Abstract
Two new sesquiterpenoids with guaiane skeletons—holosericin A (1) and holosericin B (2)—were isolated from the medicinal plant Daphne holosericea (Diels) Hamawa (Thymelaeceae). Their structures were elucidated by 1D and 2D-NMR spectroscopy, as well as HR-ESI-MS data. Compounds 1 and 2 were evaluated for inhibitory activities against acetylcholinesterase and compound 2 showed a moderate activity with 31% inhibition.
Keywords: Thymelaeaceae, Daphne holosericea, guaiane sesquiterpenoids, acetylcholinesterase inhibition
1. Introduction
Daphne is an important genus of the family Thymelaeaceae widely distributed around the world, and among which some species are used as traditional Chinese medicine [1]. Phytochemical investigations on some common Daphne species showed that the main type of natural products present were terpenoids, including sesquiterpenoids and diterpenoids [2,3,4,5,6,7] with extensive pharmacological activities as antitumor [8], antifeedant [2], antiinflammation [6], and anti-HIV-1 agents [7,9]. Daphne holosericea (Diels) Hamawa plants grow in the wet valleys of southwestern China at 2000 m above sea level [10] and its limited chemical studies have reported the presence of flavonoids [11] and phenylpropanoids [12]. In the course of our systematic chemical research on the genus Daphe [2,5,7,9] for the search for new bioactive constituents, a chemical investigation on D. holosericea have been carried out and two new sesquiterpenoids with unusual guaiane skeletons, holosericin A (1) and holosericin B (2) (Figure 1), were isolated from the EtOAc extract of this plant. Herein, we describe the isolation, structural elucidation of the new compounds, as well as their acetylcholinesterase (AChE) inhibitory activities.
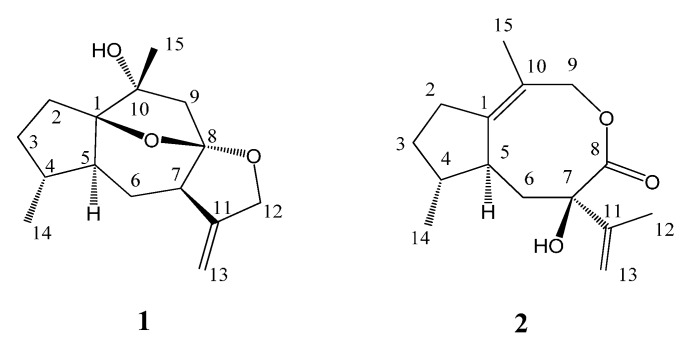
Figure 1.
Structures of compounds 1–2.
2. Results and Discussion
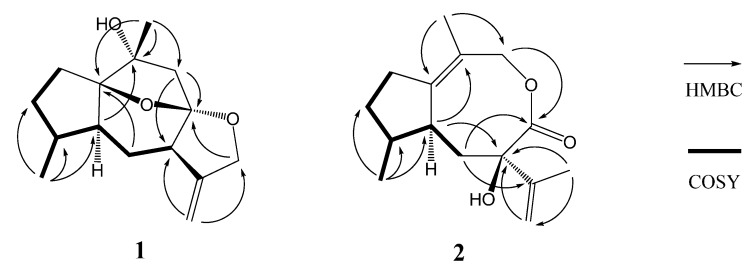
Compound 1 was obtained as a colorless oil, and its molecular formula was assigned to be C15H22O3 from its HR-EI-MS (m/z 250.1567 [M]+) and NMR data (Table 1), indicating five degrees of unsaturation. The IR spectrum revealed the presence of hydroxyl (3417 cm−1) and double bond (1597 cm−1) absorptions. The 1H-NMR spectrum (Table 1) of compound 1 exhibited signals of two methyl [δH 1.38 (3H, s, H-15) and 0.96 (3H, d, J = 6.9 Hz, H-14)], one oxygenated methylene [δH 4.56 (1H, d, J = 13.0 Hz, H-12a) and 4.48 (1H, d, J = 13.0 Hz, H-12b)], and one terminal olefinic bond [δH 4.95 (1H, d, J = 2.4 Hz, H-13a) and 4.93 (1H, d, J = 2.4 Hz, H-13b)]. The 13C-NMR (DEPT) spectroscopic data (Table 1) showed the presence of 15 carbon resonances for two methyl, six methylene (one oxygenated and one olefinic), three methine, and four quaternary carbons (one olefinic and three oxygenated), indicative of a possible sesquiterpenoid skeleton. Further comprehensive analysis of the 1D- and 2D-NMR spectra indicated that compound 1 had the same guaiane skeleton as 4β,10α-dihydroxy-5α(H)-1,11(13)-guaidien-8α,12-olide [13]. The differences between two compounds were one oxygenated quaternary carbon (C-1), one saturated methylene (C-2) and one methane (C-4) in compound 1 replacing the corresponding double bond and the oxygenated quaternary carbon in 4β,10α-dihydroxy-5α(H)-1,11(13)-guaidien-8α,12-olide. This was further proved by the 1H 1H COSY correlations of H-2/H-3, H-3/H-4, H-4/H-14, H-4/H-5, H-5/H-6, and H-6/H-7 (Figure 2), indicating the presence of the partial structures C-2-C-3-C-4(-C-14)-C-5-C-6-C-7, and HMBC correlations from H-14 to C-4 (δC 38.3) and from H-15 to C-1 (δC 93.4) and C-10 (δC 76.1) (Figure 2). The other difference was the oxygenated methylene (C-12) replacing the carbonyl to firm the epoxy group at C-8 and C-12 in compound 1, which was confirmed by HMBC correlations from H-13 to C-12 (δC 72.2) and from H-12 to C-8 (δC 110.2). The additional epoxy group between C-8 and C-1 in compound 1 was deduced based on the requirement of degrees of unsaturation, the molecular model as well as the reasonable chemical shifts of C-8 and C-1. The planar structure of compound 1, a 5/6/5/5 ring system via two oxygen bridges, was established and its stereochemistry needed to be further confirmed.
Table 1.
1H- and 13C-NMR data for compounds 1 and 2 (CDCl3).
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH, J (Hz) | δC | δH, J (Hz) | |
| 1 | 93.4 | 157.0 | ||
| 2 | 32.1 | 1.74 m, 1.61 m (α-H) | 39.7 | 1.86 m (α-H), 1.69 m |
| 3 | 31.8 | 1.60 m, 1.45 m (α-H) | 32.4 | 1.85 m, 1.55 m (α-H) |
| 4 | 38.3 | 1.96 m | 36.0 | 2.25 m |
| 5 | 39.1 | 2.38 ddd (5.0, 8.5, 13.5) | 45.5 | 2.46 m |
| 6 | 24.9 | 1.82 m (α-H), 1.21 m | 19.5 | 2.40 dd (14.0, 9.5, α-H), 2.28 dd (14.0, 6.0) |
| 7 | 49.8 | 2.50 dd (10.5, 8.0) | 84.7 | |
| 8 | 110.2 | 176.5 | ||
| 9 | 50.6 | 2.22 d (13.6), 2.03 d (13.6, α-H) | 72.8 | 4.56 s |
| 10 | 76.1 | 126.9 | ||
| 11 | 152.1 | 149.9 | ||
| 12 | 72.2 | 4.48 d (13.0, α-H), 4.56 d (13.0) | 19.6 | 1.67 s |
| 13 | 104.6 | 4.95 d (2.4), 4.93 d (2.4) | 109.3 | 4.90 s, 4.72 s |
| 14 | 14.6 | 0.96 d (6.9) | 17.5 | 1.06 d (7.5) |
| 15 | 26.5 | 1.38 s | 12.5 | 2.02 s |
Figure 2.
Key HMBC and COSY correlations of compounds 1 and 2.
