Abstract
Aspartic acid (Asp) residues in peptides and proteins are prone to isomerization to the β-form and racemization via a five-membered succinimide intermediate. These nonenzymatic reactions have relevance to aging and age-related diseases. In this paper, we report a three water molecule-assisted, six-step mechanism for the formation of succinimide from Asp residues found by density functional theory calculations. The first two steps constitute a stepwise iminolization of the C-terminal amide group. This iminolization involves a quintuple proton transfer along intramolecular and intermolecular hydrogen bonds formed by the C-terminal amide group, the side-chain carboxyl group, and the three water molecules. After a conformational change (which breaks the intramolecular hydrogen bond involving the iminol nitrogen) and a reorganization of water molecules, the iminol nitrogen nucleophilically attacks the carboxyl carbon of the Asp side chain to form a five-membered ring. This cyclization is accompanied by a triple proton transfer involving two water molecules, so that a gem-diol tetrahedral intermediate is formed. The last step is dehydration of the gem-diol group catalyzed by one water molecule, and this is the rate-determining step. The calculated overall activation barrier (26.7 kcal mol−1) agrees well with an experimental activation energy.
Keywords: aspartic acid residue, nonenzymatic reaction, succinimide intermediate, density functional theory, water catalysis, multiple proton transfer, amide-iminol tautomerism
1. Introduction
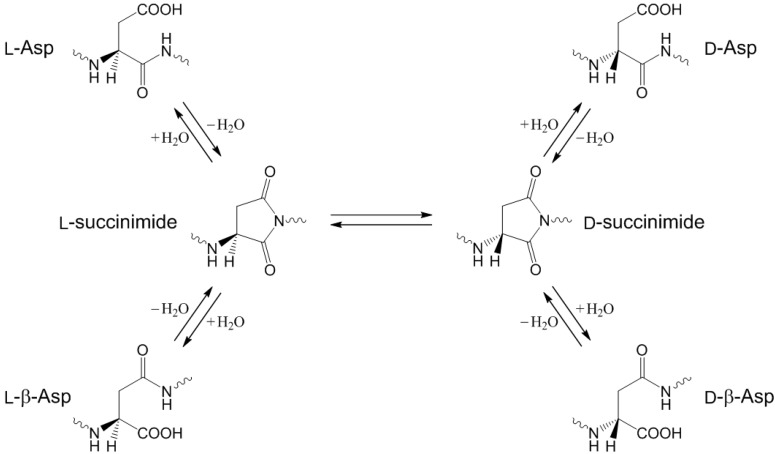
Aspartic acid (Asp or D) residues in peptides and proteins (l-Asp) are prone to isomerization to the β-form and racemization under physiological conditions [1,2,3]. These reactions occur spontaneously (i.e., nonenzymatically) and interdependently via a cyclic intermediate (succinimide) as shown in Scheme 1, giving rise to the biologically uncommon l-β-Asp, d-Asp, and d-β-Asp residues. The formation of these altered Asp residues in proteins, which can affect their three-dimensional structures and hence their properties and functions, has indeed been shown to have close relevance to aging and pathologies (especially those of age-related diseases such as cataracts and Alzheimer’s disease) [3,4,5,6,7,8,9,10]. Although the succinimide-mediated reactions of the Asp residues have been studied extensively since the pioneering work by Geiger and Clarke [1], many mechanistic details remain to be clarified.
Scheme 1.
Interdependent isomerization and racemization reactions of l-Asp residues in peptides and proteins via the succinimide intermediate.
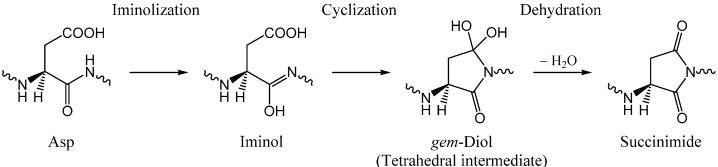
Our recent computational studies [11,12] have shown that, under physiological conditions, it is quite likely that the succinimide formation reactions from Asp residues are catalyzed by two or three water molecules. Moreover, we have shown that the reactions are likely to be initiated by iminolization (i.e., isomerization to the iminol tautomer) of the amide group on the C-terminal side of the Asp residue. The succinimide intermediates are usually thought to result from nucleophilic attack by the C-terminal amide nitrogen on the side-chain carboxyl carbon. However, amide nitrogens are generally poor nucleophiles. On the other hand, iminol nitrogens are expected to be much better nucleophiles [11,12]. We have thus proposed the novel iminolization−cyclization−dehydration mechanism as shown in Scheme 2.
Scheme 2.
The iminolization−cyclization−dehydration mechanism of the succinimide formation from an Asp residue.
However, our previous computational studies have dealt with the reaction pathway up to the gem-diol tetrahedral precursor of the succinimide product and not with the last dehydration step. Catak et al. [13] have computationally investigated a one-water-assisted mechanism where direct cyclization from the amide form to give the gem-diol intermediate is followed by dehydration. However, the calculated activation barrier of 46.1 kcal mol−1 is too high for a biological reaction.
In the present paper, we report new computational results which provide, for the first time, a qualitatively satisfactory reaction model of the entire reaction from Asp to succinimide. The reaction is assisted by three water molecules, but in a different manner from the previously proposed three water molecule-assisted mechanism [12]. In particular, the first two steps, which constitute a stepwise iminolization process, involve a quintuple proton transfer along intramolecular and intermolecular hydrogen bonds. This iminolization process is similar, except for the stepwise nature, to the previously reported two water molecule-catalyzed concerted mechanism [11].
