Abstract
Design and synthesis of triazole library antifungal agents having piperazine side chains, analogues to fluconazole were documented. The synthesis highlighted utilization of the click chemistry on the basis of the active site of the cytochrome P450 14α-demethylase (CYP51). Their structures were characterized by 1H-NMR, 13C-NMR, MS and IR. The influences of piperazine moiety on in vitro antifungal activities of all the target compounds were evaluated against eight human pathogenic fungi.
Keywords: antifungal, triazole, synthesis, piperazine
1. Introduction
Triazole compounds have been specially paid increasing attention because of their extensively medicinal applications in antimicrobial agent’s particularly antifungal therapy, and a large number of predominant triazole drugs have been successfully developed and prevalently used in the treatment of various microbial infections for many years [1]. Azoles (fluconazole, itraconazole, voriconazole, and posaconazole, Figure 1) are important antifungal drugs for the treatment of invasive fungal infections (IFIs), which continue to be a major cause of morbidity and mortality in immunocompromised orseverely ill patients [2]. However, fluconazole is not effective against invasive aspergillosis and has suffered severe drug resistance [3,4]. The increasing frequency of fungal infections and development of resistance to the current treatment highlight the need for development of new triazole derivatives possessing broader antifungal spectra and higher therapeutic indexes.
Figure 1.
Triazole antifungal agents used in clinical therapy.
Azole antifungals act by competitive inhibition of CYP51, the enzyme that catalyzes the oxidative removal of the 14α-methyl group of lanosterol to give Δ14,15-desaturated intermediates in ergosterol biosynthesis [5]. In general, the active site of CYP51 for ligand binding can be divided into four subsites: a coordination bond with iron of the heme group, the hydrophilic H-bonding region, the hydrophobic region, and the narrow hydrophobic cleft formed by the residues in the helix B'-meander 1 loop and N-terminus of helix I [6]. These compounds target the biosynthesis of ergosterol by inhibiting the cytochrome P450-dependent lanosterol 14α-demethylase (Erg11p, CYP51), encoded by the ERG11 gene, resulting in accumulation of toxic methylsterols inmembranes that may culminate in fungi static effect or fungal death [7].
Literature precedents [8,9] revealed a pharmacophore of antifungal triazoles, which contained a triazole ring linking to a dihalophenyl ring through a two carbon chain. In addition, the carbon alpha to the phenyl ring bears a hydroxyl group. Itraconazole and posaconazole which containing the group of piperazine. We intended to alter the side chains to find potent systemic antifungal compounds with a broad antifungal spectrum and less potential to develop resistance. In our previous works [10,11,12,13,14,15,16], many studies on the structure-activity relationships (SAR) of antifungal azoles have been developed, and these studies have led to new compounds endowed with better biological and pharmacological properties.
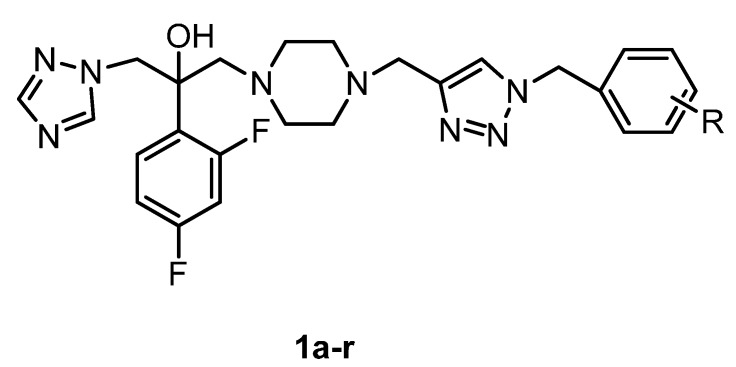
According to the above results, we designed a series of 1-(1H-1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-substituted-2-propanols (1a–r Figure 2) containing a triazole ring, a difluorophenyl group, a hydroxyl group and a side chain containing piperazine group. In our design, we systematically altered the structure of fluconazole as a platform and tried to insert the 1,2,3-triazole group into the side chain.
Figure 2.
Generic structure of the designed fluconazole analogues.
