Abstract
The genus Tephrosia, belonging to the Leguminosae family, is a large pantropical genus of more than 350 species, many of which have important traditional uses in agriculture. This review not only outlines the source, chemistry and biological evaluations of natural products from the genus Tephrosia worldwide that have appeared in literature from 1910 to December 2013, but also covers work related to proposed biosynthetic pathways and synthesis of some natural products from the genus Tephrosia, with 105 citations and 168 new compounds.
Keywords: Tephrosia, chemical constituents, proposed biosynthetic pathways, synthesis, biological activity
1. Introduction
The genus Tephrosia, belonging to the Leguminosae family, is a large pantropical genus of more than 350 species, many of which have important traditional uses [1,2]. Phytochemical investigations have revealed the presence of glucosides, rotenoids, isoflavones, chalcones, flavanones, flavanols, and prenylated flavonoids [1,2,3,4,5,6,7,8,9] of chemotaxonomic importance in the genus [10]. Moreover, bioactivity has been studied extensively, indicating that chemical constituents and extracts of the genus Tephrosia exhibited diverse bioactivities, such as insecticidal [11], antiviral [12], antiprotozoal [13], antiplasmodial [14] and cytotoxic [15] activities.
So far, the reviews on natural products isolated from the genus Tephrosia are limited [16]. To gain a comprehensive and systematic understanding of this genus, this review outlines the chemistry, proposed biosynthetic pathways, synthesis, and biological evaluations of natural products from the genus Tephrosia worldwide that have appeared in literature from 1971 to December 2013, with 105 citations and 168 new compounds from them.
2. Chemical Constituents
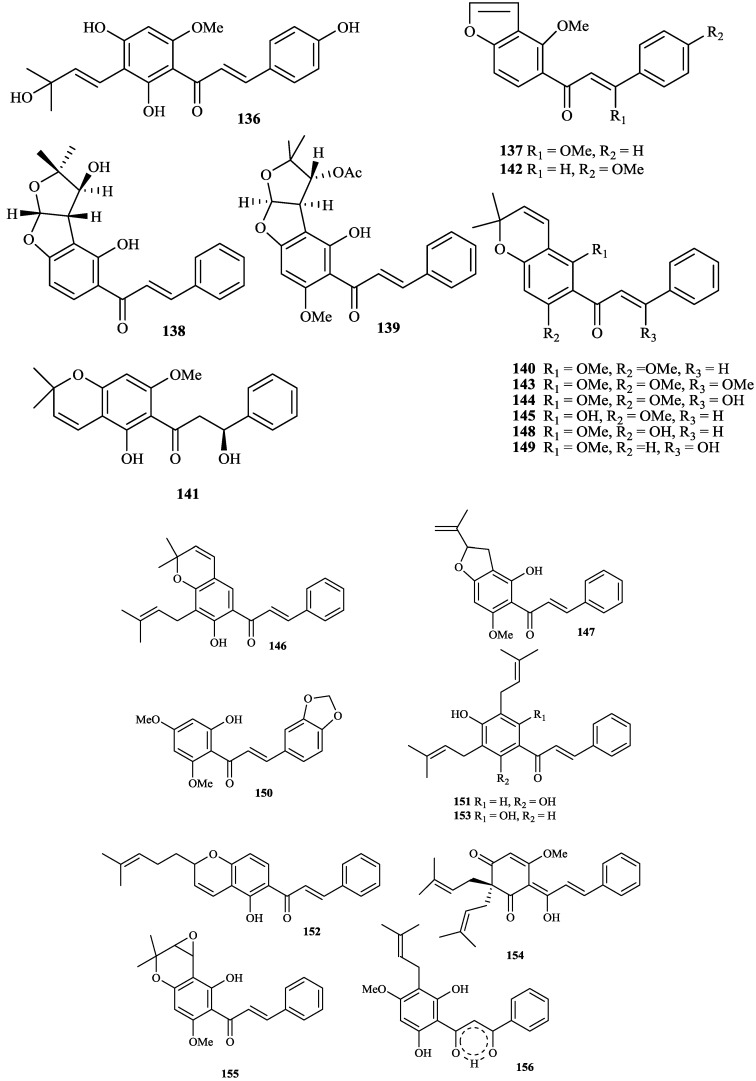
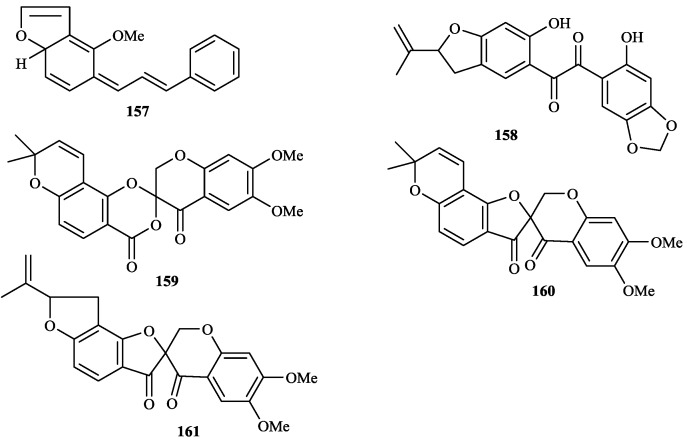
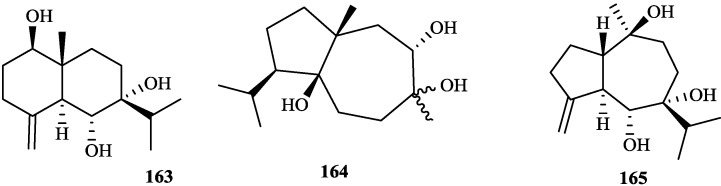
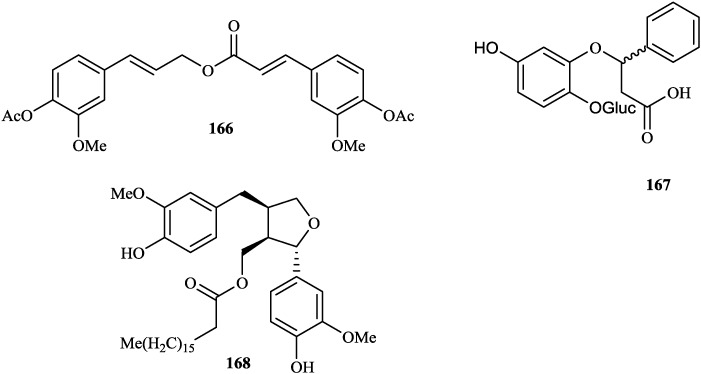
The chemical constituents of the genus Tephrosia reported since 1910 (compounds 1–168) are shown in Table 1 and Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, and Figure 10 below with their names, and their biological sources. As listed in the table and Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, flavonoids are the predominant constituents of this genus.
Table 1.
Chemical constituents from the genus Tephrosia.
| No. | Compound class and name | Source | Ref. | |||
|---|---|---|---|---|---|---|
| Flavones | ||||||
| 1 | tephroglabrin | T. purpurea | [3] | |||
| 2 | tepurindiol | T. purpurea | [3] | |||
| 3 | glabratephrin | T. apollinea | [10] | |||
| 4 | tachrosin | Tephrosia polystachyoides | [17] | |||
| 5 | staohyoidin | T. polystachyoides | [18] | |||
| 6 | tephrodin | T. polystachyoides | [18] | |||
| 7 | semiglabrin | T. semiglabra, T. apollinea | [19,20] | |||
| 8 | semiglabrinol | T. semiglabra, T. apollinea | [10,19] | |||
| 9 | tephrostachin | T. polystachyoides | [21] | |||
| 10 | emoroidone | T. emoroides | [22] | |||
| 11 | tephroapollin C | T. apollinea | [23] | |||
| 12 | tephroapollin D | T. apollinea | [23] | |||
| 13 | tephroapollin E | T. apollinea | [23] | |||
| 14 | tephroapollin F | T. apollinea | [23] | |||
| 15 | tephroapollin G | T. apollinea | [23] | |||
| 16 | multijugin | T. multijuga | [24] | |||
| 17 | multijuninol | T. multijuga | [24] | |||
| 18 | pseudosemiglabrinol | T. apollinea | [25] | |||
| 19 | (−)-pseudosemiglabrin | T. semiglabra | [26] | |||
| 20 | polystachin | T. polystachya | [27] | |||
| 21 | 5-methoxy-6,6-dimethylpyrano[2,3:7,6]flavone | T. praecans | [28] | |||
| 22 | candidin | T. candida | [29] | |||
| 23 | hookerianin | T. hookeriana | [30] | |||
| 24 | fulvinervin B | T. fulvinervis | [31] | |||
| 25 | fulvinervin C | T. fulvinervis | [32] | |||
| 26 | enantiomultijugin | T. viciodes | [33] | |||
| 27 | apollinine | T. purpurea | [34] | |||
| 28 | demethylapollinin 7-O-β-D-glucopyranoside | T. cinerea | [35] | |||
| 29 | tephropurpulin A | T. apollinea, T. purpurea | [36,37] | |||
| 30 | isoglabratephrin | T. purpurea | [37] | |||
| 31 | terpurinflavone | T. purpurea | [38] | |||
| Flavonols | ||||||
| 32 | 6-hydroxykaempferol 6-methyl ether 3-O-α-rhamno-pyranosyl(7→6)-β-galactopyranoside-7-O-α-rhamno-pyranoside | T. vogelii | [1] | |||
| 33 | 6-hydroxykaempferol 6-methyl ether 3-O-α-rhamno-pyranosyl(1→2)[α-rhamnopyranosyl(1→6)-β-galacto-pyranoside | T. vogelii | [1] | |||
| 34 | 6-hydroxykaempferol 6-methyl ether 3-O-α-rhamno-pyranosyl(1→2)[α-rhamnopyranosyl(1→ 6)]-β-galacto-pyranoside-7-O-α-rhamnopyranoside | T. vogelii | [1] | |||
| 35 | 6-hydroxykaempferol 6-methyl ether 3-O-α-rhamnopyranosyl (1→2)[(3-O-E-feruloyl)-α-rhamnopyranosyl(1→6)]-β-galacto-pyranosides | T. vogelii | [1] | |||
| 36 | 6-hydroxykaempferol 4'-methyl ether | T. candida | [39] | |||
| 37 | candidol | [40] | ||||
| 38 | candirone | T. candida | [41,42] | |||
| 39 | 7-ethoxy-3,3',4'-trihydroxyflavone | T. procumbens | [43] | |||
| Flavanonols | ||||||
| 40 | (2R,3R)-3-hydroxy-5-methoxy-6'',6''-dimethylpyrano-[2'',3'':7,8]flavanone | T. vogelii | [1] | |||
| 41 | lupinifolinol | T. lupinifolia | [44] | |||
| 42 | lupinifolinol triacetate | T. lupinifolia | [44] | |||
| Flavans | ||||||
| 43 | (2S)-4'-hydroxy-5-methoxy-6'',6''-dimethylpyrano[2'',3'':7,8]-flavanone | T. vogelii | [1] | |||
| 44 | (2S)-7-hydroxy-5-methoxy-8-prenylflavanone | T. vogelii | [1] | |||
| 45 | (2S)-5-methoxy-6'',6''-dimethy1-4'',5''-dihydrocyclopropa-[4'',5'']furano[2'',3'':7,8]flavanone | T. vogelii | [1] | |||
| 46 | (2S)-5,7-dimethoxy-8-(3-methylbut-1,3-dienyl)flavanone | T. vogelii | [1] | |||
| 47 | tephrocandidin A | T. candida | [2] | |||
| 48 | tephrocandidin B | T. candida | [2] | |||
| 49 | (+)-tephrorin A | T. purpurea | [4] | |||
| 50 | (+)-tephrorin B | T. purpurea | [4] | |||
| 51 | (2S)-5-hydroxy-7,4'-di-O-(γ,γ-dimethylallyl)flavanone | T. calophylla | [6] | |||
| 52 | 6-hydroxy-E-3-(2,5-dimethoxybenzylidine)-2',5'-dimethoxyflavanone | T. calophylla | [6] | |||
| 53 | pumilanol | T. pumila | [13] | |||
| 54 | emoroidenone | T. emoroides | [22] | |||
| 55 | tephroapollin A | T. apollinea | [23] | |||
| 56 | tephroapollin B | T. apollinea | [23] | |||
| 57 | fulvinervin A | T. fulvinervis | [30] | |||
| 58 | lupinifolin | T. lupinifolia | [44] | |||