2. Results and Discussion
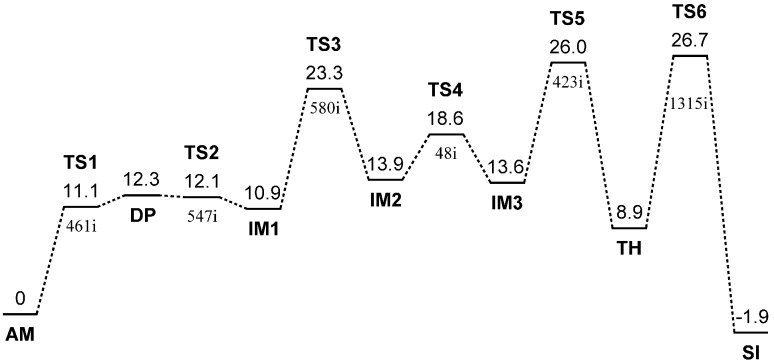
A six-step reaction pathway was found starting from the reactant complex AM (amide form). Figure 1 shows the energy diagram for the entire reaction from AM to the succinimide product SI.
Figure 1.
Energy diagram where the ZPE-corrected relative energies (kcal mol−1) with respected to AM are shown for all the optimized geometries (the total energy of AM is −913.830022 Eh). For abbreviations of the optimized geometries, see text. The single imaginary frequency (cm−1) is also shown for each TS geometry.
Note that SI is a complex including four water molecules because a water molecule is released in the last dehydration step. The six TSs are numbered consecutively and abbreviated as TS1, TS2, TS3, TS4, TS5, and TS6. DP stands for the deprotonated form in which the side-chain carboxyl group is in the dissociated form (-COO−). IM1, IM2, and IM3 are complexes between the iminol form and the three water molecules, which differ in conformation and complexation mode. TH stands for the gem-diol tetrahedral intermediate (complexed with the three water molecules). The optimized geometries (except for TS3 and TS4) are shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12, respectively. Table 1 lists the φ, ψ, and χ1 dihedral angles (Figure 13) of all the optimized geometries.
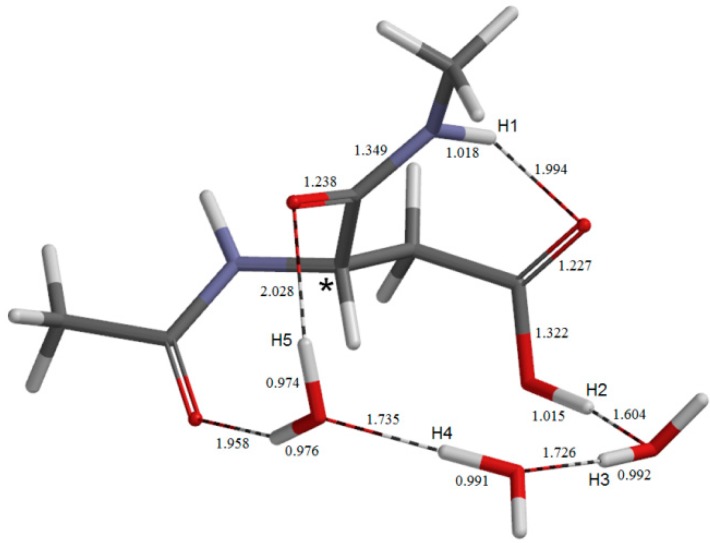
Figure 2.
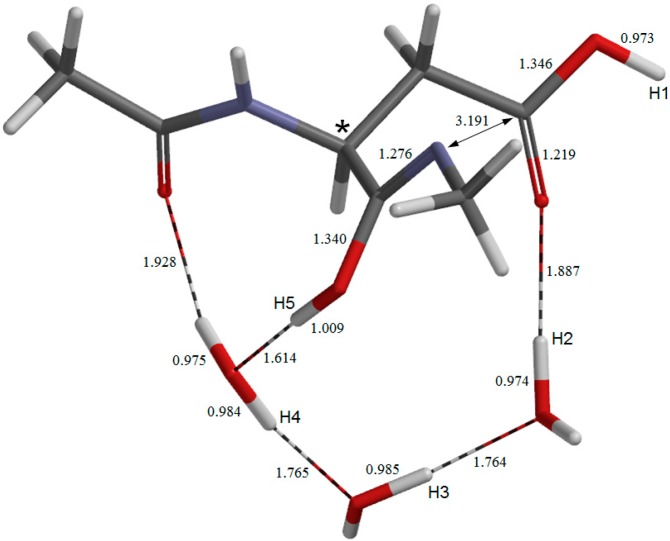
The geometry of the reactant complex AM. The α carbon atom is indicated by an asterisk. Selected bond distances are shown in Å.
Figure 3.
