Abstract
Radix isatidis (Banlangen), a famous traditional Chinese medicine, has been used for thousands of years in China due to its anti-viral activity. Through our research, we inferred that the anti-viral activity of Radix isatidis depended on the water-soluble part. Among the components of this extract, the isoquinoline derivative 1 was isolated for the first time and has shown better anti-viral activity than other constituents. In this study, to solve the problem of sourcing sufficient quantities of compound 1, a total synthesis route is described, and several analogues are also evaluated for their anti-viral activities. Among them, compound 8 shown potent anti-viral activity with an IC50 value of 15.3 µg/mL. The results suggested that isoquinoline derivatives possessed potent anti-viral activity and are worthy further development.
Keywords: Radix isatidis, isoquinoline derivative, anti-viral activity
1. Introduction
Radix isatidis had a clear anti-viral effect in the clinic and has been used for thousands of years in China [1,2]. The chemical constituents are the material basis of Radix isatidis’s activities, but due to the complexity of its constituents, the mechanism of Radix isatidis’s anti-viral activity is still unknown. The main constituents include alkaloids, nucleosides, amino acids, organic acids, flavonoids, volatile oils and polysaccharides. Our group has focused on this Traditional Chinese Medicine for several years and we inferred that the anti-viral activity of Radix isatidis depended on the water-soluble part [3,4,5]. The isoquinoline derivative 1 was isolated from Radix isatidis for the first time. The anti-viral activity of compound 1 was much better than that of the other chemical constituents isolated from Radix isatidis, which attracted our interest [3]. Now, the most important task is to get sufficient amounts of compound 1 for the further development. In this study, a total synthesis route was design and executed. Selected compounds were also evaluated for their anti-viral activities.
2. Results and Discussion
2.1. Chemistry
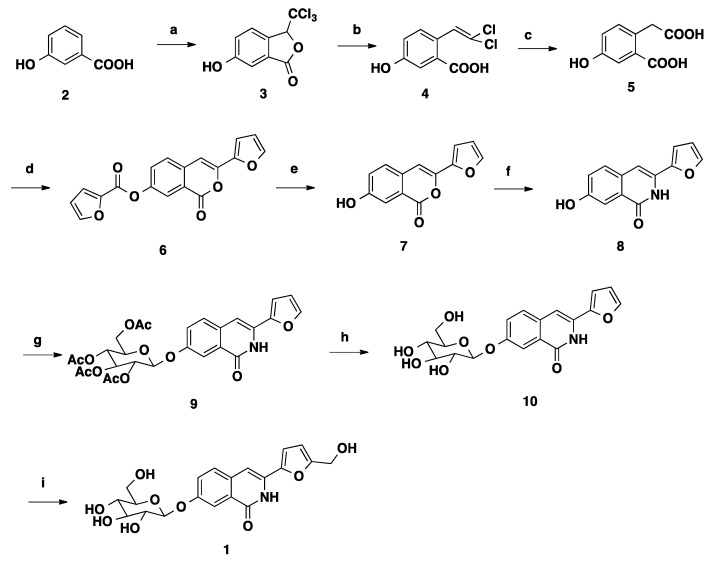
The total synthesis route was designed according to our previous research [6] and was described in Scheme 1. 3-Hydroxybenzoic acid (2) was used as starting material, and was condensed with chloral hydrate in the presence of concentrated sulfuric acid to give 6-hydroxy-3-(trichloromethyl)-iso-benzofuran-1(3H)-one (3). After reduction of 3 with Zn/HOAc, 2-(2,2-dichlorovinyl)-5-hydroxy-benzoic acid (4) was obtained. Compound 4 was treated with concentrated sulfuric acid to give 2-(carboxymethyl)-5-hydroxybenzoic acid (5), followed by condensation with 2-furoyl chloride to yield 3-(furan-2-yl)-1-oxo-1H-isochromen-7-yl furan-2-carboxylate (6). Next 3-(furan-2-yl)-7-hydroxy-1H-isochromen-1-one (7) was obtained by saponification with LiOH in aqueous THF, followed by heating with ammonium hydroxide to give 3-(furan-2-yl)-7-hydroxyisoquinolin-1(2H)-one (8). 3-(Furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (10) was obtained via glycosidation, followed by a hydroxymethylation reaction with formaldehyde in the presence of ZnCl2 as catalyst to finally yield 3-(5-(hydroxy- methyl)furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (1).
Scheme 1.
Synthesis route of 3-(5-(hydroxymethyl)furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5- trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (1).