2. Results and Discussion
2.1. Chemistry
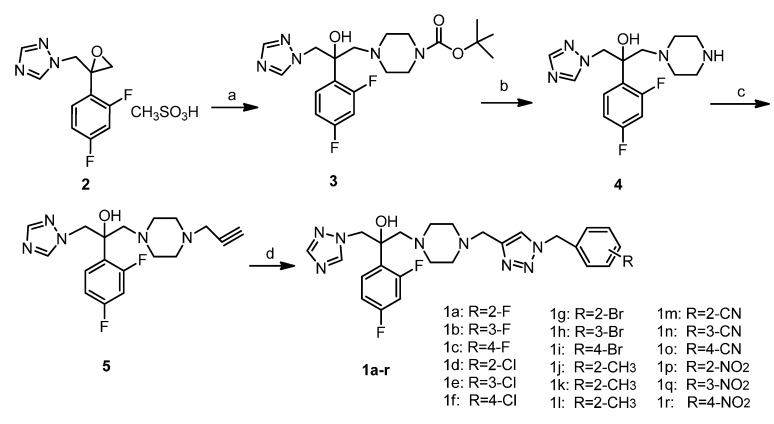
Target compounds 1a–r were synthesized according to a very efficient and straightforward synthetic route outlined in Scheme 1. Compound 3 was synthesized by ring-open reaction of oxirane 2 with N-Boc-piperazine and simultaneous Boc-deprotection was accomplished by treatment with F3CCOOH to furnish compound 4. N-alkylation was effective in the presence of KI, K2CO3 and propargyl bromide in acetonitrile at room temperature to secure compound 5. The target compounds were achieved by using click reaction [17] with variously substituted benzyl azides.
Scheme 1.
Synthesis of the target compounds 1a–r. Conditions: (a) Et3N, N-Boc-piperazine, EtOH, reflux, 6 h, 80%; (b) CF3COOH, room temperature, 1 h, 87%; (c) propargyl bromide, KI, K2CO3, CH3CN, room temperature, 5–6 h, 72%; (d) NaN3, substituted benzyl bromide, DMSO, CuSO4.5H2O, sodium ascorbate, rt, 12 h, 60%–70%.
2.2. Pharmacology
The in vitro antifungal activities of all the target compounds were evaluated against eight human pathogenic fungi, Candida albicans 14053 (C.alb.14053), Candida albicans 20352 (C.alb.20352), Candida parapsilosis (C. Par.), Cryptococcus neoformans (C.neo.), Candida glabrata (C. gla.), Aspergillus fumigates (A.fum.), Trichophyton rubrum (T.rub.), Microsporum gypseum (M. gyp.), which are often encountered clinically, and were compared with itraconazole (ICZ), voriconazole (VCZ) and fluconazole (FCZ). All of the above eight human pathogenic fungi were provided by Shanghai Changzheng Hospital; Fluconazole (FCZ), itraconazole (ICZ) and voriconazole (VCZ) served as the positive control and were obtained from their respective manufacturers.
The in vitro minimal inhibitory concentrations (MICs) of the compounds were determined by the micro-broth dilution method in 96-well microtest plates according to the methods defined by the National Committee for Clinical Laboratory Standards (NCCLS) [18]. The MIC80 was defined as the first well with an approximate 80% reduction in growth compared to the growth of the drug-free well. For assays, the title compounds to be tested were dissolved in dimethyl sulfoxide (DMSO), serially diluted in growth medium, inoculated and incubated at 35 °C Growth MIC was determined at 24 h for C. alb. and at 72 h for C. neo. The in vitro antifungal activities are summarized in Table 1. The MIC values (in μg/mL) are presented against different pathogenic fungi, in comparison with ICZ, VCZ and FCZ.
Table 1.
Antifungal activities of the target compounds in vitro (MIC80 μg/mL).
