Abstract
Artichoke (Cynara scolymus L.) is one of the world’s oldest medicinal plants with multiple health benefits. We have previously shown that artichoke leaf extracts and artichoke flavonoids upregulate the gene expression of endothelial-type nitric oxide synthase (eNOS) in human endothelial cells. Whereas NO produced by the eNOS is a vasoprotective molecule, NO derived from the inducible iNOS plays a pro-inflammatory role in the vasculature. The present study was aimed to investigate the effects of artichoke on iNOS expression in human coronary artery smooth muscle cells (HCASMC). Incubation of HCASMC with a cytokine mixture led to an induction of iNOS mRNA expression. This iNOS induction was concentration- and time-dependently inhibited by an artichoke leaf extract (1–100 µg/mL, 6 h or 24 h). Consistently, the artichoke leaf extract also reduced cytokine-induced iNOS promoter activation and iNOS protein expression. In addition, treatment of HCASMC with four well-known artichoke compounds (cynarin > cyanidin > luteolin ≈ cynaroside) led to a downregulation iNOS mRNA and protein expression, with cynarin being the most potent one. In conclusion, artichoke contains both eNOS-upregulating and iNOS-downregulating compounds. Such compounds may contribute to the beneficial effects of artichoke and may per se have therapeutic potentials.
Keywords: nitric oxide, inducible NO synthase, vascular smooth muscle cells, artichoke, Cynara scolymus L.
1. Introduction
In blood vessels, nitric oxide (NO) can be produced by the endothelial-type NO synthase (eNOS) or the inducible NO synthase (iNOS). Under physiological conditions, iNOS is absent in the vasculature and vascular NO is mainly produced by eNOS. This enzyme is constitutively expressed in the endothelium and is activated by shear stress of the flowing blood or by agonists such as bradykinin and acetylcholine. NO produced in endothelial cells can diffuse into the underlying smooth muscle cells and induce vasodilation by stimulating NO-sensitive guanylyl cyclase. Endothelial NO can also diffuse into the blood and inhibit platelet aggregation and adhesion. In addition to these antihypertensive and antithrombotic actions, eNOS-derived NO also possesses multiple anti-atherosclerotic properties, including prevention of leukocyte adhesion to vascular endothelium and leukocyte migration into the vascular wall, inhibition of low-density lipoprotein oxidation, and inhibition of vascular smooth muscle cell proliferation [1,2]. Genetic depletion of eNOS leads to exacerbation of diet-induced atherosclerosis in the apolipoprotein E-knockout mouse model. The blood pressure of eNOS knockout mice is about 30% higher than that of wild-type animals [3].
Under conditions of inflammation, sepsis, or oxidative stress, iNOS expression can be induced in blood vessels. In contrast to the regulated production of NO by eNOS, iNOS may generate large amounts of NO over long periods of time, if substrate and cofactors are not limited. This excessive NO from iNOS leads to vascular dysfunction evident as impairment of both vasoconstriction and endothelium-dependent vasorelaxation. Several mechanisms have been proposed by which iNOS impairs contractile responses, including continuous activation of the soluble guanylyl cyclase [4]; abnormal vascular calcium regulation [5]; and oxidative modification of catecholamines [6]. In parallel, the endothelium-dependent, NO-mediated vasodilation response (e.g., to acetylcholine or bradykinin) is also impaired by iNOS. This may result from reduced NO production by eNOS [4,7] or enhanced inactivation of eNOS-derivied NO by superoxide [8]. Tetrahydrobiopterin is an essential cofactor for NO production by NOS enzymes. iNOS expressed in the endothelium competes with eNOS for tetrahydrobiopterin and reduces NO production from eNOS by limiting availability of tetrahydrobiopterin for eNOS [4]. The continuous generation of NO by iNOS induced in the media can impair the signal transduction cascade that links activation of endothelial receptors to the calcium-calmodulin-dependent activation of eNOS [7]. Moreover, the reduction of endothelium-dependent relaxation may be mediated in part by reduced reactivity of smooth muscle cells to NO [9].
The consequence of this dysregulation (impaired vasomotor reactivity to both vasoconstrictor and vasodilator agonists) can be seen, for example, in septic shock. Septic shock is characterized by massive arteriolar vasodilatation, hypotension, and microvascular damage. Inappropriate vasodilation, abnormal regulation of blood flow to organs, myocardial suppression, and interference with cellular respiration all contribute to hypotension and mortality in septic shock [3,10]. Bacterial endotoxins usually initiate the symptoms and the fall in blood pressure is predominantly due to excess NO production by iNOS induced in the vascular wall. Endotoxin administration in experimental animals leads to high expression of iNOS in vascular smooth muscle cells and impairs contractile responses [11]. Inhibitors of iNOS largely restore the contractile responses to agonists in animal models of sepsis [12] and reverse the hypotension of patients in septic shock [13]. iNOS mutant mice have a blunted hypotensive response to sepsis [14]. Recently, a non-synonymous SNP in the iNOS gene has been shown to be associated with increased susceptibility to septic shock in Chinese populations [15].
The induction of iNOS in the vasculature is also associated with enhanced formation of peroxynitrite [8,16,17], a key pathogenic mechanism in conditions such as septic shock, stroke, myocardial infarction, chronic heart failure, diabetes, and atherosclerosis [18,19]. iNOS is present in human atherosclerosis plaque. Genetic deficiency of iNOS reduces atherosclerosis in apolipoprotein E-knockout mice [20]. iNOS also contributes to tissue damage after cerebral ischemia. Inhibition of iNOS by selective pharmacologic inhibitors [21], or gene deletion of iNOS [22] reduces brain damage.