Reagents and conditions: (a) Chloral hydrate (1 eq), conc. H2SO4, room temperature, 16 h; (b) Zn, HOAc, room temperature, 30 min; (c) conc. H2SO4, room temperature, 30 min; (d) 2-furoyl chloride, 200 °C, 4 h; (e) LiOH (5 eq), THF, water, room temperature, 30 min; (f) NH4OH, EtOH, 120 °C, 6 h; (g) tetraacetyl-d-glucose 2,2,2-trichloroacetimidate (2 eq), CH2Cl2, BF3-Et2O, 0 °C, 2 h; (h) CH3ONa/CH3OH, room temperature, 20 min; (i) aqueous formaldehyde, AlCl3, 90 °C, 3 h.
2.2. Biological Activity
Compounds 7, 8, 10 and 1 were first selected to study their cytotoxicity on Vero cells by an MTT assay. Based on the results of the MTT assay, non-cytotoxic concentrations of compounds were used to determine their inhibitory effects on HSV-1 replication in Vero cells by a MTT assay. The monolayers of Vero cells in 96-well plates were infected with HSV-1 at the multiplicity of infection of 100 TCID50/mL. The results are shown in Table 1.
Table 1.
TC50 and IC50 values of compounds against HSV-1.
| Compounds | IC50 (µg/mL) | TC50 (µg/mL) | TI * |
|---|---|---|---|
| 7 | >200 | - | - |
| 8 | 15.3 | 90.9 | 5.94 |
| 10 | 42.4 | 72.1 | 1.70 |
| 1 | 79.1 | 619.4 | 7.83 |
| Acyclovir | 1.4 | - | - |
* TI (Therapeutic Index) = TC50/IC50.
With the exception of compound 7, all the other compounds (8, 10 and 1) could, to different degrees, protect the Vero cells from HSV-1 infection. Compound 8 had most potent inhibitory effect against HSV-1, with a low IC50 value of 15.3 µg/mL. This result also suggested that the anti-viral activity of the aglycone derivative (compound 8) was better than that of the glucoside derivatives (compounds 10 and 1).
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Solutions
All reagents were purchased from commercial sources and were used without further purification unless otherwise noted. Melting points were determined by XT-4 melting point apparatus (Beijing Tech Instrument Co., LTD, Beijing, China) and are reported without any correction. The 1H-NMR spectra were recorded on a Bruker AV-300 instrument (Bruker, BioSpin AG, Faellanden, Switzerland) using deuterated solvents with tetramethylsilane (TMS) as internal standard. EI-MS was recorded on a Shimadzu GCMS-2010 apparatus (Shimadzu, Tokyo, Japan). Each of the target compounds was purified by silica gel (100 mesh) column chromatography (Grace, Columbia, MD, USA). Concentration and evaporation of the solvent after reaction or extraction was carried out on a rotary evaporator (Buchi Laborterchnik AG, Flawil, Switzerland) operated at reduced pressure.
3.1.2. Synthesis
6-Hydroxy-3-(trichloromethyl)isobenzofuran-1(3H)-one (3): To a solution of 3-hydroxybenzoic acid (1 g, 7.25 mmol) in concentrated sulfuric acid (3 mL) was added chloral hydrate (1.2 g, 7.25 mmol), and the mixture was stirred at room temperature for 16 h. The mixture was poured into water, and the precipitate was filtered, washed with water and dried to give compound 3 (1.4 g, 73%) as a white solid, mp 186–189 °C. MS (ESI): m/z calcd. for C9H5Cl3O3 [M+H]+ 267, found: 267.
2-(2,2-Dichlorovinyl)-5-hydroxybenzoic acid (4): To a solution of compound 3 (0.2 g, 0.75 mmol) in HOAc (1.4 mL) was added zinc powder (0.15 g, 2.3 mmol) in portions, and the mixture was then stirred at room temperature for 30 min. The mixture was poured into water, extracted with ethyl acetate, dried over Na2SO4 and filtered to give compound 4 (0.15 g, 86%) as a white solid, mp 196–199 °C. MS (EI): m/z calcd. for C9H6Cl2O3 [M]+ 232, found: 232.
2-(Carboxymethyl)-5-hydroxybenzoic acid (5): Compound 4 (0.13 g, 0.56 mmol) was added to concentrated sulfuric acid (0.3 mL) in portions and then stirred at room temperature for 30 min. The mixture was poured into water, extracted with ethyl acetate, dried over Na2SO4 and filtered to give compound 5 (50 mg, 50%) as a yellow solid, mp 214–216 °C. MS (EI): m/z calcd. for C9H8O5 [M]+ 196, found: 196.