| 59 | 5,4'-O,O-dimethyl-lupinifolin | T. lupinifolia | [44] | |||
| 60 | lupinifolin diacelate | T. lupinifolia | [44] | |||
| 61 | obovatin | T. obovata | [45] | |||
| 62 | obovatin methyl-ether | T. obovata | [45] | |||
| 63 | methylhildardtol B | T. hildebrandtii | [46] | |||
| 64 | hildgardtol B | T. hildebrandtii | [46] | |||
| 65 | hildgardtene | T. hildebrandtii | [46] | |||
| 66 | methylhildgardtol A | T. hildebrandtii | [46] | |||
| 67 | hildgardtol A | T. hildebrandtii | [46] | |||
| 68 | purpurin | T. purpurea | [47] | |||
| 69 | tephrinone | T. villosa | [48] | |||
| 70 | 5,7-dimethoxy-8-prenylflavan | T. madrensis | [49] | |||
| 71 | tephrowatsin A | T. watsoniana | [50] | |||
| 72 | tephrowatsin C | T. watsoniana | [50] | |||
| 73 | tephrowatsin B | T. watsoniana | [50] | |||
| 74 | tephrowatsin D | T. watsoniana | [50] | |||
| 75 | tephrowatsin E | T. watsoniana | [50] | |||
| 76 | nitenin | T. nitens | [51] | |||
| 77 | falciformin | T. falciformis | [52] | |||
| 78 | candidone | T. candida | [53] | |||
| 79 | quercetol A | T. quercetorum | [54] | |||
| 80 | quercetol B | T. quercetorum | [54] | |||
| 81 | quercetol C | T. quercetorum | [54] | |||
| 82 | 5,7-dimethoxy-8-(2,3-epoxy-3-methylbutyl)-flavanone | T. hamiltonii | [55] | |||
| 83 | tephroleocarpin A | T. leiocarpa | [56] | |||
| 84 | tephroleocarpin B | T. leiocarpa | [56] | |||
| 85 | spinoflavanone A | T. spinosa | [57] | |||
| 86 | spinoflavanone B | T. spinosa | [57] | |||
| 87 | maxima flavanone A | T. maxima | [58] | |||
| 88 | tepicanol A | T. tepicana | [59] | |||
| 89 | crassifolin | T. crassifolia | [60] | |||
| 90 | astraciceran | T. strigosa | [61] | |||
| 91 | (+)-apollineanin | T. apollinea | [62] | |||
| 92 | (2S)-5,4'-dihydroxy-7-O-[E-3,7-dimethyl-2,6-octadienyl]flavanone | T. villosa | [63] | |||
| Isoflavones | ||||||
| 93 | (2S)-5,4'-dihydroxy-7-O-[E-3,7-dimethyl-2,6-octa-dienyl]-8-C-[E-3,7-dimethyl-2,6-octadienyl]flavanone | T. villosa | [63] | |||
| 94 | 7,4'-dihydroxy-3',5'-dimethoxyisoflavone | T. purpurea | [5] | |||
| 95 | emoroidocarpan | T. emoroides | [22] | |||
| 96 | elongatin | T. elongate | [64] | |||
| 97 | pumilaisoflavone D | T. pumila | [65] | |||
| 98 | pumilaisoflavone C | T. pumila | [65] | |||
| 99 | barbigerone | T. barbigera | [66] | |||
| 100 | 4'-demethyltoxicarol isoflavone | T. polyphylla | [67] | |||
| 101 | maxima isoflavone D | T. maxima | [68] | |||
| 102 | maxima isoflavone E | T. maxima | [68] | |||
| 103 | maxima isoflavone F | T. maxima | [68] | |||
| 104 | maxima isoflavone G | T. maxima | [68] | |||
| 105 | viridiflorin | T. viridiflora | [69] | |||
| 106 | maxima isoflavone J | T. maxima | [70] | |||
| 107 | pumilaisoflavone A | T. pumila | [71] | |||
| 108 | pumilaisoflavone B | T. pumila | [71] | |||
| 109 | 7-O-geranylbiochanin A | T. tinctoria | [72] | |||
| 110 | 5,7-di-O-prenylbiochanin A | T. tinctoria | [73] | |||
| 111 | toxicarol | T. toxicaria | [74] | |||
| 112 | villosinol | T. villosa | [75] | |||
| 113 | villosol | T. villosa | [75] | |||
| 114 | villosin | T. villoss | [76] | |||
| 115 | villol | T. villoss | [76] | |||
| 116 | villosone | T. villoss | [76] | |||
| 117 | villinol | T. villoss | [76] | |||
| 118 | dehydrodihydrorotenone | T. candida | [77] | |||
| 119 | dihydrostemonal | T. pentaphylla | [78] | |||
| 120 | 9-demethyldihydrostemonal | T. pentaphylla | [78] | |||
| 121 | 6-acetoxydihydrostemonal | T. pentaphylla | [78] | |||
| 122 | 6a,12a-dehydro-2,3,6-trimethoxy-8-(3',3'-dimethylallyl)-9,11-dihydroxyrotenone | T. villosa | [79] | |||
| 123 | 12a-dehydro-6-hydroxysumatrol | T. villosa | [80] | |||
| 124 | 12a-hydroxyrotenone | T. uniflora | [81] | |||
| 125 | 12a-hydroxy-β-toxicarol | T. candida | [82] | |||
| 126 | tephrosol | T. villosa | [83] | |||
| 127 | tephrocarpin | T. bidwilli | [84] | |||
| 128 | hildecarpin | T. hildebrandtii | [85,86] | |||
| 129 | hildecarpidin | T. hildebrandtii | [87] | |||
| 130 | 2-methoxy-3,9-dihydroxy coumestone | T. hamiltonii | [88] | |||
| 131 | 3,4:8,9-dimethylenedioxypterocarpan | T. aequilata | [89] | |||
| 132 | tephcalostan | T. calophylla | [90] | |||
| 133 | tephcalostan B | T. calophylla | [91] | |||
| Chalcones | ||||||
| 134 | tephcalostan C | T. calophylla | [91] | |||
| 135 | tephcalostan D | T. calophylla | [91] | |||
| 136 | candidachalcone | T. candida | [2] | |||
| 137 | O-methylpongamol | T. purpurea | [3] | |||
| 138 | (+)-tephrosone | T. purpurea | [4] | |||
| 139 | (+)-tephropurpurin | T. purpurea | [5] | |||
| 140 | 2',6'-dimethoxy-4',5'-(2''2''dimethyl)-pyranochalcone | T. pulcherrima | [7] | |||
| 141 | (S)-elatadihydrochalcone | T. elata | [14] | |||
| 142 | purpuritenin | T. purpurea | [15] | |||
| 143 | praecansone A | T. praecans | [28] | |||
| 144 | praecansone B | T. praecans | [28] | |||
| 145 | obovatachalcone | T. obovata | [45] | |||
| 146 | spinochalcone C | T. spinosa | [57] | |||
| 147 | crassichalone | T. crassifolia | [60] | |||
| 148 | oaxacacin | T. woodii | [92] | |||
| 149 | 6'-demethoxypraecansone B | T. purpurea | [93] | |||
| 150 | tephrone | T. candida | [94] | |||
| 151 | spinochalcone A | T. spinosa | [95] | |||
| 152 | spinochalcone B | T. spinosa | [95] | |||
| 153 | 3',5'-diisopentenyl-2',4'-dihydroxychalcone | T. spinosa | [96] | |||
| 154 | tunicatachalcone | T. tunicate | [97] | |||
| 155 | epoxyobovatachalcone | T. carrollii | [98] | |||
| 156 | 2',6'-dihydroxy-3'-prenyl-4'-methoxy-β-hydroxychalcone | T. major | [99] | |||
| Other Flavonoids | ||||||
| 157 | purpureamethied | T. purpurea | [15] | |||
| 158 | calophione A | T. calophylla | [91] | |||
| 159 | tephrospirolactone | T. candida | [100] | |||
| 160 | tephrospiroketone I | T. candida | [100] | |||
| 161 | tephrospiroketone II | T. candida | [100] | |||
| Triterpenoid | ||||||
| 162 | oleanolic acid | T. strigosa | [61] | |||
| Sesquiterpenes | ||||||
| 163 | 1β-hydroxy-6,7α-dihydroxyeudesm-4(15)-ene | T. candida | [2] | |||
| 164 | linkitriol | T. purpurea | [34] | |||
| 165 | 1β,6α,10α-guai-4(15)-ene-6,7,10-triol | T. vogelii | [101] | |||
| Others | ||||||
| 166 | 2-propenoic acid, 3-(4-(acetyloxy) -3-methoxypheny)-3(4-actyloxy)-3-methoxyphenyl)-2-propenyl ester | T. purpurea | [34] | |||
| 167 | cineroside A | T. cinerea | [35] | |||
| 168 | (+)-lariciresinol-9'-stearate | T. vogelii | [101] | |||
Figure 1.
