Abstract
In this study, we report the antibacterial activities of six polyphenols (i.e., luteolin, quercetin, scutellarin, apigenin, chlorogenic acid, and resveratrol) against 29 clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA), and in vitro antibacterial activities of two-drug combinations. All of the MRSA strains evaluated were clinical isolates from patients with MRSA bacteremia. The antibacterial activities were determined by agar dilution method, and the two-drug antibacterial activities were determined by the checkerboard agar dilution method. It was found that luteolin, quercetin and resveratrol show obvious antibacterial activities against MRSA, and the results of two-drug antibacterial activity show either synergy or additivity, without evidences of antagonistic effects.
Keywords: methicillin-resistant Staphylococcus aureus, polyphenols, antibacterial activity, synergism
1. Introduction
MRSA (the so called “superbug” as it was originally termed) represents a worldwide threat because of its virulence and broad distribution in community and hospital settings [1]. In many hospitals, MRSA could be detected in over 80 percent of pneumonia sputum samples from severe and elderly patients in the intensive care unit (ICU) [2]. MRSA is the result of the selective pressure of currently used antibiotics, leading to high morbidity and mortality [1]. MRSA normally possesses a multidrug-resistant gene which causes it resistant to β-lactams, aminoglycosides, fluoroquinolones and macrolides [3]. Vancomycin and teicoplanin are the two glycopeptides presently used in clinics for treatment of multi-resistant infections caused by Gram-positive organisms. However, glycopeptide-resistant S. aureus (vancomycin-intermediate or resistant S. aureus, VISA or VRSA, respectively) are being found with increasing frequency around the World [4]. Therefore, there is an urgent need to develop novel active agents.
Natural products from higher plants have traditionally been regarded as an important source of antimicrobial agents and have attracted extensive attention in fundamental and clinic applications [5,6]. They are often effective in the treatment of infectious diseases while simultaneously mitigating many of the side effects that are often associated with synthetic antimicrobials [7,8]. Many efforts have been made in the isolation of pure natural products to validate their use in folk medicine and to reveal the active principle(s) [9]. Mixtures of pure phytochemicals as well as various extracts from plants have also been reported to exhibit encouraging antimicrobial activities [7,10,11,12]. Partly inspired by these efforts, there is a keen interest in studying effects of combinations of phytochemicals for antimicrobial applications. Polyphenols from fruits, vegetables and cereals, herbs and spices have shown beneficial effects on human health, and have been found to be effective antimicrobial substances against a wide variety of microorganisms [7,9,13,14,15]. However, to our knowledge, few studies have focused on the synergistic antibacterial activities of combinations of polyphenols. In the present study, we report the antibacterial activities of six polyphenols (luteolin, quercetin, scutellarin, apigenin, chlorogenic acid and resveratrol, Figure 1) against 29 clinical MRSA strains, and their in vitro in vitro synergistic activities. These six polyphenols are the main components of a traditional Chinese medicine named as “Compound Qingre Granule”. In our previous study, it was found that this medicine could exhibit antibacterial activities toward MRSA [16]. Due to the aforementioned antibacterial activities of polyphenols, we are very interesting in the antibacterial activities of the polyphenol components of this traditional Chinese medicine toward MRSA. All of these MRSA strains evaluated were clinical isolates from patients with MRSA bacteremia. To estimate antibacterial activities of each polyphenol, a minimum inhibitory concentration (MIC) was determined by the agar dilution method as recommended by the Clinical and Laboratory Standards Institute [17]. Because of poor solubility of these polyphenols in water and Mueller-Hinton broth, studies of antibacterial activities of two-drug combinations were performed by the checkerboard agar dilution method [18] to obtain a fractional inhibitory concentration (FIC) index.
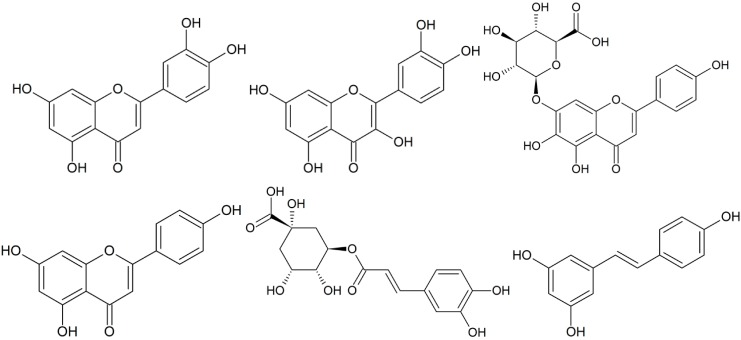
Figure 1.