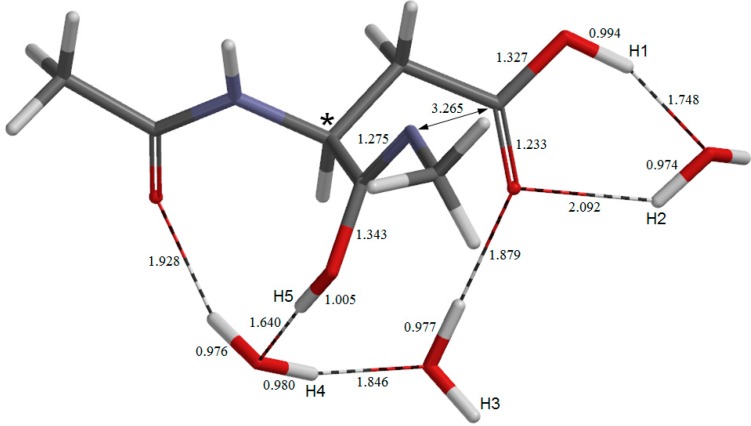
The geometry of the first-step transition state TS1. Selected interatomic distances are shown in Å.
Figure 4.
The geometry of the first intermediate DP. Selected bond distances are shown in Å.
Figure 5.
The geometry of the second-step transition state TS2. Selected interatomic distances are shown in Å.
Figure 6.
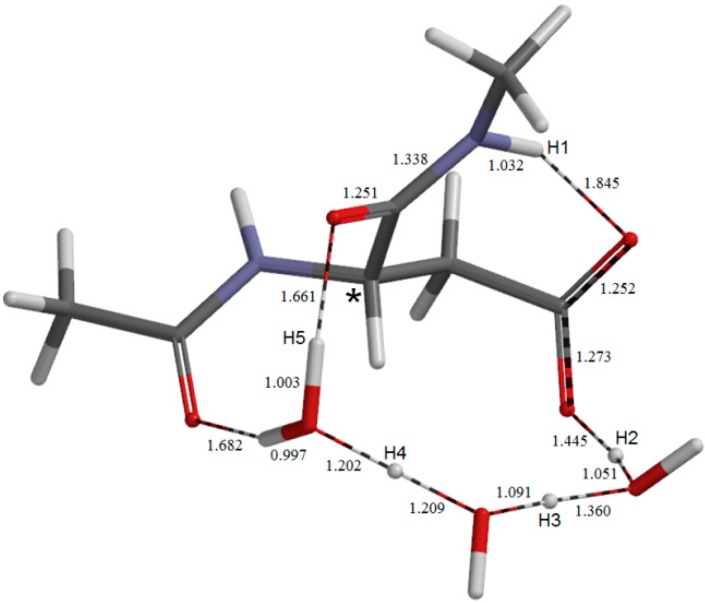
The geometry of the second intermediate IM1. Selected bond distances are shown in Å.
Figure 7.
The geometry of the third intermediate IM2. Selected bond distances are shown in Å.
Figure 8.
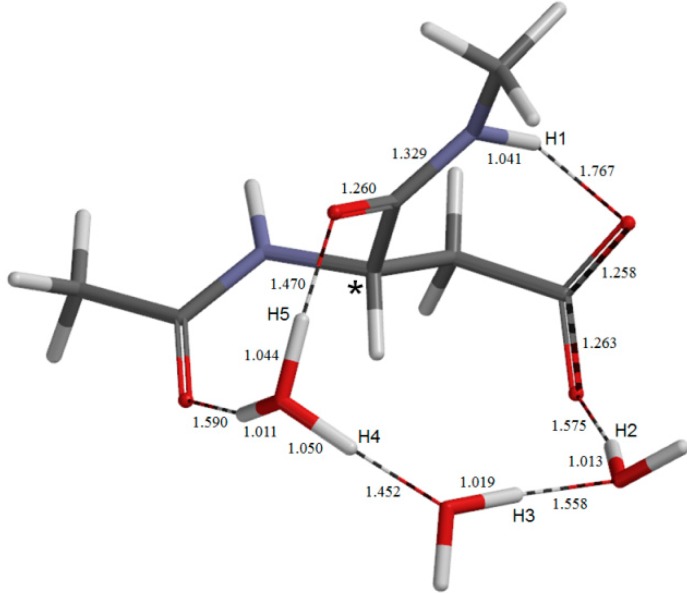
The geometry of the fourth intermediate IM3. Selected bond distances are shown in Å.
Figure 9.
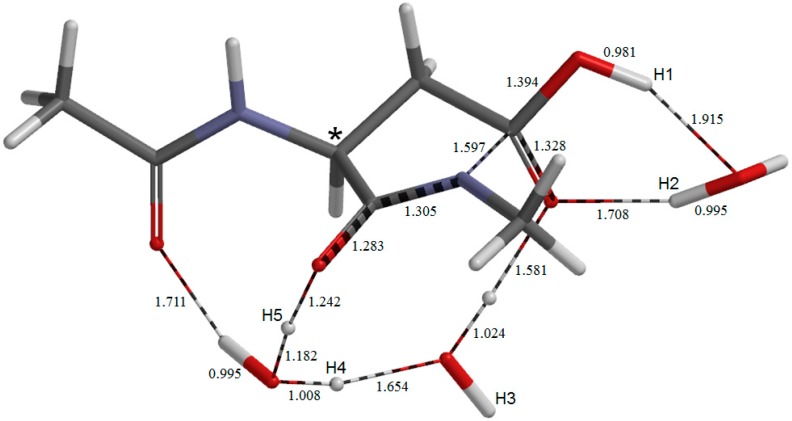
The geometry of the fifth-step transition state TS5. Selected interatomic distances are shown in Å.