Flavones from genus Tephrosia.
Figure 2.
Flavonols from genus Tephrosia.
Figure 3.
Flavanonols from genus Tephrosia.
Figure 4.
Flavans from genus Tephrosia.
Figure 5.
Isoflavones from genus Tephrosia.
Figure 6.
Chalcones from genus Tephrosia.
Figure 7.
Other flavonoids from genus Tephrosia.
Figure 8.
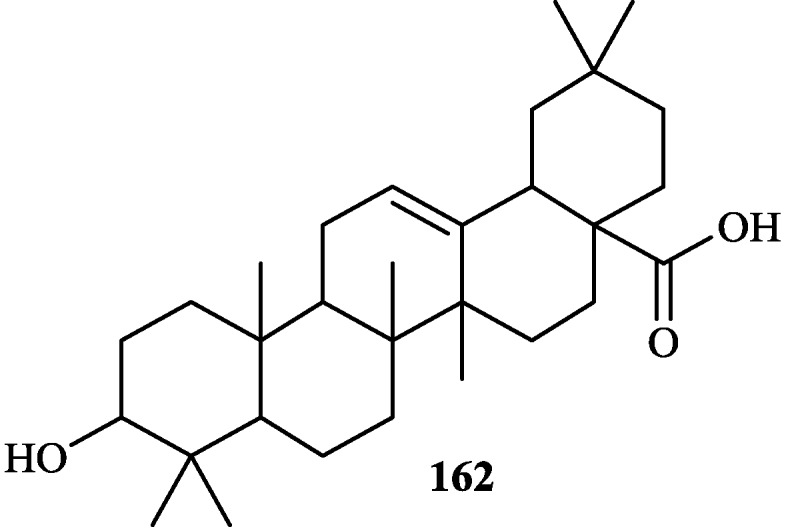
Triterpenoid from genus Tephrosia.
Figure 9.
Sesquiterpenes from genus Tephrosia.
Figure 10.
Other compounds from genus Tephrosia.
2.1. Flavonoids
Flavonoids were the most main constituents of the genus Tephrosia, even of the Leguminosae family. From the year of 1971, 161 flavonoids isolated from the genus Tephrosia are divided into several categories depending on their skeletons (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
2.1.1. Flavones
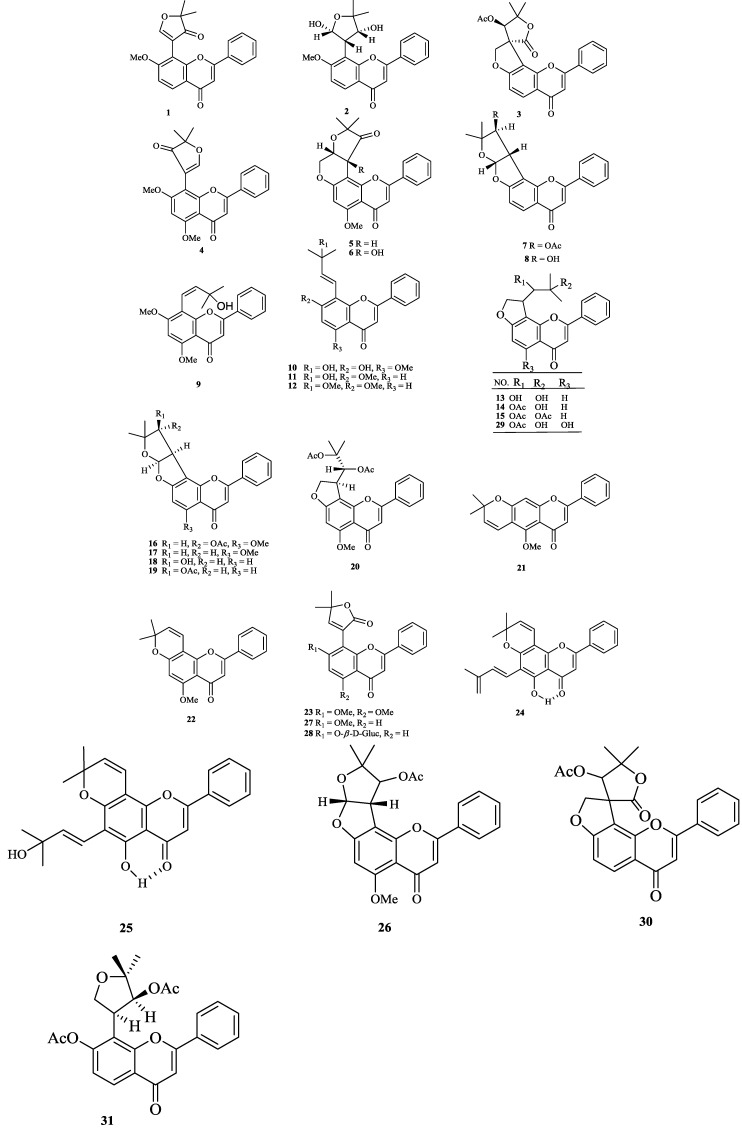
Thirty-one flavones (1–31), were isolated from T. polystachyoides, T. semiglabra, T. multijuga, T. polystachya, T. praecans, T. apollinea, T. candida, T. purpurea, T. fulvinervis, T. viciodes, T. emoroids and T. hookeriana [3,10,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
2.1.2. Flavonols
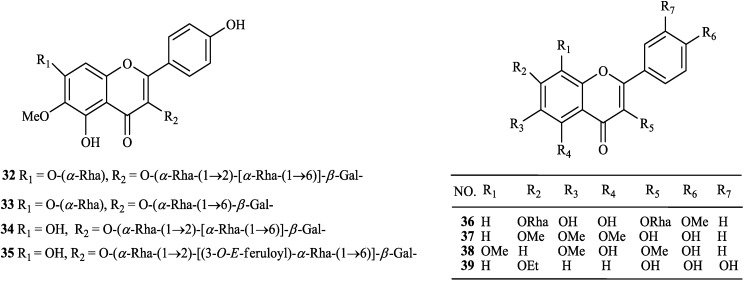
Eight flavonols (32–39), were isolated, four, i.e., 32–34 were obtained from T. vogelii [1], one, i.e., 35–38, from T. candida [39,40,41,42] and 39 from T. procumbens [43].
2.1.3. Flavanonols
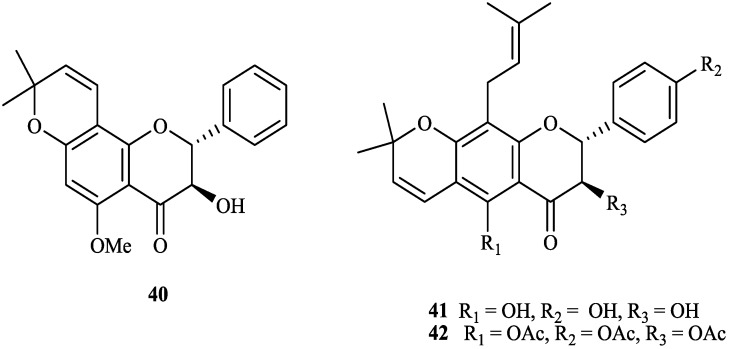
Only three flavanonols, 40, 41 and 42 were isolated from T. vogelii and T. lupinifolia, respectively [1,44].