Molecular structures of the six flavonoids luteolin, quercetin, scutellarin, apigenin, chlorogenic acid and resveratrol.
2. Results and Discussion
Screening tests of these six different polyphenols were performed against 29 clinical strains of MRSA, one reference strain of MRSA and four strains of methicillin-sensitive Staphylococcus aureus (MSSA) using the agar diffusion method. The results are shown in Table 1.
Table 1.
MICs of six polyphenols against 34 isolates of S. aureus.
| Bacteria | MIC (ug/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| luteolinn | quercetin | resveratrol | scutellarin | apigenin | chlorogenic acid | ||||||||
| 1 | MRSA ATCC43300 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 2 | SA0922 | 31.2 | 62.5 | 1000 | >2000 | >4000 | >4000 | ||||||
| 3 | SA0925 | 62.5 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 4 | SA0927 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 5 | SA0928 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 6 | SA0929 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 7 | SA0930 | 125 | 62.5 | 1000 | >2000 | >4000 | >4000 | ||||||
| 8 | SA0933 | 62.5 | 62.5 | 500 | >2000 | >4000 | >4000 | ||||||
| 9 | SA0936 | 62.5 | 62.5 | 500 | >2000 | >4000 | >4000 | ||||||
| 10 | SA0942 | 125 | 62.5 | 500 | >2000 | >4000 | >4000 | ||||||
| 11 | SA1032 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 12 | SA1039 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 13 | SA1040 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 14 | SA1053 | 62.5 | 31.2 | 1000 | >2000 | >4000 | >4000 | ||||||
| 15 | SA1054 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 16 | SA1056 | 62.5 | 125 | 250 | >2000 | >4000 | >4000 | ||||||
| 17 | SA1057 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 18 | SA1060 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 9 | SA1061 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 20 | SA1065 | 31.2 | 62.5 | 500 | >2000 | >4000 | >4000 | ||||||
| 21 | SA1131 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 22 | SA1133 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 23 | SA1134 | 62.5 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 24 | SA1140 | 62.5 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 25 | SA1143 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 26 | SA1147 | 62.5 | 62.5 | 1000 | >2000 | >4000 | >4000 | ||||||
| 27 | SA1148 | 125 | 62.5 | 250 | >2000 | >4000 | >4000 | ||||||
| 28 | SA1154 | 125 | 125 | 500 | >2000 | >4000 | >4000 | ||||||
| 29 | SA1157 | 62.5 | 31.2 | 500 | >2000 | >4000 | >4000 | ||||||
| 30 | SA1159 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 1 | MSSA ATCC29213 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 2 | SA1031 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 3 | SA1072 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
| 4 | SA1081 | 125 | 125 | 1000 | >2000 | >4000 | >4000 | ||||||
It can be seen that the MIC values of luteolin and quercetin for each of these 34 strains range from 31.25 to 125 μg/mL and the MIC values of resveratrol for each of these 34 strains range from 500 to 1000 μg/mL. However, all the MIC values of scutellarin, apigenin and chlorogenic acid are more than 2000 μg/mL. In addition, in the control experiments, no inhibition was observed, indicating that the used solvent dimethyl sulphoxide (DMSO) has no antibacterial activity against either MRSA or MSSA. These results indicate that luteolin, quercetin and resveratrol exhibit remarkable antibacterial activities against MRSA and MSSA, and there are no obvious differences in susceptibility to these three compounds against the MRSA and MSSA strains. On the other hand, scutellarin, apigenin and chlorogenic acid were found to be relatively inactive.