3-(Furan-2-yl)-1-oxo-1H-isochromen-7-yl furan-2-carboxylate (6): Compound 5 (6 g, 30.6 mmol) was added to furoyl chloride (17.5 mL) and the mixture was stirred at 200 °C for 4 h. The mixture was cooled down and purified via fresh column to give compound 6 (7.7 g, 78%) as a yellow solid, mp 178–180 °C; 1H-NMR (DMSO-d6) δ: 8.14 (s, 1H, 8-H), 8.02 (d, 1H, J = 2.28 Hz, 4-H), 7.93 (d, 1H, J = 1.08 Hz, 3'-H), 7.81 (d, 1H, J = 2.3 Hz, 6-H), 7.78 (d, 1H, J = 2.31 Hz, 5-H), 7.63 (dd, 1H, 4'-H), 7.22 (s, 1H, 5''-H), 7.03 (d, 1H, J = 3.39 Hz, 4''-H), 6.83 (dd, 1H, J = 1.71, 1.74 Hz, 3''-H), 6.72 (dd, 1H, J = 1.77, 1.80 Hz, 5'-H); MS (ESI): m/z calcd. for C18H10O6 [M+H]+ 323, found: 345 [M+Na]+.
3-(Furan-2-yl)-7-hydroxy-1H-isochromen-1-one (7): To a solution of compound 6 (7.7 g, 23.9 mmol) in MeOH (105 mL), THF (310 mL) and water (105 mL) was added LiOH (5.1 g, 120 mmol), the mixture was stirred at room temperature for 30 min. The mixture was then evaporated to give compound 7 (3.7 g, 68%) as a yellow solid, mp 173–175 °C; 1H-NMR (DMSO-d6) δ: 10.54 (s, 1H, 7-OH), 7.86 (s, 1H, 8-H), 7.48 (d, 1H, J = 2.4 Hz, 6-H), 7.38 (dd, 1H, J = 4.4, 3.3 Hz, 4'-H), 7.29 (dd, 1H, J = 1.77, 1.8 Hz, 3'-H), 7.07 (s, 1H, 4-H), 6.9 (d, 1H, J = 3.36 Hz, 5-H), 6.67 (dd, 1H, J = 1.77, 1.8 Hz, 5'-H); MS (ESI): m/z calcd. for C13H8O4 [M+H]+ 229, found: 251 [M+Na]+.
3-(Furan-2-yl)-7-hydroxyisoquinolin-1(2H)-one (8): To a solution of compound 7 (3.7 g, 16.2 mmol) in ethanol (15 mL) was added NH4OH (15 mL), the mixture was stirred at 120 °C for 6 h. The mixture was then cooled down and evaporated. The residue was added small amount of water, extracted with ethyl acetate, dried over Na2SO4 and filtered to give compound 8 (2.9 g, 79%) as a yellow solid, mp 135–137 °C; 1H-NMR (DMSO-d6) δ: 11.33 (s, 1H, 2-H), 9.99 (s, 1H, 7-OH), 7.8 (d, 1H, J = 1.2 Hz, 8-H), 7.6 (d, 1H, J = 8.58 Hz, 5-H), 7.52 (d, 1H, J = 2.52 Hz, 6-H), 7.27 (d, 1H, J = 3.42 Hz, 4'-H), 7.2 (dd, 1H, J = 2.61, 2.64 Hz, 5'-H), 6.88 (s, 1H, 4-H), 6.63 (dd, 1H, J = 1.77 Hz, 1.8, 3'-H); MS (ESI): m/z calcd. for C13H9NO3 [M+H]+ 228, found: 250 [M+Na]+.
