Abstract
Propolis is a honeybee product with broad clinical applications. Current literature describes that propolis is collected from plant resins. From a systematic database search, 241 compounds were identified in propolis for the first time between 2000 and 2012; and they belong to such diverse chemical classes as flavonoids, phenylpropanoids, terpenenes, stilbenes, lignans, coumarins, and their prenylated derivatives, showing a pattern consistent with around 300 previously reported compounds. The chemical characteristics of propolis are linked to the diversity of geographical location, plant sources and bee species.
Keywords: propolis, honeybee, flavonoids, phenypropanoids, terpenenes, plant origin
1. Introduction
Propolis is a honeybee product with a broad spectrum of biological properties [1]. As a resinous substance, propolis is prepared by the honeybees to seal the cracks, smooth walls, and to keep moisture and temperature stable in the hive all year around. Raw propolis is typically composed of 50% plant resins, 30% waxes, 10% essential and aromatic oils, 5% pollens and 5% other organic substances. It has been reported that propolis is collected from resins of poplars, conifers, birch, pine, alder, willow, palm, Baccharis dracunculifolia, and Dalbergia ecastaphyllum [2,3,4].
Propolis is widely used to prevent and treat colds, wounds and ulcers, rheumatism, sprains, heart disease, diabetes [5,6,7,8] and dental caries [9] due to its diverse biological properties such as anti-inflammatory [8,10,11,12], antimicrobial, antioxidant, antitumor [3], antiulcer and anti-HIV activities [13]. The wide application of propolis in modern medicine has drawn growing attention to its chemical composition. Many studies have revealed that the observed effects might be the result of synergistic action of its complex constituents [14,15,16].
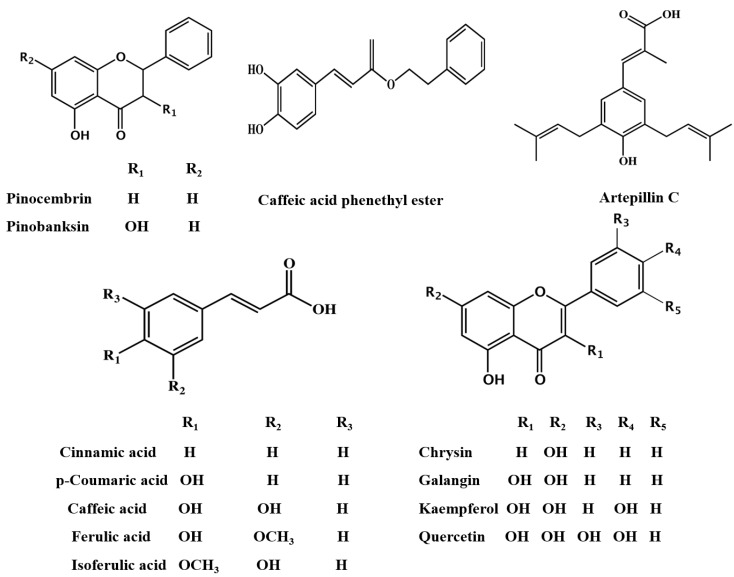
Previous reviews [3,17,18] have covered the knowledge about the chemical composition and botanical origin of propolis throughout 20th century. Until 2000, over 300 chemical components belonging to the flavonoids, terpenes, and phenolics have been identified in propolis. Some representative chemical compounds are summarized in Figure 1.
Figure 1.
Representative chemical components in propolis.
The characteristic constituents in temperate region propolis are flavonoids without B-ring substituents, such as chrysin, galangin, pinocembrin, pinobanksin. Caffeic acid phenethyl ester (CAPE) is a major constituent of temperate propolis with broad biological activities, including inhibition of nuclear factor κ-B; inhibition of cell proliferation; induction of cell cycle arrest and apoptosis. In tropical region propolis, especially Brazilian green propolis, the dominating chemical components are prenylated phenylpropanoids (e.g., artepillin C) and diterpenes. For propolis produced in the Pacific region, geranyl flavanones are the characteristic compounds which are also found in propolis from the African region [19].
The chemical composition of propolis is susceptible to the geographical location, botanical origin [20,21,22,23], and bee species [23]. In order to provide a theoretical basis for studying the chemical composition and pharmacological activity of propolis and plant sources, and controlling the quality, chemical components that were isolated for the first time from propolis between 2000 and 2012 were scouted and summarized from databases including BioMed Central, Biosis Citation Index, Medline, and PubMed.
2. Chemical Compounds in Propolis
With the development of separation and purification techniques such as high performance liquid chromatography (HPLC), thin layer chromatography [24], gas chromatography (GC), as well as identification techniques, such as mass spectroscopy (MS) [25], nuclear magnetic resonance (NMR), gas chromatography and mass spectroscopy (GC-MS) [26], more compounds have been identified in propolis for the first time; including flavonoids, terpenes, phenolics and their esters, sugars, hydrocarbons and mineral elements. In contrast, relatively common phytochemicals such as alkaloids, and iridoids have not been reported. Two hundred and forty one (241) compounds have been reported for the first time from propolis between 2000 and 2012. Their chemical category, geographical locations, and possible plant source, are summarized below.
3. Flavonoids
As the major constituents of propolis, flavonoids contribute greatly to the pharmacological activities of propolis. The quantity of flavonoids is used as a criterion to evaluate the quality of temperate propolis [27]. Flavonoids have a broad spectrum of biological properties, such as antibacterial, antiviral and anti-inflammatory effects [16,28]. According to the chemical structure, flavonoids in propolis are classified into flavones, flavonols, flavanones, flavanonols, chalcones, dihydrochalcones, isoflavones, isodihydroflavones, flavans, isoflavans and neoflavonoids. From 2000 to 2012, 112 flavonoids were identified in different type of propolis for the first time (Table 1). In addition, flavonoid glycosides that are very rare in propolis were identified; they are isorhamnetin-3-O-rutinoside [29] and flavone C-glycoside [30].
Table 1.
Flavonoids identified in propolis since 2000.
| No. | Chemical Name | Geographical Location | Reference |
|---|---|---|---|
| Flavones | |||
| 1 | Luteolin | China | [33] |
| 2 | 6-Cinnamylchrysin | China | [34] |
| 3 | 3',5-Dihydroxy-4',7-dimenthoxy flavone | Poland | [26] |
| 4 | Hexamethoxy flavone | Egypt | [35] |
| 5 | (7''R)-8-[1-(4'-Hydroxy-3'-methoxyphenyl) prop-2-en-1-yl]chrysin | Mexico | [36] |
| Flavonols | |||
| 6 | 2'-(8"-Hydroxy-3",8"-dimethyl-oct-2"-enyl)-quercetin | Solomon Island | [31] |
| 7 | 8-(8"-Hydroxy-3",8"-dimethyl-oct-2"-enyl)-quercetin | Solomon Island | [31] |
| 8 | 2'-Geranylquercetin | Solomon Island | [31] |
| 9 | Macarangin | Kenya | [37] |
| 10 | (7"R)-8-[1-(4'-Hydroxy-3'-methoxyphenyl)prop-2-en-1-yl]-galangin | Mexico | [36] |
| Flavanones | |||
| 11 | 3-O-[(S)-2-Methylbutyroyl]pinobanksin | China | [34] |
| 12 | (2S)-5,7-Dihydroxy-4'-methoxy-8-prenylflavanone | Solomon Island | [31] |
| 13 | Hesperitin-5,7-dimethyl ether | Portugal | [38] |
| 14 | Pinobanksin-5-methyl-ether-3-O-pentanoate | Portugal | [38] |
| 15 | 7-O-Prenylstrobopinin | Greek | [39] |
| 16 | 7-O-Prenylpinocembrin | Greek | [39] |
