Abstract
A new compound and seven known compounds were isolated from Murraya tetramera Huang for the first time, and they were identified with NMR and MS spectral analysis. It was confirmed that the new compound was 10-methoxy-7-methyl-2H-benzo[g]chromen-2-one (3) and the others were β-eudesmol (1), trans-3β-(1-hydroxy-1-methylethyl)-8aβ-methyl-5-methylenedecalin-2-one (2), 5,7-dimethoxy-8-[(Z)-3'-methyl-butan-1',3'-dienyl]coumarin (4), 7-geranyloxy-6-methoxycoumarin (5), 5,7-dimethoxy-8-(3-methyl-2-oxo-butyl)coumarin (6), murrangatin acetate (7) and toddalenone (8). Furthermore, the cytotoxic activity against human lung adenocarcinoma (A549), human hepatocellular carcinoma cells (SMMC-7721), human bladder tumor cells (EJ), human cervical carcinoma cells (HeLa), and human B-lineage acute lymphoblastic leukemia 1 cells (BALL-1) was evaluated for all compounds. It was found that five of them displayed various degrees of cytotoxicity against different testing targets. Compound 1 showed significant cytotoxic activity against the five cell lines (A549, SMMC-7721, EJ, Hela and BALL-1). Compounds 2 and 5 showed significant cytotoxicity against three cell lines (A549, SMMC-7721 and BALL-1). Compound 4 showed significant cytotoxicity against three cell lines (A549, EJ and BALL-1). However, compound 3 only showed fair cytotoxicity against the BALL-1 cell line. The structure-active relationships were investigated as well. These active compounds might be potential lead compounds for the treatment of cancer.
Keywords: M. tetramera, cytotoxicity, coumarin, sesquiterpene
1. Introduction
Cancer is one of the most common diseases that threaten peoples’ health. Accordingly, much effort has been invested to develop effective treatments. Chemotherapy and radiotherapy have been the primary approaches for conventional cancer treatment, but they are not always effective [1,2,3]. Traditional Chinese medicines (TCMs) are generally economical and plentiful, while showing very low toxicity or side effects in clinical practice. Hence, TCMs have been applied worldwide for the treatment of cancers [3,4,5,6]. Furthermore, TCM have been one of the most important sources for seeking new leading compounds that possess significant cytotoxicity [7,8,9].
In East Asia, the genus Murraya (family Rutaceae) has been widely used in traditional medicine. Its plants contain various alkaloids, coumarins and flavonoids [10,11]. The cytotoxicity has been investigated in some species in this genus, such as M. koenigii [12], M. paniculata [13,14], M. euchrestifolia [15] and M. exotica [16]. However, there are no reports on the cytotoxicity of M. tetramera Huang.
M. tetramera is widely distributed in the Chinese provinces of Guangxi and Yunnan. It has been used as a folk TCM for the treatment of colds, coughs, asthma, stomach disorders, rheumatism, pruritus and eczema [11,17]. In this work, we sought to isolate and identify bioactive compounds with potential cytotoxicity from M. tetramera. As a result a new coumarin and seven known compounds were isolated from M. tetramera for the first time and all the compounds were evaluated in vitro against the A549, SMMC-7721, EJ, HeLa and BALL-1 tumor cell lines. The results indicated that some of these compounds have significant cytotoxic activities against the five tested human cancer cell lines.
2. Results and Discussion
2.1. Compounds Isolated from M. tetramera
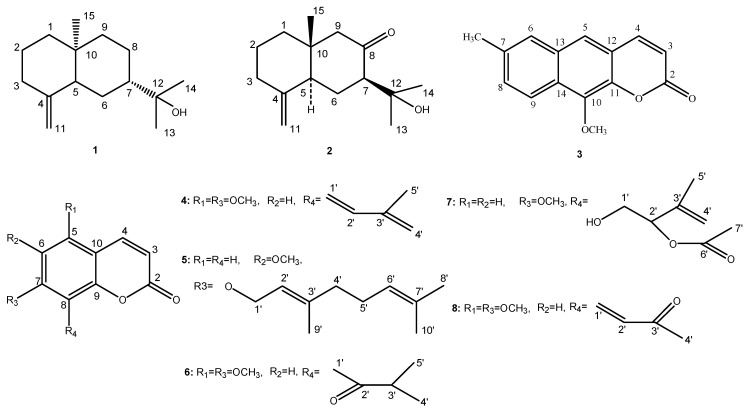
A new compound and seven known compounds were isolated from the M. tetramera for the first time. The new one was identified that 10-methoxy-7-methyl-2H-benzo[g]chromen-2-one (3) and the others were β-eudesmol (1), trans-3β-(1-hydroxy-1-methylethyl)-8aβ-methyl-5-methylenedecalin-2-one (2), 5,7-dimethoxy-8-[(Z)-3'-methylbutan-1',3'-dienyl]coumarin (4), 7-geranyloxy-6-methoxycoumarin (5), 5,7-Dimethoxy-8-(3-methyl-2-oxo-butyl)coumarin (6), murrangatin acetate (7) and toddalenone (8). Compounds 1 and 2 are sesquiterpenes and compounds 3–8 are coumarins. Their structures are shown in Figure 1.