Antibacterial activities of the two-drug combination between the three polyphenol compounds (i.e., luteolin, quercetin and resveratrol) were also evaluated by using the checkerboard agar dilution method. Drug interactions are usually classified as synergistic, additive, or antagonistic on the basis of the FIC index. The interaction is defined as: synergy, °0.5; additive effect, 0.5–1; indifference (or no effect), 1–2; antagonism, >2 [19,20,21]. The reductions in the geometric mean (GM) MICs of the drugs when they were given in combination compared to the MICs of the drugs when they were given alone were analyzed by using a paired rank test, which is a nonparametric test for comparison between two related samples. A p value of <0.05 is considered significant [19]. When luteolin was combined with quercetin, significant reductions in the GM MICs of luteolin (from 92.56 to 41.20 μg/mL (p = 0.000)), and GM MICs of quercetin (from 94.72 to 39.33 μg/mL (p = 0.000)) for the MRSA strains were observed. For this combination, synergistic effects were observed in 6.7% (2 of 30) of the interactions. Additive effects were found in 93.3% (28 of 30) of the interactions. No antagonism was observed (see Table 2). When luteolin was combined with resveratrol, significant reductions in the GM MICs of luteolin (from 92.56 to 42.17 μg/mL (p = 0.000)), and GM MICs of resveratrol (from 659.75 to 280.62 μg/mL (p = 0.000)) for the MRSA strains were observed. For this combination, synergistic effects were observed in 3.3% (1 of 30) of the interactions. Additive effects were found in 96.7% (29 of 30) of the interactions. No antagonism was observed (see Table 3). When quercetin was combined with resveratrol, significant reductions in the GM MICs of quercetin (from 94.72 to 43.16 μg/mL (p = 0.000)), and GM MICs of resveratrol (from 659.75 to 274.21 μg/mL (p = 0.000)) for the MRSA strains were observed. For this combination, synergistic effects were observed in 3.3% (1 of 30) of the interactions. Additive effects were found in 96.7% (29 of 30) of the interactions. No antagonism was observed (see Table 4). These results show the two-drug combinations between these three polyphenols exhibit either synergy or additivity without evidence of antagonistic effects, which are very encouraging. Owing to the lack of standardization in the methodology used to perform in vitro antibacterial susceptibility testing for three or more drugs combinations, we only performed studies of antibacterial activities of two-drug combinations by using the checkerboard agar dilution method.
Table 2.
FIC indexes of two-drug combination of luteolin-quercetin against 30 MRSA strains.
| Bacteria | MIC (μg/mL) of luteolin-quercetin | FIC Index | Interpretation | |
|---|---|---|---|---|
| 1 | MRSA ATCC43300 | 62.5/31.2 | 0.75 | ad |
| 2 | SA0922 | 15.6/31.2 | 1 | ad |
| 3 | SA0925 | 31.2/31.2 | 0.75 | ad |
| 4 | SA0927 | 62.5/62.5 | 1 | ad |
| 5 | SA0928 | 62.5/62.5 | 1 | ad |
| 6 | SA0929 | 31.2/31.2 | 0.5 | Syn |
| 7 | SA0930 | 62.5/31.2 | 1 | ad |
| 8 | SA0933 | 31.2/31.2 | 1 | ad |
| 9 | SA0936 | 31.2/31.2 | 1 | ad |
| 10 | SA0942 | 31.2/31.2 | 0.75 | ad |
| 11 | SA1032 | 31.2/62.5 | 0.75 | ad |
| 12 | SA1039 | 62.5/31.2 | 0.75 | ad |
| 13 | SA1040 | 62.5/62.5 | 1 | ad |
| 14 | SA1053 | 31.2/15.6 | 1 | ad |
| 15 | SA1054 | 62.5/31.2 | 0.75 | ad |
| 16 | SA1056 | 15.6/31.2 | 0.5 | syn |
| 17 | SA1057 | 62.5/62.5 | 1 | ad |
| 18 | SA1060 | 62.5/62.5 | 1 | ad |
| 19 | SA1061 | 62.5/31.2 | 0.75 | ad |
| 20 | SA1065 | 15.6/31.2 | 1 | ad |
| 21 | SA1131 | 62.5/62.5 | 1 | ad |
| 22 | SA1133 | 62.5/62.5 | 1 | ad |
| 23 | SA1134 | 31.2/62.5 | 1 | ad |
| 24 | SA1140 | 31.2/62.5 | 1 | ad |
| 25 | SA1143 | 62.5/62.5 | 1 | ad |
| 26 | SA1147 | 31.2/31.2 | 1 | ad |
| 27 | SA1148 | 31.2/31.2 | 0.75 | ad |
| 28 | SA1154 | 62.5/62.5 | 1 | ad |
| 29 | SA1157 | 31.2/15.6 | 1 | ad |
| 30 | SA1159 | 62.5/31.2 | 0.75 | ad |
Abbreviations: Syn, synergy; ad, additive effect.