2.1.4. Flavans
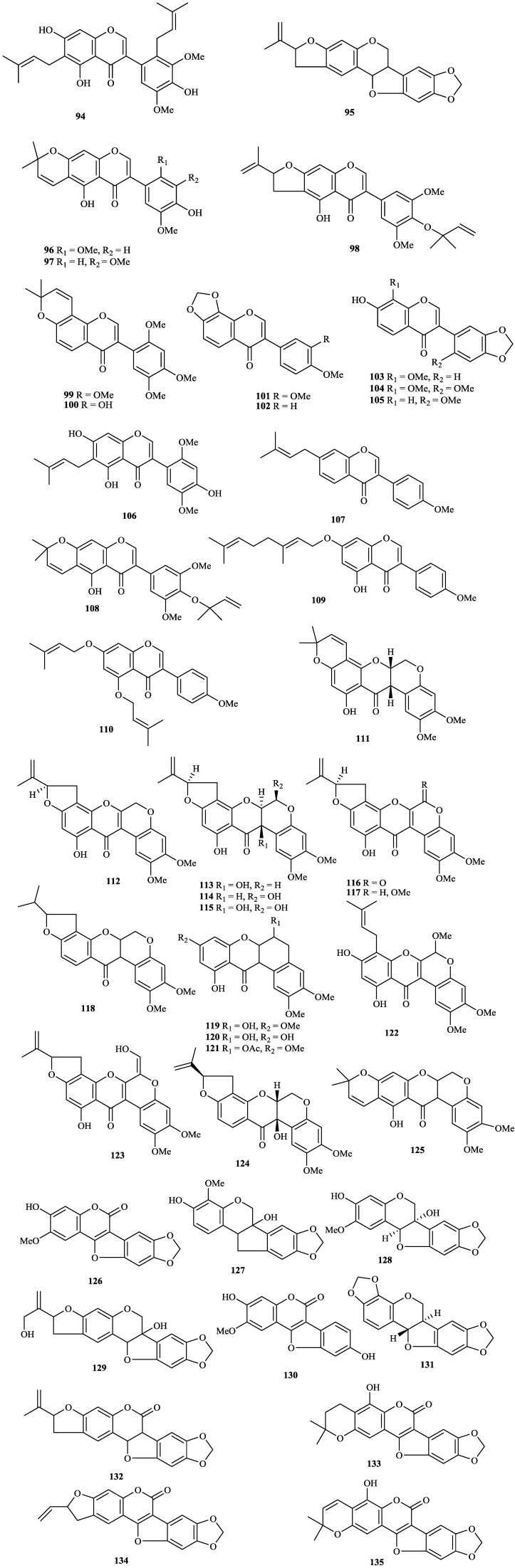
Fifty-one flavans, 43–93, were isolated from twenty-three species of the genus Tephrosia, i.e., T. obovata, T. villosa, T. madrensis, T. nitens, T. watsoniana, T. hildebrandtii, T. falciformis, T. hamiltonii, T. quercetorum, T. leiocarpa, T. spinosa, T. maxima, T. emoroides, T. tepicana, T. crassifolia, T. strigosa, T. pumila, T. calophylla, T. vogelii, T. apollinea, T. candida, T. purpurea and T. fulvinervis [1,2,4,6,13,22,23,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63].
2.1.5. Isoflavones
Forty-two isoflavones, 94–135, have been isolated and identified from this genus [5,22,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. Among them, 111–125 were identified as rotenoids [74,75,76,77,78,79,80,81,82], 94 and 126–135 were identified as coumestan derivatives [22,83,84,85,86,87,88,89,90,91].
2.1.6. Chalcones
Twenty-one chalcones, 136–156, isolated from twelve species of genus Tephrosia, i.e., T. obovata, T. praecans, T. purpurea, T. candida, T. woodii, T. spinosa, T. crassifolia, T. tunicate, T. carrollii, T. major, T. pulcherrima and T. elata [2,3,4,5,7,14,15,28,45,57,60,92,93,94,95,96,97,98,99].
2.1.7. Other Flavonoids
157 was isolated from T. purpurea seeds [15]. 158 was isolated from T. calophylla [91]. 159–161 were isolated from T. candida [100].
2.2. Triterpenoid
Only one triterpenoid has been isolated from this genus, that is 162 from T. strigosa [61].
2.3. Sesquiterpenes
Three sesquiterpenes, 163, 164 and 165 were isolated from T. candida [2], T. purpurea [33] and T. vogelii [101], respectively.
2.4. Others
166–168 have been isolated from T. purpurea [34], T. cinerea [35] and T. vogelii [101], respectively.
3. Proposed Biosynthetic Pathways and Synthesis
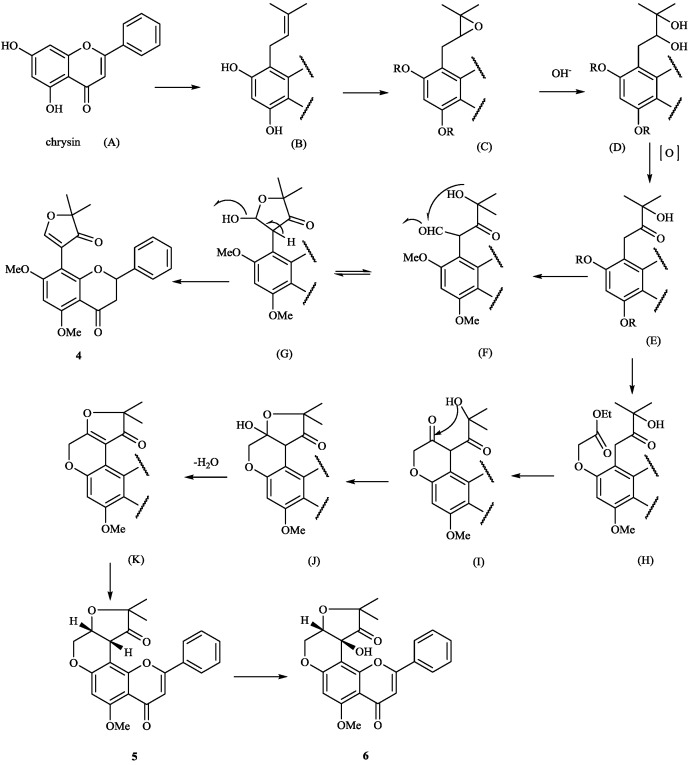
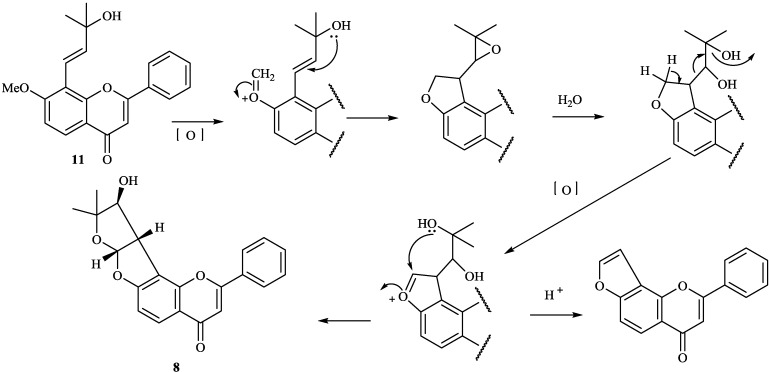
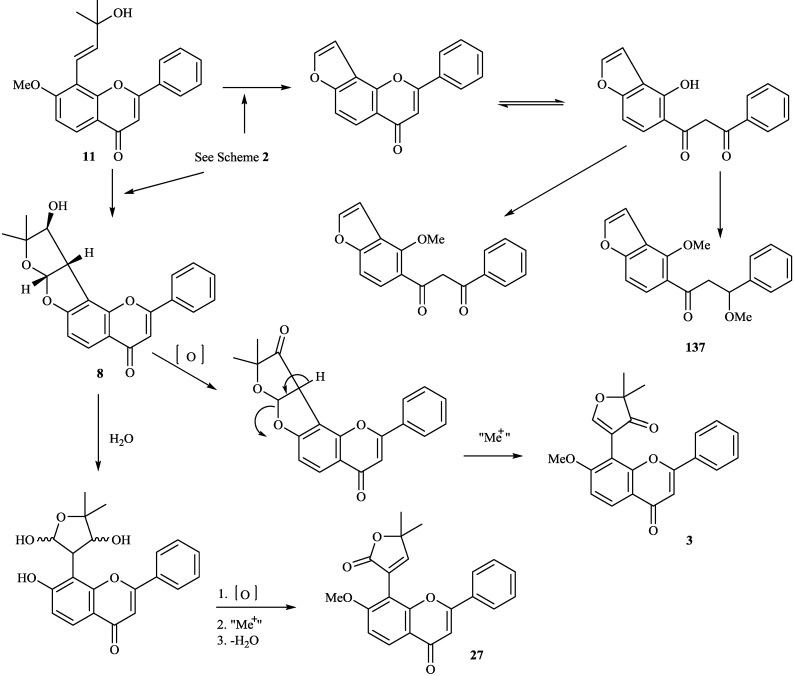
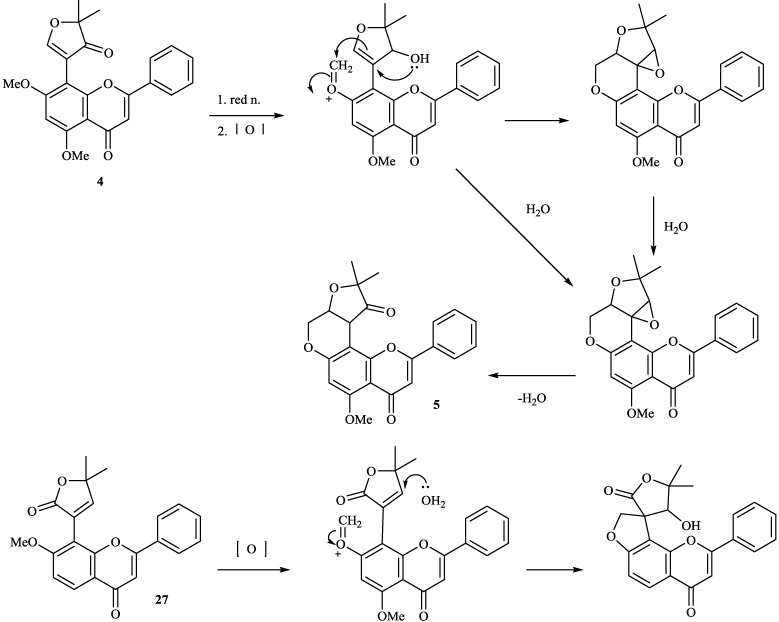
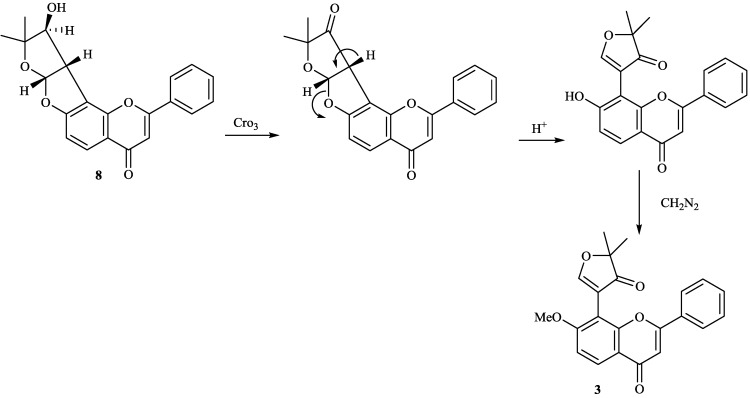
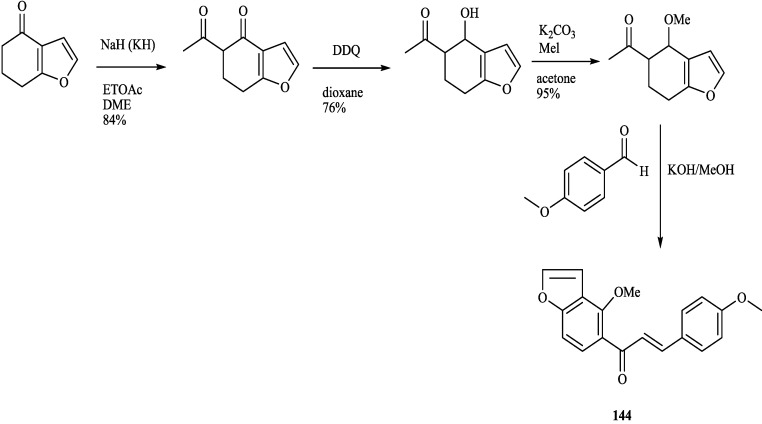
8-Substituted isoflavonoids such as toxicarol isoflavone and rotenoids are well known [3]. Compounds 4–6 from T. polystachyoides could be explained to be evolved biogenetically from naturally occurring chrysins (A) as illustrated in the Scheme 1 [102]. It would appear that the complex substituents at C-8 arise from the ability of Tephrosia species to oxidise a 7-OMe group to a ‒O+=CH2 group (Scheme 2), in the same way that closely related species of Leguminosae oxidise the 2'-OMe group of isoflavonoids to yield rotenoids [103]. A pattern that explains the various C-8 substituents in T. purpurea and T. apollinea is shown in Scheme 3. In T. polystachoides this process is taken even further and the carbon of yet another 7-OMe group is incorporated into the additional rings attached to C-7 and C-8 (Scheme 4) [3]. We could confirm the structures of compounds 7 and 8 by their conversion into semiglabrinone, isoemiglabrinone and tephroglabrin (3) as shown in Scheme 5 [3]. Purpuritenin (142) was isolated from T. purpurea has been synthesed as showed in Scheme 6 [104].
Scheme 1.
Possible biogenetic pathway of compounds 4–6 of T. polystachyoides.
Scheme 2.
Possible biogenetic pathway of compounds 8 and 11.
Scheme 3.
Possible biogenetic pathway of compounds 3, 8, 11, 27 and 137.
Scheme 4.
Possible biogenetic pathway of compounds 4, 5 and 137.
Scheme 5.
Transform of compounds 3 and 8.
Scheme 6.
The synthesis of 144.
4. Biological Activities
The chemical constituents from the genus Tephrosia have been shown to exhibit various bioactivities, such as estrogenic, antitumor, antimicrobial, antiprotozoal, and antifeedant activities [2,105].
4.1. Estrogenic Activity
Candidachalcone (136) isolated from T. candida exhibited estrogenic activity with IC50 value of 80 µM, compared with 18 µM for the natural steroid 17 β-estradiol [2].
4.2. Antitumor Activities
Calophione A (158) and tephcalostans B–D (133–135) from T. calphylla were evaluated for cytotoxicity against RAW (mouse macrophage cells) and HT-29 (colon cancer cells) cancer cell lines. 158 exhibited significant cytotoxicity with IC50 of 5.00 (RAW) and 2.90 µM (HT-29), respectively, while 133–135 showed moderated cytotoxicity against both RAW and HT-29 cell lines [91]. (+)-Tephrorins A (49) and B (50), and (+)-tephrosone (138) isolated from T. purpurea were evaluated for their potential cancer chemopreventive properties using a cell-based quinone reductase induction assay [4]. 7,4'-dihydroxy-3',5'-dimethoxyisoflavone (94), and (+)-tephropurpurin (139), were obtained as active compounds from T. purpurea, using a bioassay based on the induction of quinone reductase (QR) activity with cultured Hepa 1c1c7 mouse hepatoma cells [5].
