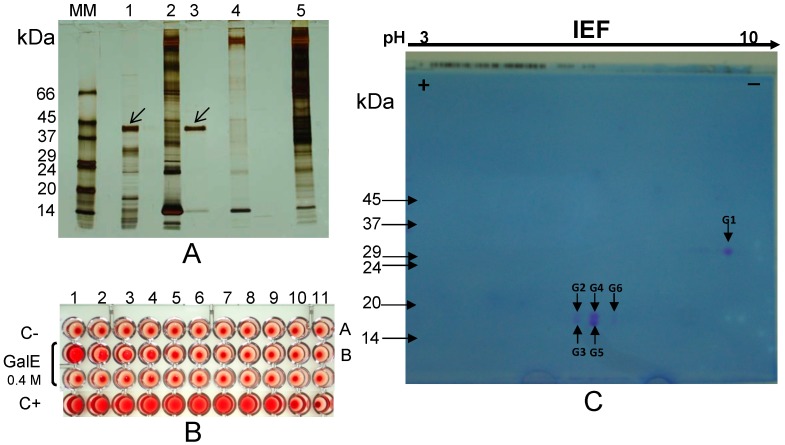
Figure 1.
(A) NR-SDS-PAGE. The albumin fraction from L. albus seeds (lane 1) was incubated with erythrocyte membranes (lane 2). A 42 kDa subunit was eluted with 0.4 M galactose (lane 3) leaving behind a final membrane fraction (lane 5). Control erythrocyte membranes were treated with galactose (lane 4); (B) Haemagglutination activity of the galactose eluate (GalE, lane 3) was apparent in the first four wells when compared with the negative control (C-; saline); (C) 2-D electrophoretic analysis (2nd dimension performed under reducing conditions) of the 42 kDa subunit reveals the presence of a 30 kDa larger polypeptide (G1) and five isoforms of a smaller (17–18 kDa) polypeptide (G2 to G6). Molecular masses of standards (lane MM) are indicated in kDa. Arrows point to the protein subunit (SDS-PAGE performed under non-reducing conditions) which bound to the erythrocyte membranes.