Figure 1.
The structures of compounds 1–8.
2.2. Chemical Structure Identification of the New Compound
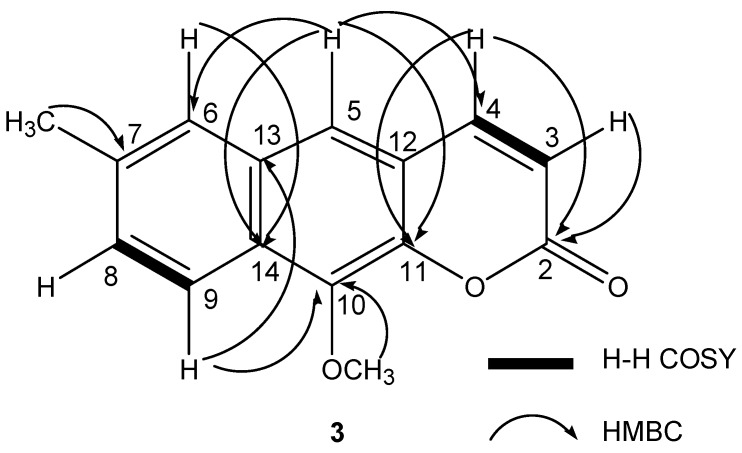
Compound 3 was obtained as colorless needles. The molecular formula was established as C15H12O3 by HR-ESI-MS, which indicted an [M+H]+ peak at m/z 241.0861 (calculated for C15H13O3, 241.0865). The 1H-NMR spectrum showed characteristic peaks of a coumarin framework at δH 8.21 (1H, d, J = 9.5 Hz, H-4), δH 6.86 (1H, s, H-5), δH 6.47 (1H, d, J = 9.5 Hz, H-3) indicative of a substituent at C-13, C-14 and C-10. Moreover, the 1H-NMR spectrum showed one methoxyl peak at δH 4.03 (3H, s, 10-OCH3) and one aromatic methyl peak at δH 2.54 (3H, s, 7-CH3). The 13C-NMR spectrum revealed the presence of fifteen carbon atoms and the characteristic coumarin framework ones at δC 161.03 (C-2) and δC 152.46 (C-11). The H-H COSY spectrum exhibited the correlations between H-3 (δH 6.47) and H-4 (δH 8.21), between H-8 (δH 7.32) and H-9 (δH 8.33). The HMBC spectrum showed correlations arising from H-3 (δH 6.47) to C-2 (δC 161.0), H-4 (δH 8.21) to C-2 (δC 161.0) and C-11 (δC 152.5), H-5 (δH 6.86) to C-6 (δC 126.0), C-4 (δC 139.4), C-14 (δC 117.1) and C-11 (δC 152.5), H-6 (δH 7.53) to C-14 (δC 117.1), 7-CH3 (δH 2.5) to C-7 (δC 126.9), H-9 (δH 8.33) to C-10 (δC 152.6) and C-13 (δC 135.8), 10-OCH3 (δH 4.03) to C-10 (δC 152.6). The H–H COSY and HMBC correlations were presented in Figure 2. On the basis of the results, the structure of compound 3 was identified as 10-methoxy-7-methyl-2H-benzo[g]chromen-2-one.
Figure 2.
The structure of compound 3 and key assignments of its COSY and HMBC correlations signals.
