Abstract
A series of novel arylpiperazine derivatives was synthesized. The in vitro cytotoxic activities of all synthesized compounds against three human prostate cancer cell lines (PC-3, LNCaP, and DU145) were evaluated by a CCK-8 assay. Compounds 9 and 15 exhibited strong cytotoxic activities against LNCaP cells (IC50 < 5 μM), and compound 8 (IC50 = 8.25 μM) possessed the most potent activity against DU145 cells. However, these compounds also exhibited cytotoxicity towards human epithelial prostate normal cells RWPE-1. The structure–activity relationship (SAR) of these arylpiperazine derivatives was also discussed based on the obtained experimental data.
Keywords: synthesis, arylpiperazine derivatives, cytotoxic activity, CCK-8, structure-activity relationship
1. Introduction
Prostate cancer is the most common non-skin cancer in men and is the second-leading cause of cancer-related deaths in the US [1]. The incidence of prostate cancer varies worldwide. Generally, the incidence rate in Occidental countries is higher than that in Asian countries [2,3]. Old age [4], ethnicity [4], family history [5,6] diet [7] hormones and other related factors [3] of this disease are the principal risk factors of developing prostate cancer. Prostate cancer mortality typically results from the metastasis to the bone and lymph nodes, as well as the progression from androgen-dependent to androgen-independent prostatic growth [8]. Clinically, localized disease is potentially curable [9] through surgery or radiotherapy to remove or destroy cancerous cells. However, metastatic prostate cancer remains essentially incurable and androgen ablation therapy has been the standard therapy [10,11]. Although various chemotherapeutic agents [12] are used solely or in combination with radiotherapy to treat advanced diseases, none of the conventional approaches to cancer therapy have been proven to be highly successful for prostate cancer. Other studies have shown once tumor cells have become hormone refractory, the standard cytotoxic agents for hormone-refractory prostate cancer (HRPC) can do little to improve the treatment outcomes or survival rates [13,14,15], although they can still relieve the pain to some extent in some patients. Therefore, inventing and developing more effective and safe anti-prostate cancer drugs are urgently needed.
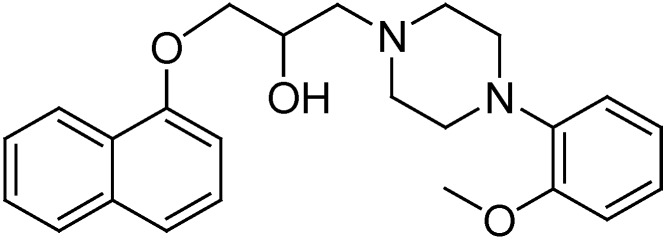
Compounds with arylpiperazine moieties have a wide range of bioactivities including antiarrhythmic [16] diuretic [17], antiallergic [18], antidepressant [19] anxiolytic [20] antipsychotic [21] antimalarial [22] and antiplasmodial properties [23]. In addition, these compounds also exhibit receptor-blocking properties [17,24,25,26,27]. Naftopidil (Figure 1), an arylpiperazine compound, is a specific α1d receptor antagonist [28,29]. Previous studies have demonstrated that naftopidil has higher affinity for the α1d-adrenergic receptor than for the α1a- and α1b-adrenergic receptor subtypes [30], and in Japan it is one of the most widely used α1-adrenergic receptor antagonists for the treatment of benign prostatic hyperplasia (BPH) [31,32].
Figure 1.
Structure of naftopidil.
Recent studies have shown that naftopidil could possibly exert an anticancer effect and inhibit prostate cancer cell growth by arresting the G1 cell cycle phase [33,34]. In addition, naftopidil can also decrease cell viability for bladder, prostate, and renal cancer cell lines [35], as well as induce apoptosis in malignant mesothelioma cell lines [36]. These findings indicate that naftopidil might be useful as an anti-cancer drug. Many studies have shown that arylpiperazine derivatives may act as potential α1a- and/or α1a- + α1d-selective ligands for the treatment of BPH. However, there have been few studies on cytotoxic activities of these compounds against human prostate cancer cells [37,38,39,40,41,42]. As part of our group’s continuing efforts to study the core framework of naftopidil [43,44,45,46], herein we report the synthesis of a series of novel arylpiperazinyl derivatives based on naftopidil (Scheme 1) to identify new anti-prostate cancer drug candidates with potential for further development. All synthesized compounds were evaluated for their cytotoxic activities against the androgen-insensitive human prostate cancer cell line PC-3, the androgen-sensitive human prostate cancer cell line LNCaP, the androgen-insensitive human prostate cancer cell line DU145, and the human prostate epithelial cell line RWPE-1. The SAR was further discussed on the basis of the obtained experimental data. Some compounds exhibited strong anti-cancer activities against the tested cancer cells and superior potency than naftopidil.
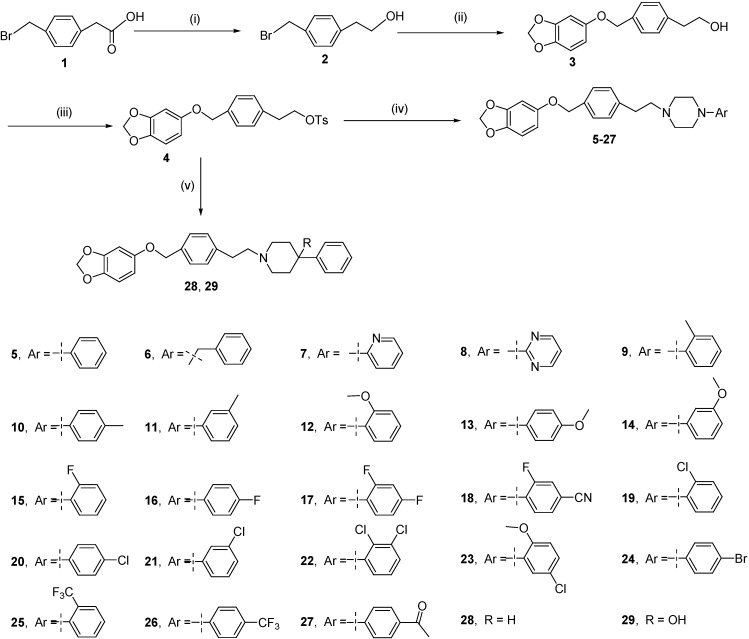
Scheme 1.
The synthesis route of compounds 5–29.
Reagents and conditions: (i) BH3.S(CH3)2, THF, 0 °C for 1 h, and room temperature for 10 h; (ii) sesamol, K2CO3, CH3CN, reflux, 16 h; (iii) TsCl, Et3N and 4-dimethylaminopyridine, Cl2CH2, 0 °C, 16 h; (iv) arylpiperazines, K2CO3, CH3CN, reflux, 16 h; (v) phenylpiperidines, K2CO3, CH3CN, reflux, 16 h.
2. Results and Discussion
2.1. Chemistry
As depicted in Scheme 1, a series of novel arylpiperazine derivatives were synthesized in four steps starting from the commercially available 2-(4-(bromomethyl)phenyl)acetic acid (1). First, compound 1 was reduced to alcohol 2 in the presence of a borane–methyl sulfide complex (2 M in tetrahydrofuran) at 0 °C for 1 h and then at room temperature for 10 h. The intermediate 2 was directly used without further purification. The nucleophilic substitution reaction of compound 2 with sesamol in the presence of potassium carbonate (K2CO3) gave compound 3 (67% yield from compound 1) after 16 h at reflux. Compound 3 was treated with 4-toluenesulfonyl chloride in the presence of triethylamine and a catalytic amount of 4-dimethylaminopyridine at 0 °C for 16 h to generate compound 4 (95% yield). Finally, the reactions of compound 4 with various arylpiperazines or phenyl-piperidines in the presence of K2CO3 at reflux for 16 h gave arylpiperazine or phenylpiperidine derivatives 5 to 29 (55% to 82% yield; Scheme 1). The structures of all the compounds (as their HCl salts) were confirmed using 1H-NMR, 13C-NMR, and elemental analyses (C, H, and N).