3-(Furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (10): To a solution of compound 8 (1.5 g, 6.6 mmol) in CH2Cl2 (100 mL) was added tetraacetyl-d-glucose 2,2,2-trichloroacetimidate (6.5 g, 13.2 mmol), the mixture was then added BF3-Et2O at 0 °C and stirred for 2 h. The mixture was added Et3N, evaporated and purified via fresh column to give a yellow solid. The solid was then added CH3ONa/CH3OH (2 mL) and the mixture was stirred at room temperature for 20 min. The precipitate was then filtered, washed with methanol, dried to give compound 10 (1.65 g, 92%) as a white solid, mp 223–226 °C; 1H-NMR (D2O) δ: 7.82 (d, 1H, J = 1.6 Hz, 8-H), 7.75 (d, 1H, J = 2.8 Hz, 5-H), 7.7 (d, 1H, J = 8.8 Hz, 6-H), 7.42 (dd, 1H, 4''-H), 7.31 (d, 1H, J = 3.2 Hz, 3''-H), 6.95 (s, 1H Hz, 4-H), 6.65 (dd, 1H, J = 1.6, 2.0 Hz, 5'-H), 5.0 (d, 1H, J = 5.4 Hz, 1''-H), 3.7 (m, 1H, 6''-H), 3.52 (m, 1H, 6''-H), 3.41 (m, 1H, 2''-H), 3.33 (m, 1H, 3''-H), 3.28 (m, 1H, 5''-H), 3.2 (t, 1H, 4''-H); MS (ESI): m/z calcd. for C19H19NO8 [M+H]+ 390, found: 390 [M+H]+.
3-(5-(Hydroxymethyl)furan-2-yl)-7-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)isoquinolin-1(2H)-one (1): To a solution of compound 10 (0.1 g, 0.26 mmol) in HCHO (5 mL) was added AlCl3 (56 mg, 0.42 mmol), the mixture was stirred at 90 °C for 3 h. The mixture was then evaporated and the residue was purified via fresh column to give compound 1 (34 mg, 31%) as a red solid, mp 232–233 °C; 1H-NMR (DMSO-d6) δ: 10.43 (1H, s, H-2), 7.25 (1H, s, H-4), 6.73 (1H, d, J = 8.5 Hz, H-5), 6.98 (1H, dd, J = 8.4, 2.1 Hz, H-6), 8.06 (1H, d, J = 2.1 Hz, H-8), 7.22 (1H, d, J = 3.2 Hz, H-3'), 6.60 (1H, d, J = 3.2 Hz, H-4'), 4.63 (2H, d, J = 5.6 Hz, H-6'), 5.52 (1H, t, J = 5.8 Hz, 6'-OH), 4.86 (1H, d, J = 7.7 Hz, H-1''), 3.21 (1H, m, H-2''), 3.28 (1H, m, H-3''), 3.15 (1H, m, H-4''), 3.27 (1H, m, H-5''), 3.63 (1H, m, H-6''), 3.45 (1H, m, H-6''); MS (ESI): m/z calcd. for C19H19NO8 [M+H]+ 390, found: 390 [M+H]+.
3.2. Anti-Virus Biological Assay
3.2.1. Materials and Solutions
96-Well plates (Corning Corporation, Corning, NY, USA); Power Wave 340 type Microplate reader (Bio-Teck Corporation, Winooski, VT, USA); BC- J80S type CO2 Incubators (BoXun Industrial Co., Ltd., Shanghai, China); 79-1 type Magnetic heating Stirrer (Jintan Medical Instrument Factory, Jintan, Jiangsu, China); 20–200 µL and 100–1000 µL Pipette (Genex Beta, Suzhou, Jiangsu, China); Centrifuge tube (Shanghai Shen’an Medical Devices Factory, Shanghai, China); SYQ-DSX-280B type Portable Stainless Steel Pressure Steam Sterilizer (Shanghai Shen’an Medical Devices Factory, Shanghai, China); Laboratory-grade ultra-pure water Instrument (EPED, Nanjing, Jiangsu, China); LDZ5-2 type Low-speed automatic balance centrifuge (Beijing Medical Centrifuge factory, Beijing, China); KQ-500DE type CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, China); Human herpes simplex virus type I (HSV-1) passage twice to enhance virulence (provided by Nanjing Medical University Virus Lab, Nanjing, Chana); Vero cells (sensitive cells to HSV) were routinely grown in RPMI1640 medium with 10% newborn calf serum (NCS) and 0.06 mg/mL penicillin, 0.1 mg/mL streptomycin, 2 mg/mL NaHCO3. Grown as a monolayer culture; 10% newborn calf serum (Lanzhou MinHai Biological Engineering Co., Ltd., a Sino-US joint venture, Lanzhou, China); culture medium RPMI (Gibco Company, Grand Island, NE, USA); Acyclovir powder (a gift of Jiangsu Provincial Hospital, Nanjing, China); 100TCID50 virus solution (prepared by Department of Microbiology and Immunology, Nanjing University of Traditional Chinese Medicine, Nanjing, China).