Collectively, eNOS and iNOS have opposite roles in the vasculature with eNOS being protective and iNOS mostly detrimental. Compounds that upregulate eNOS or downregulate iNOS are of therapeutic interest. In a previous study, we have demonstrated that artichoke leaf extracts and artichoke flavonoids enhance eNOS expression and NO production in human endothelial cells [23]. The present study shows that artichoke inhibits iNOS expression in vascular smooth muscle cells.
2. Results and Discussion
In the present study, we demonstrate for the first time that artichoke leaf extract and artichoke compounds downregulate iNOS expression human coronary artery smooth muscle cells when administered concurrently with an inflammatory stimulus.
2.1. Artichoke Leaf Extracts Downregulate iNOS Expression
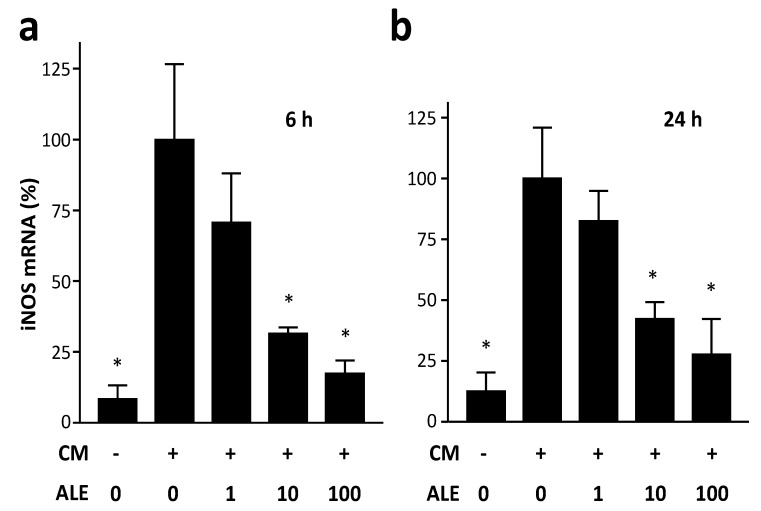
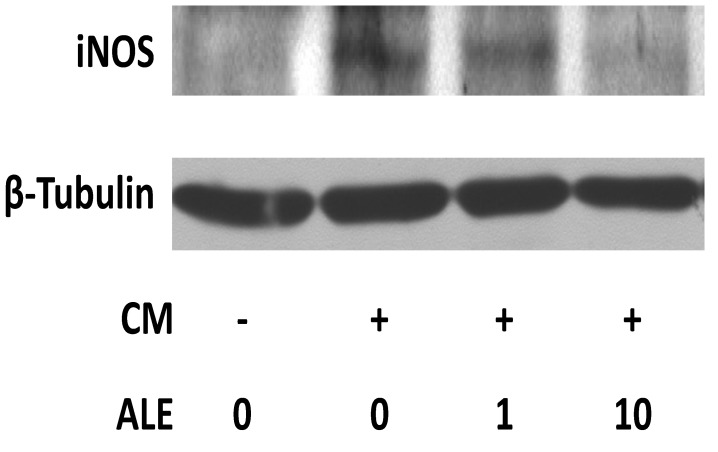
Incubation of HCASMC with the cytokine mixture (CM) led to an induction of iNOS mRNA expression. The induction of iNOS mRNA expression was more pronounced at 6 h than at 24 h (Figure 1). At both time points, the artichoke leaf extract (ALE) decreased the CM-induced iNOS expression at concentrations of 10 and 100 µg/mL. The kinetics of iNOS induction in HCASMC is likely to be similar as that in human intestinal epithelial DLD-1 cells in which the maximum of the CM-induced iNOS mRNA expression is reached at 6–12 h whereas iNOS protein expression and NO production are induced with a delay of several h [24]. Therefore, in the following experiments, iNOS mRNA expression and iNOS promoter activity were analyzed at 6 h whereas iNOS protein expression and NO production were studied at 24 h after CM treatment. At the protein level, ALE also concentration-dependently decreased the CM-induced iNOS expression (Figure 2).
Figure 1.
Artichoke leaf extracts downregulate iNOS mRNA expression. Human coronary artery smooth muscle cells were treated with the cytokine mixture (CM) or CM in combination with the artichoke leaf extract (ALE, µg/mL) for 6 h (a) or 24 h (b). Human iNOS mRNA expression was analyzed with real-time RT-PCR. * p < 0.05, compared with CM.
Figure 2.
Artichoke leaf extracts downregulate iNOS protein expression. Human coronary artery smooth muscle cells were treated with the cytokine mixture (CM) or CM in combination with an artichoke leaf extract (ALE, µg/mL) for 24 h. Western blot analyses were performed with a monoclonal anti-iNOS-antibody (top) or a monoclonal antibody to β-tubulin (bottom, for normalization). The blots shown are representative of three independent experiments with similar results.
2.2. Artichoke Leaf Extracts Reduce Cytokine-Induced NO Production
Among the three NOS isoforms, iNOS is a calcium-independent enzyme. NO production by iNOS correlates well with the expression level of the enzyme. Therefore, the inhibition of iNOS expression by ALE should result in a reduction of NO production.