2.2. Cytotoxic Activity
The synthesized target compounds 5 to 29 were evaluated for their in vitro cytotoxic activities against the three human prostate cancer cell lines (PC-3, LNCaP, and DU145), and compared with their effects on human prostate epithelial cell line RWPE-1 by CCK-8 assay [47,48,49]. The results are summarized in Table 1.
Table 1.
In vitro cytotoxicity of compounds 5–29.
| Compd. | IC50 (μM) a | |||
|---|---|---|---|---|
| PC-3 b | LNCaP b | DU145 b | RWPE-1 b | |
| 5 | >50 | 27.85 ± 1.72 | >50 | >50 |
| 6 | >50 | 27.70 ± 4.17 | 28.92 ± 0.98 | 38.78 ± 0.77 |
| 7 | >50 | 31.22 ± 0.63 | >50 | 41.05 ± 0.54 |
| 8 | >50 | 26.60 ± 0.86 | 8.25 ± 0.16 | 31.69 ± 3.14 |
| 9 | >50 | 3.47 ± 0.36 | >50 | 31.01 ± 1.57 |
| 10 | >50 | 31.94 ± 0.89 | 47.75 ± 2.02 | >50 |
| 11 | >50 | >50 | >50 | >50 |
| 12 | >50 | 30.73 ± 0.83 | >50 | >50 |
| 13 | >50 | 28.27 ± 0.49 | >50 | >50 |
| 14 | >50 | >50 | >50 | 38.95 ± 0.77 |
| 15 | >50 | 1.25 ± 0.23 | >50 | 41.01 ± 0.47 |
| 16 | >50 | 32.47 ± 1.04 | >50 | >50 |
| 17 | >50 | 26.76 ± 1.00 | 26.83 ± 0.92 | 23.94 ± 0.75 |
| 18 | 26.89 ± 1.00 | 13.11 ± 0.51 | >50 | >50 |
| 19 | >50 | 26.58 ± 0.40 | >50 | 29.85 ± 0.80 |
| 20 | >50 | >50 | >50 | >50 |
| 21 | >50 | 25.28 ± 1.55 | >50 | >50 |
| 22 | >50 | 39.41 ± 0.54 | >50 | >50 |
| 23 | >50 | 33.25 ± 2.69 | >50 | 35.89 ± 1.25 |
| 24 | >50 | 19.85 ± 1.01 | >50 | >50 |
| 25 | >50 | 32.96 ± 0.21 | >50 | 18.94 ± 3.68 |
| 26 | >50 | 26.83 ± 1.51 | >50 | >50 |
| 27 | 24.95 ± 0.68 | >50 | >50 | 26.79 ± 3.01 |
| 28 | >50 | 18.21 ± 1.88 | 24.71 ± 2.22 | 28.46 ± 2.18 |
| 29 | >50 | 42.98 ± 7.58 | 38.23 ± 2.45 | 19.73 ± 0.01 |
| naftopidil | 42.10 ± 0.79 | 22.36 ± 0.61 | 34.58 ± 0.31 | >50 |
a IC50 values are taken as means ± standard deviation from three experiments; b PC-3, LNCaP and DU145, human prostate cancer cell line; RWPE-1, the human prostate epithelial cell line.
As shown in Table 1, some compounds exhibited moderate to strong cytotoxic activities against the tested cancer cell lines, and even exhibited much better activity than naftopidil. For example, compounds 9 and 15 exhibited strong cytotoxic activities against LNCaP cells (IC50 < 5 μM) and these compounds showed excellent selective activity for LNCaP cells over the other tested cancer cells. However, compounds 9 and 15 exhibited cytotoxic effects on normal RWPE-1 human epithelial prostate cells. Moreover, compound 8 (IC50 = 8.25 μM) showed the most potent activity against DU145 cells and a marked selectivity for DU145 cells over the other tested cancer cells.
The SAR analysis revealed the following: (1) phenylpiperazine derivative 5 exhibited similar cytotoxic activities against LNCaP cells compared with benzylpiperazine derivative 6, however, these activities decreased significantly in DU145 cells; (2) compound 8 exhibited a more effective cytotoxic activity than compounds 5 and 7 against DU145 cells. These results suggest that the introduction of a pyrimidinyl moiety at the 4-position of piperazine ring was beneficial for anti-cancer activity. Moreover, compound 8 showed excellent selective activity for DU145 cells over the other tested cancer cells; (3) compound 11 lost potency (IC50 > 50 μM) against LNCaP cells compared with compounds 9 and 10 (IC50 = 3.47 and 31.94 μM, respectively). The activity profiles indicated that a methyl at the m-position on the phenyl group was inauspicious for anti-cancer activity. A similar potency profile was observed in compounds 12, 13, and 14; (4) Moreover, compounds containing an o-methyl-substituted phenyl group showed better activity for LNCaP cells than did the p-methyl-substituted group for LNCaP cells, as exemplified by compound 9 which showed significantly improved activity, while compound 10 exhibited moderate cytotoxic activity. A similar potency profile was also observed in compound 15 (IC50 = 1.25 μM) vs. 16 (IC50 = 32.47 μM), as well as 19 (IC50 = 26.58 μM) vs. 20 (IC50 > 50 μM); (5) Compared to compounds 16 and 24 (IC50 = 19.85 μM), compound 20 (IC50 > 50 μM) lost potency against LNCaP cells. The obtained results suggested that a chloro group in the p-position on the phenyl group was not favorable for anti-cancer activity; (6) Although compounds 22 (IC50 = 39.41 μM), 23 (IC50 = 33.25 μM), 25 (IC50 = 32.96 μM), and 26 (IC50 = 26.83 μM) demonstrated moderate cytotoxic activities against LNCaP cells, these compounds had improved selectivity for LNCaP cells over the other cancer cells; (7) The compounds with methyl or fluoro groups at the o-position on the phenyl group exhibited a relatively strong cytotoxicity against LNCaP cells (IC50 < 5 μM); (8) Phenylpiperidine derivatives 28 and 29 (Scheme 1) were synthesized to compare the anti-cancer activity of arylpiperazine derivatives. As shown in Table 1, compounds 28 and 29 exhibited moderate cytotoxic activities against LNCaP and DU145 cells. However, these compounds also exhibited cytotoxic effect on human prostate epithelial cell line.
3. Experimental Section
3.1. General Information
Reagents and solvents were commercially available. Solvents were dried and purified prior to use using standard procedures. Melting points were determined on a SGW X-4 micro melting point apparatus (Shanghai Precision & Scientific Instrument Co., Ltd, Shanghai, China) and are uncorrected. NMR spectra were determined on a Bruker AV-400 NB spectrometer (Faellanden, Switzerland) in DMSO-d6 using TMS as internal standard, and coupling constants (J) are in Hz. EI mass spectra were recorded on a DSQ mass spectrometer. Elemental analyses (C, H, N) were performed on an Elementar Vario EL elemental analyzer and the analytical results were within ±0.4% of the theoretical values for the formula given unless otherwise listed. Flash column chromatography was performed with silica gel (300–400 mesh, Qing Dao Ocean Chemical Factory, Qingdao, China) eluted with petroleum ether–ethyl acetate.