3.2.2. Cytotoxic Effects of Compounds on Vero Cells
Test compounds were accurately weighed, fully dissolved in fresh RPMI1640 medium, filtered for sterilization, and saved at 4 °C. Vero cells were digested by trypsin, 1640 medium containing 10% NCS was added, and a 2 × 105 /mL cell suspension was prepared. Confluent cell monolayers in 96-well multidishes (0.1 mL per well) were prepared at 37 °C, 5% CO2, and the supernatants were removed. The sample solutions were diluted to seven concentrations: 2000, 1000, 500, 100, 20, 4 and 0.8 µg/mL. The above liquid was added to 96-well plates (0.2 mL per well, each concentration with four wells), two control groups of Vero cells were also set up. MTT (0.02 mL per well) was added after culturing for 72 h at 37 °C, 5% CO2, then the plates were placed in a 37 °C 5% CO2 incubator and incubated for 4 h. The culture medium was removed, DMSO (0.2 mL per well) was added, and shaken for 5–10 min. The absorbance value was measured at the wavelength of 570–630 nm, cell survival and half toxic concentration (TC50) were calculated by the Reed-Muench method.
3.2.3. Anti-HSV-1 Activity Assay
Maximum non-toxic concentrations were chosen as the initial concentration, and then they were diluted to several concentrations for the anti-viral experiments. Four groups were set up: normal cells control, viral infection control, test drug control, positive drug control. Acyclovir was chosen as positive drug. Monolayers of Vero cells prepared in microtiter plates were infected with 100 TCID50 HSV-1 diluted in PBS, 20 µL per well, while PBS 0.02 mL per well was added in the normal cell controls. After 1 h at 37 °C 5% CO2, residual inoculum was removed and cell monolayers were washed twice with PBS. Test compounds which had been diluted in assay medium were added to give 0.2 mL per well, and each concentration has four wells. Plates were incubated at 37 °C 5% CO2 for 72 h. Then MTT (0.02 mL per well) was added after culturing for 72 h at 37 °C 5% CO2, then the plates were placed in a 37 °C 5% CO2 incubator for 4 h, the culture medium was removed, DMSO (0.2 mL per well) was added and shaken for 5–10 min, and the absorbance value was measured at a wavelength of 570–630 nm. The rate of viral suppression and IC50 were calculated by the Reed-Muench method. The therapeutic index (TI) is calculated according to the formula TI = TC50/IC50.
4. Conclusions
In conclusion, this study presented a convenient total synthesis route of a new constituent (compound 1) from Radix isatidis, which solves the problem of the availability of this compound. Also, some isoquinoline derivatives were tested for their inhibitory effects against HSV-1. The results show that compound 8 had potent anti-viral activity with an IC50 value of 15.3 µg/mL, which suggested that isoquinoline derivatives might possess potent anti-viral activity and are worthy of further development.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81473316) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/12/20906/s1.
Author Contributions
Wei Li, Tian-Lin Wang and Li-Wei He designed research; Li-Wei He, Hua-Qing Liu, Yu-Qing Chen and Jing-Yan Yang performed research and analyzed the data; Wei Li, Tian-Lin Wang and Li-Wei He wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Zhou W., Zhang X.Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen) Am. J. Chin. Med. 2013;41:743–764. doi: 10.1142/S0192415X1350050X. [DOI] [PubMed] [Google Scholar]
- 2.Liao H.F., Lu M.C., Chang H.C., Wei C.C., Kao C.H., Chen Z.H., Huang C.C., Li C. Effects of herbal medicinal medicinal formulas on suppressing viral replication and modulating immune responses. Am. J. Chin. Med. 2010;38:173–190. doi: 10.1142/S0192415X10007749. [DOI] [PubMed] [Google Scholar]
- 3.He L.W., Dong W., Yang J.Y., Li X., Li W. Chemical constituents in antiviral fraction of Isatidis Radix and their activities. Zhong Cao Yao. 2013;44:2960–2964. [Google Scholar]
- 4.He L.W., Li X., Chen J.W. Research progress of Radix isatidis against virus. Inform. Tradit. Chin. Med. 2005;22:37–40. [Google Scholar]
- 5.He L.W., Li X., Chen J.W., Sun D.D., Ju W.Z., Wang K.C. Chemical constituents from water extract of Radix isatidis. Yao Xue Xue Bao. 2006;41:1193–1196. [PubMed] [Google Scholar]
- 6.Wang T.L., Xiang H., Xu X.L., Xiao H., You Q.D. Synthesis and anti-cancer activities of novel 6-arylindenoisoquinolines. Chin. J. Med. Chem. 2012;22:268–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.