| 17 | (2R,3R)-3,5-Dihydroxy-7-methoxyflavanone 3-(2-methyl)-butyrate | Mexico | [36] |
| 18 | (2R,3R)-6[1-(4'-Hydroxy-3'-methoxyphenyl) prop-2en-1-yl] pinobanksin | Mexico | [40] |
| 19 | (2R,3R)-6[1-(4'-Hydroxy-3'-methoxyphenyl) prop-2en-1-yl]-pinobanksin-3-acetate | Mexico | [40] |
| 20 | 3',4',6-Trihydroxy-7-methoxy flavanone | Nepal | [41] |
| 21 | 5,7,3',4'-Tetrahydroxy-5'-C-geranylflavanone | Japan | [42] |
| 22 | 5,7,3',4'-Tetrahydroxy-6-C-geranylflavanone | Japan | [42] |
| 23 | 5,7,3',4'-Tetrahydroxy-2'-C-geranylflavanone | Japan | [42] |
| 24 | 5,7,3',4'-Tetrahydroxy-2'-C-geranyl-6-prenlyflavanone | Japan | [42] |
| 25 | Propolin A | Taiwan | [43] |
| 26 | Propolin B | Taiwan | [43] |
| 27 | Propolin E | Taiwan | [43] |
| 28 | Sigmoidin B | Taiwan | [43] |
| 29 | Bonannione A | Taiwan | [31] |
| 30 | Solophenol A | Solomon Island | [31] |
| 31 | Sophoraflavanone A | Solomon Island | [31] |
| 32 | (2S)-7-Hydroxyflavanone | Brazil | [44] |
| 33 | (2S)-Liquiritigenin | Brazil | [44] |
| 34 | (2S)-7-Hydroxy-6-methoxyflavanone | Brazil | [44] |
| 35 | (2S)-Naringenin | Brazil | [44] |
| 36 | (2S)-Dihydrobaicalein | Brazil | [44] |
| 37 | (2S)-Dihydrooroxylin A | Brazil | [44] |
| 38 | (2R,3R)-3,7-Dihydroxyflavanone | Brazil | [44] |
| 39 | Garbanzol | Brazil | [44] |
| 40 | (2R,3R)-3,7-Dihydroxy-6-methoxyflavanone | Brazil | [44] |
| 41 | Alnustinol | Brazil | [44] |
| 42 | (2R, 3R)-3,6,7-Trihydroxyflavanone | Nepal | [41] |
| 43 | 5-Methoxy-3-hidroxyflavanone | Portugal | [38] |
| 44 | 5,7-Dihydroxy-6-methoxy-2,3-Dihydroflavonol-3-acetate | Australia | [45] |
| Isoflavones | |||
| 45 | Odoratin | Nepal | [41] |
| 46 | 7,3',4'-Trihydroxy-5'-methoxyisoflavonoid | Nepal | [41] |
| 47 | 6,7,3'-Trihydroxy-4'-methoxyisoflavonoid | Nepal | [41] |
| 48 | 7,3'-Dihydroxy-6,5'- methoxyisoflavonoid | Nepal | [41] |
| 49 | 7-Hydroxy-4'-methoxyisoflavonoid | Cuba | [46] |
| 50 | 5,7-Dihydroxy-4'-methoxyisoflavonoid | Cuba | [46] |
| 51 | Calycosin | Brazil | [44] |
| 52 | 7,4'-Dihydroxyisoflavone | Brazil | [24] |
| 53 | Homopterocarpin | Brazil | [24] |
| 54 | Medicarpin | Brazil | [24] |
| 55 | 4',7-Dimethoxy-2'-isoflavonol | Brazil | [24] |
| Isodihydroflavones | |||
| 56 | Daidzein | Brazil | [44] |
| 57 | Formononetin | Brazil | [44] |
| 58 | Xenognosin B | Brazil | [44] |
| 59 | Biochanin A | Brazil | [44] |
| 60 | Pratensein | Brazil | [44] |
| 61 | 2'-Hydroxybiochanin A | Brazil | [44] |
| 62 | (3S)-Vestitone- | Brazil | [44] |
| 63 | (3S)-Violanone | Brazil | [44] |
| 64 | (3S)-Ferreirin | Brazil | [44] |
| 65 | (3R)-4'-Methoxy-2',3,7-trihydroxyisoflavanone | Brazil | [44] |
| 66 | Biochanin | Cuba | [25] |
| Chalcones | |||
| 67 | 3,4,2',3'-Tetrahydroxychalcone | Brazil | [30] |
| 68 | Isoliquiritigenin | Brazil | [44] |
| 69 | 4,4'-Dihydroxy-2'-methoxychalcone | Brazil | [44] |
| Dihydrochalcones | |||
| 70 | (αS)-α,2',4,4'-Tetrahydroxydihydrochalcone | Brazil | [44] |
| 71 | 2',4'-Dihydroxychalcone | Brazil | [44] |
| 72 | 2',6'-Dihydroxy-4',4-dimethoxydihydrochalcone | Canada | [47] |
| 73 | 2',4',6'-Trihydroxy-4-methoxydihydrochalcone | Canada | [47] |
| 74 | 2',6',4-Tryhydroxy-4'-methoxydihydrochalcone | Canada | [47] |
| Flavans | |||
| 75 | 8-[(E)-4-Phenylprop-2-en-1-one]-(2R,3S)-2-(3,5-dihydroxyphenyl)-3,4-dihydro-2H-2-be-nzopyran-5-methoxyl-3,7-diol, | China | [48] |
| 76 | 8-[(E)-4-Phenylprop-2-en-1-one]-(2S,3R)-2-(3,5-dihydroxyphenyl)-3,4-dihydro-2H-2-benzopyran-5-methoxyl-3,7-diol | China | [48] |
| 77 | 8-[(E)-4-Phenylprop-2-en-1-one]-(2R,3S)-2-(3-methoxyl-4-hydroxyphenyl)-3,4-dihydro-2H-2-benzopyran-5-methoxyl-3,7-diol | China | [48] |
| 78 | 3-Hydroxy-5,6-dimethoxyflavan | Mexico | [49] |
| Isoflavans | |||
| 79 | (3S)-Vestitol | Brazil | [44] |
| 80 | (3S)-Isovestitol | Brazil | [44] |
| 81 | (3S)-7-O-Methylvestitol | Brazil | [44] |
| 82 | (3S)-Mucronulatol | Brazil | [44] |
| 83 | 7,4'-Dihydroxy-2'-methoxyisoflavone | Cuba | [46] |
| 84 | Neovestitol | Cuba | [25] |
| Pterocarpins (a type of neoflavonoid) | |||
| 85 | Medicarpin | Cuba | [46] |
| 86 | 4-Hydroxymedicarpin | - | [46] |
| 87 | Homopterocarpin | Cuba | [46] |
| 88 | 4'-Methoxy-5'hydroxyvesticarpan | - | [46] |
| 89 | 3,8-Dihydroxy-9-methoxypterocarpan | Cuba | [46] |
| 90 | 3-Hydroxy-8,9-dimethoxypterocarpan | Cuba | [46] |
| 91 | 3,4-Dihydroxy-9-methoxypterocarpan | Cuba | [46] |
| 92 | 3,10-Dihydroxy-9-methoxypterocarpan | Brazil | [44] |
| 93 | 6a-Ethoxymedicarpin | Brazil | [44] |
| 94 | (6aR,11aR)-4-Methoxymedicarpin | Brazil | [44] |
| Open-chain neoflavonoids | |||
| 95 | Neoflavonoid 1 | Nepal | [50] |
| 96 | Neoflavonoid 2 | Nepal | [50] |
| 97 | Neoflavonoid 3 | Nepal | [50] |
| 98 | Neoflavonoid 4 | Nepal | [50] |
| 99 | Neoflavonoid 5 | Nepal | [50] |
| 100 | Neoflavonoid 6 | Nepal | [50] |
| 101 | Neoflavonoid 7 | Nepal | [50] |
| 102 | Neoflavonoid 8 | Nepal | [50] |
| 103 | Neoflavonoid 9 | Nepal | [50] |
| 104 | Neoflavonoid 10 | Nepal | [50] |
| 105 | (S)-3'-hydroxy-4-methoxydalbergione | Nepal | [51] |
| 106 | (S)-3',4'-dihydroxy-4-methoxydalbergione | Nepal | [51] |
| 107 | (S)-4-methoxydalbergione | Nepal | [51] |
| Other flavonoids | |||
| 108 | 2,6-Dihydroxy-2-[(4-hydroxyphenyl)methyl]-3-benzofuranone | Brazil | [44] |
| 109 | 2-(2',4'-Dihydroxyphenyl)-3-methyl-6-methoxybenzofuran | Brazil | [44] |
| 110 | 1-(3',4'-Dihydroxy-2'-methoxyphenyl)-3-(phenyl)propane | Mexico | [49] |
| 111 | (Z)-1-(2'-Methoxy-4',5'dihydroxyphenyl)-2-(3-phenyl)propene | Mexico | [49] |
Five flavones 1–5 were identified in Chinese, Polish, Egyptian and Mexican propolis. According to the geographical origin and the typical chemical compounds, the botanical origins of these propolis samples are assumed to be the genus Populus. In samples from the Solomon Islands and Kenya, researchers identified four flavonols 6–9 and confirmed that these compounds exhibited potent antibacterial activity [31]. The majority of the identified compounds were also found in the plants Macaranga, suggesting that the genus Macaranga is the likely plant source. In Pacific propolis, scientists identified many prenylated flavanones 21–31 which exhibited strong antimicrobial activity because the lipophilic prenyl group could rapidly damage the membrane and cell wall function [32]. Some flavanones 11, 13, 14, 17–19 were also identified in poplar propolis. Sherstha et al. identified three flavanonols 42–44 in Nepalese propolis, Portuguese propolis and Australian propolis, respectively.