2.3. Cytotoxic Activity of Isolated Compounds
The cytotoxicity of compounds 1–8 was evaluated against A549, SMMC-7721, EJ, HeLa and BALL-1 cancer cell lines using the Cell Counting Kit (CCK)-8 method. The results are listed in Table 1. The two sesquiterpenes showed fair cytotoxicity against the five cell lines. Moreover, compound 1 possessed stronger cytotoxic activity against A549, SMMC-7721, EJ, HeLa and BALL-1, with IC50 values of 6.70, 5.17, 31.93, 17.82 and 11.15 µg/mL, respectively. Coumarins 3–8 share the same basic skeleton with different substitution patterns, yet their cytotoxic activities varied greatly. Compound 5 exhibited potent cytotoxicity against A549, SMMC-7721 and BALL-1, with IC50 values of 7.30, 9.09 and 12.50 µg/mL. Compound 4 exhibited potent cytotoxicity against A549, EJ and BALL-1 with IC50 values of 17.04, 30.59 and 22.54 µg/mL, respectively. Compound 3 merely exhibited cytotoxic activity against BALL-1 with an IC50 value of 94.88 µg/mL. None of the tested cell lines were susceptible to compounds 6–8.
Table 1.
Cytotoxicity of compounds 1–8 from Murraya tetramera.
| Compound | IC50 (µg/mL) a | ||||
|---|---|---|---|---|---|
| A549 | SMMC-7721 | EJ | Hela | BALL-1 | |
| 1 | 6.70 ± 1.05 | 5.17 ± 0.97 | 31.93 ± 2.84 | 17.82 ± 2.34 | 11.15 ± 1.62 |
| 2 | 31.67 ± 2.36 | 35.62 ± 2.73 | 47.45 ± 3.22 | 70.61 ± 3.95 | 33.91 ± 2.78 |
| 3 | >100 | >100 | >100 | >100 | 94.88 ± 3.25 |
| 4 | 17.04 ± 0.58 | >100 | 30.59 ± 2.73 | >100 | 22.54 ± 2.03 |
| 5 | 7.30 ± 0.46 | 9.09 ± 0.51 | 38.18 ± 2.23 | 46.63 ± 2.62 | 12.50 ± 1.47 |
| 6–8 | >100 | >100 | >100 | >100 | >100 |
| DOX b | 3.53 ± 0.25 | 1.35 ± 0.28 | 5.88 ± 0.18 | 2.11 ± 0.21 | 6.99 ± 0.37 |
a Inhibitory activity was expressed as the mean ± SD of 50% inhibitory concentration of triplicate determinations and was obtained by interpolation of concentration-inhibition curve. b Doxorubicin (positive control).
The various cytotoxic activities might be related to the different substitution patterns in the chemical structures. Among compounds 3–8, compound 5 had a longer alkyl-substituent than the other compounds and it showed the most potent cytotoxic activity against the human cancer cell lines, which corresponds to the result previously described by Wang et al. [18] indicating that the length of alkyl-substituents contributed to the cytotoxicity. Interestingly, the results also showed that the compounds possessing carbonyls on the alkyl moiety had weak cytotoxic activities. Further study is needed to investigate the structure-active relationships.
3. Experimental Section
3.1. General Information
1H- and 13C-NMR and 2D-NMR spectra were recorded on Bruker Avance III NMR spectrometer with the magnetic field of 11.74 Tesla. HR-ESI-MS were obtained on a Bruker Q-TOF mass spectrometer. Silica gel (160–200 mesh) used for column chromatography and TLC (silica gel G plates) used for monitoring fractions were purchased from Qingdao Marine Chemical Plant (Qingdao, China). Sephadex LH-20 was supplied by Amersham Pharmacia Biotech (Beijing, China). Analytical grade solvents were produced by Beijing Chemical Factory (Beijing, China).
3.2. Plant Material
The branches with leaves of M. tetramera were collected in June 2012 from Xishuangbanna, Yunnan Province, China (21.13°~22.60° N latitude, 99.93°~101.83° E longitude). The plant was identified by Dr. Liu, Q.R. (College of Life Sciences, Beijing Normal University, Beijing, China) and a voucher specimen (BNU-CMH-Dushushan-2012-06-017-007) was deposited at the Herbarium (BNU) of College of Resources Sciences, Beijing Normal University.