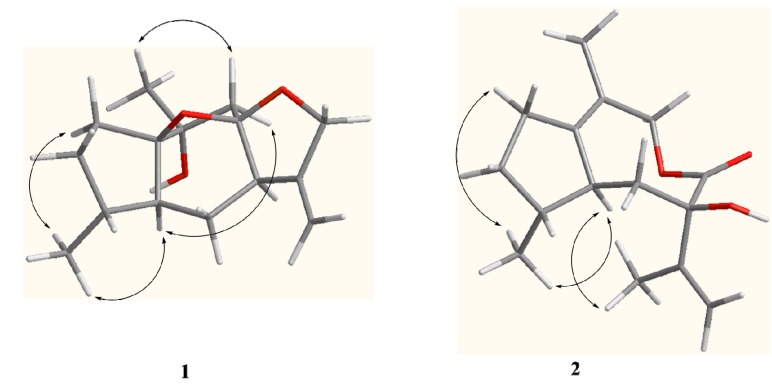
The relative configuration of compound 1 was proposed based on a ROESY experiment (Figure 3). The ROESY correlation of CH3-14 with H-5 (δH 2.38, ddd, J = 13.5, 8.5, 5.0 Hz) revealed that H-5 and CH3-14 were located at the same side and assumed to be α-orientation. The α-orientation of H-7 was determined from NOE of H-5/H-9α (δH 2.03, d, J = 13.6 Hz) and H-7/H-9α. The β-orientation of CH3-15 was deduced by the key NOE of CH3-15/H-9β (δH 2.22, d, J = 13.6 Hz). Moreover, the NOE of H-5/H-9α also indicated that the partial structure C-9-C-10 connected to C-1 was α-oriented in the five number (C-1-C-5) ring system and the 1,8-epoxy was accordingly β-oriented, which correspondingly assigned the α-orientation of 8,12-epoxy. Based on above analysis, compound 1 was elucidated as 10α-hydroxyl-1(8),8(12)-diepoxy-11(13)-guaiene and named holosericin A.
Figure 3.
Key ROESY correlations of compounds 1 and 2.
Compound 2, obtained as a colorless oil, had the same molecular formula C15H22O3 as 1 based on its HR-EI-MS (m/z 250.1575 [M]+), with five degrees of unsaturation. The IR spectrum also displayed the presence of hydroxyl (3398 cm−1), carbonyl (1643 cm−1) and olefinic bonds (1597 cm−1) absorptions. The 1H-NMR spectrum (Table 1) of compound 2 showed signals of three methyl [δH 2.20 (3H, s, H-15), 1.67 (3H, s, H-12) and 1.06 (3H, d, J = 7.5 Hz, H-14)] and one terminal olefinic bond [δH 4.90 (1H, s, H-13a) and 4.72 (1H, s, H-13b)]. The 13C-NMR (DEPT) spectra (Table 1) showed the presence of 15 carbon resonances for three methyl, five methylene, two methine, and five quaternary carbons, suggestive of a sesquiterpenoid skeleton. The assignment of compound 2 was based on the comprehensive analysis of the 1D and 2D-NMR spectroscopic data including 1H-1H COSY, HSQC, and HMBC spectra. The 1H-1H COSY correlations of H-2/H-3, H-3/H-4, H-4/H-14, H-4/H-5, and H-5/H-6 (Figure 2) indicated the presence of the partial structures C-2-C-3-C-4(-C-14)-C-5-C-6. The detail HMBC correlations (Figure 2) indicated the structure of compound 2 possessed the skeleton of 8,9-seco guaiane. The key HMBC correlation from H-9 [δH 4.56 (s)] to C-8 (δC 176.5) determined the presence of 8,9-olide group in compound 2. Thus, the planar structure of compound 2, a 5/8 ring system, was established as shown in Figure 1. The relative configurations of the stereogenic centers (C-4, C-5, and C-7) of compound 2 were determined by the ROESY experiment. The key ROESY correlation of H-5 (δH 2.46, m) with CH3-14 indicated that H-5 and CH3-14 were located at the same side and assumed to be α-orientation. The β-orientation of 7-OH was assigned from the NOE of H-5/CH3-12. Based on above evidence, compound 2 was elucidated as 8,9-seco-1(10),11(13)-guaidien-8,9-olide and named holosericin B.