4.3. Antimicrobial Activities
2',6'-Dimethoxy-4',5'-(2'',2''-dimethyl)-pyranochalcone (140) from T. pulcherrima showed significant antimicrobial activity when tested against a series of micro-organisms [7]. 3,4:8,9-Dimethylenedioxypterocarpan (131) from T. aequilata exhibited low activity against gram-positive bacteria, Bacillus subtilis and Micrococcus lutea [89]. Hildecarpin (128) from T. hildebrandtii had exhibited antifungal activity against Cladosporium cucumerinum [85,86].
4.4. Antiprotozoal Activities
Terpurinflavone (31) isolated from T. purpurea showed the highest antiplasmodial activity against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of Plasmodium falciparum with IC50 values of 3.12 ± 0.28 µM (D6) and 6.26 ± 2.66 µM (W2) [38]. The crude extract of the seedpods of T. elata showed antiplasmodial activities against D6 and W2 strains of P. falciparum with IC50 values of 8.4 ± 0.3 and 8.6 ± 1.0 µg/mL, respectively [14]. Obovatin (61) and obovatin methyl ether (62) from T. obovata [45] showed antiplasmodial activities against D6 and W2 strains of P. falciparum with IC50 values of 4.9 ± 1.7 and 6.4 ± 1.1 µg/mL, and 3.8 ± 0.3 and 4.4 ± 0.6 µg/mL, respectively [14]. (S)-Elatadihydrochalcone (141) from T. elata exhibited good antiplasmodial activity against the D6 and W2 strains of P. falciparum with IC50 values of 2.8 ± 0.3 (D6) and 5.5 ± 0.3 µg/mL (W2), respectively [14]. Tephcalostans C (134) and D (135) from T. calphylla were found to be weakly antiprotozoal activity in vitro [91]. Pumilanol (53) from T. pumila exhibited significant antiprotozoal activity against T. b. rhodensiense, T. cruzi and L. donovani with IC50 of 3.7, 3.35 and 17.2 µg/mL, respectively, but displayed high toxicity towards L-6 (IC50 of 17.12 µg/mL) rat skeletal myoblasts [13]. Tephrinone (69) from T. villosa [48] also exhibited high degree of activity and selectivity against both T. b. rhodensiense, T. cruzi and L. donovani with IC50 of 3.3 and 16.6 µg/mL [13].
4.5. Antifeedant Activities
Emoroidenone (54) from T. emoroides showed strong feeding deterrent activity against Chilo partellus larvae with a mean percentage deterrence of 66.1% at a dose of 100 µg/disc [22]. Hildecarpin (128) from T. hildebrandtii had exhibited insect antifeedant activity against the legume pod-borer Maruca testulalis, and important pest of cowpea (Vigna) [85,86].
4.6. Other Activities
(−)-Pseudosemiglabrin (19) from T. semiglabra displayed in vitro inhibitory effects on human platelet aggregation [26]. Obovatin (61), obovatin methyl-ether (62) and obovatachalcone (145) from T. obovata displayed moderate piscicidal activity against loach fish Misgurnus angullicaudatus. The TLm (median tolerance limit) values of 61, 62 and 145 were 1.25, 1.55 and 1.35 ppm, respectively [45]. Toxicarol (111) was a constituent of the South American fish poison T. toxicaria [74].
5. Conclusions
The genus Tephrosia, including ca. 400 species, with ca. 52 species being investigated worldwide, was reported to possess various chemical constituents and to display diverse bioactivities, especially antiplasmodial, estrogenic, antitumor, antimicrobial, antiprotozoal, antifeedant activities. Although the number of natural compounds was isolated from this genus, there are still many Tephrosia species that received no little attention further, phytochemical and biological studies on this genus are needed in the future. In addition, the biosynthetic pathways and synthesis of these bioactive molecules in the genus remained largely unexplored. Thus, much more chemical, biosynthetic, synthetic and biological studies should be carried out on natural compounds in Tephrosia species in order to disclose their potency, selectivity, toxicity, and availability.
Acknowledgments
We thank the authors of all the references cited herein for their valuable contributions. Financial supported for this work by grants from National Natural Science Foundation of China (No. 31100260, 31200246), Knowledge Innovation Program of Chinese Academy of Sciences (KSCX2-EW-J-28), Program of Guangzhou City (No. 12C14061559), Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences (No. 201210ZS).