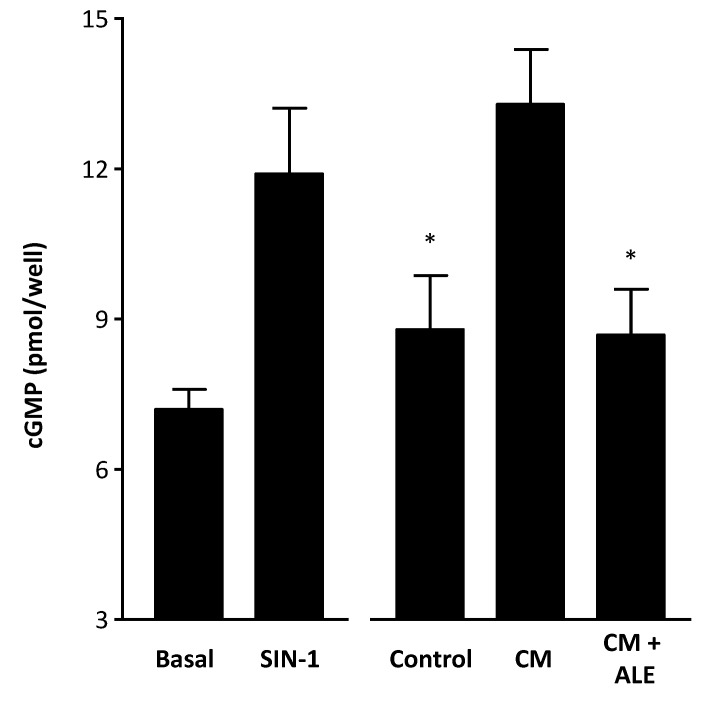
To measure NO production from HCASMC, we applied the RFL-6 reporter cell assay. These cells express relatively high level of guanylyl cyclase and produce cGMP when stimulated with NO [25]. Incubation of RFL-6 cells with conditioned media from CM-treated HCASMC led to an increase of cGMP content, a surrogate marker for NO. The increase in cGMP content was significantly reduced by ALE (Figure 3).
Figure 3.
Artichoke leaf extracts prevent CM-induced cGMP production. Human coronary artery smooth muscle cells (HCASMC) were treated with the cytokine mixture (CM) or CM in combination with the artichoke leaf extract (ALE, 10 µg/mL) for 24 h. Then, conditioned media from HCASMC was transferred to RFL-6 reporter cells. The cGMP content in the RFL-6 cells was measured by radioimmunoassay. RFL-6 cells without conditioned media from HCASMC (basal) and RFL-6 cells treated for 3 min with the NO donor linsidomine (SIN-1, 1 µM) served as negative and positive controls, respectively. * p < 0.05, compared with CM.
2.3. Artichoke Leaf Extracts Inhibit iNOS Promoter Activity
The mRNA expression of iNOS can be regulated at the transcriptional level or the post-transcriptional level (via regulation of iNOS mRNA stability) [26]. We used a stably transfected cell line as a reliable and feasible tool for studying iNOS promoter activity. The stable A549/8 cells contain a 16 kb fragment of the human iNOS promoter cloned in front of a luciferase reporter gene [27]. The luciferase activity can be used as a measure of iNOS promoter activity.
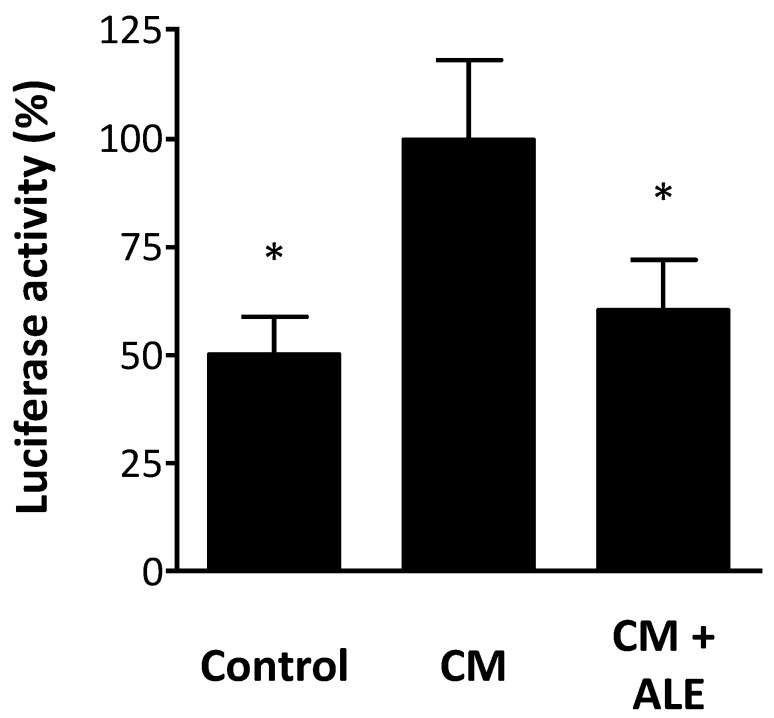
Treatment of the stable A549/8 cells with CM led to an induction of iNOS promoter activity (Figure 4). This transcriptional activation was significantly inhibited by ALE (Figure 4). Thus, the reduction of iNOS expression at mRNA and protein levels by ALE is likely to result, at least in part, from an inhibition of iNOS transcription.
Figure 4.
Artichoke leaf extracts reduce iNOS promoter activity. Human alveolar epithelium-like A549/8 cells were stably transfected with a construct containing a 16 kb fragment of the human iNOS promoter cloned in front of a luciferase reporter gene. The cells were treated with the cytokine mixture (CM) or CM in combination with an artichoke leaf extract (ALE, 10 µg/mL) for 6 h. Then, the cells were lysed, and luciferase activity was determined. The luciferase activity (normalized to protein content) was taken as a measure of iNOS promoter activity. * p < 0.05, compared with CM.
Cytokine-induced iNOS expression results from both transcriptional activation and post-transcriptional mechanisms (mRNA stabilization) [24]. This may be the reason why the induction of promoter activity (Figure 4) is less pronounced than that of mRNA expression (Figure 1). It is possible that ALE may also reduce iNOS expression partially by decreasing iNOS mRNA half-life. However, this remains an assumption at this time point as we did not study the effect of ALE on iNOS mRNA stability in the present study.