The AChE inhibitory activities of compounds 1 and 2 were determined according to the previously described method [14,15]. The known AChE inhibitor tacrine was used as positive control in this assay and showed the percentage inhibition of 57%. Compound 2 exhibited certain inhibitory activity with a 31% inhibition at the concentration of 100 µmol∙L−1, whereas compound 1 showed no activity.
3. Experimental Section
3.1. General Information
Optical rotations were recorded using a Rudolph Autopol III polarimeter (Rudolph, Hackettstown, NJ, USA). The UV spectra were measured on a Beckman DU800 spectrometer (Beckman, Brea, CA, USA). The IR spectra were obtained as KBr pellets on a Nicolet 380 FT-IR instrument (Thermo, Pittsburgh, PA, USA). The NMR spectra were recorded on a Bruker AV-500 spectrometer (Bruker, Bremen, Germany), using TMS as an internal standard. ESI-MS and HR-EI-MS were measured with an API QSTAR Pulsar 1 spectrometer (Bruker). Column chromatography was performed with silica gel (Qingdao Marine Chemical Industry Factory, Qingdao, China) and Sephadex LH-20 (Merck, Darmstadt, Germany). TLC was performed with silica gel GF254 (Qingdao Marine Chemical Industry Factory). Fractions were monitored by TLC and spots were visualized by heating after spraying with 5% H2SO4 in ethanol.
3.2. Plant Material
The stems of Daphne holosericea (Diels) Hamawa were collected in Deqin, Yunnan Province, People’s Republic of China. A voucher specimen (HUANG0010) identified by Y. Niu (Kunming Institute of Botany, Chinese Academy of Sciences) was deposited at the Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences.
3.3. Extraction and the Isolation
The dry stems of D. holosericea (2.0 kg) were powdered and extracted with EtOH (95%) under reflux for three times (3 × 6 L). The combined extracted EtOH solution was evaporated under reduced pressure, then suspended in water and partitioned successively with EtOAc (3 × 4 L) and n-BuOH (3 × 4 L). The EtOAc extract (192 g) was separated by silica gel column using a gradient solvent petroleum ether/EtOAc (8:1–1:2 10 L) to afford fractions A/E. Fraction B (16.3 g) was separated by silica gel column chromatography (CC) using a gradient solvent petroleum ether/EtOAc (15:1–1:1 8 L) to afford fractions B1-B4. Fr. B2 (2.1 g) was purified over Sephadex LH-20 column (CHCl3/MeOH 1:1 3 L) to give two subfractions B2-1 and B2-2. Subfr.B2-1 (300 mg) was subjected to repeated RP-18 column (MeOH/H2O 2:1 2 L) and was further purified by repeated silica gel CC with petroleum ether/acetone (5:1, 1.5 L) and Sephadex LH-20 (CHCl3/MeOH 1:1 2 L) to yield 1 (4.0 mg) and 2 (6.5 mg).
Holosericin A (1). Colorless oil; C15H22O3,  + 6.7 (c 0.5, MeOH); UV (MeOH) λmax (logε) 203 (3.26); IR (KBr) νmax 3417, 2924, 2852, 1597, 1420, 1083, 1032; 1H- and 13C-NMR data see Table 1; positive ESI-MS m/z [M+Na]+ 273 (100); HR-EI-MS m/z [M]+ 250.1567 (calcd. for C15H22O3, 150.1569).
+ 6.7 (c 0.5, MeOH); UV (MeOH) λmax (logε) 203 (3.26); IR (KBr) νmax 3417, 2924, 2852, 1597, 1420, 1083, 1032; 1H- and 13C-NMR data see Table 1; positive ESI-MS m/z [M+Na]+ 273 (100); HR-EI-MS m/z [M]+ 250.1567 (calcd. for C15H22O3, 150.1569).