Figure 10.
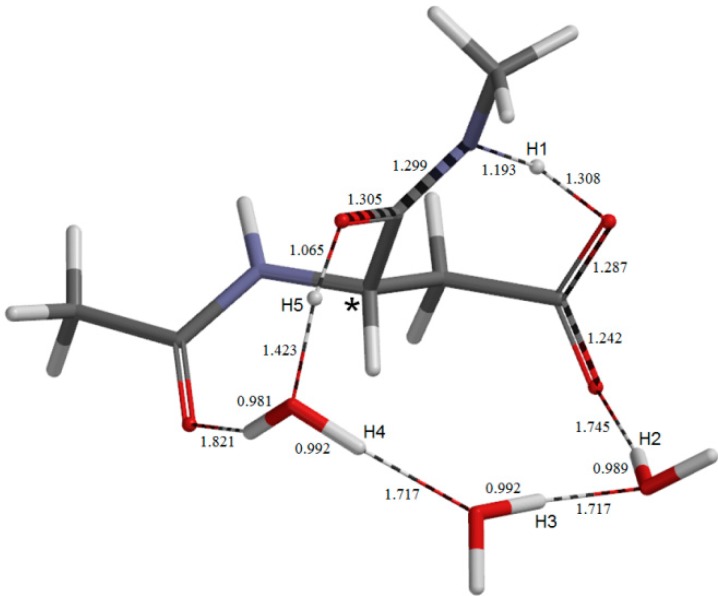
The geometry of the fifth intermediate TH. Selected bond distances are shown in Å.
Figure 11.
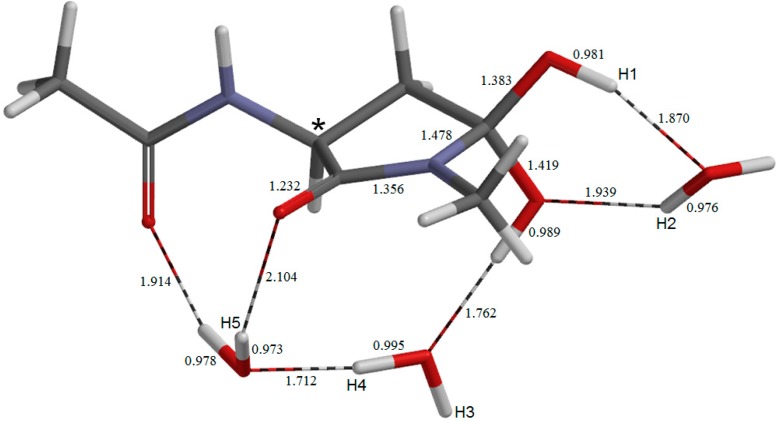
The geometry of the sixth-step transition state TS6. Selected interatomic distances are shown in Å.
Figure 12.
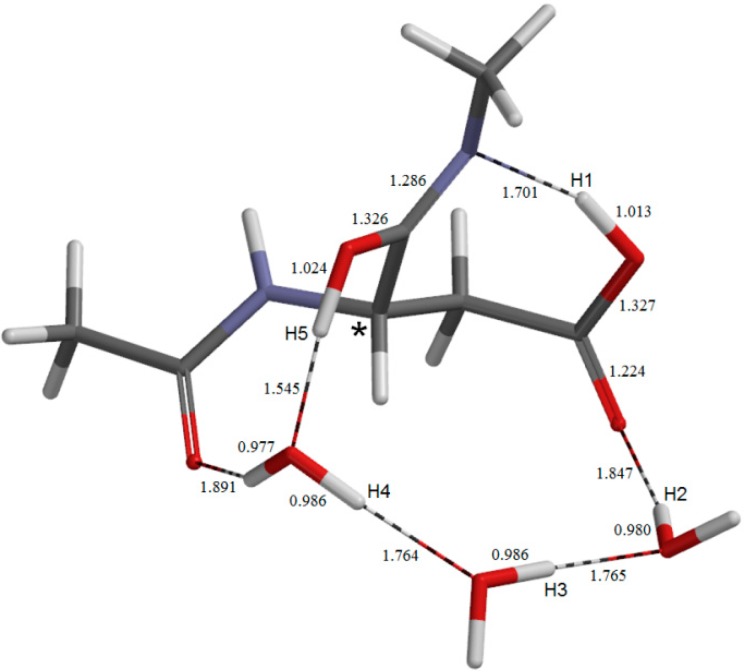
The geometry of the succinimide product SI. Selected bond distances are shown in Å.
Table 1.
The φ, ψ, and χ1 dihedral angles (°) (Figure 13) of the optimized geometries.
| Geometry | φ | ψ | χ1 |
|---|---|---|---|
| AM | −101 | −133 | −162 |
| TS1 | −102 | −126 | −166 |
| DP | −103 | −121 | −171 |
| TS2 | −112 | −118 | −179 |
| IM1 | −116 | −109 | −173 |
| TS3 | −121 | −110 | 173 |
| IM2 | −122 | −98 | −173 |
| TS4 | −115 | −98 | −174 |
| IM3 | −114 | −93 | −173 |
| TS5 | −97 | −136 | 155 |
| TH | −97 | −142 | 151 |
| TS6 | −96 | −146 | 153 |
| SI | −100 | −134 | 136 |
Figure 13.