3.1.1. 2-(4-(Bromomethyl)phenyl)ethanol (2)
To a cooled (0 °C) solution of carboxylic acid 1 (5 g, 0.021 mol) in dry tetrahydrofuran (THF, 100 mL) borane-dimethyl sulfide complex (21.9 mL, 0.042 mol, 2 M in THF) was added dropwise. The reaction mixture was stirred at 0 °C for 1 h and at room temperature for 10 h. Water (20 mL) was added slowly and extracted with ethyl acetate (3 × 100 mL). The combined organic phase was successively washed with water, brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo. The resulting residue was directly used without further purification in the following step.
3.1.2. 2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethanol (3)
To a solution of compound 2 (4 g, 18.7 mmol) in acetone (100 mL) sesamol (2.58 g, 18.7 mmol)and potassium carbonate (10.32 g, 74.8 mmol) were added, and the reaction mixture was stirred at reflux for 16 h. After cooling to ambient temperature, the reaction mixture was filtered through a Buchner funnel. After filtration the filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1/10, v/v) as eluent to afford 4.06 g of compound 3 (67% from compound 1) as a white solid. m.p. 102–103 °C; 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 7.32 (d, J = 8.0 Hz, 2H, Ar-H), 7.22 (d, J = 8.0 Hz, 2H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 4.97 (s, 2H, CH2), 4.60 (t, J = 5.2 Hz, 1H, OH), 3.60 (td, J = 7.0, 5.2 Hz, 2H, CH2), 2.72 (t, J = 7.0 Hz, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.25, 148.34, 141.64, 139.65, 135.07, 129.33, 128.09, 108.46, 106.64, 101.42, 98.55, 70.39, 62.57; MS (EI, m/z): 272 (M+), 167, 149, 135 (100%), 117, 105, 97, 79.
3.1.3. 2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl 4-methylbenzenesulfonate (4)
To a solution of compound 3 (4 g, 14.7 mmol), triethylamine (5.94 g, 58.8 mmol) and 4-dimethyl- aminopyridine (0.18 g, 1.47 mmol) in dry dichloromethane (CH2Cl2, 100 mL) at 0 °C was added dropwise a solution of 4-toluenesulfonyl chloride (4.19 g, 22.1 mmol) in CH2Cl2 (10 mL). The reaction mixture was stirred at 0 °C for 16 h. Water (20 mL) was added slowly and the reaction mixture was extracted with CH2Cl2 (3 × 100 mL). The combined organic phase was successively washed with water and brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo. The residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1/15, v/v) as eluent to afford 5.76 g (95%) of compound 4 as a white solid. m.p. 90–91 °C; 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 7.65 (d, J = 8.0 Hz, 2H, Ar-H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.15 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.44 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 4.98 (s, 2H, CH2), 4.23 (t, J = 6.4 Hz, 2H, CH2), 2.89 (t, J = 6.4 Hz, 2H, CH2), 2.40 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.22, 148.36, 145.23, 141.68, 136.78, 135.90, 132.78, 130.52, 129.28, 128.21, 127.89, 108.47, 106.64, 101.44, 98.55, 71.43, 70.27, 34.55, 21.53; MS (EI, m/z): 426 (M+), 289, 254, 155, 137, 117 (100%), 104, 91.
3.1.4. General Procedure for the Preparation of Compounds 5–29
To a solution of 4 (100 mg, 0.23 mmol) in acetonitrile (CH3CN, 10 mL) was added the corresponding arylpiperazine or phenylpiperidine (1.2 equiv) and potassium carbonate (6.0 equiv). The reaction mixture was stirred at reflux for 16 h. After cooling to ambient temperature, the reaction mixture was filtered through a Buchner funnel. After filtration the filtrate was concentrated in vacuo and the residue was purified by silica gel column chromatography using ethyl acetate/petroleum ether (1/5, v/v) as eluent to afford the corresponding products, and all compounds were recrystallized from trichloromethane and n-hexane.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-phenylpiperazine (5). Yield: 65%, m.p. 195–196 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.51 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.27 (dd, J = 8.0, 7.3 Hz, 2H, Ar-H), 7.02 (d, J = 8.0 Hz, 2H, Ar-H), 6.87 (t, J = 7.3 Hz, 1H, Ar-H ), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.82 (d, J = 10.4 Hz, 2H, CH2), 3.62 (d, J = 10.4 Hz, 2H, CH2), 3.40–3.09 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.07, 149.87, 148.26, 141.60, 137.07, 136.08, 129.49, 129.09, 128.40, 120.45, 116.40, 108.38, 106.61, 101.36, 98.49, 70.13, 56.36, 50.92, 45.80, 29.33; Anal. Calc. for C26H28N2O3·2HCl: C, 63.80; H, 6.18; N, 5.72. Found: C, 63.64; H, 6.12; N, 5.58.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-benzylpiperazine (6). Yield: 80%, m.p. 177–178 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 12.00 (s, 1H, N+H), 7.71–7.42 (m, 5H, Ar-H), 7.39 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 4.34 (s, 2H, CH2), 3.94–2.93 (m, 12H, CH2); Anal. Calc. for C27H30N2O3·2HCl: C, 64.41; H, 6.41; N, 5.56. Found: C, 63.98; H, 6.45; N, 5.31.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(pyridin-2-yl)piperazine (7). Yield: 70%, m.p. 185–186 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.92 (s, 1H, N+H), 8.13 (dd, J = 5.6, 1.2 Hz, 1H, pyridine H), 7.96 (t, J = 7.6 Hz, 1H, pyridine H), 7.41–7.30 (m, 5H, Ar-H and pyridine H ), 6.98 (t, J = 6.4 Hz, 1H, pyridine H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.44 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 4.54 (d, J = 10.6 Hz, 2H, CH2), 3.78–3.09 (m, 10H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 148.35, 141.69, 137.09, 136.19, 129.17, 128.50, 114.40, 108.47, 106.69, 101.45, 98.58, 70.22, 56.47, 50.38, 43.40, 40.66, 29.38; Anal. Calc. for C25H27N3O3·2.8HCl: C, 57.79; H, 5.78; N, 8.09. Found: C, 57.78; H, 6.06; N, 7.86.
