Abstract
Hibiscus sabdariffa has gained attention for its antioxidant activity. There are many accessions of H. sabdariffa in the world. However, information on the quantification of antioxidant compounds in different accessions is rather limited. In this paper, a liquid chromatography/quadrupole-time-of-flight mass spectrometry (LC-Q-TOF-MS) method for simultaneous determination of five antioxidant compounds (neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, rutin, and isoquercitrin) in H. sabdariffa leaves was developed. The method was validated for linearity, sensitivity, precision, repeatability and accuracy. The validated method has been successfully applied for determination of the five analytes in eight accessions of H. sabdariffa. The eight accessions of H. sabdariffa were evaluated for their antioxidant activities by DPPH free radical scavenging assay. The investigated accessions of H. sabdariffa were rich in rutin and exhibited strong antioxidant activity. The two accessions showing the highest antioxidant activities were from Cuba (No. 2) and Taiwan (No. 5). The results indicated that H. sabdariffa leaves could be considered as a potential antioxidant source for the food industry. The developed LC-Q-TOF-MS method is helpful for quality control of H. sabdariffa.
Keywords: Hibiscus sabdariffa, antioxidant activity, LC-Q-TOF-MS, method validation, DPPH, antioxidant compounds, quantitative analysis
1. Introduction
Hibiscus sabdariffa L. (family: Malvacea) is an annual herb shrub popularly known as Roselle or Sorrel [1]. The plant is commonly used as beverages and folk medicines [2]. Many H. sabdariffa accessions (samples of a crop variety collected at a specific location and time) are widely cultivated in tropical and subtropical countries. In Africa, the leaves of H. sabdariffa are usually consumed as vegetables in the preparation of soups and sauces [3]. The leaf extract has been found to possess many bioactive properties, such as anti-oxidant [1,4], anti-tumor [5], anti-hyperammonemic [4], anti-atherosclerotic [3], anti-filarial [6] and anti-hyperlipidemic activities [1,7]. The leaves of H. sabdariffa are rich in phenolics, which could be responsible for the antioxidant capacity. Yields of H. sabdariffa leaves may be about 10 t/ha. Unfortunately, only the calyces of H. sabdariffa are used widely and the leaves are usually ignored and discarded in most countries [3].
There has been growing interest in natural antioxidants found in plants because of the carcinogenic effects of synthetic antioxidants [8]. Oxidative stress causes collapse of the mitochondrial membrane potential, which is associated with many age-related diseases [9,10]. Dietary antioxidants, such as vitamin C and phenolic compounds, present in foods contribute to defense against oxidative stress [11,12]. Overall, natural antioxidants can protect the human body from free radicals and retard the lipid oxidative rancidity in foods. According to the previous studies, H. sabdariffa has long been recognized as a source of antioxidants [1,4,13]. However, the H. sabdariffa leaves from different countries could have different chemical compositions, which may influence their antioxidant activity. Our research group has identified five major antioxidant compounds (including neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, rutin, and isoquercitrin) from H. sabdariffa leaves. To the best of our knowledge, there is still no reported work on the comparison of the contents of the main antioxidant compounds in the leaves of H. sabdariffa from different accessions.
Time-of-flight tandem mass spectrometry (TOF-MS) is a powerful technology, which has been successfully applied for qualitative and quantitative analysis of the chemical constituents in medicinal plant [14,15]. The aim of this study was to develop and validate a LC-Q-TOF-MS method for determination of the contents of the five antioxidant compounds in the different H. sabdariffa accessions. In addition, total antioxidant capacity of different accessions of H. sabdariffa was evaluated by using DPPH free radical scavenging assay. This study is helpful for the full utilization of H. sabdariffa.
