., Borovickova L., Slaninova J., Lammek B. Arginine vasopressin and its analogues-the influence of position 2 modification with 3,3-diphenylalanine enantiomers. Highly potent V-2 agonists. Eur. J. Med. Chem. 2009;44:2862–2867. doi: 10.1016/j.ejmech.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Wong L.L., Verbalis J.G. Vasopressin V2 receptor antagonists. Cardiovasc. Res. 2001;51:391–402. doi: 10.1016/S0008-6363(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 5.Ohtake Y., Naito A., Hasegawa H., Kawano K., Morizono D., Taniguchi M., Tanaka Y., Matsukawa H., Naito K., Oguma T., et al. Novel vasopressin V2 receptor-selective antagonists, pyrrolo[2,1-a]quinoxaline and pyrrolo[2,1-c][1,4]benzodiazepine derivatives. Bioorg. Med. Chem. 1999;7:1247–1254. doi: 10.1016/S0968-0896(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 6.Ghali J.K., Orlandi C., Abraham W.T., Investigators C.-L.S. The efficacy and safety of lixivaptan in outpatients with heart failure and volume overload: Results of a multicentre, randomized, double-blind, placebo-controlled, parallel-group study. Eur. J. Heart Fail. 2012;14:642–651. doi: 10.1093/eurjhf/hfs051. [DOI] [PubMed] [Google Scholar]
- 7.Pang P.S., Gheorghiade M., Dihu J., Swedberg K., Khan S., Maggioni A.P., Grinfeld L., Zannad F., Burnett J.C., Ouyang J., et al. Effects of tolvaptan on physician-assessed symptoms and signs in patients hospitalized with acute heart failure syndromes: Analysis from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) trials. Am. Heart J. 2011;161:1067–1072. doi: 10.1016/j.ahj.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M., Pang P.S., O’Connor C.M., Prasad K., McMurray J., Teerlink J.R., Fiuzat M., Sabbah H., Komajda M. Clinical development of pharmacologic agents for acute heart failure syndromes: A proposal for a mechanistic translational phase. Am. Heart J. 2011;161:224–232. doi: 10.1016/j.ahj.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Veeraveedu P.T., Palaniyandi S.S., Yamaguchi K., Komai Y., Thandavarayan R.A., Sukumaran V., Watanabe K. Arginine vasopressin receptor antagonists (vaptans): Pharmacological tools and potential therapeutic agents. Drug Discov. Today. 2010;15:826–841. doi: 10.1016/j.drudis.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Matsuhisa A., Taniguchi N., Koshio H., Yatsu T., Tanaka A. Nonpeptide arginine vasopressin antagonists for both V1A and V2 Receptors: Synthesis and pharmacological properties of 4'-(1,4,5,6-tetrahydroimidazo[4,5-d][1]benzoazepine-6-carbonyl)benzanilide derivatives and 4'-(5,6-dihydro-4h-thiazolo[5,4-d][1]benzoazepine-6-carbonyl)benzanilide derivatives. Chem. Pharm. Bull. 2000;48:21–31. doi: 10.1248/cpb.48.21. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka F., Ogura T., Yamauchi T., Oishi T., Hashimoto M., Mimura Y., Makino H. Effects of OPC-216268, a vasopressin V1-receptor antagonist, on expression of growth factors from glomeruli in spontaneously hypertensive rats. Regul. Pept. 1997;72:87–95. doi: 10.1016/S0167-0115(97)01041-0. [DOI] [PubMed] [Google Scholar]
- 12.Masuda S., Hattori A., Matsumoto H., Miyazawa S., Natori Y., Mizutani S., Tsujimoto M. Involvement of the V-2 receptor in vasopressin-stimulated translocation of placental leucine aminopeptidase/oxytocinase in renal cells. Eur. J. Biochem. 2003;270:1988–1994. doi: 10.1046/j.1432-1033.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Blair J.E.A., Pang P.S., Schrier R.W., Metra M., Traver B., Cook T., Campia U., Ambrosy A., Burnett J.C., Grinfeld L., et al. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur. Heart J. 2011;32:2563–2572. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 14.Sakaida I., Yamashita S., Kobayashi T., Komatsu M., Sakai T., Komorizono Y., Okada M., Okita K., Grp A.D.A.S. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J. Int. Med. Res. 2013;41:835–847. doi: 10.1177/0300060513480089. [DOI] [PubMed] [Google Scholar]
- 15.Walcott B.P., Kahle K.T., Simard J.M. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9:65–72. doi: 10.1007/s13311-011-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.-Y., Huo T.-I., Wang S.-S., Huang H.-C., Lee F.-Y., Lin H.-C., Chuang C.-L., Lee S.-D. Diabetes diminishes the portal-systemic collateral vascular response to vasopressin via vasopressin receptor and g(alpha) proteins regulations in cirrhotic rats. PLoS One. 2013;8:e67703. doi: 10.1371/journal.pone.0067703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyader D., Patat A., Ellis-Grosse E.J., Orczyk G.P. Pharmacodynamic effects of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology. 2002;36:1197–1205. doi: 10.1053/jhep.2002.36375. [DOI] [PubMed] [Google Scholar]