2.4. Artichoke Compounds Inhibit iNOS Expression
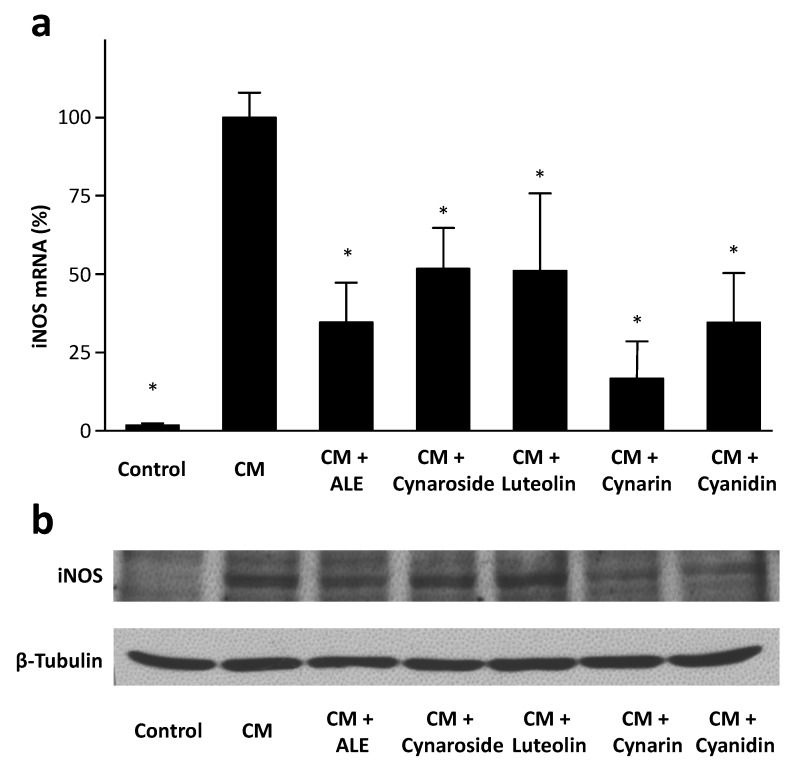
Artichoke is rich in polyphenolic compounds, with mono- and dicaffeoylquinic acids as the major chemical components [28]. The most well-known caffeoylquinic acid derivative identified in artichoke extracts (heads and leaves), even though it is not the most abundant, is cynarin [28]. Besides caffeoylquinic acid derivatives, other phenolics belonging to the flavonoid class such as the flavones (e.g., luteolin and its 7-O-glucoside, cynaroside) and the anthocyanidins (e.g., cyanidin) have been identified in artichoke tissues [28]. Therefore, we studied the effects of these four well-known artichoke compounds on iNOS expression in HCASMC.
As shown in Figure 5, all four tested compounds (cynarin > cyanidin > luteolin ≈ cynaroside) downregulated iNOS mRNA expression in HCASMC, with cynarin being the most potent one (Figure 5a). At the protein level, cynarin and cyanidin clearly reduced iNOS expression, whereas luteolin and cynaroside had only minor effects (Figure 5b).
Figure 5.
Artichoke compounds downregulate iNOS expression. Human coronary artery smooth muscle cells were treated with the cytokine mixture (CM) or CM in combination with an artichoke leaf extract (ALE, 10 µg/mL) or the artichoke compounds (10 µM each) for 6 h (a) or 24 h (b). Human iNOS mRNA expression was analyzed with real-time RT-PCR (a). * p < 0.05, compared with CM. Protein expression of iNOS was studied with Western blot analyses (b). The blots shown are representative of three independent experiments with similar results.
In our previous study, we have found that the flavone luteolin and its 7-O-glucoside cynaroside enhance eNOS expression in endothelial cells whereas the caffeoylquinic acids (cynarin and chlorogenic acid) are without effect [23]. These results, together with the data from the present study, indicate that the active constituents responsible for the artichoke effects on eNOS and iNOS are likely to be different compounds.
2.5. Potential Therapeutic Relevance
Artichoke is one of the world’s oldest medicinal plants. It has been known by the ancient Egyptians, and the ancient Greeks and Romans used it as a digestive aid. Long known as an herbal medicine, the dried leaves of artichoke have been used in folk medicine because of their choleretic and hepatoprotective activities [28].
In various pharmacological test systems, artichoke leaf extracts have shown choleretic activity [29], hepatoprotective [30,31] and prebiotic effects [32,33]. In addition, artichoke leaf extracts contain compounds with antifungal [32] and antimicrobial activities [34].
Recent studies indicate that artichoke leaf extracts may also have therapeutic potential for cardiovascular disease. The plant contains multiple compounds with high antioxidant properties [35,36]. Antioxidative effects of artichoke extracts have been shown in endothelial cells [37,38] and leukocytes [37,39] in vitro, as well as in experimental animals in vivo [40,41]. Luteolin-rich artichoke extracts and luteolin itself protect low-density lipoprotein (LDL) from oxidation in vitro [42]. In a rat model of streptozotocin-induced diabetes, an artichoke leaf extract reduced the plasma malondialdehyde and urinary 8-hydroxydeoxyguanosine levels, and increased erythrocyte glutathione levels [41].
Although it is still a matter of debate [43], there is some evidence that artichoke leaf extract may lower cholesterol levels. In randomized, double-blind, placebo-controlled clinical trials, artichoke leaf extract reduced the total cholesterol levels in hypercholesterolemic adults [44,45].