Holosericin B (2). Colorless oil; C15H22O3, 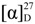 + 5.2 (c 0.5, MeOH); UV (MeOH) λmax (logε) 217 (3.76), 204 (3.73); IR (KBr) νmax 3398, 2922, 2852, 1643, 1597, 1419, 1042; 1H- and 13C-NMR data see Table 1; positive ESI-MS m/z [M+Na]+ 273 (60); HR-EI-MS m/z [M]+ 250.1575 (calcd. for C15H22O3, 150.1569).
+ 5.2 (c 0.5, MeOH); UV (MeOH) λmax (logε) 217 (3.76), 204 (3.73); IR (KBr) νmax 3398, 2922, 2852, 1643, 1597, 1419, 1042; 1H- and 13C-NMR data see Table 1; positive ESI-MS m/z [M+Na]+ 273 (60); HR-EI-MS m/z [M]+ 250.1575 (calcd. for C15H22O3, 150.1569).
3.4. Bioassay of AChE Inhibitory Activity
AChE inhibitory activity of these compounds was assayed by the spectrophotometric method [14,15]. Acetylthiocholine iodide (Sigma, St. Louis, MO, USA) was used as substrate in the assay. Compounds were dissolved in dimethyl sulfoxide (DMSO). The reaction mixture, consisting of 110 µL phosphate buffer (pH 8.0), 10 µL of tested compounds solution (2000 µmol·L−1), and 40 µL AChE solution (0.04 U/100 µL), was mixed and incubated for 20 min (30 °C). The reaction was initiated by the addition of 20 µL 5,5-dithiobis-2-nitrobenzoic acid (6.25 mmol·L−1) and 20 µL acetylthiocholine. The hydrolysis of acetylthiocholine was monitored at 405 nm after 30 min. Tacrine (Sigma-Aldrich 99%, Milwaukee, WI, USA) was used as positive control. All the reactions were done in triplicate. The percentage inhibition was calculated as follows: % inhibition = (E − S)/E × 100 (E is the activity of the enzyme without test compound and S is the activity of enzyme with test compounds).
4. Conclusions
The chemical investigation of Daphne holosericea led to the isolation of two new sesquiterpenoids with guaiane skeletons, holosericin A (1) and holosericin B (2). Holosericin A possessed a 5/6/5/5 ring system via two oxygen bridges and holosericin B was a 8,9-seco one. Evaluation of the inhibitory activities against acetylcholinesterase of the two new compounds showed holosericin B exhibited a moderate activity with 31% inhibition.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 31300294), Special Fund for Agro-scientific Research in the Public Interest (201303117), National Support Science and Technology Subject (2013BAI11B04) and the Fundamental Scientific Research Funds for CATAS (ITBB110301; ITBB140401).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/9/14266/s1.
Supplementary Files
Author Contributions
The contributions of the respective authors are as follows: Q.Y. Ma performed isolation, structure elucidation of the constituents, and prepared the manuscript. Y.C. Chen contributed to the bioassay and related manuscript preparation. S.Z. Huang contributed to checking and confirming all of the procedures of the isolation and structural identification. Z.K. Guo contributed to the MS and NMR measurements and interpretation of those spectra. H.F. Dai contributed to the interpretation of the NMR spectra and revision of this manuscript. This study was performed based on the planning of Y.X. Zhao and Y. Hua, the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Kunming Institute of Botany, Chinese Academy of Sciences . Flora of Yunnan. Science Press; Kunming, China: 1997. p. 219. [Google Scholar]