| Compd | C. alb14053 | C. alb. 20352 | C. par. | C. neo. | C. gla. | A. fum. | T. rub. | M. gyp. |
|---|---|---|---|---|---|---|---|---|
| 1a | 2 | 8 | 4 | 8 | >64 | >64 | 8 | 4 |
| 1b | 1 | 4 | 4 | 4 | 4 | >64 | 4 | 16 |
| 1c | 1 | 2 | 8 | 2 | 4 | >64 | 8 | 8 |
| 1d | 0.25 | 0.5 | 1 | 1 | 16 | >64 | 2 | 8 |
| 1e | 1 | 1 | 1 | 1 | 16 | >64 | 1 | 4 |
| 1f | 0.5 | 0.5 | 1 | 1 | >64 | >64 | 1 | 1 |
| 1g | 0.5 | 0.5 | 1 | 1 | 16 | >64 | 1 | 0.5 |
| 1h | 0.5 | 0.5 | 1 | 0.5 | 32 | >64 | 1 | 0.5 |
| 1i | 0.25 | 0.5 | 0.5 | 0.5 | >64 | >64 | 1 | 0.5 |
| 1j | 1 | 2 | 4 | 4 | >64 | >64 | 4 | 0.25 |
| 1k | 64 | 64 | 4 | 4 | >64 | >64 | 4 | 0.25 |
| 1l | 1 | 4 | 4 | 0.5 | 64 | >64 | 1 | 0.25 |
| 1m | 4 | 16 | 16 | 16 | >64 | >64 | 16 | 16 |
| 1n | 8 | 8 | 16 | 8 | >64 | >64 | 16 | 4 |
| 1o | 8 | 8 | 16 | 8 | >64 | >64 | 16 | 2 |
| 1p | 2 | 4 | 8 | 4 | >64 | >64 | 4 | 2 |
| 1q | 1 | 8 | 8 | 8 | >64 | >64 | 4 | 2 |
| 1r | 2 | 8 | 8 | 4 | 64 | >64 | 4 | 0.25 |
| ICZ | 0.0625 | 0.0625 | 0.03125 | 0.0156 | 0.5 | 2 | 0.125 | 4 |
| VCZ | 0.03125 | 0.0625 | 0.03125 | 0.0156 | 0.5 | 0.25 | 0.0625 | 0.25 |
| FCZ | 1 | 0.5 | 0.5 | 0.5 | 2 | >64 | 4 | 64 |
Abbreviations: C.alb.14053, Candida albicans14053; C.alb.20352, Candida albicans20352; C. par., Candida parapsilosis; C.neo., Cryptococcus neoformans; C. gla., Candida glabrata; A.fum., Aspergillusfumigates; T.rub., Trichophytonrubrum; M. gyp., Microsporum gypseum. ICZ, Itraconazole; VCZ, Voriconazole; FCZ, Fluconazole.
The results of antifungal activities in vitro showed that all the 18 target compounds (1a–r) were active against nearly all fungi tested to some extent except against A. fum. and C. gla. The MIC80 value of compound 1d and 1i is 4 times lower than that of FCZ against C. alb. 14053 in vitro (with the MIC80 value of 0.25 μg/mL). The MIC80 value of compound 1j, 1k, 1l and 1r is 128 times lower than that of FCZ against M. gyp. in vitro, and the same as VCZ against M. gyp. in vitro (with the MIC80 value of 0.25 μg/mL). However, most of the target compounds’ antifugal activities were not as good as the ICZ and VCZ.
3. Experimental Section
3.1. General Procedures
1H and spectra were recorded in CDCl3 unless otherwise indicated with a Bruker AC-300P spectrometer, using TMS as internal standard. ESI mass spectra were performed on an API-3000 LC-MS spectrometer. The solvents and reagents were used as received or dried prior to use as needed.
Tert-butyl-4-(2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl)piperazine-1-carboxylate (3): A mixture of compound 2 (16.65 g, 0.05 mol), CH3CH2OH (300 mL) and Et3N (25 mL), N-Boc-piperazine (13.96 g, 0.075 mol) was stirred and refluxed for 6 h. The reaction was monitored by TLC. After filtration, the filtrate was evaporated under reduced pressure. Water was added to the residue, extracted with ethylacetate twice, combinate the organic layer, washed with saturated NaHCO3 and NaCl solution twice, dried over anhydrous Na2SO4 and evaporated to get compound 3 (16.93 g, 80%).
1-(1H-1,2,4-Triazole-1-yl)-2-(2,4-difluorophenyl)-3-(piperazin-1-yl)-2-propanol (4): A mixture of compound 3 (4.23 g, 0.01 mol), Trifluoroacetic acid (11.4 g, 0.1 mol) was stirred at room temperature for 30 min. The reaction was monitored by TLC. After reaction, most of the trifluoroacetic acid was removed in a vacuum. Column chromatography of the residue afforded compound 4 as yellow oil (DCM/CH3OH, 20:1–10:1, 2.81 g, 87%).