2. Results and Discussion
2.1. Optimization of LC-Q-TOF-MS Conditions
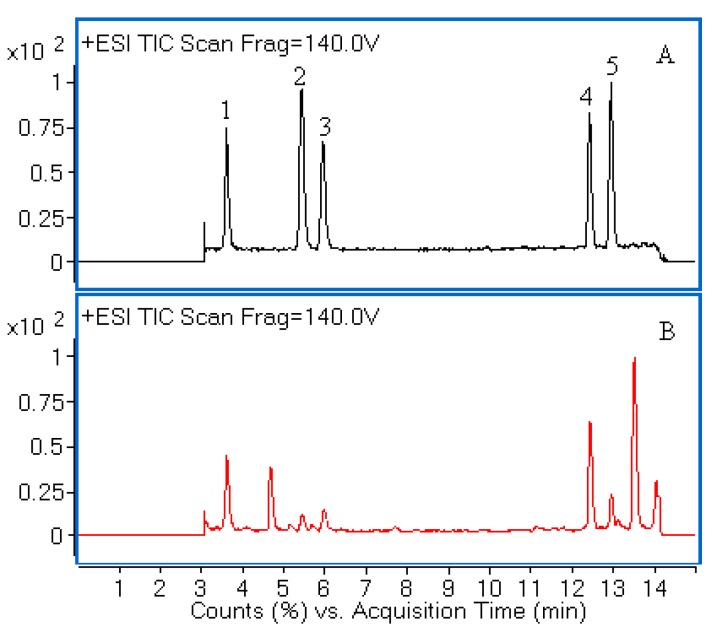
A series of preliminary experiments were carried out in order to optimize the LC-Q-TOF-MS conditions. After comparison with YMC-UltraHT pro C18 (100 mm × 2.0 mm i.d., 2 μm) and Agilent Eclipse XDB C18 (150 mm × 2.1 mm i.d., 3.5 μm) column, Agilent Eclipse Plus C18 (150 mm × 2.1 mm i.d., 1.8 μm) column gave a better separation of the analytes within 15 min. Different mobile phase compositions such as acetonitrile-water and aqueous acetonitrile-acid solvents were tested. The acidified mobile phase (0.1% formic acid) and gradient mode were necessary to achieve a satisfactory MS response and chromatographic separation. To obtain more stable product ions and high responses, MS parameters including fragmentor voltage and drying gas temperature were optimized. Moreover, the positive mode was selected for MS analysis as it had better sensitivity of ion response than that of negative mode. The typical total ion current (TIC) chromatograms were shown in Figure 1. The chemical structures of five compounds were characterized by comparing accurate mass and their retention times with those of standard compounds.
Figure 1.
Liquid chromatography/quadrupole-time-of-flight mass spectrometry (LC-Q-TOF-MS) total ion chromatograms (TIC) of a mixture of five standards (A) and the extract of H. sabdariffa (B). Peaks 1, 2, 3, 4 and 5 correspond to neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, rutin and isoquercitrin, respectively.
2.2. Method Validation
The regression equations, linear ranges, LODs and LOQs values of five analytes were performed using the developed LC-Q-TOF-MS method. As shown in Table 1, the calibration curves of the proposed method were generated by using polynomial regression. Reasonable correlation coefficient values (R2 ≥ 0.9993) indicated good correlations between the concentrations of five analytes and their peak areas within the tested ranges. In this study, LOD and LOQ values were less than 0.09 µg/mL and 0.19 µg/mL, respectively, which were small enough to meet the need for determination of the analytes in H. sabdariffa.
Table 1.
Calibration curves, linearity, limits of detection (LOD) and limits of quantification (LOQ) of the five analytes.
| Analytes | Regression Equation | Linear Range (µg/mL) | Correlation Coefficient (R2) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Neochlorogenic acid | y = −833.90·x2 + 139143.92·x + 6461.02 | 0.75–48.00 | 0.9997 | 0.05 | 0.19 |
| Chlorogenic acid | y = −1795.39·x2 + 239416.18·x − 4374.15 | 0.19–48.00 | 0.9993 | 0.05 | 0.19 |
| Cryptochlorogenic acid | y = −677.23·x2 + 119252.57·x + 2703.14 | 0.19–48.00 | 0.9998 | 0.09 | 0.19 |
| Rutin | y = −1265.45·x2 + 220749.21·x + 1297.41 | 0.09–48.00 | 0.9999 | 0.02 | 0.09 |
| Isoquercitrin | y = −1970.01·x2 + 256239.13·x + 3378.03 | 0.09–48.00 | 0.9997 | 0.02 | 0.09 |
As shown in Table 2, the repeatability present as RSD (n = 6) was between 2.77% and 4.89% of the five investigated compounds. The overall intra- and inter-day variations (RSD) of five analytes for peak areas were in the range from 1.80% to 3.10%, and 1.05% to 3.93%, respectively. Meanwhile, the retention time variations (RSD) were less than 0.21% and 0.52%, respectively (Table 2).
Table 2.
Repeatability and precision of the investigated analytes.
| Analytes | Repeatability (RSD, n = 6) % | Intra-day | Inter-day | ||
|---|---|---|---|---|---|
| (RSD, n = 6) % | (RSD, n = 6) % | ||||
| Retention Time | Peak Area | Retention Time | Peak Area | ||
| Neochlorogenic acid | 3.92 | 0.09 | 2.04 | 0.52 | 3.91 |
| Chlorogenic acid | 4.89 | 0.21 | 2.54 | 0.46 | 1.05 |
| Cryptochlorogenic acid | 4.47 | 0.15 | 1.84 | 0.47 | 1.29 |
| Rutin | 2.77 | 0.09 | 1.80 | 0.22 | 2.96 |
| Isoquercitrin | 2.81 | 0.06 | 3.10 | 0.19 | 3.93 |
The average recoveries obtained in this study ranged from 86.28% to 101.26%, while RSD were all less than 4.88% (Table 3). Therefore, the proposed LC-Q-TOF-MS method was sensitive, precise, and accurate for quantitative evaluation of the five analytes in H. sabdariffa.