3.3. Extraction and Isolation
The dried samples (2.5 kg) were extracted with petroleum ether-ethyl acetate (PE/EtOAc, 20 L) three times (each for half an hour) under ultrasound. A crude extract (100.62 g) was obtained by solvent evaporation under vacuum. The extract was fractionated by silica gel column chromatography (160–200 mesh, 10.0 × 33 cm, 1000 g), using a gradient solvent system of PE/EtOAc (100:1, 80:1, 60:1, 40:1, 20:1, 10:1, 5:1, 1:1 and EtOAc) to afford 90 fractions. Fractions with similar TLC patterns were combined. 160–200 Mesh/Fr. 29–30 (1.55 g) and 160–200 mesh/Fr. 35–37 (1.41 g) were chromatographed on a silica gel column eluting with PE/EtOAc (60:1) to obtain compound 1 (128.3 mg) and compound 3 (16.7 mg), respectively. 160–200 Mesh/Fr. 51 (0.88 g) and 160–200 mesh/Fr. 54–57 (1.17 g) were subjected to repeated silica gel column chromatography eluting with PE/EtOAC (10:1) to afford compound 2 (11.7 mg) and compound 4 (52.6 mg), respectively. 160–200 Mesh/Fr. 64 (0.41 g) and 160–200 mesh/Fr. 67–70 (3.12 g) were repeatedly subjected to silica gel column chromatography eluting with PE/EtOAc 5:1, and then purified by chromatography on a Sephadex LH-20 column with MeOH as eluent to give compound 5 (17.2 mg) and 6 (62.8 mg), respectively. Compounds 7 (33.7 mg) and 8 (27.9 mg) were obtained from 160–200 mesh/Fr. 74 (3.35 g) and 160–200 mesh/Fr. 77–78 (2.55 g) after repeatedly purification by chromatography on a silica gel column eluting with PE/EtOAc 2:1.
3.4. Characterization of Isolated Compounds
β-Eudesmol (1). White needles. 1H-NMR (500 MHz, CDCl3) δ ppm: 4.74 (1H, s, H-11a), 4.47 (1H, s, H-11b), 2.33 (1H, m, H-3a), 2.01 (1H, m, H-3b), 1.79 (1H, m, H-5), 1.65 (2H, m, H-2), 1.55 (2H, m, H-1), 1.47 (2H, m, H-9), 1.40 (1H, m, H-7), 1.28 (2H, m, H-8), 1.23 (6H, s, H-13, H-14), 1.16 (2H, m, H-6), 0.72 (3H, s, H-15). 13C-NMR (125 MHz, CDCl3) δ ppm: 151.2 (C-4), 105.3 (C-11), 73.0 (C-12), 49.8 (C-7), 49.5 (C-5), 41.9 (C-9), 41.1 (C-1), 36.9 (C-3), 35.9 (C-10), 27.2 (C-14), 27.1 (C-13), 25.0 (C-6), 23.5 (C-2), 22.4 (C-8), 16.3 (C-15) [19].
trans-3β-(1-Hydroxy-1-methylethyl)-8aβ-methyl-5-methylenedecalin-2-one (2). White powder. 1H-NMR (500 MHz, CDCl3) δ ppm: 4.86 (1H, s, H-11a), 4.55 (1H, s, H-11b), 2.50 (1H, m, H-7), 2.41 (1H, m, H-3a), 2.37 (1H, m, H-5), 2.32 (1H, d, J = 10.0 Hz, H-9a), 2.20 (1H, d, J = 10.0 Hz, H-9b), 2.10 (1H, m, H-6a), 2.10 (1H, m, H-3b), 1.71 (1H, m, H-6b), 1.66 (1H, m, H-2a), 1.56 (1H, m, H-2b), 1.52 (2H, m, H-1), 1.29 (3H, s, H-14), 1.27 (3H, s, H-13), 0.72 (3H, s, H-15) [19]. 13C-NMR (125 MHz, CDCl3) δ ppm: 214.7 (C-8), 148.5 (C-4), 107.3 (C-11), 71.5 (C-12), 58.9 (C-7), 57.1 (C-9), 48.4 (C-5), 41.2 (C-1), 40.8 (C-10), 36.6 (C-3), 28.6 (C-13), 28.1 (C-6), 25.5 (C-14), 23.0 (C-2), 17.1 (C-15) [20].