Author Contributions
In this paper, Yinning Chen was in charge of writing the manuscript; Tao Yan was responsible for drawing the structures of the compounds; Chenghai Gao was in charge of correcting the revised manuscript; Wenhao Cao was responsible for searching for the literature; Riming Huang is the corresponding author who was responsible for arranging, checking and revising the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stevenson P.C., Kite G.C., Lewis G.P., Forest F., Nyirenda S.P., Belmain S.R., Sileshi G.W., Veitch N.C. Distinct chemotypes of Tephrosia vogelii and implications for their use in pest control and soil enrichment. Phytochemistry. 2012;78:135–146. doi: 10.1016/j.phytochem.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Hegazy M.E.F., Mohamed A.E.H., El-Halawany A.M., Djemgou P.C., Shahat A.A., Pare P.W. Estrogenic activity of chemical constituents from Tephrosia candida. J. Nat. Prod. 2011;74:937–942. doi: 10.1021/np100378d. [DOI] [PubMed] [Google Scholar]
- 3.Pelter A., Ward R.S., Rao E.V., Raju N.R. 8-Substituted flavonoids and 3'-substituted 7-oxygenated chalcones from Tephrosia purpurea. J. Chem. Soc. Perkin Trans. 1. 1981;9:2491–2498. doi: 10.1039/p19810002491. [DOI] [Google Scholar]
- 4.Chang L.C., Chavez D., Song L.L., Farnsworth N.R., Pezzuto J.M., Kinghorn A.D. Absolute configuration of novel bioactive flavonoids from Tephrosia purpurea. Org. Lett. 2000;2:515–518. doi: 10.1021/ol990407c. [DOI] [PubMed] [Google Scholar]
- 5.Chang L.C., Gerhauser C., Song L., Farnsworth N.R., Pezzuto J.M., Kinghorn A.D. Activity-guided isolation of constituents of Tephrosia purpurea with the potential to induce the phase II enzyme, quinone reductase. J. Nat. Prod. 1997;60:869–873. doi: 10.1021/np970236p. [DOI] [PubMed] [Google Scholar]
- 6.Reddy R.V.N., Khalivulla S.I., Reddy B.A.K., Reddy M.V.B., Gunasekar D., Deville A., Bodo B. Flavonoids from Tephrosia calophylla. Nat. Prod. Commun. 2009;4:59–62. [PubMed] [Google Scholar]
- 7.Ganapaty S., Srilakshmi G.V.K., Pannakal S.T., Laatsch H. A pyranochalcone and prenylflavanones from Tephrosia pulcherrima (Baker) drumm. Nat. Prod. Commun. 2008;3:49–52. [Google Scholar]
- 8.Kassem M.E.S., Sharaf M., Shabana M.H., Saleh N.A.M. Bioactive flavonoids from Tephrosia purpurea. Nat. Prod. Commun. 2006;1:953–955. [Google Scholar]
- 9.Clarke G., Banerjee S.C. A glucoside from Tephrosia purpurea. J. Chem. Soc. 1910;97:1833–1837. doi: 10.1039/ct9109701833. [DOI] [Google Scholar]
- 10.Waterman P.G., Khalid S.A. The major flavonoids of the seed of Tephrosia apollinea. Phytochemistry. 1980;19:909–915. doi: 10.1016/0031-9422(80)85137-5. [DOI] [Google Scholar]
- 11.Kole R.K., Satpathi C., Chowdhury A., Ghosh M.R., Adityachaudhury N. Isolation of amorpholone, a potent rotenoid insecticide from Tephrosia candida. J. Agric. Food Chem. 1992;40:1208–1210. doi: 10.1021/jf00019a026. [DOI] [Google Scholar]
- 12.Sanchez I., Gomez-Garibay F., Taboada J., Ruiz B.H. Antiviral effect of flavonoids on the dengue virus. Phytother. Res. 2000;14:89–92. doi: 10.1002/(SICI)1099-1573(200003)14:2<89::AID-PTR569>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Ganapaty S., Pannakal S.T., Srilakshmi G.V.K., Lakshmi P., Waterman P.G., Brun R. Pumilanol, an antiprotozoal isoflavanol from Tephrosia pumila. Phytochem. Lett. 2008;1:175–178. doi: 10.1016/j.phytol.2008.09.006. [DOI] [Google Scholar]
- 14.Muiva L.M., Yenesew A., Derese S., Heydenreich M., Peter M.G., Akala H.M., Eyase F., Waters N.C., Mutai C., Keriko J.M., et al. Antiplasmodial beta-hydroxydihydrochalcone from seedpods of Tephrosia elata. Phytochem. Lett. 2009;2:99–102. doi: 10.1016/j.phytol.2009.01.002. [DOI] [Google Scholar]
- 15.Sinha B., Natu A.A., Nanavati D.D. Prenylated flavonoids from Tephrosia purpurea seeds. Phytochemistry. 1982;21:1468–1470. doi: 10.1016/0031-9422(82)80177-5. [DOI] [Google Scholar]
- 16.Touqeer S., Saeed M.A., Ajaib M. A review on the phytochemistry and pharmacology of genus Tephrosia. Phytopharmacology. 2013;4:598–637. [Google Scholar]
- 17.Smalberg T.M., Vleggaar R., de Waal H.L. Tachrosin: A new flavone from Tephrosia polystachyoides Bak F. S. Afr. J. Chem. 1971;24:1–8. [Google Scholar]
- 18.Vleggaar R., Smalberg T.M., de Waal H.L. Two new flavones from Tephrosia polystachyoides Bakf 2. Tetrahedron Lett. 1972;8:703–704. doi: 10.1016/S0040-4039(01)84415-4. [DOI] [Google Scholar]
- 19.Smalberg T.M., van den Berg A.J., Vleggaar R. Flavonoids from Tephrosia—VI: The structure of semiglabrin and semiglabrinol. Tetrahedron. 1973;29:3099–3104. doi: 10.1016/S0040-4020(01)93450-7. [DOI] [Google Scholar]
- 20.Vleggaar R., Kruger G.J., Smalberger T.M., van den Berg A.J. Flavonoids from Tephrosia. XI1. Structure of glabratephrin. Tetrahedron. 1978;34:1405–1408. doi: 10.1016/0040-4020(78)88338-0. [DOI] [Google Scholar]
- 21.Vleggaar R., Smalberg T.M., de Waal H.L. Flavonoids from Tephrosia. V. Structure of tephrostachin. S. Afr. J. Chem. 1973;26:71–73. [Google Scholar]
- 22.Machocho A.K., Lwande W., Jondiko J.I., Moreka L.V.C., Hassanali A. Threenew flavonoids from the root of Tephrosia emoroides and their antifeedant activity against the larvae of the spotted stalk Borer Chilo-Partellus Swinhoe. Pharmaceut. Biol. 1995;33:222–227. doi: 10.3109/13880209509065367. [DOI] [Google Scholar]
- 23.El-Razek M.H.A., Mohamed A.E.H.H., Ahmed A. Prenylated flavonoids, from Tephrosia apollinea. Heterocycles. 2007;71:2477–2490. doi: 10.3987/COM-07-11089. [DOI] [Google Scholar]
- 24.Vleggaar R., Smalberger T.M., van den Berg A.J. Flavonoids from Tephrosia. IX. Structure of multijugin and multijuginol. Tetrahedron. 1975;31:2571–2573. doi: 10.1016/0040-4020(75)80271-7. [DOI] [Google Scholar]
- 25.Ahmad S. Natural occurrence of Tephrosia flavones. Phytochemistry. 1986;25:955–958. [Google Scholar]
- 26.Jonathan L.T., Gbeassor M., Che C.T., Fong H.H.S., Farnsworth N.R., Lebreton G.C., Venton D.L. Pseudosemiglabrin, a platelet-aggregation inhibitor from Tephrosia semiglabra. J. Nat. Prod. 1990;53:1572–1574. doi: 10.1021/np50072a029. [DOI] [PubMed] [Google Scholar]
- 27.Vleggaar R., Smalberger T.M., van Aswegen J.L. Flavonoids from Tephrosia. X. Structure of polystachin. S. Afr. J. Chem. 1978;31:47–50. [Google Scholar]
- 28.Camele G., Dellemonache F., Dellemonache G., Marinibettolo G.B. Three new flavonoids from Tephrosia praecans. Phytochemistry. 1980;19:707–709. doi: 10.1016/0031-9422(80)87050-6. [DOI] [Google Scholar]
- 29.Chibber S.S., Dutt S.K. Candidin, a pyranoflavone from Tephrosia candida seeds. Phytochemistry. 1981;20:1460–1460. doi: 10.1016/0031-9422(81)80074-X. [DOI] [Google Scholar]
- 30.Prabhakar P., Vanangamudi A., Gandhidasan R., Raman P.V. Hookerianin: A flavone from Tephrosia hookeriana. Phytochemistry. 1996;43:315–316. doi: 10.1016/0031-9422(96)00188-4. [DOI] [Google Scholar]
- 31.Rao E.V., Venkataratnam G., Vilain C. Flavonoids from Tephrosia fulvinervis. Phytochemistry. 1985;24:2427–2430. doi: 10.1016/S0031-9422(00)83056-3. [DOI] [Google Scholar]
- 32.Venkataratnam G., Rao E.V., Vilain C. Fulvinervin C, a flavone from Tephrosia fulvinervis. Phytochemistry. 1986;25:1507–1508. doi: 10.1016/S0031-9422(00)81327-8. [DOI] [Google Scholar]
- 33.Gomezgaribay F., Quijano L., Hernandez C., Rios T. Flavonoids from Tephrosia species. IX. Enantiomultijugin, a flavone from Tephrosia viciodes. Phytochemistry. 1992;31:2925–2926. [Google Scholar]