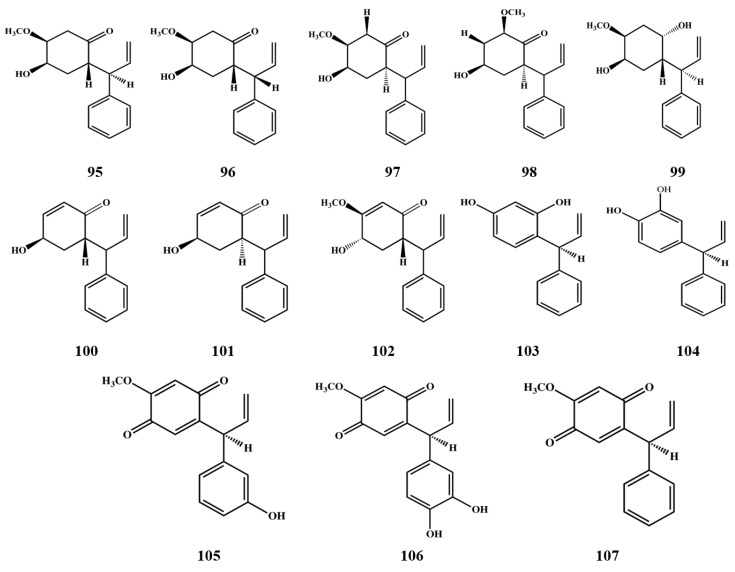
Red Brazilian propolis is a new type of propolis that has attracted wide attention. Researchers identified many compounds typically found in resinous exudates of leguminous plant (Dalbergia ecastophyllum) including 10 flavanones 32–41, four isoflavones 51–55, 11 isodihydroflavones 56–65, three chalcones 67–69, two dihydrochalcones 70–71. Three dihydrochalcones 72–74 that are considered to be characteristic for the bud exudates of Tacamahaca poplars were found in Canadian samples for the first time. Sha et al. and Lotti et al. identified some flavans 75–78 with high cytotoxic activity in Chinese and Mexican propolis [48,49]. Piccinelli et al. identified two isoflavones: 7-hydroxy-4'-methoxyisoflavonoid and 5,7-dihydroxy- 4'-methoxy isoflavonoids in red Cuban propolis, although their plant source has not been confirmed. They presumably originated from Leguminous plants, which is the same botanical origin of red Brazilian propolis [46]. At the same time, isoflavanes 79–84 and pterocarpins 85–94 were also found in the two types of red propolis. In samples from Nepal, 14 unique open-chain neoflavonoids 95–107 (Figure 2) were identified, which are used as markers of the plant source of this type of propolis.
Figure 2.
Open-chain neoflavonoids in propolis.
Among the compounds isolated from Nepalese propolis, (S)-4-methoxydalbergione and obtusaquinol were reported as constituents of Dalbergia and Machaerium woods, but some neoflavonoids such as cearoin and 9-hydroxy-6,7-dimethoxydalbergiquinol were identified only in Dalbergia species [50]. Other flavonoids 108–111 found in Brazilian and Mexican propolis, respectively, are listed in Table 1.
4. Terpenoids
Although volatiles only represent 10% of the propolis constituents, they account for the characteristic resinous odor and contribute to the pharmacological effects of propolis. As the major compounds among the volatile substances, terpenoids play an important role in distinguishing premium propolis from inferior or fake propolis and they exhibit antioxidant, antimicrobial, and other biological activities.
Monoterpenes isolated from propolis include acyclic, monocyclic, dicyclic monoterpenes and their derivatives. The primary acyclic and monocyclic monoterpenes are myrcenes, p-menthanes and cineoles, respectively. The dicyclic monoterpenes in propolis are classified into five groups: thujanes, caranes, pinanes, fenchanes and camphenes. Sesquiterpenes are the most abundant chemical components in propolis. According to the number of the rings, sesquiterpenes fall into four categories: acyclic, monocyclic, dicyclic and tricyclic. The main acyclic sesquiterpenes in propolis are the derivatives of farnesane. There are four types of monocyclic sesquiterpenes, five types of dicyclic sesquiterpenes and ten types of tricyclic sesquiterpenes in propolis. Cembrane, labdane, abietane, pimarane, and totarane are reported to be the major diterpenes in propolis, and some of these are proven to have a broad spectrum of pharmacological properties. The tetracyclic triterpenes in propolis are lanostanes and cycloartane and the pentacyclic triterpenes are oleanane, ursane and lupane.
One monoterpene (trans-β-terpineol) and three sesquiterpenes (γ-elemene, α-ylangene, valencene) with valuable biological activities were identified in Brazilian propolis [52]. In Turkish propolis, a few sesquiterpenes 119–123 were identified; and there was no direct evidence to determine the correct plant source of the each type of Turkish propolis [53]. Popova et al. identified the usual “Mediterranean” diterpenes in samples from Greece, together with some diterpenes (Table 2) that are deemed as characteristic oleoresin components of different Coniferae (mainly Pinaceae and Cupressaceae) plants [29], although their plant source was considered to be the Cupressaceae because Greek propolis contained ferruginol, totarol, oxygenated ferruginol and totarol derivatives, and sempervirol, which are typically found in Cupressaceae plant, but not in Pinaceae. Some triterpenes belonging to the lupane (154–156), lanostane (157–158), oleanane (159–161), ursane (162–164) and other types (165–170) were found in Brazilian, Cuban, Greek, Burmese and Egyptian propolis for the first time.
Table 2.
Terpenes identified in propolis since 2000.
| No. | Chemical Name | Geographical Location | Reference |
|---|---|---|---|
| Monoterpenes | |||
| 112 | trans-β-Terpineol | Greece | [54] |
| 113 | Linalool | Brazil | [52] |
| 114 | Camphor | Iran | [55] |
| Sesquiterpenes | |||
| 115 | Junipene | Greece | [54] |
| 116 | γ-Elemene | Brazil | [52] |
| 117 | α-Ylangene | Brazil | [52] |
| 118 | Valencene | Brazil | [52] |
| 119 | 8-βH-Cedran-8-ol | Turkey | [53] |
| 120 | 4-βH,5α-Eremophil-1(10)-ene | Turkey | [53] |
| 121 | α-Bisabolol | Turkey | [23] |
| 122 | α-Eudesmol | Turkey | [23] |
| 123 | α-Cadinol | Turkey | [23] |
| 124 | Patchoulene | Indonesia | [56] |
| Diterpenes | |||
| 125 | Manoyl oxide | Greece | [57] |
| 126 | Ferruginol | Greece | [57] |
| 127 | Ferruginolone | Greece | [57] |
| 128 | 2-Hydroxyferruginol | Greece | [57] |
| 129 | 6/7-Hydroxyferruginol | Greece | [57] |
| 130 | Sempervirol | Greece | [57] |
| 131 | Abietic acid | Greece | [57] |
| 132 | 18-Succinyloxyabietadiene | Greece | [57] |
| 133 | 18-Succinyloxyhydroxyabietatriene | Greece | [57] |
| 134 | 18-Hydroxyabieta-8,11,13-triene | Greece | [57] |
| 135 | Imbricataloic acid | Greece | [57] |
| 136 | Imbricatoloic acid | Greece | [57] |
| 137 | Diterpenic acid | Greece | [57] |
| 138 | Neoabietic acid | Greece | [57] |
| 139 | Labda-8(17),12,13-triene | Greece | [57] |
| 140 | Hydroxydehydroabietic acid | Greece | [57] |
| 141 | Dihydroxyabieta-8,11,13-triene | Greece | [57] |
| 142 | 13(14)-Dehydrojunicedric acid | Greece | [57] |
| 143 | Dehydroabietic acid | Greece | [57] |
| 144 | 18-Hydroxyabieta-8,11,13-triene | Greece | [57] |
| 145 | Junicedric acid | Greece | [29] |
| 146 | 14,15-Dinor-13-oxo-8(17)-labden-19-oic acid | Greece | [29] |
| 147 | tran-Communal | Greece | [29] |
| 148 | Palmitoyl isocupressic acid | Greece | [29] |
| 149 | Oleoyl isocupressic acid | Greece | [29] |
| 150 | 13-Hydroxy-8(17),14-labdadien-19-oic acid | Greece | [29] |
| 151 | 15-Oxolabda-8(17),13(E)-dien-19-oic acid | Greece | [29] |
| 152 | Pimaric acid | Greece | [29] |
| 153 | Totarolone | Greece | [29] |
| Triterpenes | |||
| 154 | Lupeol alkanoates | Brazil | [58] |
| 155 | Lupeol | Brazil | [58] |
| 156 | Lupeol acetate | Cuba | [59] |
| 157 | Lanosterol acetate | Egypt | [35] |
| 158 | Lanosterol | Cuba | [59] |
| 159 | Germanicol acetate | Cuba | [59] |
| 160 | Germanicol | Cuba | [59] |
| 161 | β-Amyrin acetate | Cuba | [59] |
| 162 | β-Amyrone | Cuba | [59] |
| 163 | α-Amyrin acetate | Cuba | [59] |
| 164 | α-Amyrone | Cuba | [59] |
| 165 | 24-Methylene-9,19-ciclolanostan-3β-ol | Brazil | [58] |
| 166 | (22Z,24E)-3-Oxocycloart-22,24-dien-26-oic acid | Burma | [60] |
| 167 | (24E)-3-Oxo-27,28-dihydroxycycloart-24-en-26-oic acid | Burma | [60] |
| 168 | 3,4-seco-Cycloart-12-hydroxy-4(28),24-dien-3-oicacid | Greece | [29] |
| 169 | Cycloart-3,7-dihydroxy-24-en-28-oic acid | Greece | [29] |
| 170 | 3-Oxo-triterpenic acid methyl ester | Egypt | [61] |
5. Phenolics
Brazilian green propolis is rich in phenylpropanoids including cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid and their derivatives. Among these substances, prenylated cinnamic acids turn out to be a salient chemical feature and have a consanguineous bearing on antimicrobial activity of green propolis. In recent years, researchers identified a series of phenylpropanoid derivatives 171–180 in Brazilian propolis. Meanwhile, some caffeic acid derivatives 182–183 and isoferulic acid derivative 184 were also identified in poplar propolis by GC-MS. Chlorogenic acid is abundant in Brazilian propolis of floral origin from Citrus spp. [62]. Three quinic acid derivatives 185–187 were identified in this type of propolis.