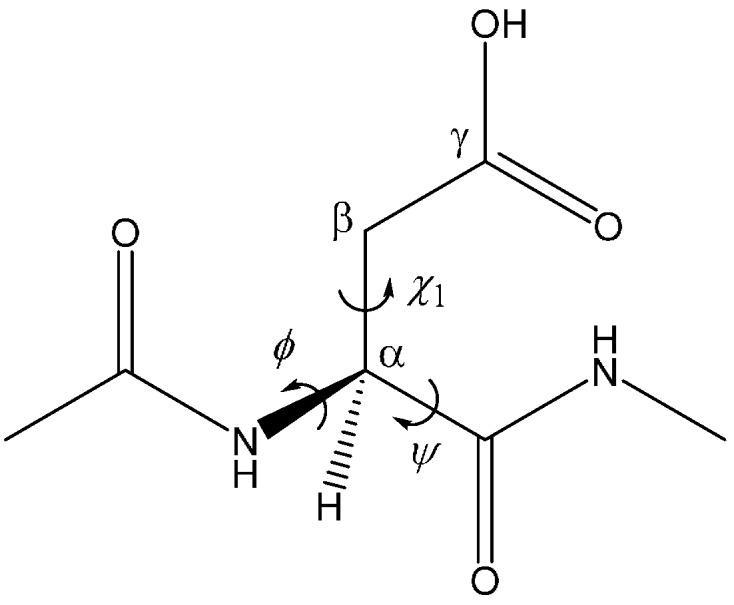
The model compound used in the present study (Ace−Asp−Nme). The φ (C−N−Cα−C) and ψ (N−Cα−C−N) dihedral angles which characterize the backbone conformation and the χ1 dihedral angle (N−Cα−Cβ−Cγ) which characterizes the side-chain conformation are indicated.
2.1. Iminolization
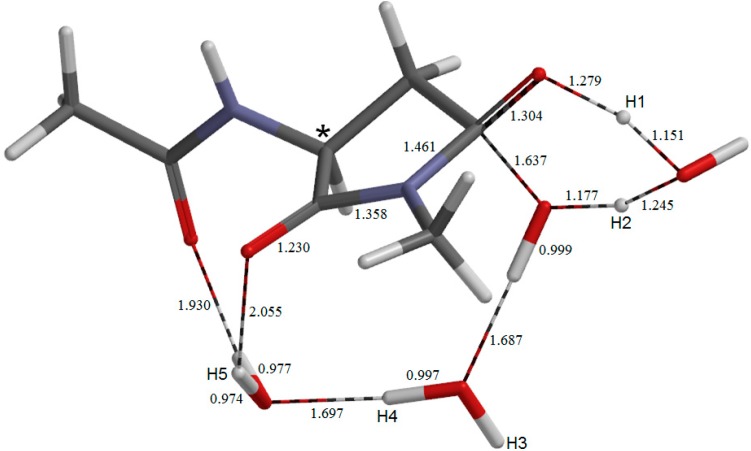
The geometry of the reactant complex AM is shown in Figure 2, where five hydrogen atoms relevant to the following discussion are labeled as H1, H2, H3, H4 and H5. The φ, ψ, and χ1 dihedral angles of AM are −101°, −133°, and −162°, respectively (Table 1). In AM, a wire of the three water molecules forms a hydrogen-bond bridge between the backbone carbonyl oxygen and the side-chain OH hydrogen (H2) of the Asp residue. There is also an intramolecular hydrogen bond (1.994 Å) between the NH hydrogen (H1) of the C-terminal amide group and the side-chain C=O oxygen. Thus, there exists a large cyclic structure formed by the C-terminal amide group, the side-chain carboxyl group, and the three water molecules. Moreover, one water molecule (the left one in Figure 2) also forms an additional hydrogen bond (1.958 Å) to the oxygen of the acetyl group (which mimics the neighboring residue on the N-terminal side).
The first two steps in Figure 1 constitute a stepwise iminolization from AM to give IM1 (Figure 6). TS1 (Figure 3) and TS2 (Figure 5) are the TSs of the first and second steps, respectively, and DP (Figure 4) is the intermediate lying between TS1 and TS2. In the first step, a triple proton transfer involving H2, H3, and H4 occurs to give DP. The side-chain carboxyl group (-COOH) becomes deprotonated (-COO−) by this step. Concomitantly, the left water molecule in Figure 2 is protonated leading to the formation of an oxonium ion H3O+. As may be seen from Figure 3, this triple proton transfer is asynchronous (though concerted); more specifically, transfers of H2 and H3 are followed by that of H4. In the second step, a concerted double proton transfer to give IM1 occurs. More specifically, H1 is transferred to the COO− group along the intramolecular hydrogen bond, and H5 in the H3O+ ion is transferred to the oxygen of the C-terminal amide group. This double proton transfer is also asynchronous; transfer of H5 precedes that of H1, and so TS2 possesses an ‘O-protonated amide group’. No other pathways connecting AM and IM1 were found.