2-(4-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)piperazin-1-yl)pyrimidine (8). Yield: 65%, m.p. 181–182 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.36 (s, 1H, N+H), 8.45 (d, J = 4.4 Hz, 2H, pyrimidine H), 7.39 (d, J = 7.5 Hz, 2H, Ar-H), 7.29 (d, J = 7.5 Hz, 2H, Ar-H), 6.85–6.37 (m, 4H, Ar-H and pyrimidine H), 5.94 (s, 2H, CH2), 5.00 (s, 2H, CH2), 4.70 (d, J = 11.4 Hz, 2H, CH2), 3.64–3.05 (m, 10H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 161.07, 158.61, 154.14, 145.81, 141.68, 137.02, 136.20, 129.16, 128.49, 111.79, 108.46, 106.69, 101.45, 98.57, 70.20, 56.59, 50.85, 29.41; Anal. Calc. for C24H26N4O3·1.5HCl: C, 60.92; H, 5.86; N, 11.84. Found: C, 60.67; H, 5.98; N, 11.61.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-o-tolylpiperazine (9). Yield: 80%, m.p. 202–203 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.16 (s, 1H, N+H), 7.42 (d, J = 8.1 Hz, 2H, Ar-H), 7.32 (d, J = 8.1 Hz, 2H, Ar-H), 7.10 (m, 4H, Ar-H), 6.81 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.44 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.96 (s, 2H, CH2), 5.01 (s, 2H, CH2), 3.62 (d, J = 10.9 Hz, 2H, CH2), 3.43–3.08 (m, 10H, CH2), 2.28 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 155.55, 151.59, 149.74, 143.08, 138.51, 137.58, 133.84, 132.86, 130.58, 129.89, 128.55, 125.62, 120.84, 109.85, 108.08, 102.83, 99.96, 71.59, 57.99, 53.31, 50.02, 30.88, 19.26; Anal. Calc. for C27H30N2O3·2HCl: C, 64.41; H, 6.41; N, 5.56. Found: 64.63; H, 6.45; N, 5.44.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-p-tolylpiperazine (10). Yield: 70%, m.p. 184–185 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.30 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.08 (d, J = 8.3 Hz, 2H, Ar-H), 6.92 (d, J = 8.3 Hz, 2H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.75 (d, J = 9.2 Hz, 2H, CH2), 3.62 (d, J = 9.2 Hz, 2H, CH2), 3.40–3.07 (m, 8H, CH2), 2.22 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.15, 148.34, 147.73, 141.69, 137.12, 136.17, 130.02, 129.62, 129.18, 128.49, 116.79, 108.47, 106.71, 101.45, 98.58, 70.22, 56.48, 51.05, 46.41, 29.44, 20.51; Anal. Calc. for C27H30N2O3·2HCl: C, 64.41; H, 6.41; N, 5.56. Found: C, 64.40; H, 6.43; N, 5.36.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-m-tolylpiperazine (11). Yield: 82%, m.p. 171–172 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.61 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.15 (t, J = 7.6 Hz, 1H, Ar-H), 6.93–6.62 (m, 5H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.80 (d, J = 11.2 Hz, 2H, CH2), 3.62 (d, J = 11.2 Hz, 2H, CH2), 3.41–3.06 (m, 8H, CH2), 2.27 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.17, 149.89, 148.35, 141.69, 138.78, 137.18, 136.15, 129.42, 129.17, 128.49, 121.45, 117.19, 113.80, 108.47, 106.70, 101.45, 98.58, 70.23, 56.45, 50.97, 46.01, 29.41, 21.80; Anal. Calc. for C27H30N2O3·2HCl: C, 64.41; H, 6.41; N, 5.56. Found: C, 64.54; H, 6.44; N, 5.44.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2-methoxyphenyl)piperazine (12). Yield: 75%, m.p. 173–174 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.50 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.31 (d, J = 8.0 Hz, 2H, Ar-H), 7.07–6.88 (m, 4H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.01 (s, 2H, CH2), 3.80 (s, 3H, OCH3), 3.62 (d, J = 10.8 Hz, 2H, CH2), 3.51 (d, J = 10.8 Hz, 2H, CH2), 3.39–3.10 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 152.31, 148.35, 141.69, 139.69, 137.17, 136.17, 129.18, 128.49, 124.06, 121.33, 118.80, 112.50, 108.47, 106.70, 101.45, 98.58, 70.22, 56.62, 55.88, 51.52, 47.36, 29.43; Anal. Calc. for C27H30N2O4·2HCl: C, 62.43; H, 6.21; N, 5.39. Found: C, 62.37; H, 6.19; N, 5.25.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(4-methoxyphenyl)piperazine (13). Yield: 70%, m.p. 174–175 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.62 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.31 (d, J = 8.0 Hz, 2H, Ar-H), 7.05 (d, J = 9.0 Hz, 2H, Ar-H), 6.89 (d, J = 9.0 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.71 (s, 3H, OCH3), 3.66 (t, J = 9.0 Hz, 4H, CH2), 3.40–3.10 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.30, 153.66, 147.84, 142.69, 141.19, 136.63, 135.66, 128.68, 127.98, 118.48, 114.47, 107.96, 106.19, 100.95, 98.08, 69.72, 55.88, 55.25, 50.41, 47.06, 28.93; Anal. Calc. for C27H30N2O4·1.9HCl: C, 62.87; H, 6.23; N, 5.43. Found: C, 62.90; H, 6.20; N, 5.25.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(3-methoxyphenyl)piperazine (14). Yield: 72%, m.p. 171–172 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.56 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.17 (t, J = 8.0 Hz, 1H, Ar-H), 6.80 (d, J = 8.4 Hz, 1H, Ar-H), 6.69 (d, J = 2.4 Hz, 1H, Ar-H), 6.60 (dd, J = 8.4, 2.0 Hz, 1H, Ar-H), 6.55 (t, J = 2.0 Hz, 1H, Ar-H), 6.50–6.37 (m, 2H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.83 (d, J = 11.2 Hz, 2H, CH2), 3.74 (s, 3H, OCH3), 3.61 (d, J = 11.2 Hz, 2H, CH2), 3.41–3.06 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 160.46, 153.86, 151.00, 148.05, 141.39, 136.88, 135.86, 130.03, 128.87, 128.19, 108.60, 108.17, 106.40, 105.53, 102.39, 101.15, 98.28, 69.93, 56.15, 55.18, 50.65, 45.51, 29.11; Anal. Calc. for C27H30N2O4·2HCl: C, 62.43; H, 6.21; N, 5.39. Found: C, 62.41; H, 6.19; N, 5.27.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2-fluorophenyl)piperazine (15). Yield: 65%, m.p. 187–188 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.46 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.23–6.96 (m, 4H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.63 (d, J = 9.7 Hz, 2H, CH2), 3.50 (d, J = 9.7 Hz, 2H, CH2), 3.42–3.09 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 156.27, 153.89, 148.08, 141.42, 138.50, 138.42, 136.83, 135.91, 128.91, 128.22, 125.18, 125.16, 123.65, 123.58, 119.80, 116.44, 116.24, 108.20, 106.44, 101.18, 98.31, 69.95, 56.31, 51.05, 47.16, 29.13; Anal. Calc. for C26H27FN2O3·2HCl: C, 61.54; H, 5.76; N, 5.52. Found: C, 61.38; H, 5.56; N, 5.33.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(4-fluorophenyl)piperazine (16). Yield: 60%, m.p. 196–197 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.55 (s, 1H, N+H), 7.40 (d, J = 7.9 Hz, 2H, Ar-H), 7.30 (d, J = 7.9 Hz, 2H, Ar-H), 7.15–7.00 (m, 4H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.74 (d, J = 8.8 Hz, 2H, CH2), 3.62 (d, J = 8.8 Hz, 2H, CH2), 3.40–3.08 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 157.75, 155.40, 153.64, 147.83, 146.32, 141.17, 136.65, 135.64, 128.65, 127.97, 117.90, 117.82, 115.55, 115.33, 107.95, 106.18, 100.94, 98.06, 69.71, 55.90, 50.51, 46.07, 28.90; Anal. Calc. for C26H27FN2O3·1.9HCl: C, 61.99; H, 5.78; N, 5.56. Found: C, 62.02; H, 5.77; N, 5.41.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2,4-difluorophenyl)piperazine (17). Yield: 67%, m.p. 169–170 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.42 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.28–7.00 (m, 3H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.63 (d, J = 8.6 Hz, 2H, CH2), 3.43 (d, J = 8.6 Hz, 2H, CH2), 3.40–3.09 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 158.98, 158.93, 158.86, 156.58, 156.47, 156.24, 156.12, 153.84, 148.04, 141.38, 136.77, 135.88, 135.37, 135.34, 135.28, 135.25, 128.86, 128.18, 120.82, 120.80, 120.74, 120.70, 111.50, 111.47, 111.28, 111.26, 108.15, 106.38, 105.24, 104.98, 104.73, 101.14, 98.26, 69.90, 56.23, 51.03, 47.44, 29.10; Anal. Calc. for C26H26F2N2O3·1.25HCl: C, 62.70; H, 5.51; N, 5.62. Found: C, 62.85; H, 5.61; N, 5.42.