Table 3.
Recovery test of the five compounds in H. sabdariffa leaves (n = 3).
| Analytes | Original Amount (µg) | Added (µg) | Found (µg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Neochlorogenic acid | 594.63 | 412.50 | 1007.11 | 99.99 | 3.88 |
| 594.63 | 825.00 | 1317.16 | 87.58 | 1.38 | |
| Chlorogenic acid | 83.37 | 45.00 | 122.13 | 86.28 | 1.95 |
| 83.37 | 90.00 | 166.31 | 92.23 | 4.32 | |
| Cryptochlorogenic acid | 166.01 | 100.00 | 262.57 | 96.56 | 4.76 |
| 166.01 | 200.00 | 355.90 | 94.94 | 3.13 | |
| Rutin | 726.71 | 562.50 | 1296.29 | 101.26 | 4.11 |
| 726.71 | 1125.00 | 1793.28 | 94.81 | 4.03 | |
| Isoquercitrin | 147.97 | 112.50 | 259.52 | 99.15 | 4.88 |
| 147.97 | 225.00 | 345.92 | 87.98 | 0.83 |
2.3. Quantitative Analysis
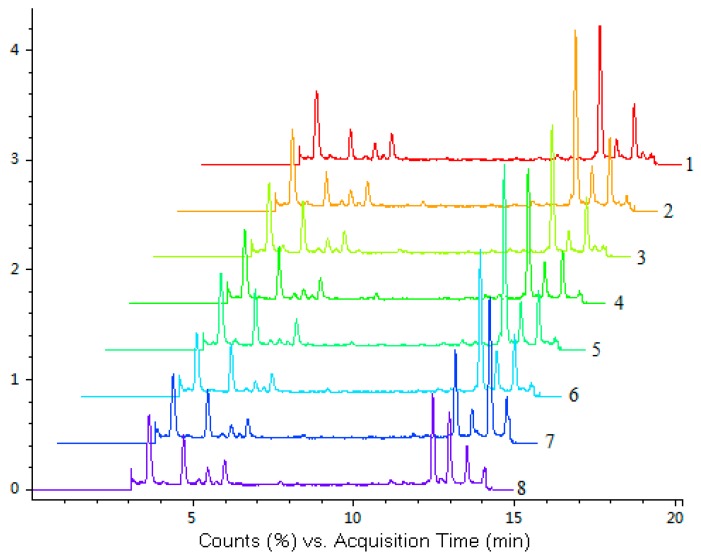
The developed LC-Q-TOF-MS method was subsequently applied to simultaneous quantification of the five investigated compounds in eight accessions of H. sabdariffa. The fingerprint chromatograms of eight samples of H. sabdariffa are shown in Figure 2.
Figure 2.
LC-Q-TOF-MS total ion chromatograms (TIC) of eight samples of H. sabdariffa. Different samples (No. 1–8) are listed in Table 4.
The identification of the investigated compounds was performed by comparison of their retention time and accurate MS with those of reference standards. The molecular ion peaks and the MS data were shown in Table 5.
Table 5.
MS data of the five compounds from H. sabdariffa by LC-Q-TOF-MS.
| No. | RT (min) | [M+H]+ | Main Fragments | Calculated m/z | Error | Formula | Identification | |
|---|---|---|---|---|---|---|---|---|
| mDa | ppm | |||||||
| 1. | 3.62 | 355.1023 | 163.0390 | 355.1024 | 0.10 | 0.21 | C16H18O9 | Neochlorogenic acid |
| 2. | 5.44 | 355.1026 | 163.0391 | 355.1024 | −0.65 | −0.20 | C16H18O9 | Chlorogenic acid |
| 3. | 5.96 | 355.1032 | 163.0395 | 355.1024 | −0.20 | −0.48 | C16H18O9 | Cryptochlorogenic acid |
| 4. | 12.42 | 611.1612 | 465.1025 | 611.1607 | −0.60 | −0.91 | C27H30O16 | Rutin |
| 5. | 12.95 | 465.1029 | 303.0500 | 465.1028 | −0.10 | −0.31 | C21H20O12 | Isoquercitrin |
The contents of five analytes in H. sabdariffa are presented in Table 6. The results showed that the content of each analyte varied greatly among different accessions of H. sabdariffa. The variations (RSD) in the content of five analytes were in the range from 10.5% to 54.9%, which could lead to the variation of antioxidant effects. Among the tested samples, the sample No. 5 from Taiwan had the highest contents of the five analytes, while the sample No. 7 from Senegal had the lowest amount. There was no significant difference in the content between the sample No. 5 and sample No. 2. Therefore, the two accessions of H. sabdariffa from Cuba and Taiwan had higher contents of five marker compounds compared to the other tested samples.