- 2.Huang S.Z., Li X.N., Ma Q.Y., Dai H.F., Li L.C., Cai X.H., Liu Y.Q., Zhou J., Zhao Y.X. Daphnauranols A-C, new antifeedant sesquiterpenoids with a 5/6/7 ring system from Daphne aurantiaca. Tetrahedron Lett. 2014;55:3693–3696. doi: 10.1016/j.tetlet.2014.05.007. [DOI] [Google Scholar]
- 3.Li L.Z., Gao P.Y., Peng Y., Wang L.H., Yang J.Y., Wu C.F., Zhang Y., Song S.J. Daphnane-type diterpenoids from the flower buds of Daphne genkwa. Helv. Chim. Acta. 2010;93:1172–1179. doi: 10.1002/hlca.200900359. [DOI] [Google Scholar]
- 4.Bang K.K., Yun C.Y., Lee C., Jin Q.H., Lee J.W., Jung S.H., Lee D., Lee M.K., Hong J.T., Kim Y., et al. Melanogenesis inhibitory daphnane diterpenoids from the flower buds of Daphne genkwa. Bioorg. Med. Chem. Lett. 2013;23:3334–3337. doi: 10.1016/j.bmcl.2013.03.096. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y.X., Huang S.Z., Ma Q.Y., Mei W.L., Dai H.F. Two new daucane sesquiterpenoids from Daphne aurantiaca. Molecules. 2012;17:10046–10051. doi: 10.3390/molecules170910046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang S., Shen Y.H., Feng Y., Tian J.M., Liu X.H., Xiong Z., Zhang W.D. Terpenoids from Daphne aurantiaca and their potential anti-inflammatory activity. J. Nat. Prod. 2010;73:532–535. doi: 10.1021/np9005053. [DOI] [PubMed] [Google Scholar]
- 7.Huang S.Z., Zhang X.J., Li X.Y., Kong L.M., Jiang H.Z., Ma Q.Y., Liu Y.Q., Hu J.M., Zheng Y.T., Li Y., et al. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry. 2012;75:99–107. doi: 10.1016/j.phytochem.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Xu W.C., Shen J.G., Jiang J.Q. Phytochemical and biological studies of the plants from the genus Daphne. Chem. Biodivers. 2011;8:1215–1233. doi: 10.1002/cbdv.201000117. [DOI] [PubMed] [Google Scholar]
- 9.Huang S.Z., Zhang X.J., Li X.Y., Jiang H.Z., Ma Q.Y., Wang P.C., Liu Y.Q., Hu J.M., Zheng Y.T., Zhou J., et al. Phenols with anti-HIV activity from Daphne acutiloba. Planta Med. 2012;78:182–185. doi: 10.1055/s-0031-1280263. [DOI] [PubMed] [Google Scholar]
- 10.Editorial committee of flora of China, Chinese Academy of Sciences . Flora of China. Science Press; Beijing, China: 1999. p. 344. [Google Scholar]
- 11.Chen Y.Q., Su J., Shen Y.H., Zhang W., Liang S., Zhang W.D., Kong L.Y. Flavonoids from Daphne holosericea. Chem. Nat. Comp. 2009;45:542–545. doi: 10.1007/s10600-009-9359-1. [DOI] [Google Scholar]
- 12.Chen Y.Q., Su J., Shen Y.H., Zhang W., Hu X.J., Xu W.Z., Zhang W.D., Kong L.Y. Studies on chemical constituents of Daphne holosericea (Diels) Hamaya. Chin. Pharm. J. 2008;19:1453–1456. [Google Scholar]
- 13.Li X.W., Weng L., Gao X., Zhao Y., Pang F., Liu J.H., Zhang H.F., Hu J.F. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorg. Med. Chem. Lett. 2011;21:366–372. doi: 10.1016/j.bmcl.2010.10.138. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S.S., Ma Q.Y., Zou X.S., Dai H.F., Huang S.Z., Luo Y., Yu Z.F., Luo H.R., Zhao Y.X. Chemical constituents and their in vitro acetylcholinesterase inhibitory activities from the fungus Amauroderma amoiensis. Planta Med. 2013;79:87–91. doi: 10.1055/s-0032-1327951. [DOI] [PubMed] [Google Scholar]
- 15.Adewusi E.A., Fouche G., Steenkamp V. Cytotoxicity and acetylcholinesterase inhibitory activity of an isolated crinine alkaloid from Boophane disticha (Amaryllidaceae) J. Ethnopharmacol. 2012;143:572–578. doi: 10.1016/j.jep.2012.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.