A previous study from our laboratory shows that artichoke leaf extracts upregulate eNOS expression in human endothelial cells [23]. Increased NO production by artichoke extracts has also been shown in porcine aortic endothelial cells [46]. In a randomized, placebo-controlled trial, concentrated artichoke leaf juice significantly lowered blood pressure (by ≈ 3 mmHg after 12 weeks) in patients with mild hypertension [47].
The present study demonstrates that ALE, cynarin and cyanidin inhibit iNOS expression in vascular smooth muscle cells when administered concurrently with an inflammatory stimulus. We did not analyze whether the compounds in ALE have any effect on iNOS expression without an inflammatory stimulus. The cynarin concentration in the ALE we used is not known; but it could be in similar ranges as that in methanolic extracts of artichoke (≈1.5%) [28]. Based on this assumption, the cynarin concentration of 100 µg/mL ALE would be ≈3 µM, a concentration that is relevant to the effect of cynarin on iNOS expression. We are aware that we have only studied a few candidate compounds in the present study, and we may have missed a number of active compounds. It should also be reminded that the effects of artichoke extracts cannot be attributed to one or two single compounds. Rather, the in vivo effect of a plant extract is more likely to result from multiple active compounds that act additively or synergistically. It is also possible that some compounds contained in ALE may have antagonistic effects, or complex and unpredictable effects worthy of future study.
The therapeutic potential of cynarin needs to be further investigated. Several issues should be considered in future studies including the bioavailability of the compound and the specificity of its action. After ingestion of artichoke extracts, cynarin and other caffeoylquinic acids are not found in human plasma. However, caffeoylquinic acid metabolites (such as caffeic acid, ferulic acid, isoferulic acid, dihydrocaffeic acid, and dihydroferulic acid) are detected in considerable concentrations [48,49]. It is still unknown whether the metabolites of cynarin are effective in inhibiting iNOS expression. In addition to its effect on iNOS, cynarin has antioxidative activities [39] and immuno-suppressive effects [50]. Recent studies indicate that cynarin may have the potential to serve as a chemosensitizing agent by reversing P-glycoprotein-mediated multidrug resistance [31]; but it may also induce drug-drug interactions by inhibiting organic anion transporters [51]. Therefore, animal studies are required to test the therapeutic potential and possible side effects of cynarin in vivo.
Anthocyanidins (e.g., cyanidin) and their glycosides (i.e., anthocyanins) are responsible for the brilliant color of fruits and flowers and are widely ingested by humans [52]. Cyanidin and its glycosides show antioxidant, anti-inflammatory, and antimutagenic effects [52]. Cyanidin 3-O-β-d-glucoside reduces cytokine-induced iNOS and cyclooxygenase-2 (COX-2) expression in intestinal cells [53] and macrophages [54]. Moreover, cyanidin 3-O-β-d-glucoside has been shown to reduce ochratoxin-induced iNOS expression in vivo [55]. Orally administered cyanidin 3-O-β-d-glucoside is metabolized to cyanidin and protocatechuic acid by intestinal microflora [7] and these metabolites are detectable in blood and urine [56]. Cyanidin itself has also been shown to suppresses phorbol ester-induced COX-2 and iNOS expression in human colon adenocarcinoma cell line HT-29 cells [57] and in RAW 264.7 macrophages [56]. In an air pouch model of inflammation in mouse skin, both cyanidin 3-O-β-d-glucoside and cyanidin inhibit carrageenan-induced iNOS and COX2 expression, as well as the production of inflammatory cytokines after oral application [56]. These results are compatible with the present study and support the concept that cyanidin may have therapeutic potentials.
3. Experimental
3.1. Cell Culture
Primary human coronary artery smooth muscle cells (HCASMC) were purchased from PromoCell (Heidelberg, Germany) and cultured in Smooth Muscle Cell Growth Medium 2 (PromoCell). For iNOS induction, HCASMC were incubated with a triple cytokine mixture (CM) containing IFN-γ (100 U/mL), IL-1β (50 U/mL) and TNF-α (10 ng/mL) [58]. To study the effects of artichoke, the cells were treated with CM in combination with LI-220 or artichoke compounds. LI-220 was an aqueous artichoke leaf extract (ALE) provided by Lichtwer Pharma AG (Berlin, Germany). Cynarin(1,5-dicaffeoylquinic acid; CAS number 30964-13-7) and cynaroside (luteolin-7-O-glucoside; CAS number 68321-11-9) were obtained from AppliChem (Darmstadt, Germany). Luteolin (CAS number 491-70-3) was from Calbiochem/Merck Millipore (Darmstadt, Germany) and cyanidin chloride (CAS number 528-58-5) was from Carl Roth (Karlsruhe, Germany).
3.2. Real-Time RT-PCR for iNOS mRNA Analyses
Human iNOS mRNA expression was analyzed with quantitative real-time RT-PCR using an iCycler iQ System (Bio-Rad, Munich, Germany). Total RNA was isolated from HCASMC by guanidinium thiocyanatephenol-chloroform extraction. Total RNA (0.5 µg) was used for real-time RT-PCR analysis with the QuantiTect Probe RT-PCR kit (QIAGEN, Hilden, Germany). For real-time PCR, the following oligonucleotides served as sense and antisense primers and Taqman hybridization probes: iNOS, sense 5'- TGC AGA CAC GTG CGT TAC TCC-3', antisense 5'- GGT AGC CAG CAT AGC GGA TG-3', probe 5'-TGG CAA GCA CGA CTT CCG GGT G-3'; Pol2a (the large subunit of RNA polymerase II), sense 5'- GCA CCA CGT CCA ATG ACA T-3', antisense 5'-GTG CGG CTG CTT CCA TAA-3', probe 5'-TAC CAC GTC ATC TCC TTT GAT GGC TCC TAT-3' [58]. Pol2a was detected for normalization.