- 34.Khalafalah A.K., Yousef A.H., Esmail A.M., Abdelrazik M.H., Hegazy M.E., Mohamed A.E. Chemical constituents of Tephrosia purpurea. Pharmacogn. Res. 2010;2:72–75. doi: 10.4103/0974-8490.62951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldini M., Montoro P., Macchia M., Pizza C., Piacente S. Profiling of phenolics from Tephrosia cinerea. Planta Med. 2011;77:1861–1864. doi: 10.1055/s-0030-1271190. [DOI] [PubMed] [Google Scholar]
- 36.Khalafallah A.K., Suleiman S.A., Yousef A.H., El-kanzi N.A.A., Mohamed A.E.H.H. Prenylated flavonoids from Tephrosia apollinea. Chin. Chem. Lett. 2009;20:1465–1468. doi: 10.1016/j.cclet.2009.05.025. [DOI] [Google Scholar]
- 37.Hegazy M.E.F., Abd El-Razek M.H., Nagashima F., Asakawa Y., Pare P.W. Rare prenylated flavonoids from Tephrosia purpurea. Phytochemistry. 2009;70:1474–1477. doi: 10.1016/j.phytochem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Juma W.P., Akala H.M., Eyase F.L., Muiva L.M., Heydenreich M., Okalebo F.A., Gitu P.M., Peter M.G., Walsh D.S., Imbuga M., et al. Terpurinflavone: An antiplasmodial flavone from the stem of Tephrosia purpurea. Phytochem. Lett. 2011;4:176–178. doi: 10.1016/j.phytol.2011.02.010. [DOI] [Google Scholar]
- 39.Sarin J.P.S., Singh S., Garg H.S., Khanna N.M., Dhar M.M. Flavonol glycoside with anticancer activity from Tephrosia candida. Phytochemistry. 1976;15:232–234. [Google Scholar]
- 40.Dutt S.K., Chibber S.S. Candidol, a flavonol from Tephrosia candida. Phytochemistry. 1983;22:325–326. doi: 10.1016/S0031-9422(00)80128-4. [DOI] [Google Scholar]
- 41.Parmar V.S., Jain R., Simonsen O., Boll P.M. Isolation of candirone—A novel pentaoxygenation pattern in a naturally-occurring 2-phenyl-4H-1-benzopyran-4-one from Tephrosia candida. Tetrahedron. 1987;43:4241–4247. doi: 10.1016/S0040-4020(01)83467-0. [DOI] [Google Scholar]
- 42.Horie T., Kawamura Y., Kobayashi T., Yamashita K. Revised structure of a natural flavone from Tephrosia candida. Phytochemistry. 1994;37:1189–1191. doi: 10.1016/S0031-9422(00)89555-2. [DOI] [Google Scholar]
- 43.Venkataratnam G., Rao E.V., Vilain C. Flavonoids of Tephrosia procumbens—Revised structure for praecansone A and conformation of praecansone B. J. Chem. Soc. Perkin Trans. 1. 1987;12:2723–2727. doi: 10.1039/p19870002723. [DOI] [Google Scholar]
- 44.Smalberg T.M., Vleggaar R., Weber J.C. Flavonoids from Tephrosia. VII: Constitution and absolute-configuration of lupinifolin and lupinifolinol, two flavanones from Tephrosia lupinifolia Burch (Dc) Tetrahedron. 1974;30:3927–3931. [Google Scholar]
- 45.Chen Y.L., Wang Y.S., Lin Y.L., Munakata K., Ohta K. Obovatin, obovatin methyl-ether and obovatachalcone, new piscicidal flavonoids from Tephrosia obovata. Agric. Biol. Chem. Tokyo. 1978;42:2431–2432. doi: 10.1271/bbb1961.42.2431. [DOI] [Google Scholar]
- 46.Dellemonache F., Labbiento L., Marta M., Lwande W. 4-β-substituted flavans from Tephrosia hildebrandtii. Phytochemistry. 1986;25:1711–1713. doi: 10.1016/S0031-9422(00)81241-8. [DOI] [Google Scholar]
- 47.Gupta R.K., Krishnamurti M., Parthasarathi J. Purpurin, a new flavanone from Tephrosia purpurea seeds. Phytochemistry. 1980;19:1264–1264. doi: 10.1016/0031-9422(80)83109-8. [DOI] [Google Scholar]
- 48.Rao P.P., Srimannarayana G. Tephrinone, a new flavanone from Tephrosia villosa. Curr. Sci. India. 1981;50:319–320. [Google Scholar]
- 49.Gomez F., Quijano L., Garcia G., Calderon J.S., Rios T. A prenylated flavan from Tephrosia madrensis. Phytochemistry. 1983;22:1305–1306. doi: 10.1016/0031-9422(83)80255-6. [DOI] [Google Scholar]
- 50.Gomez F., Quijano L., Calderon J.S., Rodriquez C., Rios T. Prenylflavans from Tephrosia watsoniana. Phytochemistry. 1985;24:1057–1059. doi: 10.1016/S0031-9422(00)83182-9. [DOI] [Google Scholar]
- 51.Gomez F., Calderon J., Quijano L., Cruz O., Rios T. Nitenin—A new flavan from Tephrosia nitens Beth. Chem. Ind. 1984;17:632–632. [Google Scholar]
- 52.Khan H.A., Chandrasekharan I., Ghanim A. Falciformin, a flavanone from pods of Tephrosia falciformis. Phytochemistry. 1986;25:767–768. doi: 10.1016/0031-9422(86)88049-9. [DOI] [Google Scholar]
- 53.Ganguly A., Bhattacharyya P., Bhattacharyya A., Adityachaudhury N. Synthesis of Candidone—A new flavanone isolated from Tephrosia candida. Indian J. Chem. B. 1988;27:462–463. [Google Scholar]
- 54.Gomezgaribay F., Quijano L., Calderon J.S., Morales S., Rios T. Flavonoids from Tephrosia species. VI. Prenylflavanols from Tephrosia quercetorum. Phytochemistry. 1988;27:2971–2973. doi: 10.1016/0031-9422(88)80699-X. [DOI] [Google Scholar]
- 55.Hussaini F.A., Shoeb A. A new epoxyflavanone from Tephrosia hamiltonii. Planta Med. 1987;2:220–221. doi: 10.1055/s-2006-962679. [DOI] [PubMed] [Google Scholar]
- 56.Gomezgaribay F., Quijano L., Rios T. Flavonoids from Tephrosia species. VII. Flavanones from Tephrosia leiocarpa. Phytochemistry. 1991;30:3832–3834. doi: 10.1016/0031-9422(91)80129-O. [DOI] [Google Scholar]
- 57.Rao E.V., Prasad Y.R. Prenylated flavonoids from Tephrosia spinosa. Phytochemistry. 1993;32:183–185. [Google Scholar]
- 58.Rao E.V., Prasad Y.R., Murthy M.S.R. A prenylated flavanone from Tephrosia maxima. Phytochemistry. 1994;37:111–112. doi: 10.1016/0031-9422(94)85007-0. [DOI] [Google Scholar]
- 59.Gomez-Garibay F., Calderon J.S., Quijano L., Tellez O., Olivares M.D., Rios T. Flavonoids from Tephrosia species part 8—An unusual prenyl biflavanol from Tephrosia tepicana. Phytochemistry. 1997;46:1285–1287. [Google Scholar]
- 60.Gomez-Garibay F., Calderon J.S., Arciniega M.D., Cespedes C.L., Tellez-Valdes O., Taboada J. Flavonoids from Tephrosia species part 9—An unusual isopropenyldihydrofuran biflavanol from Tephrosia crassifolia. Phytochemistry. 1999;52:1159–1163. doi: 10.1016/S0031-9422(99)00336-2. [DOI] [Google Scholar]
- 61.Rao E.V., Sridhar P. Chemical examination of Tephrosia strigosa. Indian J. Chem. B. 1999;38:872–873. [Google Scholar]
- 62.Hisham A., John S., Al-Shuaily W., Asai T., Fujimoto Y. (+)-Apollineanin: A new flavanone from Tephrosia apollinea. Nat. Prod. Res. 2006;20:1046–1052. doi: 10.1080/14786410500399714. [DOI] [PubMed] [Google Scholar]
- 63.Madhusudhana J., Reddy R.V.N., Reddy B.A.K., Reddy M.V.B., Gunasekar D., Deville A., Bodo B. Two new geranyl flavanones from Tephrosia villosa. Nat. Prod. Res. 2010;24:743–749. doi: 10.1080/14786410903020560. [DOI] [PubMed] [Google Scholar]
- 64.Smalberger T.M., Vleggaar R., Weber J.C. Flavonoids from Tephrosia. VIII: Structure of elongatin, an isoflavone from Tephrosia elongata E Mey. Tetrahedron. 1975;31:2297–2301. [Google Scholar]
- 65.Yenesew A., Dagne E., Waterman P.G. Flavonoids from the seed pods of Tephrosia pumila. Phytochemistry. 1989;28:1291–1292. doi: 10.1016/0031-9422(89)80240-7. [DOI] [Google Scholar]
- 66.Vilain C. Barbigerone, a new pyranoisoflavone from seeds of Tephrosia barbigera. Phytochemistry. 1980;19:988–989. doi: 10.1016/0031-9422(80)85162-4. [DOI] [Google Scholar]
- 67.Dagne E., Mammo W., Sterner O. Flavonoids of Tephrosia polyphylla. Phytochemistry. 1992;31:3662–3663. doi: 10.1016/0031-9422(92)83754-M. [DOI] [Google Scholar]
- 68.Rao E.V., Murthy M.S.R., Ward R.S. Nine isoflavones from Tephrosia maxima. Phytochemistry. 1984;23:1493–1501. doi: 10.1016/S0031-9422(00)80493-8. [DOI] [Google Scholar]
- 69.Gomez F., Calderon J.S., Quijano L., Dominguez M., Rios T. Viridiflorin, an isoflavone from Tephrosia viridiflora. Phytochemistry. 1985;24:1126–1128. doi: 10.1016/S0031-9422(00)83209-4. [DOI] [Google Scholar]
- 70.Murthy M.S.R., Rao E.V. Maxima isoflavone J: A new O-prenylated isoflavone from Tephrosia maxima. J. Nat. Prod. 1985;48:967–968. doi: 10.1021/np50042a015. [DOI] [Google Scholar]