1-(1H-1,2,4-Triazole-1-yl)-2-(2,4-difluorophenyl)-3-(4-(prop-2-yn-1-yl)piperazin-1-yl)-2-propanol (5): A mixture of compound 4 (3.23 g, 0.01 mol), propargyl bromide (2.36 g, 0.02 mol), KI (166 mg, 0.001 mol), K2CO3 (3.45 g, 0.025 mol), and CH3CN (50 mL) was stirred at room temperature for 6 h. The reaction was monitored by TLC. After reaction, we filtrated off the solid and washed with CH3CN. The filtrate was concentrated in a vacuum. Column chromatography of the residue afforded compound 5 as a brown oil (DCM/CH3OH, 20:1–10:1, 2.60 g, 72%).
3.2. General Procedure for the Preparation of the Compounds 1a–r
A mixture of NaN3 (100 mg, 1.4 mmol), 2-fluorobenzyl bromide (200 mg, 1.2 mmol) and DMSO (15 mL) was stirred at room temperature for 6 h. Then the compound 5 (217 mg, 0.6 mmol) was added, sodium ascorbate (20 mg), CuSO4.5H2O (25 mg) and H2O (1 mL) was added, then stirred at room temperature for another 2 h. The reaction was monitored by TLC, then NH3.H2O was added carefully and extracted with ethylacetate. The organic layer was acidificated with dilute hydrochloric acid, then the aqueous layer was adjusted pH about 7.0 by saturation sodium bicarbonate, then extracted with ethylacetate, washed with water, NaHCO3 and NaCl, and dried with Na2SO4. It was then concentrated in a vacuum. Column chromatography of the residue afforded compound 1a as yellow oil. (212 mg, 69%).1H-NMR (300 MHz, CDCl3) δ: 8.17 (s, 1H), 7.81 (s, 1H), 7.58–7.55 (m, 2H), 7.46–7.34 (m, 2H), 7.32–7.27 (m, 1H), 7.19–7.09 (m, 1H), 6.85–6.78 (m, 2H), 5.58 (s, 2H), 4.53 (s, 2H), 3.62 (s, 2H), 3.10–2.63 (m, 2H), 2.41–2.27 (m, 8H); 13C NMR (75 MHz, CDCl3) δ: 163.7, 158.7, 151.3, 147.5, 144.1, 139.1, 132.5, 130.1, 129.9, 129.8, 129.7, 127.7, 124.3, 123.7, 119.1, 118.5, 114.1, 111.4, 104.1, 71.7, 62.1, 56.3, 54.1, 53.1, 51.3; IR(KBr): 3380, 2923, 2821, 2100, 1615, 1498, 1458, 1273, 1137, 1048, 1015, 966, 852, 675, 616, 515 cm−1; ESI-MS, m/z calcd. for C25H27F3N8O 512.2, found [M+H]+ 513.5.
The target compounds 1b–r were synthesized by the same operation procedure of the compound 1a.
4. Conclusions
In summary, a novel series of antifungal agents have been designed and synthesized by chemical methods. In vitro antifungal activity assay indicated that most of the compounds showed antifungal activities against both systemic pathogenic fungi. Several compounds show high in vitro antifungal activity with a broad spectrum, which were valuable for further evaluation.
Acknowledgments
This work was supported by Creativity and Innovation Training Program of Second Military Medical University (No. ZD2013029, MS2012047), the National Basic Research Program of China (2013CB531606, 2013CB531601), National Science and Technology Major Projects of China Grant (2013ZX10004612001, 2011ZXJ09201-12), Chinese natural science fund (No. 31270180) and Shanghai Key Laboratory of Molecular Medical Mycology Fund (14DZ2272900).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/8/11333/s1.