Energetically, the intermediate DP corresponds to a very shallow minimum on the potential energy surface. Indeed, after the ZPE correction, its energy becomes even slightly higher than those of TS1 and TS2 as shown in Figure 1. Before the ZPE correction, the energies of TS1 and TS2 are higher than DP by 0.97 and 2.05 kcal mol−1, respectively. It may be said that TS1, DP, and TS2 constitute a very broad, plateau-like transition state ‘region’ [14]. The ZPE-corrected relative energies of DP and IM1 with respected to AM are 12.3 and 10.9 kcal mol−1, respectively. Although the backward reaction from IM1 to AM is almost barrierless, it is rather surprising that the iminol tautomer can be said to be energetically accessible under the physiological temperature of 37 °C. The hydrogen bond involving the acetyl oxygen becomes very short (1.590 Å) in DP (Figure 4), indicating that it plays an important role in stabilizing the H3O+ ion and hence DP. It is also interesting to note that the hydrogen bond involving the iminol hydrogen (H5) is considerably short (1.545 Å, Figure 6). Short hydrogen bonds involving iminol hydrogens were also noted in previous computational studies [11,12]. Moreover, the changes in the φ, ψ, and χ1 dihedral angles on going from AM to IM1 are modest (Table 1) (the largest change by 24° is observed in ψ). This may suggest that the iminolization by the three water molecule-catalyzed mechanism as shown here can occur even in a structurally-constrained region in a peptide chain.
2.2. Change in Conformation and Complexation Mode in the Iminol Form
It seems that a direct cyclization from IM1 is inhibited by the intramolecular hydrogen bond between the iminol nitrogen and the side-chain carboxyl hydrogen (H1) both structurally and electronically [11]. This hydrogen bond is broken by the trans-to-cis conformational change in the carboxyl group (O=C−O−H), i.e., rotation about the C−O single bond. This occurs via TS3 (geometry not shown) with a somewhat high local activation barrier (12.4 kcal mol−1). The resulting IM2 (Figure 7) is higher in energy than IM1 by 3 kcal mol−1. The O=C−O−H dihedral angles in IM1, TS3, and IM2 are −166°, −83°, and 0°, respectively. Also notable is that rotation about the Cβ−Cγ bond strongly couples with the OH rotation; the Cα−Cβ−Cγ=O dihedral angles in IM1, TS3, and IM2 are 102°, 51°, and 18°, respectively.
IM2 is converted to the third iminol minimum IM3 (Figure 8) via TS4 (geometry not shown) with a local activation barrier of 4.7 kcal mol−1. In this conversion, one water molecule (the right one in Figure 7) moves to a new position where it forms two hydrogen bonds with the carboxyl group (see Figure 8). This water molecule acts later as a catalyst in the dehydration of the gem-diol species. IM3 is lower in energy than IM2 by 0.3 kcal mol−1. The distance between the iminol nitrogen and the carboxyl carbon is 3.265 Å in IM3 (this distance was 3.191 Å in IM2). In the present study, we investigated the cyclization from IM3. The changes in the φ, ψ, and χ1 dihedral angles on going from IM1 to IM3 are again modest (Table 1). However, cumulative changes result in a total change by 40° in ψ when compared between AM and IM3. This is cancelled, however, by the change in the next cyclization step.
2.3. Cyclization to Form the Tetrahedral Intermediate
The cyclization from IM3 to form the tetrahedral intermediate TH (Figure 10) proceeds via TS5 (Figure 9) with a local activation barrier of 12.4 kcal mol−1. The relative energies of TS5 and TH with respect to AM are 26.0 and 8.9 kcal mol−1, respectively. The distance of the forming N−C bond in TS5 is 1.597 Å. Concomitantly with the N−C bond formation, a triple proton transfer occurs from the iminol oxygen to the carboxyl C=O oxygen mediated by the two water molecules, so that the resulting species (TH) is a gem-diol (a π-bond reorganization form O−C=N to O=C−N also accompanies). As may be seen from Figure 9, proton transfers from the water molecules are preceded by the other major changes in geometry. In TH, one water molecule forms hydrogen bonds to both of the OH groups in the gem-diol moiety. This water molecule acts as a catalyst in the next last step (dehydration to give the succinimide product SI). The other two water molecules form a hydrogen-bond bridge between the α-carbonyl group and one of the OH groups. Although no other pathways connecting IM3 and TH were found, the possibility of multiple pathways may remain.
The ψ dihedral angle changes by 49° in the cyclization step (Table 1). However, the net change by 9° from AM is much less significant. On the other hand, a large change in χ1 by 36° is observed in the cyclization step. As a result, χ1 changes by 47° on going from AM to TH.
2.4. Dehydration of the Tetrahedral Intermediate
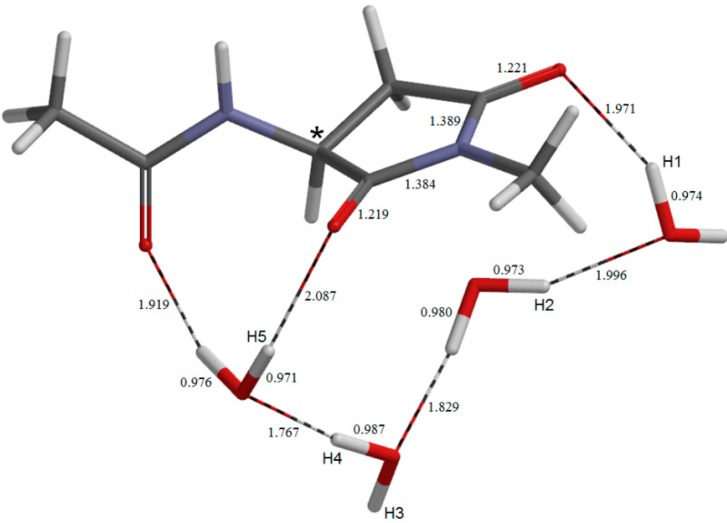
The dehydration of the gem-diol group of TH leading to the succinimide product SI (Figure 12) occurs via TS6 (Figure 11) with a local activation barrier of 17.8 kcal mol−1. This step involves a double proton transfer mediated by the water molecule hydrogen-bonded to the gem-diol group, accompanying the scission of one of the two C−O bonds. The distance of the breaking C−O bond is 1.637 Å in TS6, which becomes 2.948 Å in SI.