Another class of phenolics, stilbenes, are not very common in plants. In 2010, Petrova et al. identified two geranylstilbenes; schweinfurthin A (188) and schweinfurthin B (189) in propolis produced in Kenya. Macaranga schweinfurthii is the only plant source of these two geranylstilbenes to this date [37]. In 2012, another stilbene, 5-farnesyl-3'-hydroxyresveratrol (190) was identified in Solomon Island propolis, which is also present in Macaranga plants [31]. These results suggest that Macaranga is probably the plant source of the propolis from Kenya and Solomon Island. However, many stilbenes 191–202, especially prenylated stilbenes, were identified in Australian Kangaroo Island propolis, which makes this type of propolis a stronger scavenging activity towards DPPH free radical than Brazilian propolis [63], suggesting the source of stilbenes is not limited to only a few plants.
Lignans as main chemical compounds in tropical propolis have attracted a worldwide research interest. In the past 12 years, researchers identified three lignans 206–208 in Kenyan and Brazilian propolis. As shown in the Table 3, other phenolic compounds and derivatives were identified in propolis from Brazil (209–219), Indonesia (220–229), France (230), Iran (231–239) and Malta (240–241). Among these chemicals, nemorosone (215) is the exclusive and principal component of Clusia rosea floral resins, indicating that Clusia spp. is the plant origin of the brown propolis [64]. Tschimgin (232), tschimganin (233), ferutinin (236), tefernin (237) identified in Iranian propolis are the characteristic compositions of the Ferula species, which is considered as another plant source of Iranian propolis besides poplar.
Table 3.
Phenolics identified in propolis since 2000.
| No. | Chemical Name | Geographical Location | Reference |
|---|---|---|---|
| Phenylpropanoids | |||
| 171 | cis-3-Methoxy-4-hydroxycinnamic acid | Brazil | [65] |
| 172 | trans-3-Methoxy-4-hydroxycinnamic acid | Brazil | [65] |
| 173 | 3-Prenyl cinnamic acid allyl ester | Brazil | [66] |
| 174 | p-Methoxycinnamic acid | Brazil | [66] |
| 175 | Dihydrocinnamic acid | Brazil | [66] |
| 176 | 3-Prenyl-4-hydroxycinnamic acid | Brazil | [67] |
| 177 | 3,5-Diprenyl-4-hydroxycinnamic acid | Brazil | [67] |
| 178 | 3-Methyl-2-butenyl isoferulate | Brazil | [66] |
| 179 | 3-Methyl-3-butenyl caffeate | Brazil | [66] |
| 180 | Hexadecyl caffeate | Brazil | [66] |
| 181 | Methyl(E)-4-(4'-hydroxy-3'-methylbut-(E)-2'-enyloxy) cinnamate | Australia | [63] |
| 182 | Tetradecenyl caffeate (isomer) | Egypt | [35] |
| 183 | Tetradecenyl caffeate | Egypt | [35] |
| 184 | 2-Methyl-2-butenyl ferulate | Uruguay | [68] |
| Chlorogenic acids | |||
| 185 | 4-Feruoyl quinic acid | Brazil | [62] |
| 186 | 5-Ferruoyl quinic acid | Brazil | [33] |
| 187 | 3,4,5-tri-O-Caffeoylquinic acid | Brazil | [69] |
| Stilbenes | |||
| 188 | Schweinfurthin A | Kenya | [37] |
| 189 | Schweinfurthin B | Kenya | [37] |
| 190 | 5'-Farnesyl-3'-hydroxyresveratrol | Solomon Island | [31] |
| 191 | 5,4'-Dihydroxy-3'-methoxy-3-prenyloxy-E-stilbene. | Australia | [63] |
| 192 | 3,5,3',4'-Tetrahydroxy-2-prenyl-E-stilbene | Australia | [63] |
| 193 | 3,5,4'-Trihydroxy-3'-methoxy-2-prenyl-E-stilbene | Australia | [63] |
| 194 | 5,3',4'-Trihydroxy-3-methoxy-2-prenyl-E-stilbene | Australia | [63] |
| 195 | 5,4'-Dihydroxy-3,3'-dimethoxy-2-prenyl-E-stilbene | Australia | [63] |
| 196 | 5,4'-Dihydroxy-3-prenyloxy-E-stilbene | Australia | [63] |
| 197 | 3',4'-Dihydroxy-E-stilbene | Australia | [63] |
| 198 | 3',4'-Dihydroxy-3,5-dimethoxy-E-stilbene | Australia | [63] |
| 199 | Diprenylated dihydrostilbene | Australia | [63] |
| 200 | 3,5-Dihydroxy-2-prenyl-E-stilbene | Australia | [63] |
| 201 | 4-Prenyldihydroresveratrol | Australia | [63] |
| 202 | 3-Prenylresveratrol | Australia | [63] |
| 203 | (+)-Pinoresinol dimethyl ether | Brazil | [44] |
| 204 | (+)-Pinoresinol | Brazil | [44] |
| 205 | (+)-Syringaresinol | Brazil | [44] |
| Lignans | |||
| 206 | Tetrahydrojusticidin B | Kenya | [37] |
| 207 | 6-Methoxydiphyllin | Kenya | [37] |
| 208 | Phyllam ricin C | Kenya | [37] |
| Other phenolics | |||
| 209 | 8-(Methyl-butanechromane)-6-propenoic acid | Brazil | [70] |
| 210 | 3-Hydroxy-2,2-dimethyl-8-prenylchromane-6-propenoic acid | Brazil | [70] |
| 211 | 2,2-Dimethyl-8-prenylchromene-6-propenoic acid | Brazil | [70] |
| 212 | 2,2-Dimethylchromene-6-propenoic acid | Brazil | [70] |
| 213 | 2,2-Dimethyl-6-carboxyethnyl-2H-1-benzopyran | Brazil | [70] |
| 214 | 2,2-Dimethyl-6-carboxyethenyl-8-prenyl-2H-1-benzopyran | Brazil | [70] |
| 215 | Nemorosone | Brazil | [9] |
| 216 | 7-epi-clusianone | Brazil | [9] |
| 217 | Xanthochymol | Brazil | [9] |
| 218 | Gambogenone | Brazil | [9] |
| 219 | Hyperibone A | Brazil | [71] |
| 220 | 5-Pentadecylresorcinol | Indonesia | [72] |
| 221 | 5-(8'Z,11'Z-Heptadecadienyl)-resorcinol | Indonesia | [72] |
| 222 | 5-(11'Z-Heptadecenyl)-resorcinol | Indonesia | [72] |
| 223 | 5-Heptadecylresorcinol | Indonesia | [72] |
| 224 | 1,3-Bis(trimethylsilylloxy)-5,5-proylbenzene | Indonesia | [56] |
| 225 | 3,4-Dimethylthioquinoline | Indonesia | [56] |
| 226 | 4-Oxo-2-thioxo-3-thiazolidinepropionic acid | Indonesia | [56] |
| 227 | D-glucofuranuronic acid | Indonesia | [56] |
| 228 | Dofuranuronic acid | Indonesia | [56] |
| 229 | 3-Quinolinecarboxamine | Indonesia | [56] |
| 230 | Baccharin | France | [73] |
| 231 | Suberosin | Iran | [55] |
| 232 | Tschimgin | Iran | [55] |
| 233 | Tschimganin | Iran | [55] |
| 234 | Bornyl p-hydroxybenzoate | Iran | [55] |
| 235 | Bornyl vanillate | Iran | [55] |
| 236 | Ferutinin | Iran | [55] |
| 237 | Tefernin | Iran | [55] |
| 238 | Ferutinol p-hydroxybenzoate | Iran | [55] |
| 239 | Ferutinol vanillate | Iran | [55] |
| 240 | 2-Acetoxy-6-p-methoxybenzoyl jaeschkeanadiol | Malta | [74] |
| 241 | 2-Acetoxy-6-p-hydroxybenzoyl jaeschkeanadiol | Malta | [74] |
6. Sugars
The question about the origin of sugars in propolis has not been solved yet. Nectar and honey are thought to be the sources of glucose, fructose and sucrose. Others suggest that they come from hydrolyzed flavonoid glycosides in propolis. In addition, mucilages containing numerous sugars, sugar alcohols and acids were listed among potential propolis sugar sources by Crane [75]. In the propolis originated from the Canary Islands and Malta, many sugars, sugar alcohols and uronic acids were identified, supporting the claim that plant mucilages were the source of these compounds [74]. In Egyptian propolis, many sugars, sugar alcohols and uronic acids were identified by GC-MS. Among these substances, galactitol, gluconic acid, galacturonic acid and 2-O-glycerylgalactose were identified in propolis for the first time [61].
7. Hydrocarbons
Hydrocarbons are other basic components of propolis. In recent years, alkanes, alkenes, alkadienes, monoesters, diesters, aromatic esters, fatty acids and steroids have been identified in many types of propolis such as Egyptian propolis [35], Brazilian propolis [65] and Anatolian propolis [76]. Comparing the compositions of Brazilian propolis waxes and comb waxes which were produced by the same colony, no difference was found to allow a distinction, suggesting a common origin for both wax sources [77]. This result not only illustrates that propolis waxes are secreted by bees [78], but also indicates that the composition of propolis waxes and comb waxes is only dependent on genetic factors of the bees, not plant sources.