3.3. Western Blot for iNOS Protein Analyses
Western blot analyses were performed with total protein samples (30 µg each) from HCASMC. Protein samples were separated on a Bis-Tris gel and transferred to a nitrocellulose membrane. Blots were blocked in 5% milk powder in TBST (10 mM Tris-HCl, pH 7.4, 150 mM NaCl with 0.1% Tween 20) for one h at room temperature. The primary antibody (a monoclonal anti-iNOS-antibody, R&D Systems, Wiesbaden, Germany) was diluted in the same solution used for blocking at 4 °C overnight. Blots were then washed in TBST and incubated with a horseradish peroxidase-conjugated secondary antibody diluted in 5% milk in TBST for one h. After washing in TBST and then in TBS, the immunocomplexes were developed using an enhanced horseradish peroxidase/luminol chemiluminescence reagent (PerkinElmer Life and Analytical Sciences, Boston, MA, USA) according to the manufacturer’s instructions [59].
3.4. Reporter Cell Assay for Determination of NO Production
NO production by HCASMC was bioassayed with RFL-6 rat lung fibroblasts as reporter cells [25,60]. HCASMC were treated with CM (with or without ALE) for 24 h. For determination of NO production, HCASMC and RFL-6 cells were washed twice with Locke’s solution (154.0 mM NaCl, 5.6 mM KCl, 2.0 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5.6 mM glucose, 10.0 mM HEPES, pH 7.4). Then, HCASMC were incubated with Locke’s solution containing 200 U/mL SOD and 100 mM L-arginine, and RFL-6 cells with Locke’s solution containing 0.6 mM of the phosphodiesterase inhibitor IBMX for 20 min. After the preincubation, HCASMC were incubated for 2 min at 37 °C in 1 mL of Locke’s solution containing 200 U/mL SOD, 0.3 mM IBMX and 100 mM L-arginine. Then, the conditioned media containing the NO released from HCASMC were transferred to the RFL-6 cells, and another incubation of 2 min at 37 °C was performed. The reaction was stopped by aspiration of the solution, adding 1 mL of ice-cold 50 mM sodium acetate, pH 4.0, and rapidly freezing the cells with liquid nitrogen. The cGMP content of the RFL-6 samples was determined by radioimmunoassay as described [25,60].
3.5. Reporter Gene Assay for Determination of iNOS Promoter Activity
The human alveolar epithelium-like A549/8 cells were stably transfected with a construct containing a 16 kb fragment of the human iNOS promoter cloned in front of a luciferase reporter gene [27]. This cell line was used as a tool for analyzing iNOS promoter activity. The cells were treated with CM in the presence or absence of ALE. Then, cells were lysed in 1 × Passive Lysis Buffer (Promega). Firefly luciferase activity was determined using the Dual Luciferase Assay Kit (Promega). Protein concentrations of the extracts were determined by Bradford reagent using BSA as standard. Luciferase activity normalized to protein content of the extracts was used as a determinant of iNOS promoter activity [27].
3.6. Statistics
Data are presented as means with the standard deviation (SD). Statistical analyses were performed by one-way ANOVA with Bonferroni post-test. Differences were considered as significant when p < 0.05.
4. Conclusions
Artichoke is a medicinal plant with multiple health benefits. In the present study, we have found a new effect of artichoke leaf extracts and artichoke compounds (cynarin and cyanidin): inhibition of iNOS expression in vascular smooth muscle cells. The therapeutic potential of cynarin and cyanidin, however, needs to be verified in future studies in vivo.
Acknowledgments
The A549/8 cell line containing a 16 kb fragment of the human iNOS promoter was generated by the laboratory of Hartmut Kleinert, Department of Pharmacology, Mainz, Germany. This work was supported partly by Lichtwer Pharma AG, Berlin, Germany (now part of Klosterfrau Healthcare Group).
Author Contributions
N.X., A.P., U.W, and G.R. performed the experiments and analyzed the data. U.F. and H.L. designed the study. N.X. and H.L. wrote the manuscript.
Conflictts of Interest
This study was partly supported by Lichtwer Pharma AG, Berlin, Germany (now part of Klosterfrau Healthcare Group).
Footnotes
Sample Availability: Samples of the compounds (cynarin, cyanidin, luteolin and cynaroside) are available from the authors.