- 71.Dagne E., Dinku B., Gray A.I., Waterman P.G. Pumilaisoflavone A and Pumilaisoflavone B from the seed pods of Tephrosia pumila. Phytochemistry. 1988;27:1503–1505. doi: 10.1016/0031-9422(88)80224-3. [DOI] [Google Scholar]
- 72.Reddy B.A.K., Khalivulla S.I., Gunasekar D. A new prenylated isoflavone from Tephrosia tinctoria. Indian J. Chem. B. 2007;46:366–369. doi: 10.1080/10286020802217630. [DOI] [PubMed] [Google Scholar]
- 73.Khalivulla S.I., Reddy B.A.K., Gunasekar D., Blond A., Bodo B., Murthy M.M., Rao T.P. A new di-O-prenylated isoflavone from Tephrosia tinctoria. J. Asian Nat. Prod. Res. 2008;10:953–955. doi: 10.1080/10286020802217630. [DOI] [PubMed] [Google Scholar]
- 74.Clark E.P. Toxicarol. A constituent of the South American fish poison Cracca (Tephrosia) toxicaria. J. Am. Chem. Soc. 1930;52:2461–2464. [Google Scholar]
- 75.Sarma P.N., Srimannarayana G., Rao N.V.S. Constitution of villosol and villosinol, twonew rotenoids from Tephrosia villosa (Linn) pods. Indian J. Chem. B. 1976;14:152–156. [Google Scholar]
- 76.Krupadanam G.L.D., Sarma P.N., Srimannarayana G., Rao N.V.S. New C-6 oxygenated rotenoids from Tephrosia villosa—Villosin, villosone, villol and villinol. Tetrahedron Lett. 1977;24:2125–2128. [Google Scholar]
- 77.Roy M., Bhattacharya P.K., Pal S., Chowdhuri A., Adityachaudhury N. Dehydrodihydrorotenone and flemichapparin B in Tephrosia candida. Phytochemistry. 1987;26:2423–2424. doi: 10.1016/S0031-9422(00)84741-X. [DOI] [Google Scholar]
- 78.Dagne E., Yenesew A., Waterman P.G. Flavonoids and isoflavonoids from Tephrosia fulvinervis and Tephrosia pentaphylla. Phytochemistry. 1989;28:3207–3210. doi: 10.1016/0031-9422(89)80308-5. [DOI] [Google Scholar]
- 79.Prashant A., Krupadanam G.L.D. A new prenylated dehydrorotenoid from Tephrosia villosa seeds. J. Nat. Prod. 1993;56:765–766. doi: 10.1021/np50095a015. [DOI] [Google Scholar]
- 80.Prashant A., Krupadanam G.L.D. Dehydro-6-hydroxyrotenoid and lupenone from Tephrosia villosa. Phytochemistry. 1993;32:484–486. doi: 10.1016/S0031-9422(00)95025-8. [DOI] [Google Scholar]
- 81.Abreu P.M., Luis M.H. Constituents of Tephrosia uniflora. Nat. Prod. Lett. 1996;9:81–86. doi: 10.1080/10575639608044930. [DOI] [Google Scholar]
- 82.Andrei C.C., Viera P.C., Fernandes J.B., daSilva M.F.D.F., Fo E.R. Dimethylchromene rotenoids from Tephrosia candida. Phytochemistry. 1997;46:1081–1085. doi: 10.1016/S0031-9422(97)00405-6. [DOI] [Google Scholar]
- 83.Rao P.P., Srimannarayana G. Tephrosol, a new coumestone from the roots of Tephrosia villosa. Phytochemistry. 1980;19:1272–1273. doi: 10.1016/0031-9422(80)83114-1. [DOI] [Google Scholar]
- 84.Ingham J.L., Markham K.R. Tephrocarpin, a pterocarpan phytoalexin from Tephrosia bidwilli and a structure proposal for acanthocarpan. Phytochemistry. 1982;21:2969–2972. doi: 10.1016/0031-9422(80)85079-5. [DOI] [Google Scholar]
- 85.Lwande W., Bentley M.D., Hassanali A. The structure of hildecarpin, an insect antifeedant 6a-hydroxypterocarpan from the roots of Tephrosia hildebrandtii Vatke. Int. J. Trop. Insect Sci. 1986;7:501–503. doi: 10.1017/S1742758400009723. [DOI] [Google Scholar]
- 86.Lwande W., Hassanali A., Njoroge P.W., Bentley M.D., Delle Monache F., Jondiko J.I. A new 6a-hydroxypterocarpan with insect antifeedant and antifungal properties from the roots of Tephrosia hildebrandtii Vatke. Int. J. Trop. Insect Sci. 1985;6:537–541. doi: 10.1017/S1742758400004379. [DOI] [Google Scholar]
- 87.Lwande W., Bentley M.D., Macfoy C., Lugemwa F.N., Hassanali A., Nyandat E. A new pterocarpan from the roots of Tephrosia hildebrandtii. Phytochemistry. 1987;26:2425–2426. doi: 10.1016/S0031-9422(00)84742-1. [DOI] [Google Scholar]
- 88.Rajani P., Sarma P.N. A coumestone from the roots of Tephrosia hamiltonii. Phytochemistry. 1988;27:648–649. doi: 10.1016/0031-9422(88)83168-6. [DOI] [Google Scholar]
- 89.Tarus P.K., Machocho A.K., Lang’at-Thoruwa C.C., Chhabra S.C. Flavonoids from Tephrosia aequilata. Phytochemistry. 2002;60:375–379. doi: 10.1016/S0031-9422(02)00078-X. [DOI] [PubMed] [Google Scholar]
- 90.Kishore P.H., Reddy M.V.B., Gunasekar D., Murthy M.M., Caux C., Bodo B. A new coumestan from Tephrosia calophylla. Chem. Pharm. Bull. (Tokyo) 2003;51:194–196. doi: 10.1248/cpb.51.194. [DOI] [PubMed] [Google Scholar]
- 91.Ganapaty S., Srilakshmi G.V.K., Pannakal S.T., Rahman H., Laatsch H., Brun R. Cytotoxic benzil and coumestan derivatives from Tephrosia calophylla. Phytochemistry. 2009;70:95–99. doi: 10.1016/j.phytochem.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Dominguez X.A., Tellez O., Ramirez G. Mixtecacin, a prenylated flavanone and oaxacacin its chalcone from the roots of Tephrosia woodii. Phytochemistry. 1983;22:2047–2049. doi: 10.1016/0031-9422(83)80042-9. [DOI] [Google Scholar]
- 93.Rao E.V., Raju N.R. Two flavonoids from Tephrosia purpurea. Phytochemistry. 1984;23:2339–2342. doi: 10.1016/S0031-9422(00)80547-6. [DOI] [Google Scholar]
- 94.Chibber S.S., Dutt S.K. Tephrone, a new chalcone from Tephrosia candida seeds. Curr. Sci. India. 1982;51:933–934. [Google Scholar]
- 95.Rao E.V., Prasad Y.R. Two chalcones from Tephrosia spinosa. Phytochemistry. 1992;31:2121–2122. doi: 10.1016/0031-9422(92)80376-P. [DOI] [Google Scholar]
- 96.Sharma V.M., Rao P.S. A prenylated chalcone from the roots of Tephrosia spinosa. Phytochemistry. 1992;31:2915–2916. doi: 10.1016/0031-9422(92)83666-M. [DOI] [Google Scholar]
- 97.Andrei C.C., Ferreira D.T., Faccione M., de Moraes L.A.B., de Carvalho M.G., Braz R. C-prenylflavonoids from roots of Tephrosia tunicata. Phytochemistry. 2000;55:799–804. doi: 10.1016/S0031-9422(00)00371-X. [DOI] [PubMed] [Google Scholar]
- 98.Gomez-Garibay F., Arciniega M.D.O., Cespedes C.L., Taboada J., Calderon J.S. Chromene chalcones from Tephrosia carrollii and the revised structure of oaxacacin. Z. Naturforsch. C. 2001;56:969–972. doi: 10.1515/znc-2001-11-1210. [DOI] [PubMed] [Google Scholar]
- 99.Gomez-Garibay F., Tellez-Valdez O., Moreno-Torres G., Calderon J.S. Flavonoids from Tephrosia major. A new prenyl-β-hydroxychalcone. Z. Naturforsch. C. 2002;57:579–583. doi: 10.1515/znc-2002-7-805. [DOI] [PubMed] [Google Scholar]
- 100.Andrei C.C., Vieira P.C., Fernandes J.B., da Silva M.F., Rodrigues Fo E. New spirorotenoids from Tephrosia candida. Z. Naturforsch. C. 2002;57:418–422. doi: 10.1515/znc-2002-5-602. [DOI] [PubMed] [Google Scholar]
- 101.Wei H.H., Xu H.H., Xie H.H., Xu L.X., Wei X.Y. Sesquiterpenes and lignans from Tephrosia vogelii. Helv. Chim. Acta. 2009;92:370–374. doi: 10.1002/hlca.200800272. [DOI] [Google Scholar]
- 102.Jain A.C., Gupta R.C. Possible biogenesis of novel type of flavones from Tephrosia polystachyoides. Curr. Sci. India. 1978;47:770–770. [Google Scholar]
- 103.Crombie L., Dewick P.M., Whiting D.A. Biosynthesis of rotenoids—Chalcone, isoflavone, and rotenoid stages in formation of amorphigenin by Amorpha fruticosa seedlings. J. Chem. Soc. Perkin Trans. 1. 1973;12:1285–1294. doi: 10.1039/p19730001285. [DOI] [Google Scholar]
- 104.Lee Y.R., Morehead A.T. A new route for the synthesis of furanoflavone and furanochalcone natural products. Tetrahedron. 1995;51:4909–4922. doi: 10.1016/0040-4020(95)98689-F. [DOI] [Google Scholar]
- 105.Belmain S.R., Amoah B.A., Nyirend S.P., Kamanula J.F., Stevenson P.C. Highly variable insect control efficacy of Tephrosia vogelii Chemotypes. J. Agric. Food Chem. 2012;60:10055–10063. doi: 10.1021/jf3032217. [DOI] [PubMed] [Google Scholar]