8. Mineral Elements
Trace elements (Ca, K, Mg, Na, Al, B, Ba, Cr, Fe, Mn, Ni, Sr and Zn) and toxic elements (As, Cd, Hg and Pb) were discovered by atomic emission/absorption spectrometry in propolis samples collected from different Croatian regions [79]. Br, Co, Cr, Fe, Rb, Sb, Sm and Zn were identified in different Argentinean propolis by neutron activation analysis. These studies show that the trace element profiles can be useful for propolis identification according to their location [80].
9. The Chemical Categories Reported in Propolis
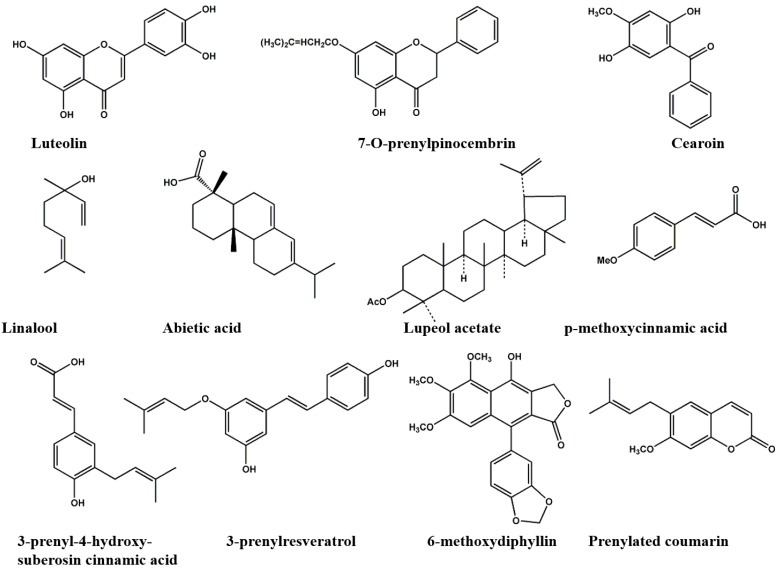
The chemical categories reported in propolis during 2000 and 2012 are summarized in Figure 3 and Table 4, indicating consistency with the categories reported previously (Figure 1). It is well recognized that the chemical composition of herbal medicines are affected by many environmental factors while maintaining their genetic characteristics [81]. Similar effects to propolis can be expected from environmental factors. However, bee species needs to be considered together with geographical factors and plant sources.
Figure 3.
Representative chemical components identified in propolis since 2000.
Table 4.
The chemical categories reported in propolis since 2000.
| Chemical Category | Example Compound | Geographical Origin | Plant Source | Bee Species | References |
|---|---|---|---|---|---|
| Flavonoids | Luteolin | Australia, Brazil, Burma, Canada, Chinese, Cuba, Egypt, Greece, Japan, Kenya, Mexico, Nepal, Poland, Portugal, Solomon Island, Taiwan | Populus, Macaranga, Dalbergia | Apis mellifera | [26,31,34,36,37,38,39,41,42,43,44,45,46,47,61] |
| Prenylated flavanones | 7-O-prenylpino-cembrin | Greece, Japan | Apis mellifera | [39,42] | |
| Neo-flavonoids | Cearoin | Nepal | Dalbergia | Apis mellifera | [50] |
| Monoterpenes Sesquiterpenes Diterpenes | Linalool abietic acid | Brazil, Greece, Indonesia, Iran, Malta, Turkey | Ferula Pinaceae Cupressaceae | Apis mellifera | [37,52,53,55,56,74] |
| Triterpenes | Lupeol acetate | Burma, Brazil, Cuba, Egypt, Greece | Apis mellifera | [29,35,58,59,60] | |
| Phenylpropanoids and esters | p-Methoxycinnamic acid | Australia, Brazil, Egypt, Uruguay | Citrus | Apis mellifera | [61,63,66,68] |
| Prenylated phenylpropanoids | 3-Prenyl-4-hydroxycinnamic acid | Brazilian Green propolis | Baccharies | Africanized Apis mellifera | [67] |
| Stilbenes and prenylated stilbenes | 3-Prenylresveratrol | Australia, Brazil, Greece, Indonesia, Kenya | Macaranga | Apis mellifera | [31,37,44,63,72] |
| Lignans | 6-Methoxydiphyllin | Kenya | Apis mellifera | [37] | |
| Coumarins | Prenylated coumarin suberosin | Iran | Apis mellifera | [55] |
10. Bee Species and Propolis
We propose that species, subspecies and varieties of bees have a major impact on the chemical components and quality of propolis. The genus Apis contains 10 generally recognized species. Honeybee, A. mellifera, is widely spread in Europe, Ural Mountains, Africa, and Asia. All other recognised Apis species are of Asian distribution. About 25 subspecies have been recognized for A. mellifera, based on morphometry, behaviour and biogeography [82], belonging to three or four major subspecies groups [83].
The most popular species of honeybee is the European honeybee, Apies mellifera. It has been shown that varieties of bee affect the antibacterial activity of propolis collected from the same apiary; A. mellifera carnica hives showed weaker antibacterial activity than that of A. mellifera anatolica and A. mellifera caucasica. The three honeybee races used neither the same nor the single plant source [23]. In another type of propolis, geopropolis, produced by stingless bee species, Melipona scutellaris, benzophenones, but no flavonoids, have been identified as the major compounds [84]; However, geopropolis produced by Melipona fasciculate contains high concentrations of polyphenols, flavonoids, triterpenoids, saponins, and even tannins [85].
Although different species of honeybee prefer different plants, the chemical profile of propolis that is produced by the same species is not always same. Brazilian green and red propolis both originate from Africanized A. mellifera [65,86], but these propolis are rich in prenylated phenylpropanoids and isoflavonoids respectively. The differences are due to the plants, namely B. dracunculifolia and Dalbergia ecastophyllum, which are used by bees as resin sources. In cerumen propolis from stingless bees (Tetragonula carbonaria), C-methylated flavanones, terpenic acids and phenolic acids, such as gallic acid, diterpenic acids of pimaric and abietic type are the predominant chemicals, but it lacks the characteristic flavonoids and prenylated phenolics found in propolis from honeybees species in Australia [87,88]. Therefore, the variant chemical composition of propolis depends on the bees’ preferences of botanical sources and the species and varieties of bees [89,90,91].
11. The Geographical Origins of Propolis
Propolis collected from many countries have demonstrated chemical profiles similar to the poplar type propolis: China [92], Korea, Croatia [93], different regions of Taiwan [43,94,95], New Zealand [96] and Africa [35]. Poplar tree (Populus nigra L. and P. alba L) is common in Europe, and is used to name the common type of propolis that is rich in flavonoids and phenylpropanoids. However, flavonoids are not restricted to poplar; furthermore, in areas where poplars are not native plants, such as Australia and equatorial regions of South America, bees will seek other plants to produce propolis, which contain the flavonoids of the poplar type propolis [36].
Propolis from the tropical zone, Brazilian green and red propolis, are respectively rich in prenylated derivatives of p-coumaric acid, and some isoflavonoids that are different from the ones found in poplar type propolis [3,97]. In addition, propolis from Solomon Island, Burma, Greek, Japan are characterized by the geranylated and prenylated flavonoids (Table 1).
12. The Plant Sources of Propolis
The current opinion is that propolis is collected from resins of trees such as poplars and conifers, and therefore propolis is sometimes classified after the name of the source plant [2,3,4]. The plant source is identified by observing the collection activities of bees, and comparing the chemical profiles of propolis and plant materials. Other researchers found that honeybees collect plant material by cutting fragments of vegetative tissues, so the anatomical characteristics of plant tissue in the propolis can be used as evidence of propolis origin [65].
As mentioned in the last section, Populus species are considered to be the main plant origin of propolis all over the world, especially in the temperate zone. Most propolis collected from Europe, North America, non-tropical region of Asia, New Zealand [3] and even Africa (mainly the east area of Nile Delta region) [35] contains the characteristic poplar chemical profile: high level of flavanones, flavones, low phenolic and their esters [98].
In the tropical and subtropical area, there are few poplar trees. Honeybees have to search for new plant source for propolis. For the propolis collected from southeast of Brazil, Baccharis dracunculifolia turns out to be the main botanical source [66,99]. Artepillin C as the salient chemical composition makes it easy to distinguish this propolis from other types of propolis. It is reported that propolis from Venezuela, Amazon and Cuba contains prenylated benzophenones, which is originated from the exudates of Clusia flower [9,100].
Macaranga plants have been demonstrated to be the plant source of Taiwan [95], Okinawan [101] that was classified as Pacific propolis [3]. High concentration of diterpenoids in Mediterranean propolis may originate from Cupressus plants for Sicilian, Cretan propolis [29] and Maltese propolis [74], Pinus plants for Greek propolis [39]. In Kangaroo Island (Australia), bees collect propolis from the sticky exudate on the stem shoots and seed pods of an endemic Australian plant, Acacia paradoxa [45]. Red Brazilian propolis and Nepalese propolis have various biologically active neoflavonoids that primarily come from the genus Dalbergia [24,50].