10-Methoxy-7-methyl-2H-benzo[g]chromen-2-one (3). Colorless needles. HR-ESI-MS m/z: 241.0861 [M+H]+ (calcd. for C15H13O3, 241.0865). 1H-NMR (500 MHz, CDCl3) δ ppm: 8.33 (1H, d, J = 8.5 Hz, H-9), 8.21 (1H, d, J = 9.5 Hz, H-4), 7.53 (1H, s, H-6), 7.32 (1H, d, J = 8.5 Hz, H-8), 6.86 (1H, s, H-5), 6.47 (1H, d, J = 9.5 Hz, H-3), 4.03 (3H, s, 10-OCH3), 2.54 (3H, s, 7-CH3). 13C-NMR (125 MHz, CDCl3) δ ppm: 161.0 (C-2), 152.6 (C-10), 152.5 (C-11), 139.5 (C-7), 139.4 (C-4), 135.8 (C-13), 126.9 (C-8), 126.0 (C-6), 122.3 (C-9), 117.1 (C-14), 114.2 (C-3), 108.1 (C-12), 100.3 (C-5), 55.8 (10-OCH3), 21.9 (7-CH3).
5,7-Dimethoxy-8-[(Z)-3'-methylbutan-1',3'-dienyl]coumarin (4). Colorless needles. 1H-NMR (500 MHz, CDCl3) δ ppm: 8.01 (1H, d, J = 9.5 Hz, H-4), 6.42 (1H, d, J = 12.0 Hz, H-1'), 6.34 (1H, s, H-6), 6.17 (1H, d, J = 12.0 Hz, H-2'), 6.17 (1H, d, J = 9.5 Hz, H-3), 4.87 (2H, d, J = 12.0 Hz, H-4'), 3.98 (3H, s, 7-OCH3), 3.94 (3H, s, 5-OCH3), 1.63 (3H, s, H-5'). 13C-NMR (125 MHz, CDCl3) δ ppm: 161.4 (C-2), 160.4 (C-7), 156.2 (C-5), 152.9 (C-9), 142.6 (C-3'), 138.6 (C-4), 136.7 (C-1'), 117.1 (C-2'), 116.9 (C-4'), 111.1 (C-3), 108.1 (C-8), 103.6 (C-10), 90.1 (C-6), 56.0 (7-OCH3), 55.9 (5-OCH3), 20.7 (C-5') [21].
7-Geranyloxy-6-methoxycoumarin (5). Brown needles. 1H-NMR (500 MHz, CDCl3) δ ppm: 7.65 (1H, d, J = 9.5 Hz, H-4), 6.87 (1H, s, H-5), 6.84 (1H, s, H-8), 6.29 (1H, d, J = 9.5 Hz, H-3), 5.50 (1H, t, H-2'), 5.08 (1H, t, H-6'), 4.71 (2H, d, J = 6.0 Hz, H-1'), 3.93 (3H, s, 6-OCH3), 2.13 (2H, m, H-5'), 2.10 (2H, m, H-4'), 1.79 (3H, s, H-9'), 1.67 (3H, s, H-8'), 1.61 (3H, s, H-10'). 13C-NMR (125 MHz, CDCl3) δ ppm: 161.5 (C-2), 152.1 (C-7), 150.0 (C-9), 146.7 (C-6), 143.3 (C-4), 142.1 (C-3'), 131.9 (C-7'), 123.6 (C-6'), 118.5 (C-2'), 113.3 (C-3), 111.3 (C-10), 108.1 (C-5), 101.2 (C-8), 66.3 (C-1'), 56.4 (6-OCH3), 39.5 (C-4'), 26.2 (C-5'), 25.6 (C-8'), 17.7 (C-10'), 16.9 (C-9') [22].
5,7-Dimethoxy-8-(3-methyl-2-oxobutyl)coumarin (6). Colorless crystals. 1H-NMR (500 MHz, CDCl3) δ ppm: 8.01 (1H, d, J = 9.5 Hz, H-4), 6.34 (1H, s, H-6), 6.14 (1H, d, J = 9.5 Hz, H-3), 3.95 (3H, s, 5-OCH3), 3.93 (2H, s, H-1'), 3.89 (3H, s, 7-OCH3), 2.81 (1H, m, H-3'), 1.22 (3H, s, H-4'), 1.20 (3H, s, H-5'). 13C-NMR (125 MHz, CDCl3) δ ppm: 211.3 (C-2'), 161.3 (C-2), 161.2 (C-7), 156.2 (C-5), 153.9 (C-9), 138.9 (C-4), 110.9 (C-3), 104.1 (C-8), 103.8 (C-10), 90.2 (C-6), 56.0 (7-OCH3), 55.9 (5-OCH3), 40.7 (C-3'), 34.3 (C-1'), 18.5 (4'-CH3, 5'-CH3) [23].