Supplementary Files
Author Contributions
Yanwei Wang and Kehan Xu contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Gadhave P.P., Dighe N.S., Pattan S.R., Deotarse P., Musmade D.S., Shete R. Current biological and synthetic profile of triazoles: A review. Annals Biol. Res. 2010;1:82–89. [Google Scholar]
- 2.Groll A.H., Lumb J. New developments in invasive fungal disease. Future Microbiol. 2012;7:179–184. doi: 10.2217/fmb.11.154. [DOI] [PubMed] [Google Scholar]
- 3.Casalinuovo I.A., di Francesco P., Garaci E. Fluconazole resistance in Candida albicans: A review of mechanisms. Eur. Rev. Med. Pharmacol. 2004;8:69–77. [PubMed] [Google Scholar]
- 4.Hoffman H.L., Ernst E.J., Klepser M.E. Novel triazole antifungal agents. Expert Opin. Inv. Drug. 2000;9:593–605. doi: 10.1517/13543784.9.3.593. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama Y., Yoshida Y., Sato R. Yeast cytochrome P-450 catalyzing lanosterol 14 alpha-demethylation. II. Lanosterol metabolism by purified P-450(14)DM and by intact microsomes. J. Biol. Chem. 1984;259:1661–1666. [PubMed] [Google Scholar]
- 6.Sheng C.Q., Zhang W.N., Ji H.T., Song Y.L., Zhang M., Zhou Y.J., Lu J.G., Zhu J. Design, synthesis and antifungal activity of novel triazole derivatives. Chin. Chem. Lett. 2004;15:404–407. [Google Scholar]
- 7.Kelly S., Arnoldi A., Kelly D. Molecular genetic analysis of azole antifungal mode of action. Biochem. Soc. Trans. 1993;21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 8.Sheng C.Q., Zhang W.N., Ji H.T., Zhang M., Song Y.L., Xu H., Zhu J., Miao Z.Y., Jiang Q.F., Yao J.Z., et al. Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J. Med. Chem. 2006;49:2512–2525. doi: 10.1021/jm051211n. [DOI] [PubMed] [Google Scholar]
- 9.Boyle F.T., Gilman D.J., Gravestock M.B., Wardleworth J.M. Synthesis and structure-activity relationships of a novel antifungal agent, ICI 195,739. Ann. NY Acad. Sci. 1988;544:86–100. doi: 10.1111/j.1749-6632.1988.tb40391.x. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y., Yu S., Li R., Zhao Q., Li X., Wu M., Huang T., Chai X., Hu H., Wu Q. Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl) propyl dithiocarbamates. Eur. J. Med. Chem. 2014;74:366–374. doi: 10.1016/j.ejmech.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Yu S., Wang L., Wang Y., Song Y., Cao Y., Jiang Y., Sun Q., Wu Q. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives containing the 1,2,3-triazole group. RSC Advan. 2013;3:13486–13490. doi: 10.1039/c3ra41310a. [DOI] [Google Scholar]
- 12.Yu S., Chai X., Wang N., Cui H., Zhao Q., Hu H., Zou Y., Sun Q., Wu Q. Synthesis and antifungal activity of the novel triazole compounds. Med. Chem. Commun. 2013;4:704–708. doi: 10.1039/c3md20086h. [DOI] [Google Scholar]
- 13.Wang N., Chai X., Chen Y., Zhang L., Li W., Gao Y., Bi Y., Yu S., Meng Q. Synthesis, antifungal activity, and molecular docking studies of novel triazole derivatives. Med. Chem. 2013;9:384–388. doi: 10.2174/1573406411309030009. [DOI] [PubMed] [Google Scholar]
- 14.Chai X., Yu S., Jiang Y., Zou Y., Wu Q., Zhang D., Jiang Y., Cao Y., Sun Q. Design, synthesis, and biological evaluation of novel 1,2,4-triazole derivatives as antifungal agent. Arch. Pharm. Res. 2012;35:1895–1901. doi: 10.1007/s12272-012-1105-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang B.G., Yu S.C., Chai X.Y., Yan Y.Z., Hu H.G., Wu Q.Y. Design synthesis and biological evaluation of 3-substituted triazole derivatives. Chin. Chem. Lett. 2011;22:519–522. doi: 10.1016/j.cclet.2010.11.029. [DOI] [Google Scholar]
- 16.Yu S., Chai X., Hu H., Yan Y., Guan Z., Zou Y., Sun Q., Wu Q. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14 alpha-demethylase. Eur. J. Med. Chem. 2010;45:4435–4445. doi: 10.1016/j.ejmech.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X.J., Li H.Y., You L.F., Tang Y., Hsung R.P. Copper salt-catalyzed azide-[3 + 2] cycloadditions of ynamides and bis-ynamides. Adv. Synth. Catal. 2006;348:2437–2442. doi: 10.1002/adsc.200600404. [DOI] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved standard. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2002. Document M27-A2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.