Table 6.
Contents (µg/g) of five antioxidant compounds in different H. sabdariffa leaves (n = 3). Sample No. corresponds to Table 4. Relative standard deviation (RSD) were obtained based on the contents of eight samples. Total—the sum of the five investigated compounds. a, b, c, d, e—the same letter in column indicates homogeneous groups obtained by the ANOVA (p = 0.05).
| No. | Contents (µg/g) (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | Rutin | Isoquercitrin | Total | |
| 1 | 6875.0 ± 251.6 | 975.0 ± 27.2 | 2318.8 ± 79.8 | 12860.4 ± 258.5 | 966.7 ± 28.9 | 23995.9 bc |
| 2 | 7633.3 ± 41.6 | 993.8 ± 43.8 | 2181.3 ± 18.8 | 19356.3 ± 409.7 | 2270.8 ± 78.2 | 32435.5 a |
| 3 | 6577.1 ± 50.1 | 879.2 ± 9.6 | 1991.7 ± 73.2 | 12556.3 ± 151.7 | 1029.2 ± 23.7 | 23033.5 cd |
| 4 | 6912.5 ± 136.2 | 645.8 ± 20.1 | 1952.1 ± 68.6 | 12560.4 ± 534.4 | 1998.0 ± 78.8 | 24068.8 bc |
| 5 | 7512.5 ± 304.1 | 350.0 ± 16.5 | 2356.3 ± 109.5 | 20472.9 ± 653.0 | 2518.8 ± 109.5 | 33210.5 a |
| 6 | 5545.8 ± 103.9 | 677.1 ± 26.0 | 1635.4 ± 68.6 | 14058.3 ± 237.4 | 2341.7 ± 23.7 | 24258.3 b |
| 7 | 5979.2 ± 191.4 | 800.0 ± 38.0 | 1637.5 ± 65.9 | 7245.8 ± 196.1 | 1466.7 ± 34.4 | 17129.2 e |
| 8 | 6841.7 ± 469.1 | 1052.1 ± 65.7 | 2202.1 ± 78.2 | 7845.8 ± 219.6 | 4702.1 ± 78.6 | 22643.8 d |
| RSD % | 10.5 | 29.2 | 13.9 | 35.4 | 54.9 | |
2.4. Antioxidant Activity
All the samples were evaluated for their antioxidant activities by DPPH free radical scavengingassay. Bamboo leaves were used as a comparison. As shown in Table 7, there was a good linear relationship (R2 ≥ 0.9897) between the free radical scavenging rate and the final concentration of H. sabdariffa leaves in the ranges 37.33–320.00 µg/mL (dry weight basis for leaf). The IC50 value was used as a significant indicator of antioxidant ability. Moreover, the lower the IC50 value, the higher the antioxidant activity of samples. In terms of IC50 values, the capacity of DPPH radical scavenging activity was in a decreasing order: 2 > 5 > 1 > 6 > 4 > 3 > 8 > 7 (Table 7). According to the Section 3.1, all the accessions had grown under the same conditions, such as the cultivated soil, cultivated method and local climate. The leaves of H. sabdariffa were collected at the same time. Therefore, the different antioxidant activity of H. sabdariffa leaves may be due to the differences among these accessions. Furthermore, the differences in the antioxidant activity can be explained by the differences of the contents of antioxidant compounds.
Table 7.
Antioxidant activity of the eight accessions of H. sabdariffa. Sample No. corresponds to Table 4.