Murrangatin acetate (7). Colorless needles. 1H-NMR (500 MHz, CDCl3) δ ppm: 7.64 (1H, d, J = 9.5 Hz, H-4), 7.42 (1H, d, J = 8.5 Hz, H-5), 6.90 (1H, d, J = 8.5 Hz, H-6), 6.28 (1H, d, J = 9.5 Hz, H-3), 5.75 (1H, d, J = 8.0 Hz, H-2'), 5.50 (1H, t, H-1'), 4.77 (2H, m, H-4'), 4.02 (3H, s, 7-OCH3), 3.60 (1H, d, J = 10.0 Hz, 1'-OH), 2.16 (3H, s, H-7'), 1.77 (3H, s, H-5'). 13C-NMR (125 MHz, CDCl3) δ ppm: 170.9 (C-6'), 160.2 (C-2), 160.0 (C-7), 152.7 (C-9), 143.7 (C-4), 140.8 (C-3'), 128.8 (C-5), 115.8 (C-8), 114.9 (C-4'), 113.5 (C-3), 113.1 (C-10), 107.8 (C-6), 79.5 (C-2'), 68.2 (C-1'), 56.3 (7-OCH3), 21.2 (C-7'), 18.6 (C-5') [24].
Toddalenone (8). Colorless needles. 1H-NMR (500 MHz, CDCl3) δ ppm: 8.00 (1H, d, J = 9.5 Hz, H-4), 7.95 (1H, d, J = 16.5 Hz, H-1'), 7.25 (1H, d, J = 16.5 Hz, H-2'), 6.35 (1H, s, H-6), 6.21 (1H, d, J = 9.5 Hz, H-3), 4.02 (3H, s, 7-OCH3), 4.01 (3H, s, 5-OCH3), 2.42 (3H, s, H-4'). 13C-NMR (125 MHz, CDCl3) δ ppm: 199.9 (C-3'), 163.1 (C-7), 160.4 (C-2), 158.3 (C-5), 155.0 (C-9), 138.5 (C-4), 131.8 (C-1'), 129.8 (C-2'), 111.4 (C-3), 104.7 (C-8), 103.8 (C-10), 90.2 (C-6), 56.2 (7-OCH3), 56.1 (5-OCH3), 27.6 (4'-CH3) [25].
3.5. Cytotoxicity Assay
The cytotoxicity of compounds 1–8 was measured by the standard CCK-8 method [26]. Human lung adenocarcinoma (A549), human hepatocellular carcinoma cells (SMMC-7721), human bladder tumor cells (EJ), human cervical carcinoma cells (Hela), and human B-lineage acute lymphoblastic leukemia 1 cells (BALL-1) were purchased from the Chinese Academy of Medical Sciences (Beijing, China). Doxorubicin (DOX, adriamycin, Actavis Italy S.p.A., Beijing, China) was the positive control. All cell lines were cultured in RPMI 1640 (Sigma, St. Louis, MO, USA) medium supplemented with 10% fetal bovine serum (GIBCO Inc., Grand Island, NY, USA), 100 IU/mL penicillin (Flow Lab, Beijing, China) and 100 μg/mL streptomycin (Flow Lab) at 37 °C, 5% CO2 and 90% humidity. The cell suspension was dispensed into a 96-well plates at 100 μL per well (adherent cells were 6 × 103 per well, suspension cells were 5 × 104 per well). After 4–6 h preincubation in the incubator (Forma Series ΙΙ Water Jacket, Waltham, MA, USA) to allow cellular attachment, various concentrations of test solution were added and cells were incubated for 48 h. At the end of the incubation, CCK-8 reagent (Dojindo, Kumamoto, Japan, 10 μL) was added into each well followed by further incubation for 2 h. The optical density (OD) was recorded at 450 nm using a microplate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA). Each determination represented the average mean of six replicates. The 50% inhibitory concentration (IC50) values were calculated by the line equation of the dose-dependent curves.