4-(4-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)piperazin-1-yl)-3-fluorobenzonitrile (18). Yield: 75%, m.p. 182–183 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.54 (s, 1H, N+H), 7.77 (dd, J = 13.1, 1.2 Hz, 1H, Ar-H), 7.63 (dd, J = 8.4, 1.2 Hz, 1H, Ar-H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.25 (t, J = 8.4 Hz, 1H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.72 (d, J = 12.0 Hz, 2H, CH2), 3.64 (d, J = 12.0 Hz, 2H, CH2), 3.45–3.09 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.39, 153.64, 151.94, 147.83, 142.35, 141.17, 136.53, 135.68, 129.90, 128.65, 127.98, 120.02, 119.92, 119.77, 118.07, 107.95, 106.17, 103.59, 100.94, 98.06, 69.69, 56.02, 50.43, 46.10, 28.88; Anal. Calc. for C27H26FN3O3·1HCl: C, 65.38; H, 5.49; N, 8.47. Found: C, 65.12; H, 5.45; N, 8.24.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2-chlorophenyl)piperazine (19). Yield: 78%, m.p. 176–177 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.27 (s, 1H, N+H), 7.46 (dd, J = 8.0, 1.6 Hz, 1H, Ar-H), 7.41 (d, J = 8.0 Hz, 2H, Ar-H), 7.35 (td, J = 8.0, 1.6 Hz, 1H, Ar-H), 7.31 (d, J = 8.0 Hz, 2H, Ar-H), 7.23 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H), 7.12 (td, J = 8.0, 1.2 Hz, 1H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.01 (s, 2H, CH2), 3.66 (d, J = 8.4 Hz, 2H, CH2), 3.44 (d, J = 8.4 Hz, 2H, CH2), 3.41–3.08 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 148.35, 147.88, 141.70, 137.08, 136.20, 130.93, 129.20, 128.72, 128.50, 128.04, 125.29, 121.52, 108.47, 106.70, 101.46, 98.58, 70.22, 56.59, 51.66, 48.15, 29.51; Anal. Calc. for C26H27ClN2O3·1.25HCl: C, 62.89; H, 5.73; N, 5.64. Found: C, 62.95; H, 5.70; N, 5.47.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(4-chlorophenyl)piperazine (20). Yield: 70%, m.p. 164–165 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.64 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.33–7.26 (m, 4H, Ar-H), 7.03 (d, J = 9.0 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.83 (d, J = 11.4 Hz, 2H, CH2), 3.62 (d, J = 11.4 Hz, 2H, CH2), 3.40–3.09 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 153.66, 148.37, 147.84, 141.19, 136.66, 135.65, 128.76, 128.66, 127.98, 123.52, 117.47, 107.96, 106.19, 100.95, 98.08, 69.72, 55.91, 50.33, 45.16, 28.89; Anal. Calc. for C26H27ClN2O3·2HCl: C, 59.61; H, 5.58; N, 5.35. Found: C, 59.66; H, 5.59; N, 5.19.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(3-chlorophenyl)piperazine (21). Yield: 65%, m.p. 177–178 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.60 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.25 (t, J = 8.4 Hz, 1H, Ar-H), 7.06 (t, J = 2.4 Hz, 1H, Ar-H), 6.97 (dd, J = 8.4, 2.4 Hz, 1H, Ar-H), 6.87 (dd, J = 7.6, 1.2 Hz, 1H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.90 (d, J = 11.6 Hz, 2H, CH2), 3.60 (d, J = 11.6 Hz, 2H, CH2), 3.40–3.07 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 151.27, 148.35, 141.69, 137.16, 136.16, 134.42, 131.08, 129.17, 128.49, 119.66, 115.76, 114.64, 108.47, 106.70, 101.45, 98.58, 70.22, 56.42, 50.77, 45.31, 29.40; Anal. Calc. for C26H27ClN2O3·2HCl: C, 59.61; H, 5.58; N, 5.35. Found: C, 59.55; H, 5.58; N, 5.18.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2,3-dichlorophenyl)piperazine (22). Yield: 60%, m.p. 177–178 °C (HCl salt). 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 10.78 (s, 1H, N+H), 7.41–7.30 (m, 6H, Ar-H), 7.22 (dd, J = 7.2, 2.8 Hz, 1H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.66 (d, J = 10.0 Hz, 2H, CH2), 3.27–3.04 (m, 10H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 155.53, 151.34, 149.73, 143.08, 140.49, 137.62, 134.60, 130.60, 130.50, 129.89, 127.95, 127.19, 121.74, 109.86, 108.09, 102.83, 99.95, 71.58, 57.99, 53.07, 49.66, 30.95; Anal. Calc. for C26H26Cl2N2O3·1HCl: C, 59.84; H, 5.21; N, 5.37. Found: C, 60.04; H, 5.27; N, 5.16.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(5-chloro-2-methoxyphenyl)piperazine (23). Yield: 65%, m.p. 177–178 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.46 (s, 1H, N+H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.05 (dd, J = 8.7, 2.4 Hz, 1H, Ar-H), 6.99 (d, J = 8.7 Hz, 1H, Ar-H), 6.94 (d, J = 2.4 Hz, 1H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 3.80 (s, 3H, OCH3), 3.60 (d, J = 10.8 Hz, 2H, CH2), 3.55 (d, J = 10.8 Hz, 2H, CH2), 3.39–3.08 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 151.12, 148.35, 141.69, 141.14, 137.14, 136.18, 129.18, 128.49, 124.99, 122.98, 118.68, 113.74, 108.47, 106.70, 101.45, 98.58, 70.22, 56.55, 56.27, 51.37, 47.01, 29.43; Anal. Calc. for C27H29ClN2O4·1.8HCl: C, 59.33; H, 5.68; N, 5.12. Found: C, 59.26; H, 5.79; N, 4.87.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(4-bromophenyl)piperazine (24). Yield: 72%, m.p. 173–174 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.62 (s, 1H, N+H), 7.43–7.35 (m, 4H, Ar-H), 7.29 (d, J = 8.0 Hz, 2H, Ar-H), 6.97 (d, J = 9.0 Hz, 2H, Ar-H), 6.79 (d, J = 8.5 Hz, 1H, Ar-H), 6.67 (d, J = 2.5 Hz, 1H, Ar-H), 6.42 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.94 (s, 2H, CH2), 4.99 (s, 2H, CH2), 3.82 (d, J = 11.3 Hz, 2H, CH2), 3.60 (d, J = 11.3 Hz, 2H, CH2), 3.38–3.07 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.16, 149.23, 148.35, 141.69, 137.17, 136.16, 132.14, 129.17, 128.49, 118.40, 111.72, 108.47, 106.70, 101.45, 98.58, 70.22, 56.42, 50.79, 45.53, 29.40; Anal. Calc. for C26H27BrN2O3·2HCl: C, 54.95; H, 5.14; N, 4.93. Found: C, 54.93; H, 5.15; N, 4.76.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(2-(trifluoromethyl)phenyl)piperazine (25). Yield: 75%, m.p. 171–172 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.35 (s, 1H, N+H), 7.60–7.56 (m, 6H, Ar-H), 7.31 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.01 (s, 2H, CH2), 3.63 (d, J = 11.2 Hz, 2H, CH2), 3.45–3.34 (m, 4H, CH2), 3.23–3.07 (m, 6H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 154.17, 150.95, 148.35, 141.69, 137.11, 136.18, 134.37, 129.21, 128.49, 127.59, 127.54, 126.58, 124.91, 108.47, 106.70, 101.45, 98.58, 70.22, 56.49, 51.95, 50.22, 29.55; Anal. Calc. for C27H27F3N2O3·1HCl: C, 62.25; H, 5.42; N, 5.38. Found: C, 62.41; H, 5.40; N, 5.24.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-(4-(trifluoromethyl)phenyl)piperazine (26). Yield: 55%, m.p. 176–177 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.45 (s, 1H, N+H), 7.57 (d, J = 8.8 Hz, 2H, Ar-H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.17 (d, J = 8.8 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 4.03 (d, J = 11.6 Hz, 2H, CH2), 3.64 (d, J = 11.6 Hz, 2H, CH2), 3.43–3.06 (m, 8H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 153.64, 151.95, 147.83, 141.18, 136.60, 135.66, 128.66, 127.98, 126.28, 114.88, 107.95, 106.18, 100.94, 98.06, 69.70, 55.94, 50.19, 44.21, 28.93; Anal. Calc. for C27H27F3N2O3·1.25HCl: C, 61.18; H, 5.37; N, 5.28. Found: C, 61.49; H, 5.42; N, 5.19.