| Samples | Regression Equation | R2 | IC50 (µg/mL, Dry Weight Basis for Leaf) |
|---|---|---|---|
| 1 | y = 0.2776·x + 5.1522 | 0.9932 | 161.91 |
| 2 | y = 0.2767·x + 7.3187 | 0.9897 | 154.65 |
| 3 | y = 0.2190·x + 5.1677 | 0.9945 | 204.72 |
| 4 | y = 0.2092·x + 8.1093 | 0.9937 | 200.29 |
| 5 | y = 0.2730·x + 6.9611 | 0.9931 | 157.65 |
| 6 | y = 0.2117·x + 9.2249 | 0.9944 | 192.61 |
| 7 | y = 0.1818·x + 5.2144 | 0.9987 | 247.44 |
| 8 | y = 0.1729·x + 14.2790 | 0.9935 | 207.73 |
| Bamboo leaves | y = 0.2497·x + 0.7810 | 0.9908 | 197.67 |
| Rutin | y = 10.492·x + 2.3650 | 0.9941 | 4.54 |
| Neochlorogenic acid | y = 9.9885·x - 0.2967 | 0.9992 | 5.04 |
| Chlorogenic acid | y = 9.6473·x + 1.5983 | 0.9982 | 5.02 |
| Cryptochlorogenic acid | y = 9.5822·x − 0.4380 | 0.9980 | 5.26 |
| Isoquercitrin | y = 12.059·x + 7.5899 | 0.9947 | 3.52 |
As seen from Table 6 to Table 7, the content of rutin was higher than that of other investigated compounds. Meanwhile, rutin exhibited strong DPPH radical scavenging activity and its IC50 value was 4.54 ug/mL. Rutin is one of the most important dietary flavonoid that is widely consumed from plant-derived foods. Rutin has significant pharmacological activities, such as antioxidation and health benefits. Over 130 registered therapeutic medicinal preparations are containing rutin in their formulations [16]. The strong antioxidant ability of rutin has been proven by several studies, especially for free radical scavenging activity [17,18,19]. Therefore, the high antioxidant activity of the leaves of H. sabdariffa was associated with increased concentrations of the investigated compounds. In most of the tested samples, the five investigated analytes were considered as the major antioxidant compounds. Besides these five compounds, there are also several unknown compounds that possess antioxidant activity in H. sabdariffa [20].
As to antioxidant activity of H. sabdariffa, several studies have focus on its flower [21,22]. In contrast with the leaves, the calyces of H. sabdariffa are rich in hydroxycitric acid and hibiscus acid [23]. Previous studies have indicated that both the total content of flavonoid and radical scavenging activity of H. sabdariffa leaves were higher than those of H. sabdariffa flowers [3,13]. Furthermore, the H. sabdariffa leaf extracts possessed stronger antioxidant capacity than mulberry leaf extracts, which are rich in polyphenolic compounds [3,24].
In China, antioxidant of bamboo leaves (AOB) has been listed in the national standards (standard No.GB2760) in 2004. AOB as a kind of food antioxidant has been used in edible oil, meat product, aquatic product and puffed food as a kind of natural antioxidant [25]. In our previous study, the methanolic extract of Bambusa textilis possessed the highest antioxidant activity among the selected bamboo species [26]. As seen from Table 7, the IC50 value of bamboo leaves (B. textilis) was 197.67 µg/mL, which was similar with the IC50 values of H. sabdariffa leaves. These results indicated that H. sabdariffa leaves could be considered as a potential antioxidant source for the food industry. Further studies on the antioxidant mechanism and safety evaluation of H. sabdariffa leaves are needed [27].
3. Experimental
3.1. Chemicals and Materials
HPLC grade acetonitrile, methanol and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was obtained from a Pall purification system (Purelab Plus, Pall, Port Washington, NY, USA). 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other analytical-grade chemicals were purchased from Beijing Chemical Works (Beijing, China). Neochlorogenic acid (1), chlorogenic acid (2), cryptochlorogenic acid (3), and isoquercitrin (5) standards were purchased from Beijing Equation Biotechnology Co., Ltd. (Beijing, China). The standards of rutin (4) were purchased from Acros Organics (Morris Plains, NJ, USA). Their purities were above 97%. Chemical structures of the five investigated compounds were shown in Figure 3.
Figure 3.
Chemical structures of the five investigated compounds including neochlorogenic acid (1), chlorogenic acid (2), cryptochlorogenic acid (3), rutin (4) and isoquercitrin (5).
The bamboo leaves were collected from Nanchang, Jiangxi Province, China and authenticated by Jiusheng Peng from Jiangxi Academy of Forestry. Bamboo leaves were dried in the shade, ground to powder, and stored at −20 °C. All the tested H. sabdariffa accessions were obtained from United States Department of Agriculture-Agricultural Research Service (USDA-ARS) Plant Genetic Resources Conservation Unit in Griffin, Georgia. Then, the seeds of all the accessions were planted in a greenhouse in Southern University Horticultural Farm at the same time on 27 March 2012. The leaf samples of all accessions were collected from top 5th, 6th, and 7th leaves on 27 June 2012. The leaves of H. sabdariffa were obtained from Southern University and A & M College, Baton Rouge, LA, USA, and were identified by Kit L. Chin (Southern University Agricultural Research and Extension Center). A total of 8 accessions of H. sabdariffa were included in this study (Table 4).
Table 4.