References
- 1.Li H., Forstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 2.Li H., Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Liu V.W., Huang P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunnett C.A., Lund D.D., McDowell A.K., Faraci F.M., Heistad D.D. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 2005;25:1617–1622. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 5.Chen S.J., Li S.Y., Shih C.C., Liao M.H., Wu C.C. NO contributes to abnormal vascular calcium regulation and reactivity induced by peritonitis-associated septic shock in rats. Shock. 2010;33:473–478. doi: 10.1097/SHK.0b013e3181bea334. [DOI] [PubMed] [Google Scholar]
- 6.Shelkovnikov S., Gonick H.C. Peroxynitrite but not nitric oxide donors destroys epinephrine: HPLC measurement and rat aorta contractility. Life Sci. 2004;75:2765–2773. doi: 10.1016/j.lfs.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Kessler P., Bauersachs J., Busse R., Schini-Kerth V.B. Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler. Thromb. Vasc. Biol. 1997;17:1746–1755. doi: 10.1161/01.ATV.17.9.1746. [DOI] [PubMed] [Google Scholar]
- 8.Virdis A., Colucci R., Fornai M., Blandizzi C., Duranti E., Pinto S., Bernardini N., Segnani C., Antonioli L., Taddei S., et al. Cyclooxygenase–2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: Role of inducible nitric-oxide synthase and oxidative stress. J. Pharmacol. Exp. Ther. 2005;312:945–953. doi: 10.1124/jpet.104.077644. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi D., D'Uscio L.V., Wambi C., Weiler D., Kovesdi I., O'Brien T., Katusic Z.S. Inhibitory effect of recombinant iNOS gene expression on vasomotor function of canine basilar artery. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2560–H2566. doi: 10.1152/ajpheart.00415.2002. [DOI] [PubMed] [Google Scholar]
- 10.Forstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julou-Schaeffer G., Gray G.A., Fleming I., Schott C., Parratt J.R., Stoclet J.C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am. J. Physiol. 1990;259:H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- 12.Pittner A., Nalos M., Asfar P., Yang Y., Ince C., Georgieff M., Bruckner U.B., Radermacher P., Froba G. Mechanisms of inducible nitric oxide synthase (iNOS) inhibition-related improvement of gut mucosal acidosis during hyperdynamic porcine endotoxemia. Intensiv. Care Med. 2003;29:312–316. doi: 10.1007/s00134-002-1577-y. [DOI] [PubMed] [Google Scholar]
- 13.Petros A., Lamb G., Leone A., Moncada S., Bennett D., Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc. Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 14.MacMicking J.D., Nathan C., Hom G., Chartrain N., Fletcher D.S., Trumbauer M., Stevens K., Xie Q.W., Sokol K., Hutchinson N., et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Feng K., Yue M., Lu X., Zheng Q., Zhang H., Zhai Y., Li P., Yu L., Cai M., et al. A non-synonymous SNP in the NOS2 associated with septic shock in patients with sepsis in Chinese populations. Hum. Genet. 2013;132:337–346. doi: 10.1007/s00439-012-1253-4. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y., Zweier J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marfella R., di Filippo C., Esposito K., Nappo F., Piegari E., Cuzzocrea S., Berrino L., Rossi F., Giugliano D., D’Amico M. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes. 2004;53:454–462. doi: 10.2337/diabetes.53.2.454. [DOI] [PubMed] [Google Scholar]
- 18.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaudet L., Rosenblatt-Velin N., Pacher P. Role of peroxynitrite in the cardiovascular dysfunction of septic shock. Curr. Vasc. Pharmacol. 2013;11:196–207. [PubMed] [Google Scholar]
- 20.Kuhlencordt P.J., Chen J., Han F., Astern J., Huang P.L. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E–knockout mice. Circulation. 2001;103:3099–3104. doi: 10.1161/01.CIR.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 21.Iadecola C., Zhang F., Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am. J. Physiol. 1995;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C., Zhang F., Casey R., Nagayama M., Ross M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Xia N., Brausch I., Yao Y., Forstermann U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004;310:926–932. doi: 10.1124/jpet.104.066639. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Pascual F., Hausding M., Ihrig-Biedert I., Furneaux H., Levy A.P., Forstermann U., Kleinert H. Complex contribution of the 3'-untranslated region to the expressional regulation of the human inducible nitric-oxide synthase gene. Involvement of the RNA-binding protein HuR. J. Biol. Chem. 2000;275:26040–26049. doi: 10.1074/jbc.M910460199. [DOI] [PubMed] [Google Scholar]
- 25.Ishii K., Sheng H., Warner T.D., Forstermann U., Murad F. A simple and sensitive bioassay method for detection of EDRF with RFL-6 rat lung fibroblasts. Am. J. Physiol. 1991;261:H598–H603. doi: 10.1152/ajpheart.1991.261.2.H598. [DOI] [PubMed] [Google Scholar]
- 26.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric. Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Hausding M., Witteck A., Rodriguez-Pascual F., von Eichel-Streiber C., Forstermann U., Kleinert H. Inhibition of small G proteins of the rho family by statins or clostridium difficile toxin B enhances cytokine-mediated induction of NO synthase II. Br. J. Pharmacol. 2000;131:553–561. doi: 10.1038/sj.bjp.0703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lattanzio V., Kroon P.A., Linsalata V., Cardinali A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods. 2009;1:131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 29.Saenz Rodriguez T., Garcia Gimenez D., de la Puerta Vazquez R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine. 2002;9:687–693. doi: 10.1078/094471102321621278. [DOI] [PubMed] [Google Scholar]
- 30.Kucukgergin C., Aydin A.F., Ozdemirler-Erata G., Mehmetcik G., Kocak-Toker N., Uysal M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biol. Trace. Elem. Res. 2010;135:264–274. doi: 10.1007/s12011-009-8484-9. [DOI] [PubMed] [Google Scholar]
- 31.Angelini A., di Pietro R., Centurione L., Castellani M.L., Conti P., Porreca E., Cuccurullo F. Inhibition of P-glycoprotein-mediated transport by S-adenosylmethionine and cynarin in multidrug-resistant human uterine sarcoma MES-SA/Dx5 cells. J. Biol. Regul. Homeost. Agents. 2012;26:495–504. [PubMed] [Google Scholar]