1-(4-(4-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)piperazin-1-yl)phenyl)ethanone (27). Yield: 60%, m.p. 183–184 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.22 (s, 1H, N+H), 7.86 (d, J = 8.8 Hz, 2H, Ar-H), 7.40 (d, J = 8.0 Hz, 2H, Ar-H), 7.30 (d, J = 8.0 Hz, 2H, Ar-H), 7.08 (d, J = 8.8 Hz, 2H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.68 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.00 (s, 2H, CH2), 4.09 (d, J = 11.6 Hz, 2H, CH2), 3.64 (d, J = 11.6 Hz, 2H, CH2), 3.36–3.10 (m, 8H, CH2), 2.48 (s, 3H, CH3); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 195.72, 153.63, 152.54, 147.82, 141.17, 136.52, 135.67, 130.00, 128.65, 127.97, 127.78, 113.87, 107.94, 106.17, 100.93, 98.05, 69.68, 55.94, 50.25, 43.92, 28.94, 26.13; Anal. Calc. for C28H30N2O4·1.5HCl: C, 65.52; H, 6.19; N, 5.46. Found: C, 65.78; H, 6.47; N, 5.31.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-phenylpiperidine (28). Yield: 75%, m.p. 183–184 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.04 (s, 1H, N+H), 7.59–7.08 (m, 9H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.44 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.01 (s, 2H, CH2), 3.64 (d, J = 11.6 Hz, 2H, CH2), 3.30–3.00 (m, 6H, CH2), 2.90–2.78 (m, 1H, CH), 2.10–2.08 (m, 4H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 153.85, 148.04, 144.41, 141.38, 136.98, 135.83, 128.86, 128.70, 128.18, 126.71, 108.16, 106.39, 101.14, 98.27, 69.91, 56.73, 52.08, 29.93, 29.26; Anal. Calc. for C27H29NO3·1HCl: C, 71.75; H, 6.69; N, 3.10. Found: C, 71.25; H, 6.69; N, 2.98.
1-(2-(4-((Benzo[d][1,3]dioxol-5-yloxy)methyl)phenyl)ethyl)-4-phenylpiperidin-4-ol (29). Yield: 80%, m.p. 194–195 °C (HCl salt); 1H-NMR (400 MHz, DMSO-d6) δ in ppm: 11.11 (s, 1H, N+H), 7.55–7.20 (m, 9H, Ar-H), 6.80 (d, J = 8.5 Hz, 1H, Ar-H), 6.69 (d, J = 2.5 Hz, 1H, Ar-H), 6.43 (dd, J = 8.5, 2.5 Hz, 1H, Ar-H), 5.95 (s, 2H, CH2), 5.47 (s, 1H, OH), 5.00 (s, 2H, CH2), 3.49 (d, J = 11.0 Hz, 2H, CH2), 3.43–3.06 (m, 8H, CH2), 1.82 (d, J = 11.0 Hz, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ in ppm: 153.65, 147.88, 147.83, 141.17, 136.77, 135.61, 128.67, 128.03, 127.95, 126.77, 124.51, 107.95, 106.18, 100.93, 98.07, 69.71, 68.00, 56.32, 48.25, 34.96, 29.17; Anal. Calc. for C27H29NO4·1HCl: C, 69.29; H, 6.46; N, 2.99. Found: C, 69.20; H, 6.45; N, 2.85.
3.2. In Vitro Cytotoxic Assay
3.2.1. Cell Culture
PC-3 and RWPE-1 cells were cultured in Dulbecco’s modification Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA), 100 U/mL penicillin and 0.1 mg/mL streptomycin (Invitrogen). DU145 cells were cultured in RPMI1640 media supplemented with 10% fetal bovine serum (FBS, Hyclone), 100 U/mL penicillin and 0.1 mg/mL streptomycin (Invitrogen). LNCaP cells were cultured in F12 media supplemented with 10% fetal bovine serum (FBS, Hyclone), 100 U/mL penicillin and 0.1 mg/mL streptomycin (Invitrogen). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
3.2.2. Assessment of Antitumor Activity by CCK-8 Assay
Cell proliferation was measured with the Cell Counting Kit-8 (CCK-8) assay kit (Dojindo Corp., Kumamoto, Japan). Cells were harvested during logarithmic growth phase and seeded in 96-well plates at a density of 1 × 105 cells/mL, and cultured at 37 °C in a humidified incubator (5% CO2) for 24 h, followed by exposure to various concentrations of compounds tested for 24 h. Subsequently 10 μL of CCK-8 (Dojindo) was added to each well, the cells were then incubated for an additional 1 h at 37 °C to convert WST-8 into formazan. Cell growth inhibition was determined by measuring the absorbance (Abs) at λ = 450 nm using amicroplate reader. Three independent experiments were performed. Cell growth inhibition was calculated according to the following equation:
| Growth inhibition = (1 − OD of treated cells/OD of control cells) × 100% |
The half maximal inhibitory concentrations (IC50) were obtained from linear regression analysis of the concentration-response curves plotted for each tested compound.
4. Conclusions
In summary, this study reported the synthesis and biological evaluation against three human prostate cancer cells and human prostate epithelial cells of a novel class of arylpiperazine derivatives. The results showed that the majority of the compounds exhibited excellent selective activity for LNCaP cells over the other tested cancer cells. The compounds with methyl (9) or fluoro (15) groups at the o-position on the phenyl group demonstrated a relatively strong cytotoxicity against LNCaP cells. It would be of interest to develop arylpiperazine derivatives for the treatment of the corresponding tumors. Designing more efficient derivatives from arylpiperazines based on the current study may successfully lead to the development of a potent anti-cancer agent. Further research involving other class of arylpiperazine derivatives, and preparation of analogs with aryl groups instead of a 1,3-benzodioxolyl group are in progress.
Acknowledgments
The project was supported by the Technological Innovation Project of Colleges and Universities in Guangdong Province (No. cx2d1127), the China Postdoctoral Science Foundation (Nos. 2013M542165 and 2013M531837), the National Science Foundation of Guangdong Province (No. S2013040014088) and the Guangzhou Postdoctoral Scientific Research Foundation (Nos. Q130 and Q074).