Investigated leaves of eight different accessions of H. sabdariffa. The plant identification (PI) numbers of accession label were assigned by the United States Department of Agriculture-Agricultural Research Service (USDA-ARS).
| No. | Country (Seed Source) | Accession Label |
|---|---|---|
| 1 | India | PI-180026 |
| 2 | Cuba | PI-207920 |
| 3 | Sudan | PI-267778 |
| 4 | Nigeria | PI-268100 |
| 5 | Taiwan | PI-273388 |
| 6 | Nigeria | PI-274245 |
| 7 | Senegal | PI-275413 |
| 8 | Sudan | PI-496938 |
3.2. Sample Preparation
Dried leaf powder (0.20 g) was accurately weighted and placed in a 40 mL amber glass bottle with polytetrafluoroethylene (PTFE) screw cap. After adding 25 mL of 70% (v/v) methanol to the glass bottle, the mixture was extracted in an ultrasonic cleaning bath (KQ-500, 500 W, Kunshan Ultrasonic Instruments Co., Ltd., Kunshan, China) for 30 min at room temperature. 1 mL of supernatant was transferred into a 5 mL volumetric flask and diluted to volume with 70% (v/v) methanol. The final solution was filtered through a syringe filter membrane (0.45 µm). The filtrate was stored at −20 °C for further analysis.
3.3. Chromatographic Conditions
Chromatographic analysis was carried out on an Agilent Series 1290 system (Agilent Technologies, Santa Clara, CA, USA), equipped with a diode array detector (DAD), a quaternary pump and an autosampler. Chromatographic separation was performed on an Agilent Eclipse Plus C18 (150 mm × 2.1 mm i.d., 1.8 μm) at 30 °C. The mixture of (A) acetonitrile and (B) 0.1% (v/v) formic acid in water was chosen as mobile phase. The gradient elution program was as follows: 0–8 min, 90%–85% B; 8–14 min, 85%–70% B; 14–15 min, 70%–10% B. The flow rate was set at 0.25 mL/min. A post-run equilibrium time of 5 min and an injection volume of 2 μL were used for all samples.
3.4. Mass Spectrometry
The quantitative analysis of the five compounds was performed using Q-TOF-MS system (model 6540, Agilent Technologies, Santa Clara, CA, USA) equipped with a jet stream ESI interface. The TOF/MS system was operated in positive mode and the mass analysis conditions were set as follows: drying gas (N2) flow rate, 10 L/min; drying gas temperature, 350 °C; fragmentor voltage, 140 V; nebulizer, 45 psi; capillary voltage, 4000 V; skimmer voltage, 65 V; sheath gas temperature, 250 °C; nozzle voltage, 500 V; octopole RF voltage, 750 V. Mass spectra were acquired in the m/z range of 100 to 800. The MS data were collected in a MS scan mode. The molecular masses of the precursor ions were accurately detected using two reference masses (121.050873 and 922.009798). During the analysis, the reference masses were infused to calibrate the MS system. All the operations and analysis of data were controlled using MassHunter B.04.00 software (Agilent Technologies, Santa Clara, CA, USA).
3.5. Calibration Curves and Limits of Detection
Stock solution of the five analytes was diluted to appropriate concentrations for construction of calibration curves. The calibration curves were constructed by plotting the peak area (EIC signal of MS) versus the concentration of each analyte. For each analyte, at least seven concentrations of the solution were analyzed. According to ICH guideline [28], the signal-to-noise (S/N) method was selected for testing limits of detection (LOD) and limits of quantification (LOQ). A signal-to-noise ratio (S/N) of three is used for evaluating LOD and signal-to-noise ratio of ten is accepted for evaluating LOQ. The LOD and LOQ were determined by injecting a series of diluted standard solutions until the signal-to-noise ratio (S/N) for the standards reached a 3:1 ratio for LOD and 10:1 for LOQ, respectively.
3.6. Accuracy, Precision and Repeatability
The spike recovery test was carried out to evaluate the accuracy of the method. Known amounts of each standard solution at low and high concentration levels were mixed with certain amount (0.10 g) of sample No. 7. Then the mixtures were extracted and analyzed using the developed method. Three replicates were prepared for each level.
| Recovery (%) = (amount found − original amount)/spiked amount × 100 | (1) |
Intra- and inter-day variations were applied to evaluate the precision of the developed method. For intra-day precision, the solution of sample No. 7 was analyzed for six replicates within one day, while for inter-day test, the same sample was examined in duplicates for consecutive three days. The relative standard deviation (RSD) was taken as a measurement of precision. Six independent samples (No. 7) were extracted and analyzed in parallel by the developed method for the measurement of repeatability.