- 32.Lopez-Molina D., Navarro-Martinez M.D., Rojas Melgarejo F., Hiner A.N., Chazarra S., Rodriguez-Lopez J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.) Phytochemistry. 2005;66:1476–1484. doi: 10.1016/j.phytochem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Costabile A., Kolida S., Klinder A., Gietl E., Bauerlein M., Frohberg C., Landschutze V., Gibson G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010;104:1007–1017. doi: 10.1017/S0007114510001571. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X., Zhang H., Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004;52:7272–7278. doi: 10.1021/jf0490192. [DOI] [PubMed] [Google Scholar]
- 35.Llorach R., Espin J.C., Tomas-Barberan F.A., Ferreres F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002;50:3458–3464. doi: 10.1021/jf0200570. [DOI] [PubMed] [Google Scholar]
- 36.Wang M., Simon J.E., Aviles I.F., He K., Zheng Q.Y., Tadmor Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.) J. Agric. Food Chem. 2003;51:601–608. doi: 10.1021/jf020792b. [DOI] [PubMed] [Google Scholar]
- 37.Zapolska-Downar D., Zapolski-Downar A., Naruszewicz M., Siennicka A., Krasnodebska B., Koldziej B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002;71:2897–2808. doi: 10.1016/S0024-3205(02)02136-7. [DOI] [PubMed] [Google Scholar]
- 38.Juzyszyn Z., Czerny B., Pawlik A., Drozdzik M. The effect of artichoke (Cynara scolymus L.) extract on ROS generation in HUVEC cells. Phytother. Res. 2008;22:1159–1161. doi: 10.1002/ptr.2385. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Garcia F., Adzet T., Canigueral S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radic. Res. 2000;33:661–665. doi: 10.1080/10715760000301171. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Escrig A., Dragsted L.O., Daneshvar B., Pulido R., Saura-Calixto F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. J. Agric. Food Chem. 2003;51:5540–5545. doi: 10.1021/jf030047e. [DOI] [PubMed] [Google Scholar]
- 41.Magielse J., Verlaet A., Breynaert A., Keenoy B.M., Apers S., Pieters L., Hermans N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014;58:211–215. doi: 10.1002/mnfr.201300282. [DOI] [PubMed] [Google Scholar]
- 42.Brown J.E., Rice-Evans C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 43.Wider B., Pittler M.H., Thompson-Coon J., Ernst E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane. Database. Syst. Rev. 2013;3:CD003335. doi: 10.1002/14651858.CD003335.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Bundy R., Walker A.F., Middleton R.W., Wallis C., Simpson H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine. 2008;15:668–675. doi: 10.1016/j.phymed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Rondanelli M., Giacosa A., Opizzi A., Faliva M.A., Sala P., Perna S., Riva A., Morazzoni P., Bombardelli E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013;64:7–15. doi: 10.3109/09637486.2012.700920. [DOI] [PubMed] [Google Scholar]
- 46.Grande S., Bogani P., de Saizieu A., Schueler G., Galli C., Visioli F. Vasomodulating potential of mediterranean wild plant extracts. J. Agric. Food Chem. 2004;52:5021–5026. doi: 10.1021/jf049436e. [DOI] [PubMed] [Google Scholar]
- 47.Roghani-Dehkordi F., Kamkhah A.F. Artichoke leaf juice contains antihypertensive effect in patients with mild hypertension. J. Diet. Suppl. 2009;6:328–341. doi: 10.3109/19390210903280207. [DOI] [PubMed] [Google Scholar]
- 48.Wittemer S.M., Ploch M., Windeck T., Muller S.C., Drewelow B., Derendorf H., Veit M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine. 2005;12:28–38. doi: 10.1016/j.phymed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Azzini E., Bugianesi R., Romano F., di Venere D., Miccadei S., Durazzo A., Foddai M.S., Catasta G., Linsalata V., Maiani G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: A pilot study. Br. J. Nutr. 2007;97:963–969. doi: 10.1017/S0007114507617218. [DOI] [PubMed] [Google Scholar]
- 50.Dong G.C., Chuang P.H., Chang K.C., Jan P.S., Hwang P.I., Wu H.B., Yi M., Zhou H.X., Chen H.M. Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of T-cell receptor. Pharm. Res. 2009;26:375–381. doi: 10.1007/s11095-008-9754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Sweet D.H. Interaction of natural dietary and herbal anionic compounds and flavonoids with human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11) Evid. Based Complement. Alternat. Med. 2013;2013:612527. doi: 10.1155/2013/612527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galvano F., La Fauci L., Lazzarino G., Fogliano V., Ritieni A., Ciappellano S., Battistini N.C., Tavazzi B., Galvano G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004;15:2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Serra D., Paixao J., Nunes C., Dinis T.C., Almeida L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS One. 2013;8:e73001. doi: 10.1371/journal.pone.0073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q., Xia M., Liu C., Guo H., Ye Q., Hu Y., Zhang Y., Hou M., Zhu H., Ma J., et al. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008;83:176–184. doi: 10.1016/j.lfs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 55.Sorrenti V., di Giacomo C., Acquaviva R., Bognanno M., Grilli E., D’Orazio N., Galvano F. Dimethylarginine dimethylaminohydrolase/nitric oxide synthase pathway in liver and kidney: Protective effect of cyanidin 3-O-beta-d-glucoside on ochratoxin-A toxicity. Toxins (Basel) 2012;4:353–363. doi: 10.3390/toxins4050353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Min S.W., Ryu S.N., Kim D.H. Anti-inflammatory effects of black rice, cyanidin-3-O-beta-d-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010;10:959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Kim J.M., Kim J.S., Yoo H., Choung M.G., Sung M.K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008;56:8427–8433. doi: 10.1021/jf801342p. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt N., Pautz A., Art J., Rauschkolb P., Jung M., Erkel G., Goldring M.B., Kleinert H. Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes. Biochem. Pharmacol. 2010;79:722–732. doi: 10.1016/j.bcp.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia N., Strand S., Schlufter F., Siuda D., Reifenberg G., Kleinert H., Forstermann U., Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Li H., Forstermann U. Structure-activity relationship of staurosporine analogs in regulating expression of endothelial nitric-oxide synthase gene. Mol. Pharmacol. 2000;57:427–435. doi: 10.1124/mol.57.3.427. [DOI] [PubMed] [Google Scholar]