Author Contributions
Conceived and designed the experiments: Mu Yuan, Hong Chen and Xue Liang. Hong Chen, Xue Liang, Fang Xu, Bingbing Xu, Biyun Huang, and Xuelan He performed most experiments. Hong Chen and Xue Liang wrote the paper. Mu Yuan read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Greenlee R.T., Murray T., Hill-Harmon M.B., Thun M.J. Cancer statistics, 2001. CA: Cancer J. Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Quinn M., Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: International comparisons. BJU Int. 2002;90:162–173. doi: 10.1046/j.1464-410X.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 3.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society . Cancer Facts & Figures 2003. American Cancer Society; Atlanta, GA, USA: 2003. [Google Scholar]
- 5.Steinberg G.D., Carter B.S., Beaty T.H., Childs B., Walsh P.C. Family history and the risk of prostate cancer. Prostate. 1990;17:337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 6.Bratt O. Hereditary prostate cancer: Clinical aspects. J. Urol. 2002;168:906–913. doi: 10.1016/S0022-5347(05)64541-7. [DOI] [PubMed] [Google Scholar]
- 7.Whittemore A., Kolonel L.N., Wu A.H., John E.M., Gallagher R.P., Howe G.R., Burch J.D., Hankin J., Dreon D.M., West D.W. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J. Natl. Cancer Inst. 1995;87:652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs J.T. Role of androgens in prostatic cancer. Vitam. Horm. 1994;49:433–502. doi: 10.1016/s0083-6729(08)61152-8. [DOI] [PubMed] [Google Scholar]
- 9.Frydenberg M., Stricker P.D., Kaye K.W. Prostate cancer diagnosis and management. Lancet. 1997;349:1681–1687. doi: 10.1016/S0140-6736(96)07393-X. [DOI] [PubMed] [Google Scholar]
- 10.Akduman B., Crawford E.D. The management of high risk prostate cancer. J. Urol. 2003;169:1993–1998. doi: 10.1097/01.ju.0000046241.95508.15. [DOI] [PubMed] [Google Scholar]
- 11.Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002;167:948–951. doi: 10.1016/S0022-5347(02)80307-X. [DOI] [PubMed] [Google Scholar]
- 12.Beedassy A., Cardi G. Chemotherapy in advanced prostate cancer. Semin. Oncol. 1999;26:428–438. [PubMed] [Google Scholar]
- 13.Denmeade S.R., Lin X.S., Isaacs J.T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Tang D.G., Porter A.T. Target to apoptosis: A hopeful weapon for prostate cancer. Prostate. 1997;32:284–293. doi: 10.1002/(sici)1097-0045(19970901)32:4<284::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Craft N., Chhor C., Tran C., Belldegrun A., DeKernion J., Witte O.N., Said J., Reiter R.E., Sawyers C.L. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59:5030–5036. [PubMed] [Google Scholar]
- 16.Szkaradek N., Rapacz A., Pytka K., Filipek B., Siwek A., Cegła M., Marona H. Synthesis and preliminary evaluation of pharmacological properties of some piperazine derivatives of xanthone. Bioorg. Med. Chem. 2013;21:514–522. doi: 10.1016/j.bmc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Cecchetti V., Fravolini A., Schiaffella F., Tabarrini O., Bruni G., Segret G. o-Chlorobenzenesulfonamidic derivatives of (aryloxy)propanolamines as beta-blocking/diuretic agents. J. Med. Chem. 1993;36:157–161. doi: 10.1021/jm00053a020. [DOI] [PubMed] [Google Scholar]
- 18.Walsh D.A., Chen Y.H., Green J.B., Nolan J.C., Yannit J.M. The synthesis and antiallergy activity of 1-(aryloxy)-4-(4-arylpiperazinyl)-2-butanol derivatives. J. Med. Chem. 1990;33:1823–1827. doi: 10.1021/jm00168a044. [DOI] [PubMed] [Google Scholar]
- 19.Seo H.J., Park E.J., Kim M.J., Kang S.Y., Lee S.H., Kim H.J., Lee K.N., Jung M.E., Lee M., Kim M.S., et al. Design and synthesis of novel arylpiperazine derivatives containing the imidazole core targeting 5-HT(2A) receptor and 5-HT transporter. J. Med. Chem. 2011;54:6305–6318. doi: 10.1021/jm200682b. [DOI] [PubMed] [Google Scholar]
- 20.Kikumoto R., Tobe A., Fukami H., Egawa M. Synthesis and antianxiety activity of (omega-piperazinylalkoxy)indan derivatives. J. Med. Chem. 1983;26:246–250. doi: 10.1021/jm00356a024. [DOI] [PubMed] [Google Scholar]
- 21.Jaen J.C., Wise L.D., Heffner T.G., Pugsley T.A., Meltzed L.T. Dopamine autoreceptor agonists as potential antipsychotics. 1. (Aminoalkoxy)anilines. J. Med. Chem. 1988;31:1621–1625. doi: 10.1021/jm00403a022. [DOI] [PubMed] [Google Scholar]
- 22.Cross R.M., Namelikonda N.K., Mutka T.S., Luong L., Kyle D.E., Manetsch R. Synthesis, antimalarial activity, and structure-activity relationship of 7-(2-phenoxyethoxy)-4(1H)-quinolones. J. Med. Chem. 2011;54:8321–8327. doi: 10.1021/jm200718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarkson C., Musonda C.C., Chibale K., Campbella W.E., Smitha P. Synthesis of totarol amino alcohol derivatives and their antiplasmodial activity and cytotoxicity. Bioorg. Med. Chem. 2003;11:4417–4422. doi: 10.1016/s0968-0896(03)00491-7. [DOI] [PubMed] [Google Scholar]
- 24.Leopoldo M., Lacivita E., Passafiume E., Contino M., Colabufo N.A., Berardi F., Perrone R. 4-[omega-[4-arylpiperazin-1-yl]alkoxy]phenyl)imidazo[1,2-a]pyridine derivatives: Fluorescent high-affinity dopamine D3 receptor ligands as potential probes for receptor visualization. J. Med. Chem. 2007;50:5043–5047. doi: 10.1021/jm070721+. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Sassano M.F., Zheng L.Y., Setola V., Chen M., Bai X., Frye S.V., Wetsel W.C., Roth B.L., Jin J. Structure-functional selectivity relationship studies of β-arrestin-biased dopamine D₂ receptor agonists. J. Med. Chem. 2012;55:7141–7153. doi: 10.1021/jm300603y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeiro L.A., da Silva Ferreira M., da Silva L.L., Castro H.C., Miranda A.L., Silva C.L., Noël F., Nascimento J.B., Araújo C.V., Tibiriçá E., et al. Discovery of LASSBio-772, a 1,3-benzodioxole N-phenylpiperazine derivative with potent alpha 1A/D-adrenergic receptor blocking properties. Eur. J. Med. Chem. 2011;46:3000–3012. doi: 10.1016/j.ejmech.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Baran M., Kepczynska E., Zylewski M., Siwek A., Bednarski M., Cegla M.T. Studies on novel pyridine and 2-pyridone derivatives of N-arylpiperazine as α-adrenoceptor ligands. Med. Chem. 2014;10:144–153. doi: 10.2174/0929867320999131122114922. [DOI] [PubMed] [Google Scholar]