Table 3.
FIC indexes of two-drug combination of luteolin-resveratrol against 30 MRSA strains.
| Bacteria | MIC (μg/mL) of luteolin-resveratrol | FIC Index | Interpretation | |
|---|---|---|---|---|
| 1 | MRSA ATCC43300 | 62.5/500 | 1 | ad |
| 2 | SA0922 | 15.6/500 | 1 | ad |
| 3 | SA0925 | 31.2/500 | 1 | ad |
| 4 | SA0927 | 62.5/125 | 0.75 | ad |
| 5 | SA0928 | 62.5/250 | 1 | ad |
| 6 | SA0929 | 62.5/125 | 0.75 | ad |
| 7 | SA0930 | 62.5/500 | 1 | ad |
| 8 | SA0933 | 31.2/250 | 1 | ad |
| 9 | SA0936 | 31.2/250 | 1 | ad |
| 10 | SA0942 | 31.2/250 | 0.75 | ad |
| 11 | SA1032 | 62.5/500 | 1 | ad |
| 12 | SA1039 | 62.5/500 | 1 | ad |
| 13 | SA1040 | 62.5/500 | 1 | ad |
| 14 | SA1053 | 31.2/500 | 1 | ad |
| 15 | SA1054 | 62.5/250 | 0.75 | ad |
| 16 | SA1056 | 15.6/62.5 | 0.5 | Syn |
| 17 | SA1057 | 62.5/125 | 0.75 | ad |
| 18 | SA1060 | 62.5/500 | 1 | ad |
| 19 | SA1061 | 62.5/500 | 1 | ad |
| 20 | SA1065 | 15.6/125 | 0.75 | ad |
| 21 | SA1131 | 62.5/500 | 1 | ad |
| 22 | SA1133 | 62.5/250 | 1 | ad |
| 23 | SA1134 | 31.2/250 | 1 | ad |
| 24 | SA1140 | 31.2/125 | 1 | ad |
| 25 | SA1143 | 31.2/250 | 0.75 | ad |
| 26 | SA1147 | 31.2/500 | 1 | ad |
| 27 | SA1148 | 31.2/125 | 0.75 | ad |
| 28 | SA1154 | 62.5/250 | 1 | ad |
| 29 | SA1157 | 31.2/250 | 1 | ad |
| 30 | SA1159 | 62.5/500 | 1 | ad |
Abbreviations: Syn, synergy; ad, additive effect.
Table 4.
FIC indexes of two-drug combination of quercetin-resveratrol against 30 MRSA strains.
| Bacteria | MIC (μg/mL) of quercetin-resveratrol | FIC Index | Interpretation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | MRSA ATCC43300 | 62.5/500 | 1 | ad | |||||
| 2 | SA0922 | 15.6/500 | 0.75 | ad | |||||
| 3 | SA0925 | 62.5/500 | 1 | ad | |||||
| 4 | SA0927 | 62.5/125 | 0.75 | ad | |||||
| 5 | SA0928 | 62.5/250 | 1 | ad | |||||
| 6 | SA0929 | 62.5/250 | 1 | ad | |||||
| 7 | SA0930 | 62.5/500 | 1 | ad | |||||
| 8 | SA0933 | 31.2/125 | 0.75 | ad | |||||
| 9 | SA0936 | 31.2/250 | 1 | ad | |||||
| 10 | SA0942 | 31.2/250 | 1 | ad | |||||
| 11 | SA1032 | 62.5/500 | 1 | ad | |||||
| 12 | SA1039 | 62.5/500 | 1 | ad | |||||
| 13 | SA1040 | 62.5/125 | 0.75 | ad | |||||
| 14 | SA1053 | 15.6/500 | 1 | ad | |||||
| 15 | SA1054 | 62.5/250 | 0.75 | ad | |||||
| 16 | SA1056 | 15.6/62.5 | 0.375 | Syn | |||||
| 17 | SA1057 | 62.5/125 | 0.75 | ad | |||||
| 18 | SA1060 | 62.5/500 | 1 | ad | |||||
| 19 | SA1061 | 62.5/500 | 1 | ad | |||||
| 20 | SA1065 | 31.2/250 | 1 | ad | |||||
| 21 | SA1131 | 62.5/500 | 1 | ad | |||||
| 22 | SA1133 | 62.5/250 | 1 | ad | |||||
| 23 | SA1134 | 62.5/250 | 1 | ad | |||||
| 24 | SA1140 | 31.2/250 | 0.75 | ad | |||||
| 25 | SA1143 | 31.2/250 | 0.75 | ad | |||||
| 26 | SA1147 | 31.2/500 | 1 | ad | |||||
| 27 | SA1148 | 31.2/125 | 1 | ad | |||||
| 28 | SA1154 | 62.5/250 | 1 | ad | |||||
| 29 | SA1157 | 15.6/250 | 1 | ad | |||||
| 30 | SA1159 | 62.5/250 | 0.75 | ad | |||||
Abbreviations: Syn, synergy; ad, additive effect.