3.7. DPPH Free Radical Scavenging Activity
The free radical scavenging activity of the crude extracts was assessed by using the method described by Koolen and with a slight modification [29]. The final concentration of DPPH solution in this study was 26.67 ug/mL for 0.068 mM solution, which could give a moderate absorbance based on Beer’s law [30]. Briefly, an aliquot (1 mL) of each plant sample with different concentrations (corresponding to a 0.96 mg, 0.80 mg, 0.48 mg, 0.16 mg, 0.11 mg of dried plant leaves) was added to DPPH solution (40 ug/mL, 2 mL) in methanol. An aliquot (1 mL) of each standard compound with different concentrations (2.50 ug/mL, 5.00 ug/mL, 10.00 ug/mL, 15.00 ug/mL, 20.00 ug/mL) was added to DPPH solution (40 ug/mL, 2 mL). The mixture was incubated with vigorous shaking for 30 min at 37 °C. The absorbance was measured at 517 nm. IC50 value (concentration providing 50% inhibition) was calculated using a calibration curve. The percentage inhibition was measured using the following equation:
| DPPH inhibition (%) = (Acontrol − Asample)/Acontrol × 100 | (2) |
where Acontrol was the absorbance of control sample and Asample was the absorbance of sample with the crude extract.
3.8. Statistical Aanlysis
Statistical significance was performed applying one-way ANOVA followed by Duncan’s test at p = 0.05, using SPSS Statistics version 17.0 (SPSS Inc., Chicago, IL, USA).
4. Conclusions
A powerful analytical method LC-Q-TOF-MS was developed to quantify five antioxidant compounds in eight accessions of H. sabdariffa. The method was validated and proven to be simple, sensitive and reliable. Comparative analysis of all samples indicated that the leaves of H. sabdariffa were rich in rutin and exhibited strong antioxidant activity. The contents of five antioxidant compounds in H. sabdariffa leaves were related with their antioxidant activity. Therefore, the five investigated compounds could be the predominant contributors to the antioxidant activity. The two accessions showing the highest antioxidant activities were from Cuba (No. 2) and Taiwan (No. 5). In addition to their traditional usage in food and medicine, the leaves of H. sabdariffa may be considered as a potential antioxidant source for the food industry.
Acknowledgments
The authors would like to acknowledge the financial support from the National Science & Technology Pillar Program in the Twelfth Five-year Plan Period (No. 2012BAD23B03) and USDA-NIFA grants #2008-38814-04772 and 2012-38821-20092.
Author Contributions
J.W. performed all experimental work and data analysis. X.C. was responsible for performing most of the experiment and analysis. H.J. performed sample preparation. Y.Q. participated in the design of the study and carried out sample preparation. K.C. collected and identified all the tested accessions. Y.Y., as project leader, contributed to editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 2, 3, 4 and 5 are available from the authors.
References
- 1.Ochani P.C., D’Mello P. Antioxidant and antihyperlipidemic activity of Hibiscus sabdariffa Linn. leaves and calyces extracts in rats. Indian J. Exp. Biol. 2009;47:276–282. [PubMed] [Google Scholar]
- 2.Lin T., Lin H., Chen C., Lin M., Chou M., Wang C. Hibiscus sabdariffa extract reduces serum cholesterol in men and women. Nutr. Res. 2007;27:140–145. doi: 10.1016/j.nutres.2007.01.007. [DOI] [Google Scholar]
- 3.Chen J., Wang C., Wang C., Sheu J., Lin C., Lin H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chem. 2013;141:397–406. doi: 10.1016/j.foodchem.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Essa M.M., Subramanian P. Hibiscus sabdariffa affects ammonium chloride-induced hyperammonemic rats. Evid. Based Complement. Altern. Med. 2007;4:321–325. doi: 10.1093/ecam/nel087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin H., Chan K., Sheu J., Hsuan S., Wang C., Chen J. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012;132:880–891. doi: 10.1016/j.foodchem.2011.11.057. [DOI] [Google Scholar]
- 6.Saxena K., Dube V., Kushwaha V., Gupta V., Lakshmi M., Mishra S., Gupta S., Arora A., Lakshmi V., Sharma R.K., et al. Antifilarial efficacy of Hibiscus sabdariffa on lymphatic filarial parasite Brugia malayi. Med. Chem. Res. 2011;20:1594–1602. [Google Scholar]
- 7.Gosain S., Ircchiaya R., Sharma P.C., Thareja S., Kalra A., Deep A., Bhardwaj T.R. Hypolipidemic effect of ethanolic extract from the leaves of Hibiscus sabdariffa L. in hyperlipidemic rats. Acta Pol. Pharm. 2010;67:179–184. [PubMed] [Google Scholar]