The relative energy of TS6 with respect to the reactant complex AM is 26.7 kcal mol−1, and this corresponds to the overall activation barrier of the entire reaction shown in Figure 1. Namely, the rate-determining step is predicted to be the last dehydration step. However, since the energy difference between TS5 (the cyclization TS) and TS6 is small (0.7 kcal mol−1), factors not considered in the present study may make the cyclization step to be rate-determining.
Experimentally, an Arrhenius plot has recently been reported for the succinimide formation in a human αA-crystallin peptide TVLDSGISEVR [15], from which the activation energy can be calculated to be 25.6 kcal mol−1. The calculated activation barrier agrees satisfactorily with this experimental value, suggesting that the water-catalyzed mechanism presented here, or one similar to it, actually operates under physiological conditions.
The rate constant is determined not only by the activation energy but also by the pre-exponential factor. The above Arrhenius plot gives a relatively low value of 3.1 × 1010 s−1 for the pre-exponential factor. This may partially be due to the requirement that three water molecules must align for the reaction to be initiated. On the other hand, we can expect some enthalpy−entropy compensation in the complex formation between the reactant and three water molecules [16].
In TS6, one water molecule acts as a proton-relay catalyst, while the other two water molecules are ‘spectators’. However, these spectators form relatively short hydrogen bonds of 1.687 and 1.697 Å in TS6 (see Figure 11). Therefore, they may have some contribution to stabilizing TS6.
Experimentally, succinimide formation from Asp residues is endothermic [17]. However, SI was calculated to be 1.9 kcal mol−1 lower than the reactant complex AM. This may be due partly to an unbalanced description of hydration, because there are four explicit water molecules in SI while there are three in AM.
Changes in the φ, ψ, and χ1 dihedral angles are also modest in the dehydration step (Table 1). When compared between the reactant complex AM and the product complex SI, φ and ψ are almost unchanged, while χ1 changes by 62°. The latter large change in conformation can be a bottleneck when the reaction occurs in a structurally constrained environment.
3. Experimental Section
Figure 13 shows the model compound (Ace−Asp−Nme, where Ace = acetyl and Nme = NHCH3) used in the present study. This compound has previously been used in related computational studies by us [11,12] and Catak et al. [13,18]. Note that the Asp side chain is protonated. This is because only the protonated form is thought to undergo the nucleophilic attack by the backbone nitrogen to form the five-membered ring [2,11,12,17,19]. If we take a pKa value of 3.9 for the Asp side-chain carboxyl group, only one of every 3,000 Asp residues is protonated at the physiological pH of 7.4 [2]. The entire reaction was investigated starting from a reactant complex in which a wire of three water molecules forms a bridge between the backbone carbonyl oxygen and the side-chain OH hydrogen of the Asp residue by hydrogen bonding.
As in our previous studies [11,12,20], all calculations were performed by the density functional theory (DFT) with the B3LYP functional and the 6-31+G(d,p) basis set using Spartan’08 [21]. Energy-minimum and transition state (TS) geometries were located in vacuum without any constraints. Vibrational frequency calculations were performed for all the optimized geometries to confirm them as energy minima (with no imaginary frequency) or TSs (with a single imaginary frequency) and to correct the relative energies for the zero-point energy (ZPE). The connectivity of all the optimized geometries as shown in Figure 1 was confirmed by intrinsic reaction coordinate (IRC) calculations and geometry optimizations. Successful IRC calculations were followed by full geometry optimization. When an IRC was not calculated successfully, the atoms were slightly displaced from the TS geometry along the transition vector (i.e., the vibration with an imaginary frequency), followed by full geometry optimization.
4. Conclusions
By DFT calculations, we have been successful for the first time in showing a single reaction model which can qualitatively describe the entire multi-step process of succinimide formation from Asp residues. This model corresponds to the iminolization−cyclization−dehydration mechanism which we have recently proposed, and includes three catalytic water molecules which mediate multiple proton transfers. More specifically, the iminolization, cyclization, and dehydration steps are catalyzed by three, two, and one water molecule(s), respectively. The iminolization occurs in two steps where five protons are transferred along intramolecular and intermolecular hydrogen bonds. The cyclization and dehydration steps involve proton transfers along intermolecular hydrogen bonds only. The last dehydration step was predicted to be rate-determining (although the energy difference between the cyclization and dehydration TSs is small), and the calculated activation barrier agreed well with an experimental activation energy [15]. The results of the present and previous calculations strongly suggest that water molecules have crucial catalytic effects in the succinimide formation from aspartic acid residues in peptides and proteins. Although the effect of bulk water was not considered in the present study, the energetic results were rather satisfactory. This may suggest that the bulk water does not largely affect the energetics of the water-catalyzed succinimide formation from Asp residues. This point will be addressed in our future work.