However, some of plant sources are just surmised by observing the bees’ foraging behaviors, not comparing chemical identity of secondary plant metabolites in propolis and in the plant source. For example, Eucalyptus species are considered as the source plant in Australia, south Anatolia (Turkey) [102], Ismailia (Egypt) [61] and Brazil, but no real proof has been presented for this origin. Therefore, it still needs further study to compare chemical compounds in propolis and the plants, in order to confirm the exact botanic origin.
13. Summary and Future Perspectives
The biological activities of propolis are attributed to a variety of major chemical constituents including phenolic acids, phenolic acid esters, flavonoids, and terpenoids, such as CAPE, artepillin C, caffeic acid, chrysin, and galangin quercetin, apigenin, kaempferol, pinobanksin 5-methyl ether, pinobanksin, pinocembrin, pinobanksin 3-acetate.
Over 500 compounds have been identified in propolis from many countries up to 2012. They belong to flavonoids, phenylpropanoids, terpenoids, stilbenes, lignans, coumarins and their prenylated derivatives. However, other common chemical components such as alkaloids, iridoids have not been reported in propolis. This characteristic is often explained by the plant sources.
We recommend that bee varieties and subspecies need to be considered together with geographical factors and plant species around the beehive in future studies on propolis. The priorities of future research lie on the influence of species and behaviour on propolis, together with feeding experiments to identify the plant part source, which will advance our understanding of the chemistry and quality of propolis, as well as honey bee biology. Characterization of propolis from various locations and plant sources is warranted to define acceptable quantitative standards for different types of propolis. Furthermore, the biological activities of each type of propolis need to be correlated with their chemical composition, and eventually, standardized products should be used in clinical studies.
Acknowledgments
This work was supported by the Grant from the National Natural Science Foundation of China (No. 31272512) and the earmarked fund for Modern Agro-industry Technology Research System from the Ministry of Agriculture of China (CARS-45).
Author Contributions
S.H.: conception, data collection, and manuscript preparation; C.P.Z.: review of the manuscript; K.W.: data collection; G.Q.L.: manuscript preparation and review of the manuscript; F.L.H.: conception and reciew of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mello B.C.B.S., Hubinger M.D. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvents in different pH values. Int. J. Food Sci. Technol. 2012;47:2510–2518. doi: 10.1111/j.1365-2621.2012.03129.x. [DOI] [Google Scholar]
- 2.Kosalec I., Bakmaz M., Pepeljnjak S., Vladimir-Knezevic S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004;54:65–72. [PubMed] [Google Scholar]
- 3.Bankova V.S., de Castro S.L., Marcucci M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. doi: 10.1051/apido:2000102. [DOI] [Google Scholar]
- 4.Burdock G. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem. Toxicol. 1998;36:347–363. doi: 10.1016/S0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 5.Li Y.J., Chen M.L., Xuan H.Z., Hu F.L. Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid. Based Complement. Alternat. Med. 2012;2012:981896. doi: 10.1155/2012/981896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W., Chen M.L., Shou Q.Y., Li Y.H., Hu F.L. Biological activities of Chinese propolis and Brazilian propolis on streptozotocin-induced type 1 diabetes mellitus in rats. Evid. Based Complement. Alternat. Med. 2011;2011:468529. doi: 10.1093/ecam/neq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W., Li Y.H., Chen M.L., Hu F.L. Protective effects of Chinese and Brazilian propolis treatment against hepatorenal lesion in diabetic rats. Hum. Exp. Toxicol. 2011;30:1246–1255. doi: 10.1177/0960327110387456. [DOI] [PubMed] [Google Scholar]
- 8.Hu F.L., Hepburn H.R., Li Y.H., Chen M., Radloff S.E., Daya S. Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J. Ethnopharmacol. 2005;100:276–283. doi: 10.1016/j.jep.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 9.De Castro Ishida V.F., Negri G., Salatino A., Bandeira M.F.C.L. A new type of Brazilian propolis: Prenylated benzophenones in propolis from Amazon and effects against cariogenic bacteria. Food Chem. 2011;125:966–972. doi: 10.1016/j.foodchem.2010.09.089. [DOI] [Google Scholar]
- 10.Wang K., Ping S., Huang S., Hu L., Xuan H.Z., Zhang C.P., Hu F.L. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a Ffavonoid-rich ethanol extract from Chinese propolis (poplar type) Evid. Based Complement. Alternat. Med. 2013;2013:127672. doi: 10.1155/2013/127672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuan H.Z., Zhao J., Miao J.Y., Li Y.J., Chu Y.F., Hu F.L. Effect of Brazilian propolis on human umbilical vein endothelial cell apoptosis. Food Chem. Toxicol. 2011;49:78–85. doi: 10.1016/j.fct.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Xuan H.Z., Zhu R.L., Li Y.J., Hu F.L. Inhibitory effect of Chinese propolis on phosphatidylcholine-specific phospholipase C activity in vascular endothelial cells. Evid. Based Complement. Alternat. Med. 2010;2011:985278. doi: 10.1155/2011/985278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito J., Chang F.R., Wang H.K., Park Y.K., Ikegaki M., Kilgore N., Lee K.H. Anti-AIDS agents. 48. 1 Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001;64:1278–1281. doi: 10.1021/np010211x. [DOI] [PubMed] [Google Scholar]
- 14.Amoros M., Simoes C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 15.Sforcin J.M., Orsi R.O., Bankova V. Effect of propolis, some isolated compounds and its source plant on antibody production. J. Ethnopharmacol. 2005;98:301–305. doi: 10.1016/j.jep.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Bueno-Silva B., Alencar S.M., Koo H., Ikegaki M., Silva G.V., Napimoga M.H., Rosalen P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from brazilian red propolis. J. Agric. Food Chem. 2013;61:4546–4550. doi: 10.1021/jf305468f. [DOI] [PubMed] [Google Scholar]
- 17.Ghisalberti E. Propolis: A review. Bee World. 1979;60:59–84. [Google Scholar]
- 18.Marcucci M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. doi: 10.1051/apido:19950202. [DOI] [Google Scholar]
- 19.Fernandes-Silva C., Freitas J., Salatino A., Salatino M. Cytotoxic activity of six samples of Brazilian propolis on Sea Urchin (Lytechinus variegatus) Eggs. Evid. Based Complement. Altern. Med. 2013;2013:619361. doi: 10.1155/2013/619361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salatino A., Fernandes-Silva C.C., Righi A.A., Salatino M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011;28:925–936. doi: 10.1039/c0np00072h. [DOI] [PubMed] [Google Scholar]
- 21.Toreti V.C., Sato H.H., Pastore G.M., Park Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Alternat. Med. 2013;2013:697390. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bankova V.S. Recent trends and important developments in propolis research. Evid. Based Complement. Alternat. Med. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silici S., Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005;99:69–73. doi: 10.1016/j.jep.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Alencar S., Oldoni T., Castro M., Cabral I., Costa-Neto C., Cury J., Rosalen P., Ikegaki M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. J. Ethnopharmacol. 2007;113:278–283. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Campo Fernandez M., Cuesta-Rubio O., Rosado Perez A. GC-MS determination of isoflavonoids in seven red Cuban propolis samples. J. Agric. Food Chem. 2008;56:9927–9932. doi: 10.1021/jf801870f. [DOI] [PubMed] [Google Scholar]
- 26.Maciejewicz W. Isolation of flavonoid aglycones from propolis by a column chromatography method and their identification by GC-MS and TLC methods. J. Liq. Chromatogr. Relat. Technol. 2001;24:1171–1179. doi: 10.1081/JLC-100103439. [DOI] [Google Scholar]
- 27.Zhang C., Huang S., Wei W., Ping S., Shen X., Li Y., Hu F. Development of High-Performance Liquid Chromatographic for Quality and Authenticity Control of Chinese Propolis. J. Food Sci. 2014;79:C1315–C1322. doi: 10.1111/1750-3841.12510. [DOI] [PubMed] [Google Scholar]