4. Conclusions
A new compound and seven known compounds were isolated from the M. tetramera for the first time. They were two sesquiterpenes (compounds 1 and 2) and six coumarins (compounds 3–8). All the compounds were tested for their in vitro cytotoxic activities against the HeLa, K562, A549, H1299 and SMMC-7721 tumor cell lines. It was found that compounds with similar structures displayed various degrees of cytotoxicity. Compound 1 showed stronger cytotoxic activity against the five cell lines (A549, SMMC-7721, EJ, HeLa and BALL-1) compared to compound 2. Compound 5 showed potent cytotoxicity against three cell lines (A549, SMMC-7721 and BALL-1). Compound 4 showed promising cytotoxicity against three cell lines (A549, EJ and BALL-1). Compound 3 only showed cytotoxicity against the BALL-1 cell line. None of the tested cell lines were susceptible towards compounds 6–8. This phenomenon might be related with the different substitution patterns in their chemical structures. Compounds having long alkyl-substituents exhibited significant potent cytotoxic activity against the human cancer cell lines, whereas compounds which had carbonyl groups on the alkyl substituents showed weak cytotoxicity. All these active compounds might be promising lead compounds for anti-cancer agents. However, further study is needed to unravel the mechanisms of their cytotoxic activity.
Acknowledgments
This project was supported by State Key Laboratory of Earth Surface Processes and Resource Ecology, National Natural Science Foundation of China (No. 81374069) and Beijing Municipal Natural Science Foundation (No. 7142116).
Author Contributions
Chun-Xue You, Kai Yang, Cheng-Fang Wang, Wen-Juan Zhang and Ying Wang made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. Chun-Xue You was involved in drafting the manuscript. Jiao Han and Zhu-Feng Geng carried out the 1H-NMR, 13C-NMR, 2D-NMR spectra and the elemental analyses. Li Fan, Shu-Shan Du and Zhi-Wei Deng were involved in revising the manuscript for important intellectual content and offered the necessary guidance to Chun-Xue You to carry out the synthesis and characterization experiments. All authors have read and approve of the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Pan L., Chai H., Kinghorn A.D. The continuing search for antitumor agents from higher plants. Phytochem. Lett. 2010;3:1–8. doi: 10.1016/j.phytol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao W., Liu L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 4.Hou J., Sun T., Hu J., Chen S., Cai X., Zou G. Chemical composition, cytotoxic and antioxidant activity of the leaf essential oil of Photinia serrulata. Food Chem. 2007;103:355–358. doi: 10.1016/j.foodchem.2006.07.060. [DOI] [Google Scholar]
- 5.Ma X., Zheng C., Hu C., Rahman K., Qin L. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2011;138:314–332. doi: 10.1016/j.jep.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 6.Rahman S., Hasnat A., Hasan C.M., Rashid M.A., Ilias M. Pharmacological evaluation of Bangladeshi medicinal plants-a review. Pharm. Biol. 2001;39:1–6. doi: 10.1076/phbi.39.1.1.5939. [DOI] [Google Scholar]
- 7.Yang H., Cho H., Sim S.H., Chung Y.K., Kim D., Sung S.H., Kim J., Kim Y.C. Cytotoxic terpenoids from Juglans sinensis leaves and twigs. Bioorg. Med. Chem. Lett. 2012;22:2079–2083. doi: 10.1016/j.bmcl.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Znati M., Jannet H., Cazaux S., Bouajila J. Chemical composition, biological and cytotoxic activities of plant extracts and compounds isolated from Ferula lutea. Molecules. 2014;19:2733–2747. doi: 10.3390/molecules19032733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasul A., Ma T.H. In vitro cytotoxic screening of 300 selected Chinese medicinal herbs against human gastric adenocarcinoma SGC-7901 cells. Afr. J. Pharm. Pharmacol. 2012;6:592–600. [Google Scholar]