Author Contributions
O.T. performed the DFT calculations and wrote the manuscript. All the authors examined and discussed the results, and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Geiger T., Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- 2.Stephenson R.C., Clarke S. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J. Biol. Chem. 1989;264:6164–6170. [PubMed] [Google Scholar]
- 3.Ritz-Timme S., Collins M.J. Racemization of aspartic acid in human proteins. Aging Res. Rev. 2002;1:43–59. doi: 10.1016/S0047-6374(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 4.Clarke S. Aging as war between chemical and biochemical processes: Protein methylation and the recognition of age-damaged proteins for repair. Aging Res. Rev. 2003;2:263–285. doi: 10.1016/S1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 5.Fujii N. d-Amino acid in elderly tissues. Biol. Pharm. Bull. 2005;28:1585–1589. doi: 10.1248/bpb.28.1585. [DOI] [PubMed] [Google Scholar]
- 6.Truscott R.J.W. Are ancient proteins responsible for the age-related decline in health and fitness? Rejuvenation Res. 2010;13:83–89. doi: 10.1089/rej.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii N., Takemoto L.J., Momose Y., Matsumoto S., Hiroki K., Akaboshi M. Formation of four isomers at the Asp-151 residue of aged human αA-crystallin by natural aging. Biochem. Biophys. Res. Commun. 1999;265:746–751. doi: 10.1006/bbrc.1999.1748. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N., Matsumoto S., Hiroki K., Takemoto L. Inversion and isomerization of Asp-58 residue in human αA-crystallin from normal aged lenses and cataractous lenses. Biochim. Biophys. Acta. 2001;1549:179–187. doi: 10.1016/S0167-4838(01)00258-8. [DOI] [PubMed] [Google Scholar]
- 9.Fujii N., Kawaguchi T., Sasaki H., Fujii N. Simultaneous stereoinversion and isomerization at the Asp-4 residue in βB2-crystallin from the aged human eye lenses. Biochemistry. 2011;50:8628–8635. doi: 10.1021/bi200983g. [DOI] [PubMed] [Google Scholar]
- 10.Hooi M.Y.S., Truscott R.J.W. Racemization and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age. 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi O., Oda A. Amide−iminol tautomerization of the C-terminal peptide groups of aspartic acid residues. Two-water-assisted mechanism, cyclization from the iminol tautomer leading to the tetrahedral intermediate of succinimide formation, and implication to peptide group hydrogen exchange. In: Jones J.E., Morano D.M., editors. Tyrosine and Aspartic Acid: Properties, Sources and Health Benefits. Nova Science Publishers; New York, NY, USA: 2012. pp. 131–147. [Google Scholar]
- 12.Takahashi O., Kirikoshi R. Intramolecular cyclization of aspartic acid residues assisted by three water molecules: A density functional theory study. Comput. Sci. Discov. 2014;7 doi: 10.1088/1749-4699/7/1/015005. [DOI] [Google Scholar]
- 13.Catak S., Monard G., Aviyente V., Ruiz-López M.F. Deamidation of asparagine residues: Direct hydrolysis versus succinimide-mediated deamidation mechanism. J. Phys. Chem. A. 2009;113:1111–1120. doi: 10.1021/jp808597v. [DOI] [PubMed] [Google Scholar]
- 14.Schweiger S., Rauhut G. Double proton transfer reactions with plateau-like transition state regions: Pyrazole–trifluoroacetic acid clusters. J. Phys. Chem. A. 2006;110:2816–2820. doi: 10.1021/jp0569842. [DOI] [PubMed] [Google Scholar]
- 15.Aki K., Fujii N., Fujii N. Kinetics of isomerization and inversion of aspartate 58 of αA-crystallin peptide mimics under physiological conditions. PLoS One. 2013;8:e58515. doi: 10.1371/journal.pone.0058515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunitz J.D. Win some, lose some: Enthalpy–entropy compensation in weak intermolecular interactions. Chem. Biol. 1995;2:709–712. doi: 10.1016/1074-5521(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 17.Capasso S. Thermodynamic parameters of the reversible isomerization of aspartic residues via a succinimide derivative. Thermochim. Acta. 1996;286:41–50. doi: 10.1016/0040-6031(96)02937-1. [DOI] [Google Scholar]
- 18.Catak S., Monard G., Aviyente V., Ruiz-López M.F. Reaction mechanism of deamidation of asparaginyl residues in peptides: Effect of solvent molecules. J. Phys. Chem. A. 2006;110:8354–8365. doi: 10.1021/jp056991q. [DOI] [PubMed] [Google Scholar]
- 19.Yi L., Beckley N., Gikanga B., Zhang J., Wang Y.J., Chih H., Sharma V.K. Isomerization of Asp–Asp motif in model peptides and a monoclonal antibody Fab fragment. J. Pharm. Sci. 2013;102:947–959. doi: 10.1002/jps.23423. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi O. Two-water-assisted racemization of the succinimide intermediate formed in proteins. A computational model study. Health. 2013;5:2018–2021. doi: 10.4236/health.2013.512273. [DOI] [Google Scholar]
- 21.Spartan’08. Wavefunction, Inc.; Irvine, CA, USA: 2008. [Google Scholar]