- 28.Nijveldt R.J., van Nood E., van Hoorn D.E., Boelens P.G., van Norren K., van Leeuwen P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 29.Popova M., Chinou I., Marekov I., Bankova V. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry. 2009;70:1262–1271. doi: 10.1016/j.phytochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 30.Righi A.A., Alves T.R., Negri G., Marques L.M., Breyer H., Salatino A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011;91:2363–2370. doi: 10.1002/jsfa.4468. [DOI] [PubMed] [Google Scholar]
- 31.Inui S., Shimamura Y., Masuda S., Shirafuji K., Moli R.T., Kumazawa S. A new prenylflavonoid isolated from propolis collected in the Solomon Islands. Biosci. Biotechnol. Biochem. 2012;76:1038–1040. doi: 10.1271/bbb.120021. [DOI] [PubMed] [Google Scholar]
- 32.Raghukumar R., Vali L., Watson D., Fearnley J., Seidel V. Antimethicillin-resistant Staphylococcus aureus (MRSA) activity of 'pacific propolis' and isolated prenylflavanones. Phytother. Res. 2010;24:1181–1187. doi: 10.1002/ptr.3096. [DOI] [PubMed] [Google Scholar]
- 33.Cao Y., Wang Y., Yuan Q. Analysis of flavonoids and phenolic acid in propolis by capillary electrophoresis. Chromatographia. 2004;59:135–140. [Google Scholar]
- 34.Usia T., Banskota A.H., Tezuka Y., Midorikawa K., Matsushige K., Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J. Nat. Prod. 2002;65:673–676. doi: 10.1021/np010486c. [DOI] [PubMed] [Google Scholar]
- 35.Hegazi A.G., El Hady F.K.A. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z. Naturforsch. C. 2002;57:395–402. doi: 10.1515/znc-2002-3-432. [DOI] [PubMed] [Google Scholar]
- 36.Li F., Awale S., Tezuka Y., Esumi H., Kadota S. Study on the constituents of Mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J. Nat. Prod. 2010;73:623–627. doi: 10.1021/np900772m. [DOI] [PubMed] [Google Scholar]
- 37.Petrova A., Popova M., Kuzmanova C., Tsvetkova I., Naydenski H., Muli E., Bankova V. New biologically active compounds from Kenyan propolis. Fitoterapia. 2010;81:509–514. doi: 10.1016/j.fitote.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Falcão S.I., Vilas-Boas M., Estevinho L.M., Barros C., Domingues M.R., Cardoso S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2010;396:887–897. doi: 10.1007/s00216-009-3232-8. [DOI] [PubMed] [Google Scholar]
- 39.Melliou E., Chinou I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004;70:515–519. doi: 10.1055/s-2004-827150. [DOI] [PubMed] [Google Scholar]
- 40.Li F., He Y.M., Awale S., Kadota S., Tezuka Y. Two new cytotoxic phenylallylflavanones from Mexican propolis. Chem. Pharm. Bull. 2011;59:1194–1196. doi: 10.1248/cpb.59.1194. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha S.P., Narukawa Y., Takeda T. Chemical constituents of Nepalese propolis (II) Chem. Pharm. Bull. 2007;55:926–929. doi: 10.1248/cpb.55.926. [DOI] [PubMed] [Google Scholar]
- 42.Kumazawa S., Goto H., Hamasaka T., Fukumoto S., Fujimoto T., Nakayama T. A new prenylated flavonoid from propolis collected in Okinawa, Japan. Biosci. Biotechnol. Biochem. 2004;68:260–262. doi: 10.1271/bbb.68.260. [DOI] [PubMed] [Google Scholar]
- 43.Chen C.N., Wu C.L., Shy H.S., Lin J.K. Cytotoxic prenylflavanones from Taiwanese propolis. J. Nat. Prod. 2003;66:503–506. doi: 10.1021/np0203180. [DOI] [PubMed] [Google Scholar]
- 44.Li F., Awale S., Tezuka Y., Kadota S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg. Med. Chem. 2008;16:5434–5440. doi: 10.1016/j.bmc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Tran V.H., Duke R.K., Abu-Mellal A., Duke C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry. 2012;81:126–132. doi: 10.1016/j.phytochem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Piccinelli A.L., Campo Fernandez M., Cuesta-Rubio O., Márquez Hernández I., de Simone F., Rastrelli L. Isoflavonoids isolated from Cuban propolis. J. Agric. Food Chem. 2005;53:9010–9016. doi: 10.1021/jf0518756. [DOI] [PubMed] [Google Scholar]
- 47.Christov R., Trusheva B., Popova M., Bankova V., Bertrand M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat. Prod. Res. 2006;20:531–536. doi: 10.1080/14786410500056918. [DOI] [PubMed] [Google Scholar]
- 48.Sha N., Guan S.-H., Lu Z.-Q., Chen G.-T., Huang H.-L., Xie F.-B., Yue Q.-X., Liu X., Guo D.-A. Cytotoxic constituents of Chinese propolis. J. Nat. Prod. 2009;72:799–801. doi: 10.1021/np900118z. [DOI] [PubMed] [Google Scholar]
- 49.Lotti C., Campo Fernandez M., Piccinelli A.L., Cuesta-Rubio O., Hernández I.M., Rastrelli L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010;58:2209–2213. doi: 10.1021/jf100070w. [DOI] [PubMed] [Google Scholar]
- 50.Awale S., Shrestha S.P., Tezuka Y., Ueda J.Y., Matsushige K., Kadota S. Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J. Nat. Prod. 2005;68:858–864. doi: 10.1021/np050009k. [DOI] [PubMed] [Google Scholar]
- 51.Shrestha S.P., Narukawa Y., Takeda T. Chemical constituents of Nepalese propolis: Isolation of new dalbergiones and related compounds. J. Nat. Med. 2007;61:73–76. doi: 10.1007/s11418-006-0024-8. [DOI] [Google Scholar]
- 52.Oliveira A.P., Franca H., Kuster R., Teixeira L., Rocha L. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010;16:121–130. doi: 10.1590/S1678-91992010005000007. [DOI] [Google Scholar]
- 53.Kartal M., Kaya S., Kurucu S. GC-MS analysis of propolis samples from two different regions of Turkey. Z. Naturforsch. C. 2002;57:905–909. doi: 10.1515/znc-2002-9-1025. [DOI] [PubMed] [Google Scholar]
- 54.Melliou E., Stratis E., Chinou I. Volatile constituents of propolis from various regions of Greece-Antimicrobial activity. Food Chem. 2007;103:375–380. doi: 10.1016/j.foodchem.2006.07.033. [DOI] [Google Scholar]
- 55.Trusheva B., Todorov I., Ninova M., Najdenski H., Daneshmand A., Bankova V. Antibacterial mono-and sesquiterpene esters of benzoic acids from Iranian propolis. Chem. Cent. J. 2010;4:8. doi: 10.1186/1752-153X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiryowidagdo S., Simanjuntak P., Heffen W.L. Chemical composition of propolis from different regions in Java and their cytotoxic activity. Am. J. Biochem. Biotechnol. 2009;5:180. doi: 10.3844/ajbbsp.2009.180.183. [DOI] [Google Scholar]
- 57.Popova M.P., Graikou K., Chinou I., Bankova V.S. GC-MS profiling of diterpene compounds in Mediterranean propolis from Greece. J. Agric. Food Chem. 2010;58:3167–3176. doi: 10.1021/jf903841k. [DOI] [PubMed] [Google Scholar]
- 58.Pereira A.S., Nascimento E.A., Aquino Neto F. Lupeol alkanoates in Brazilian propolis. Z. Naturforsch. C. 2002;57:721–726. doi: 10.1515/znc-2002-7-829. [DOI] [PubMed] [Google Scholar]
- 59.Márquez Hernández I., Cuesta-Rubio O., Campo Fernández M., Rosado Pérez A., Montes de Oca Porto R., Piccinelli A.L., Rastrelli L. Studies on the constituents of yellow Cuban propolis: GC-MS determination of triterpenoids and flavonoids. J. Agric. Food Chem. 2010;58:4725–4730. doi: 10.1021/jf904527n. [DOI] [PubMed] [Google Scholar]
- 60.Li F., Awale S., Zhang H., Tezuka Y., Esumi H., Kadota S. Chemical constituents of propolis from Myanmar and their preferential cytotoxicity against a human pancreatic cancer cell line. J. Nat. Prod. 2009;72:1283–1287. doi: 10.1021/np9002433. [DOI] [PubMed] [Google Scholar]
- 61.El Hady F.K.A., Hegazi A.G. Egyptian propolis: 2. Chemical composition, antiviral and antimicrobial activities of East Nile Delta propolis. Extraction. 2000;57:386–394. doi: 10.1515/znc-2002-3-431. [DOI] [PubMed] [Google Scholar]
- 62.Dos Santos Pereiraa A., de Miranda Pereirab A.F., Trugob L.C., de Aquino Netoa F.R. Distribution of Quinic Acid Derivatives and Other Phenolic Compounds in Brazilian Propolis. Z. Naturforsch. C. 2003;58:590–593. doi: 10.1515/znc-2003-7-824. [DOI] [PubMed] [Google Scholar]
- 63.Abu-Mellal A., Koolaji N., Duke R.K., Tran V.H., Duke C.C. Prenylated cinnamate and stilbenes from Kangaroo Island propolis and their antioxidant activity. Phytochemistry. 2012;77:251–259. doi: 10.1016/j.phytochem.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Camargo M.S., Prieto A.M., Resende F.A., Boldrin P.K., Cardoso C.R., Fernández M.F., Molina-Molina J.M., Olea N., Vilegas W., Cuesta-Rubio O. Evaluation of estrogenic, antiestrogenic and genotoxic activity of nemorosone, the major compound found in brown Cuban propolis. BMC Complement. Altern. Med. 2013;13:1–8. doi: 10.1186/1472-6882-13-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teixeira É.W., Negri G., Meira R.M., Salatino A. Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evid. Based Complement. Alternat. Med. 2005;2:85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salatino A., Teixeira É.W., Negri G. Origin and chemical variation of Brazilian propolis. Evid. Based Complement. Alternat. Med. 2005;2:33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcucci M., Ferreres F., García-Viguera C., Bankova V., De Castro S., Dantas A., Valente P., Paulino N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001;74:105–112. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 68.Kumazawa S., Hayashi K., Kajiya K., Ishii T., Hamasaka T., Nakayama T. Studies of the constituents of Uruguayan propolis. J. Agric. Food Chem. 2002;50:4777–4782. doi: 10.1021/jf020279y. [DOI] [PubMed] [Google Scholar]