- 28.Dellabella M., Milanese G., Muzzonigro G. Efficacy of tamsulosin in the medical management of juxtavesical ureteral stones. J. Urol. 2003;170:2202–2205. doi: 10.1097/01.ju.0000096050.22281.a7. [DOI] [PubMed] [Google Scholar]
- 29.Morita T., Wada I., Saeki H., Tsuchida S., Weiss R.M. Ureteral urine transport: Changes in bolus volume, peristaltic frequency, intraluminal pressure and volume of flow resulting from autonomic drugs. J. Urol. 1987;137:132–135. doi: 10.1016/s0022-5347(17)43904-8. [DOI] [PubMed] [Google Scholar]
- 30.Takei R., Ikegaki I., Shibata K., Tsujimoto G., Asano T. Naftopidil, a novel alpha1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human alpha1-adrenoceptors. Jpn. J. Pharmacol. 1999;79:447–454. doi: 10.1254/jjp.79.447. [DOI] [PubMed] [Google Scholar]
- 31.Nishino Y., Masue T., Miwa K., Takahashi Y., Ishihara S., Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: A randomized crossover study. BJU Int. 2006;97:747–751. doi: 10.1111/j.1464-410X.2006.06030.x. [DOI] [PubMed] [Google Scholar]
- 32.Kojima Y., Sasaki S., Kubota Y., Hayase M., Hayashi Y., Shinoura H., Tsujimoto G., Kohri K. Expression of alpha1-adrenoceptor subtype mRNA as a predictor of the efficacy of subtype selective alpha1-adrenoceptor antagonists in the management of benign prostatic hyperplasia. J. Urol. 2008;179:1040–1046. doi: 10.1016/j.juro.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 33.Hori Y., Ishii K., Kanda H., Iwamoto Y., Nishikawa K., Soga N., Kise H., Arima K., Sugimura Y. Naftopidil, a selective {alpha}1-adrenoceptor antagonist, suppresses human prostate tumor growth by altering interactions between tumor cells and stroma. Cancer Prev. Res (Phila). 2011;4:87–96. doi: 10.1158/1940-6207.CAPR-10-0189. [DOI] [PubMed] [Google Scholar]
- 34.Kanda H., Ishii K., Ogura Y., Imamura T., Kanai M., Arima K., Sugimura Y. Naftopidil, a selective alpha-1 adrenoceptor antagonist, inhibits growth of human prostate cancer cells by G1 cell cycle arrest. Int. J. Cancer. 2008;122:444–451. doi: 10.1002/ijc.23095. [DOI] [PubMed] [Google Scholar]
- 35.Gotoh A., Nagaya H., Kanno T., Nishizaki T. Antitumor action of α(1)-adrenoceptor blockers on human bladder, prostate and renal cancer cells. Pharmacology. 2012;90:242–246. doi: 10.1159/000342797. [DOI] [PubMed] [Google Scholar]
- 36.Masachika E., Kanno T., Nakano T., Gotoh A., Nishizaki T. Naftopidil induces apoptosis in malignant mesothelioma cell lines independently of α1-adrenoceptor blocking. Anticancer Res. 2013;33:887–894. [PubMed] [Google Scholar]
- 37.Li S., Chiu G., Pulito V.L., Liu J., Connolly P.J., Middleton S.A. 1-Arylpiperazinyl-4-cyclohexylamine derived isoindole-1,3-diones as potent and selective alpha-1a/1d adrenergic receptor ligands. Bioorg. Med. Chem. Lett. 2007;17:1646–1650. doi: 10.1016/j.bmcl.2006.12.111. [DOI] [PubMed] [Google Scholar]
- 38.Chiu G., Li S., Connolly P.J., Pulito V., Liu J., Middleton S.A. Arylpiperazinyl)cyclo-hexylsufonamides: Discovery of alpha(1a/1d)-selective adrenergic receptor antagonists for the treatment of Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms (BPH/LUTS) Bioorg. Med. Chem. Lett. 2007;17:3292–3297. doi: 10.1016/j.bmcl.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Kuo G.H., Prouty C., Murray W.V., Pulito V., Jolliffe L., Cheung P., Varga S., Evangelisto M., Shaw C. Design, synthesis and biological evaluation of pyridine-phenylpiperazines: A novel series of potent and selective alpha1a-adrenergic receptor antagonist. Bioorg. Med. Chem. 2000;8:2263–2275. doi: 10.1016/s0968-0896(00)00151-6. [DOI] [PubMed] [Google Scholar]
- 40.Khatuya H., Hutchings R.H., Kuo G.H., Pulito V.L., Jolliffe L.K., Li X., Murray W.V. Arylpiperazine substituted heterocycles as selective alpha(1a) adrenergic antagonists. Bioorg. Med. Chem. Lett. 2002;12:2443–2446. doi: 10.1016/s0960-894x(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 41.Konkel M.J., Wetzel J.M., Cahir M., Craig D.A., Noble S.A., Gluchowski C. Synthesis and structure-activity relationship of fluoro analogues of 8-{2-[4-(4-methoxyphenyl)piperazin-1yl]ethyl}-8-azaspiro[4.5]decane-7,9-dione as selective alpha(1d)-adrenergic receptor antagonists. J. Med. Chem. 2005;48:3076–3079. doi: 10.1021/jm0491391. [DOI] [PubMed] [Google Scholar]
- 42.Romeo G., Materia L., Modica M.N., Pittalà V., Salerno L., Siracusa M.A., Manetti F., Botta M., Minneman K.P. Novel 4-phenylpiperidine-2,6-dione derivatives. Ligands for α₁-adrenoceptor subtypes. Eur. J. Med. Chem. 2011;46:2676–2690. doi: 10.1016/j.ejmech.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 43.Huang J.J., Huang Y.J., Zhu L., Yuan M. Design, synthesis and α1-adrenoreceptor blocking activity of new arylpiperazines containing acetophenone substituents. Pharmazie. 2014;69:1–7. [PubMed] [Google Scholar]
- 44.Liu X.W., Zhang Y.Y., Yuan M., Sun Y.X. Determination of naftopidil enantiomers in rat plasma using chiral solid phases and pre-column derivatization high-performance liquid chromatography. J. Chromatogr. B. 2012;907:140–145. doi: 10.1016/j.jchromb.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X., Chen M.S., Huang B.Y., Ji H., Yuan M. Comparative Molecular Field Analysis (CoMFA) and Comparative Molecular Similarity Indices Analysis (CoMSIA) studies on α(1A)-adrenergic receptor antagonists based on pharmacophore molecular alignment. Int. J. Mol. Sci. 2011;12:7022–7037. doi: 10.3390/ijms12107022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X., Yuan M., Huang B.Y., Ji H., Zhu L. Ligand-based pharmacophore model of N-Aryl and N-Heteroaryl piperazine alpha 1A-adrenoceptors antagonists using GALAHAD. J. Mol. Graph. Model. 2010;29:126–136. doi: 10.1016/j.jmgm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Kaspers G.J., Veerman A.J., Pieters R., van Zantwijk C.H., Smets L.A., van Wering E.R., van der Does-Van Den Berg A. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997;90:2723–2729. [PubMed] [Google Scholar]
- 48.Kaspers G.J., Pieters R., van Zantwijk C.H., VanWering E.R., van der Does-Van Den Berg A., Veerman A.J. Prednisolone resistance in childhood acute lymphoblastic leukemia: Vitro-vivo correlations and cross-resistance to other drugs. Blood. 1998;92:259–266. [PubMed] [Google Scholar]
- 49.Ding J., Huang S.L., Wu S.Q., Zhao Y.J., Liang L.H., Yan M.X., Ge C., Yao J., Chen T.Y., Wan D.F., et al. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat. Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]