3. Experimental Section
3.1. Chemicals
Polyphenols and other chemicals were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). Polyphenols were solubilized in DMSO.
3.2. Bacterial Strains
A total of 32 clinical S. aureus. strains (MRSA and MSSA) selected from 32 individual patients attending the Beijing Friendship Hospital (Beijing, China) from 2010 to 2012 were studied. All of these isolates were obtained from blood. These strains were identified to the species level by conventional methods (colony morphology, Gram stain characteristics, coagulase reactions). The MRSA strains were defined on the basis of the occurrence of the mecA gene and of their resistance to methicillin and oxacillin, according to the guidelines of the National Committee for Clinical Laboratory Standards (M100-S9). Of 32 strains, 29 strains were mecA-positive, and three strains mecA-negative. ATCC 29213 (a MSSA strain) and ATCC 43300 (a MRSA strain) were used as the reference control strains.
Strains were stored frozen in glycerol broth at −70 °C and subcultured to ensure purity before testing. Test strains, grown overnight at 37 °C in sterile Mueller-Hinton (MH) broth, were resuspended in 0.9% saline to a density equivalent to a 0.5 McFarland standard and then diluted 1:10 in sterile MH broth. MH agar plates containing two-fold serial dilutions of antibacterial agents were inoculated with the final suspensions using an inoculator (Eppendorf Co., Hamburg, Germany) which delivered approximately 104 CFU per spot and incubated at 37 °C for 18–20 h.
3.3. Determination of Antibacterial Activity
To estimate the antibacterial activity of individual polyphenol, a MIC was determined by the agar dilution method in MH agar plates, as recommended by the Clinical and Laboratory Standards Institute. Because of poor solubility of these polyphenols in water and MH broth, studies of antibacterial activities of two-drug combinations were performed by the checkerboard agar dilution method to obtain a FIC index. In brief, two-fold serial dilutions of the polyphenol drug were totally solubilized in DMSO, and then were prepared to give initial concentrations four times the MICs of the respective polyphenol alone as determined in individual susceptibility tests. Then, combinations of polyphenol drugs were added with one drug diluted along the x axis and the other drug diluted along the y axis. Thus, for a given range of dilutions every possible combination of drug concentrations was achieved [22]. The media, inocula and conditions were the same as those used for MIC tests. In the control experiments, the same amount of DMSO that was contained in these agents was added into the MH agar to check the antibacterial effect of DMSO in the absence of polyphenols. All experiments were performed in triplicate.
4. Conclusions
In conclusion, the results presented in this study have provided useful information on the antibacterial activities of several naturally occurring plant polyphenols against MRSA strains. Luteolin, quercetin and resveratrol exhibit remarkable antibacterial activities against MRSA and MSSA, while scutellarin, apigenin and chlorogenic acid are relatively inactive. Furthermore, the two-drug combinations between luteolin, quercetin and resveratrol exhibit either synergy or additivity without evidence of antagonistic effects, which are very encouraging. Although the lowest values of the MIC exhibited by luteolin or quercetin is relatively higher (31.2 μg/mL) than those of frequently used antibiotics, of which the MIC is generally in the <10 μg/mL range [23], however, the values of the MIC for each polyphenol when used in two-drug combination could drop 2 or 4-fold for all MRSA strains.
Acknowledgments
This work was supported by the Capital Medical Development Fund (SF-2009-I-11), Beijing Municipal Bureau of Traditional Chinese Medicine and the Scientific Research Fund, Beijing Friendship Hospital (2011-5).