- 8.Sasaki Y.F., Kawaguchi S., Kamaya A., Ohshita M., Kabasawa K., Iwama K., Taniguchi K., Tsuda S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002;519:103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 9.Salminen A., Ojala J., Kaarniranta K., Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 2012;69:2999–3013. doi: 10.1007/s00018-012-0962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facecchia K., Fochesato L.A., Ray S.D., Stohs S.J., Pandey S. Oxidative toxicity in neurodegenerative diseases: Role of mitochondrial dysfunction and therapeutic strategies. J. Toxicol. 2011;2011:683728. doi: 10.1155/2011/683728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeza G., Amigo-Benavent M., Sarri B., Goya L., Mateos R., Bravo L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014;62:1038–1046. doi: 10.1016/j.foodres.2014.05.035. [DOI] [Google Scholar]
- 12.Dominguez-Perles R., Mena P., Garcia-Viguera C., Moreno D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. Nutr. 2014;54:1076–1091. doi: 10.1080/10408398.2011.626873. [DOI] [PubMed] [Google Scholar]
- 13.Mohd-Esa N., Hern F.S., Ismail A., Yee C.L. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122:1055–1060. [Google Scholar]
- 14.Liu S.P., An J.T., Wang R., Li Q. Simultaneous quantification of five bioactive components of Acanthopanax senticosus and its extract by ultra performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry. Molecules. 2012;17:7903–7913. doi: 10.3390/molecules17077903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y.X., Liu S.P., Jin Z., Qin J.F., Jiang Z.Y. Qualitative and quantitative analysis of Andrographis paniculata by rapid resolution liquid chromatography/time-of-flight mass spectrometry. Molecules. 2013;18:12192–12207. doi: 10.3390/molecules181012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chua L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Chat O.A., Najar M.H., Mir M.A., Rather G.M., Dar A.A. Effects of surfactant micelles on solubilization and DPPH radical scavenging activity of rutin. J. Colloid Interface Sci. 2011;355:140–149. doi: 10.1016/j.jcis.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Cho Y.J., Bae I.Y., Inglett G.E., Lee S. Utilization of tartary buckwheat bran as a source of rutin and its effect on the rheological and antioxidant properties of wheat-based products. Ind. Crops Prod. 2014;61:211–216. doi: 10.1016/j.indcrop.2014.07.003. [DOI] [Google Scholar]
- 19.Kerdudo A., Dingas A., Fernandez X., Faure C. Encapsulation of rutin and naringenin in multilamellar vesicles for optimum antioxidant activity. Food Chem. 2014;159:12–19. doi: 10.1016/j.foodchem.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Medina I.C., Beltran-Debon R., Molina V.M., Alonso-Villaverde C., Joven J., Menendez J.A., Segura-Carretero A., Fernandez-Gutierrez A. Direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J. Sep. Sci. 2009;32:3441–3448. doi: 10.1002/jssc.200900298. [DOI] [PubMed] [Google Scholar]
- 21.Farombi E.O., Fakoya A. Free radical scavenging and antigenotoxic activities of natural phenolic compounds in dried flowers of Hibiscus sabdariffa L. Mol. Nutr. Food Res. 2005;49:1120–1128. doi: 10.1002/mnfr.200500084. [DOI] [PubMed] [Google Scholar]
- 22.Sayago-Ayerdi S.G., Arranz S., Serrano J., Goni I. Dietary fiber content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage. J. Agric. Food Chem. 2007;55:7886–7890. doi: 10.1021/jf070485b. [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Arroyo S., Rodríguez-Medina I.C., Beltrán-Debón R.L., Pasini F., Joven J., Micol V., Segura-Carretero A., Fernández-Gutiérrez A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011;44:1490–1495. doi: 10.1016/j.foodres.2011.03.040. [DOI] [Google Scholar]
- 24.Yang M.Y., Huang C.N., Chan K.C., Yang Y.S., Peng C.H., Wang C.J. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J. Agric. Food Chem. 2011;59:1985–1995. doi: 10.1021/jf103661v. [DOI] [PubMed] [Google Scholar]
- 25.Lu B., Wu X., Tie X., Zhang Y., Zhang Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem. Toxicol. 2005;43:783–792. doi: 10.1016/j.fct.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Wang J., Yue Y.D., Tang F., Sun J. TLC screening for antioxidant activity of extracts from fifteen bamboo species and identification of antioxidant flavone glycosides from leaves of Bambusa textilis McClure. Molecules. 2012;17:12297–12311. doi: 10.3390/molecules171012297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I., Heinrich M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014;165:424–443. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Validation of Analytical Procedures: Text and Methodology Q2 (R1) (2005), ICH Harmonised Tripartite Guideline, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. [(accessed on 14 November 2014)]. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- 29.Koolen H.H.F., Da Silva F.M.A., Gozzo F.B.C., de Souza A.Q.L., de Souza A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013;51:467–473. doi: 10.1016/j.foodres.2013.01.039. [DOI] [Google Scholar]
- 30.Scherer R., Godoy H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009;112:654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]