- 69.Matsui T., Ebuchi S., Fujise T., Abesundara K.J., Doi S., Yamada H., Matsumoto K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3, 4, 5-tri-O-caffeoylquinic acid. Biol. Pharm. Bull. 2004;27:1797–1803. doi: 10.1248/bpb.27.1797. [DOI] [PubMed] [Google Scholar]
- 70.Marcucci M.C., Ferreres F., Custódio A.R., Ferreira M., Bankova V.S., García-Viguera C., Bretz W.A. Evaluation of phenolic compounds in Brazilian propolis from different geographic regions. Z. Naturforsch. C. 2000;55:76–81. doi: 10.1515/znc-2000-1-215. [DOI] [PubMed] [Google Scholar]
- 71.Castro M.L., Nascimento A.M., Ikegaki M., Costa-Neto C.M., Alencar S.M., Rosalen P.L. Identification of a bioactive compound isolated from Brazilian propolis type 6. Bioorg. Med. Chem. 2009;17:5332–5335. doi: 10.1016/j.bmc.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 72.Trusheva B., Popova M., Koendhori E.B., Tsvetkova I., Naydenski C., Bankova V. Indonesian propolis: Chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2011;25:606–613. doi: 10.1080/14786419.2010.488235. [DOI] [PubMed] [Google Scholar]
- 73.Hegazi A.G., Abd El Hady F., Abd Allah F. Chemical composition and antimicrobial activity of European propolis. Z. Naturforsch. C. 2000;55:70–75. doi: 10.1515/znc-2000-1-214. [DOI] [PubMed] [Google Scholar]
- 74.Popova M., Trusheva B., Antonova D., Cutajar S., Mifsud D., Farrugia C., Tsvetkova I., Najdenski H., Bankova V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011;126:1431–1435. doi: 10.1016/j.foodchem.2010.11.130. [DOI] [Google Scholar]
- 75.Crane E. Beekeeping: Science, Practice and World Recourses. Heinemann; London, UK: 1988. [Google Scholar]
- 76.Uzel A., Sorkun K., Önçağ Ö., Çoğulu D., Gençay Ö. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005;160:189–195. doi: 10.1016/j.micres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Negri G., Marcucci C., Salatino A., Salatino M.L.F. Comb and propolis waxes from Brazil. J. Braz. Chem. Soc. 2000;11:453–457. doi: 10.1590/S0103-50532000000500004. [DOI] [Google Scholar]
- 78.Negri G. Hydrocarbons and monoesters of propolis waxes. Apidologie. 1998;29:305–314. doi: 10.1051/apido:19980401. [DOI] [Google Scholar]
- 79.Cvek J., Medid-Saric M., Vitali D., Vedrina-Dragojevik I., Smit Z., Tomic S. The content of essential and toxic elements in Croatian propolis samples and their tinctures. J. Apicult. Res. 2008;47:35–45. doi: 10.3896/IBRA.1.47.1.06. [DOI] [Google Scholar]
- 80.Cantarelli M.A., Caminia J.M., Pettenati E.M., Marchevsky E.J., Pellerano R.G. Trace mineral content of Argentinean raw propolis by neutron activation analysis (NAA): Assessment of geographical provenance by chemometrics. LWT Food Sci. Technol. 2011;44:256–260. doi: 10.1016/j.lwt.2010.06.031. [DOI] [Google Scholar]
- 81.Razmovski-Naumovski V., Tongkao-on W., Kimble B., Qiao V.L., Beilun L., Li K.M., Roufogalis B., Depo Y., Meicun Y., Li G.Q. Multiple chromatographic and chemometric methods for quality standardisation of Chinese herbal medicines. World Sci. Technol. 2010;12:99–106. doi: 10.1016/S1876-3553(11)60003-3. [DOI] [Google Scholar]
- 82.Arias M.C., Sheppard W.S. Phylogenetic relationships of honey bees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 2005;37:25–35. doi: 10.1016/j.ympev.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Arias M.C., Sheppard W.S. Molecular phylogenetics of honey bee subspecies (Apis mellifera L.) inferred from mitochondrial DNA sequence. Mol. Phylogenet. Evol. 1996;5:557–566. doi: 10.1006/mpev.1996.0050. [DOI] [PubMed] [Google Scholar]
- 84.Da Cunha M.G., Franchin M., de Carvalho Galvão L.C., de Ruiz A.L., de Carvalho J.E., Ikegaki M., de Alencar S.M., Koo H., Rosalen P.L. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013;13:23. doi: 10.1186/1472-6882-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutra R.P., Abreu B.V., Cunha M.S., Batista M.C., Torres L.M., Nascimento F.R., Ribeiro M.N., Guerra R.N. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014;62:2549–2557. doi: 10.1021/jf404875v. [DOI] [PubMed] [Google Scholar]
- 86.Daugsch A., Moraes C.S., Fort P., Park Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid. Based Complement. Alternat. Med. 2008;5:435–441. doi: 10.1093/ecam/nem057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massaro F.C., Brooks P.R., Wallace H.M., Russell F.D. Cerumen of Australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften. 2011;98:329–337. doi: 10.1007/s00114-011-0770-7. [DOI] [PubMed] [Google Scholar]
- 88.Massaro C., Katouli M., Grkovic T., Vu H., Quinn R., Heard T., Carvalho C., Manley-Harris M., Wallace H., Brooks P. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae) Fitoterapia. 2014;95:247–257. doi: 10.1016/j.fitote.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 89.Leonhardt S., Zeilhofer S., Blüthgen N., Schmitt T. Stingless bees use terpenes as olfactory cues to find resin sources. Chem. Sens. 2010;35:603–611. doi: 10.1093/chemse/bjq058. [DOI] [PubMed] [Google Scholar]
- 90.Leonhardt S.D., Blüthgen N. A sticky affair: Resin collection by Bornean stingless bees. Biotropica. 2009;41:730–736. doi: 10.1111/j.1744-7429.2009.00535.x. [DOI] [Google Scholar]
- 91.Leonhardt S., Blüthgen N., Schmitt T. Smelling like resin: Terpenoids account for species-specific cuticular profiles in Southeast-Asian stingless bees. Insectes Sociaux. 2009;56:157–170. doi: 10.1007/s00040-009-0007-3. [DOI] [Google Scholar]
- 92.Ahn M.R., Kumazawa S., Usui Y., Nakamura J., Matsuka M., Zhu F., Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007;101:1383–1392. doi: 10.1016/j.foodchem.2006.03.045. [DOI] [Google Scholar]
- 93.Kosalec I., Bakmaz M., Pepeljnjak S. Analysis of propolis from the continental and Adriatic regions of Croatia. Acta Pharm. 2003;53:275–285. [PubMed] [Google Scholar]
- 94.Chen C.N., Weng M.S., Wu C.L., Lin J.K. Comparison of Radical Scavenging Activity, Cytotoxic Effects and Apoptosis Induction in Human Melanoma Cells by Taiwanese Propolis from Different Sources. Evid. Based Complement. Alternat. Med. 2004;1:175–185. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang W.J., Huang C.H., Wu C.L., Lin J.K., Chen Y.W., Lin C.L., Chuang S.E., Huang C.Y., Chen C.N. Propolin G, a prenylflavanone, isolated from Taiwanese propolis, induces caspase-dependent apoptosis in brain cancer cells. J. Agric. Food Chem. 2007;55:7366–7376. doi: 10.1021/jf0710579. [DOI] [PubMed] [Google Scholar]
- 96.Markham K.R., Mitchell K.A., Wilkins A.L., Daldy J.A., Yinrong L. HPLC and GC-MS identification of the major organic constituents in New Zealand propolis. Phytochemistry. 1996;42:205–211. doi: 10.1016/0031-9422(96)83286-9. [DOI] [Google Scholar]
- 97.Trusheva B., Popova M., Bankova V., Simova S., Marcucci M.C., Miorin P.L., Pasin F.R., Tsvetkova I. Bioactive constituents of Brazilian red propolis. Evid. Based Complement. Altern. Med. 2006;3:249–254. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohammadzadeh S., Shariatpanahi M., Hamedi M., Ahmadkhaniha R., Samadi N., Ostad S.N. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007;103:1097–1103. doi: 10.1016/j.foodchem.2006.10.006. [DOI] [Google Scholar]
- 99.Kumazawa S., Yoneda M., Shibata I., Kanaeda J., Hamasaka T., Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem. Pharm. Bull. 2003;51:740–742. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- 100.Trusheva B., Popova M., Naydenski H., Tsvetkova I., Gregorio Rodriguez J., Bankova V. New polyisoprenylated benzophenones from Venezuelan propolis. Fitoterapia. 2004;75:683–689. doi: 10.1016/j.fitote.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Kumazawa S., Nakamura J., Murase M., Miyagawa M., Ahn M.-R., Fukumoto S. Plant origin of Okinawan propolis: Honeybee behavior observation and phytochemical analysis. Naturwissenschaften. 2008;95:781–786. doi: 10.1007/s00114-008-0383-y. [DOI] [PubMed] [Google Scholar]
- 102.Silici S., Ünlü M., Vardar-Ünlü G. Antibacterial activity and phytochemical evidence for the plant origin of Turkish propolis from different regions. World J. Microbiol. Biotechnol. 2007;23:1797–1803. doi: 10.1007/s11274-007-9430-7. [DOI] [PubMed] [Google Scholar]