Author Contributions
Y.L. Su performed the experiments, collected and analyzed the data, and wrote the paper. S. W. Zhang designed the project, analyzed the results and revised the paper. L.Y. Ma, Y. Wen and H. Wang gave helps in the experiments. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds six polyphenoals are available from the authors.
References
- 1.Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 2.Zuo G.-Y., Li Y., Han J., Wang G.-C., Zhang Y.-L., Bian Z.-Q. Antibacterial and synergy of berberines with antibacterial agents against clinical multi-drug resistant isolates of methicillin-resistant Staphylococcus aureus (MRSA) Molecules. 2012;17:10322–10330. doi: 10.3390/molecules170910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein F.W. Combating resistance in a challenging, changing environment. Clin. Microbiol. Infect. 2007;13:2–6. doi: 10.1111/j.1469-0691.2007.01721.x. [DOI] [PubMed] [Google Scholar]
- 4.Zuo G.Y., Wang G.C., Zhao Y.B., Xu G.L., Hao X.Y., Han J., Zhao Q. Screening of Chinese medicinal plants for inhibition against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) J. Ethnopharmacol. 2008;120:287–290. doi: 10.1016/j.jep.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J.-Y., Guan M., Zhu J.-L., Wang C.-T., Su L., Zhang X.-J. Facile and material-independent fabrication of poly(luteolin) coatings and their unimpaired antibacterial activity against Staphylococcus aureus after steam sterilization treatments. Polym. Chem. 2014;5:4211–4214. doi: 10.1039/c4py00407h. [DOI] [Google Scholar]
- 6.Chang S., Sievert D.M., Hageman J.C., Boulton M.L., Tenover F.C., Downes F.P., Shah S., Rudrik J.T., Pupp G.R., Brown W.J., et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 7.Cushnie T.P.T., Lamb A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Abreu A.C., McBain A.J., Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012;29:1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 10.Al-Habib A., Al-Saleh E., Safer A.-M., Afzal M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA) J. Toxicol. Sci. 2010;35:357–364. doi: 10.2131/jts.35.357. [DOI] [PubMed] [Google Scholar]
- 11.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011;10(Suppl. 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo G.-Y., Zhang X.-J., Yang C.-X., Han J., Wang G.-C., Bian Z.-Q. Evaluation of traditional Chinese medicinal plants for anti-MRSA activity with reference to the treatment record of infectious diseases. Molecules. 2012;17:2955–2967. doi: 10.3390/molecules17032955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su X., Howell A.B., D’Souza D.H. Antibacterial effects of plant-derived extracts on methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2012;9:573–578. doi: 10.1089/fpd.2011.1046. [DOI] [PubMed] [Google Scholar]
- 14.Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mun S.-H., Joung D.-K., Kim Y.-S., Kang O.-H., Kim S.-B., Seo Y.-S., Kim Y.C., Lee D.S., Shin D.W., Kweon K.T., et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20:714–718. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y.-Y., Wang H., Zhang S.-W., Wang B.-E. Inhibition of methicillin-resistant Staphylococcus aureus by the compound Qingre granules. Chin. Med. J. (Engl. Ed.) 2010;123:1017–1020. [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard-Ninth ed. CLSI; Wayne, PA, USA: 2012. [Google Scholar]
- 18.Lorian V. Antibiotics in Laboratory Medicine. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2005. [Google Scholar]
- 19.Perea S., Gonzalez G., Fothergill A.W., Kirkpatrick W.R., Rinaldi M.G, Patterson T.F. In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob. Agents Chemother. 2002;46:3039–3041. doi: 10.1128/AAC.46.9.3039-3041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo G.-Y., Li Y., Wang T., Han J., Wang G.-C., Zhang Y.-L., Pan W.D. Synergistic antibacterial and antibiotic effects of bisbenzylisoquinoline alkaloids on clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) Molecules. 2011;16:9819–9826. doi: 10.3390/molecules16129819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odds F. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 22.Chow A.W., Wong J., Bartlett K.H. Synergistic interactions of ciprofloxacin and extended-spectrum β-lactams or aminoglycosides against multiply drug-resistant Pseudomonas maltophilia. Antimicrob. Agents Chemother. 1988;32:782–784. doi: 10.1128/AAC.32.5.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. CLSI; Wayne, PA, USA: 2013. Twenty-Third Informational Supplement CLSI document M100-S23. [Google Scholar]