- 10.Kong Y.C., Ng K.H., But P.P.H., Li Q., Yu S.X., Zhang H.T., Cheng K.F., Soejarto D.D., Kan W.S., Waterman P.G. Sources of the anti-implantation alkaloid yuehchukene in the genus Murraya. J. Ethnopharmacol. 1986;15:195–200. doi: 10.1016/0378-8741(86)90155-8. [DOI] [PubMed] [Google Scholar]
- 11.Editorial Committee of Flora of China . Flora of China. Science Press; Beijing, China: 1997. p. 145. [Google Scholar]
- 12.Tantapakul C., Phakhodee W., Laphookhieo S., Ritthiwigrom T., Cheenpracha S. Cytotoxic carbazole alkaloids from the stems of Murraya koenigii. Chem. Nat. Compd. 2014;50:186–188. [Google Scholar]
- 13.Sukari M.A., Riyanto S., Ali A.M., Yusof U.K., Haron M.J., Ahmad F.B.H. Isolation of flavonoids from Murraya paniculata L. Orient. J. Chem. 2001;17:27–30. [Google Scholar]
- 14.Riyanto S., Sukari M.A., Rahmani M., Ali A.M., Aimi D.N. Isolation and identification of compounds in petroleum extract of Murraya paniculata (L.) brands cortex. Maj. Farm. Indones. 1999;10:95–103. [Google Scholar]
- 15.Itoigawa M., Kashiwada Y., Ito C., Furukawa H., Tachibana Y., Bastow K.F., Lee K.H. Antitumor agents. 203. Carbazole alkaloid murrayaquinone A and related synthetic carbazolequinones as cytotoxic agents. J. Nat. Prod. 2000;63:893–897. doi: 10.1021/np000020e. [DOI] [PubMed] [Google Scholar]
- 16.Bishay D.W., El-Sayyad S.M., Abdel-Hafiz M.A., Achenbach H., Desoky E.K. Phytochemical study of Murraya exotica L. cultivated in Egypt. III. Coumarins and cycloartenols of the leaves. Bull. Pharm. Sci. Assiut Univ. 1988;11:105–121. [Google Scholar]
- 17.Ya Q.K., Lu W.J., Chen J.Y., Tan X. Study on the chemical constituent from Murraya tetramera Huang. Guangxi Sci. 2010;17:347–348. [Google Scholar]
- 18.Wang X.F., Ohlin C.A., Lu Q.H., Fei Z.F., Hu J., Dyson P.J. Cytotoxicity of ionic liquids and precursor compounds towards human cell line HeLa. Green Chem. 2007;9:1191–1197. [Google Scholar]
- 19.Duan J., Wang L., Qian S., Su S., Tang Y. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008;31:965–969. doi: 10.1007/s12272-001-1252-z. [DOI] [PubMed] [Google Scholar]
- 20.Torii S., Inlkuchi T. Functionalization of trans-Decalin. IV. A stereoselective synthesis of dl-β-costol, dl-arctiol, and the related eudesmane type sesquiterpenes. Chem. Soc. Jpn. 1980;53:2642–2646. [Google Scholar]
- 21.Kinoshita T., Firman K. Prenylcoumarin derivatives from the leaves of an indonesian medicinal plant Murraya paniculata (Rutaceae) Chem. Pharm. Bull. 1996;44:1261–1262. [Google Scholar]
- 22.Maes D., van Syngel K., de Kimpe N. Synthesis of artekeiskeanin A: A new coumarin monoterpene ether from Artemisia keiskeana. Heterocycles. 2007;74:927–930. doi: 10.3987/COM-07-S(W)1. [DOI] [Google Scholar]
- 23.Macias F.A., Massanet G.M., Rodriguez-luis F., Salva J. I3C-NMR of coumarios III*-simple coumarins. Magn. Reson. Chem. 1989;27:892–904. doi: 10.1002/mrc.1260270913. [DOI] [PubMed] [Google Scholar]
- 24.Mesquita S.G., Martinez M.F., Romoff P., Fávero O.A., Lieber S.R., Lago J.H.G. Constituintes químicos das folhas de Murraya paniculata(Rutaceae) Rev. Bras. Farmacogn.Braz. J. Pharmacogn. 2008;18:563–568. doi: 10.1590/S0102-695X2008000400011. [DOI] [Google Scholar]
- 25.Kinoshita T., Wu J.B., Ho F.C. The isolation of a prenylcoumarin of chemotaxonomic significance from Murraya paniculata var omphalocarpa. Phytochemistry. 1996;43:125–128. doi: 10.1016/0031-9422(96)00255-5. [DOI] [Google Scholar]
- 26.Wu S., Ji Y., Zhu J., Zhao Y., Xia G., Hu Y., Hu J. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids. 2009;74:761–765. doi: 10.1016/j.steroids.2009.04.005. [DOI] [PubMed] [Google Scholar]