- 18.Kazory A. Hyponatremia in heart failure: Revisiting pathophysiology and therapeutic strategies. Clin. Cardiol. 2010;33:322–329. doi: 10.1002/clc.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasta J.F., Chiong J.R., Christian R., Friend K., Lingohr-Smith M., Lin J., Cassidy I.B. Update on tolvaptan for the treatment of hyponatremia. Expert Rev. Pharmacoecon. Outcomes Res. 2012;12:399–410. doi: 10.1586/erp.12.30. [DOI] [PubMed] [Google Scholar]
- 20.Bettari L., Fiuzat M., Felker G.M., O’Connor C.M. Significance of hyponatremia in heart failure. Heart Fail. Rev. 2012;17:17–26. doi: 10.1007/s10741-010-9193-3. [DOI] [PubMed] [Google Scholar]
- 21.Aronson D., Verbalis J.G., Mueller M., Krum H., Investigators D. Short- and long-term treatment of dilutional hyponatraemia with satavaptan, a selective arginine vasopressin V-2-receptor antagonist: The DILIPO study. Eur. J. Heart Fail. 2011;13:327–336. doi: 10.1093/eurjhf/hfq226. [DOI] [PubMed] [Google Scholar]
- 22.Gross P. Treatment of hyponatremia. Intern. Med. 2008;47:885–891. doi: 10.2169/internalmedicine.47.0918. [DOI] [PubMed] [Google Scholar]
- 23.Artom N., Oddo S., Pende A., Ottonello L., Giusti M., Dallegri F. Syndrome of inappropriate antidiuretic hormone secretion and Ibuprofen, a rare association to be considered: Role of tolvaptan. Case Rep. Endocrinol. 2013;2013:818259–818259. doi: 10.1155/2013/818259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotas G., Kimbaris A., Varvounis G. Synthesis of a novel pyrrolo[1,2-c][1.3]benzodiazepine analogue of VPA-985. Tetrahedron. 2011;67:7805–7810. doi: 10.1016/j.tet.2011.07.083. [DOI] [Google Scholar]
- 25.Crombie A.L., Antrilli T.M., Campbell B.A., Crandall D.L., Failli A.A., He Y., Kern J.C., Moore W.J., Nogle L.M., Trybulski E.J. Synthesis and evaluation of azabicyclo 3.2.1 octane derivatives as potent mixed vasopressin antagonists. Bioorg. Med. Chem. Lett. 2010;20:3742–3745. doi: 10.1016/j.bmcl.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 26.Ponthieux S., Cabot J., Mouillac B., Seyer R., Barberis C., Carnazzi E. Design of benzophenone-containing photoactivatable linear vasopressin antagonists: Pharmacological and photoreactive properties. J. Med. Chem. 2005;48:3379–3388. doi: 10.1021/jm040871+. [DOI] [PubMed] [Google Scholar]
- 27.Kakefuda A., Suzuki T., Tobe T., Tsukada J., Tahara A., Sakamoto S., Tsukamoto S. Synthesis and pharmacological evaluation of 5-(4-biphenyl)-3-methyl-4-phenyl-1,2,4-triazole derivatives as a novel class of selective antagonists for the human vasopressin V-1A receptor. J. Med. Chem. 2002;45:2589–2598. doi: 10.1021/jm010544r. [DOI] [PubMed] [Google Scholar]
- 28.Carnazzi E., Aumelas A., Mouillac B., Breton C., Guillou L., Barberis C., Seyer R. Design, synthesis and pharmacological characterization of a potent radioiodinated and photoactivatable peptidic oxytocin antagonist. J. Med. Chem. 2001;44:3022–3030. doi: 10.1021/jm010125u. [DOI] [PubMed] [Google Scholar]
- 29.Kondo K., Ogawa H., Shinohara T., Kurimura M., Tanada Y., Kan K., Yamashita H., Nakamura S., Hirano T., Yamamura Y., et al. Novel design of nonpeptide AVP V-2 receptor agonists: Structural requirements for an agonist having 1-(4-aminobenzoyl)-2,3,4,5-tetrahydro-1H-1-benzazepine as a template. J. Med. Chem. 2000;43:4388–4397. doi: 10.1021/jm000108p. [DOI] [PubMed] [Google Scholar]
- 30.Albright J.D., Reich M.F., Delos Santos E.G., Dusza J.P., Sum F.W., Venkatesan A.M., Coupet J., Chan P.S., Ru X., Mazandarani H., et al. 5-fluoro-2-methyl-N- 4-(5H-pyrrolo 2,1-c 1,4 benzodiazepin-10(11H)-ylca rbonyl)-3-chlorophenyl benzamide (VPA-985): An orally active arginine vasopressin antagonist with selectivity for V-2 receptors. J. Med. Chem. 1998;41:2442–2444. doi: 10.1021/jm980179c. [DOI] [PubMed] [Google Scholar]
- 31.Mu S., Xie X.-S., Niu D., Zhang D.-S., Liu D.-K., Liu C.-X. Synthesis and biological evaluation of novel derivatives of desloratadine. Chin. Chem. Lett. 2013;24:531–534. [Google Scholar]
- 32.Geha R.S., Meltzer E.O. Desloratadine: A new, nonsedating, oral antihistamine. J. Allergy Clin. Immunol. 2001;107:751–762. doi: 10.1067/mai.2001.114239. [DOI] [PubMed] [Google Scholar]
- 33.Xie X.-S., Mu S., Tan C.-B., Zhou Z.-X., Liu D.-K., Xu W.-R. Synthesis and diuretic activity of novel amide derivatives of desloratadine. Chin. J. Synth. Chem. 2014;22 (In Chinese) in press. [Google Scholar]