Abstract
Background
Copper associated hepatitis (CAH) has been increasingly recognized in dogs, and speculation exists that hereditary defects in copper metabolism have been exacerbated by increased environmental copper exposure. However, no broad epidemiological investigations have been performed to investigate quantitative hepatic copper concentrations ([Cu]H) over time in both dogs that are (predisposed breed [PB]), and are not (non‐predisposed breed [NPB]), considered at‐risk for CAH.
Objectives
To investigate [Cu]H in dogs and explore temporal, demographic, and histologic associations spanning 34 years.
Animals
546 archived liver specimens.
Methods
Retrospective study. Searches of the Michigan State University Veterinary Diagnostic Laboratory database identified dogs that had undergone hepatic histopathologic assessment. Cases with archived tissue were reviewed and classified by breed, time period, and presence or absence of hepatitis. Inductively coupled plasma mass spectrometry was used to determine [Cu]H.
Results
In time period 2009–2015, median [Cu]H were 101 μg/g and 313 μg/g greater than median [Cu]H in time period 1982–1988 for NPB and PB dogs, respectively (P < .001 for both comparisons). The proportion of dogs with [CU]H > 300 μg/g increased in NPB (28% to 49%) and PB dogs (48% to 71%) during these periods (P = .002 for both comparisons). Median [Cu]H in dogs with hepatitis increased 3‐fold over time in both NPB (P = .004) and PB populations (P < .001).
Conclusions and Clinical Importance
The frequent recognition of CAH in recent years is likely due to the observed increases in [Cu]H over time. Importantly, effects are not limited to PB dogs.
Keywords: Labrador retrievers, West Highland White Terriers, Doberman Pinschers, Dalmatians, trace elements, hepatitis
Abbreviations
- [Cu]H
quantitative hepatic copper concentrations
- AAFCO
Association of American Feed Control Officials
- CAH
copper‐associated hepatitis
- ICP‐MS
inductively coupled plasma mass spectrometry
- NPB
non‐predisposed breed
- PB
predisposed breed.
1. INTRODUCTION
Copper is an essential micronutrient in all eukaryotic species. It is a cofactor for hundreds of enzymatic reactions throughout the body which are involved in diverse processes such as cellular respiration, red blood cell production, free radical defense, collagen synthesis, and iron metabolism, amongst others.1, 2, 3, 4, 5 Although essential for life, excessive tissue copper can overwhelm the body's copper storage and transport proteins.6 The subsequent accumulation of free intracellular copper, especially in its cupric form, results in oxidative stress, cell death, and inflammation.4, 6, 7 The liver is frequently affected in states of copper overload, and progressive hepatic copper accumulation can result in cirrhosis and death.7
In dogs, this syndrome is frequently termed copper‐associated hepatitis (CAH).7 Pathologic hepatic copper accumulation was first reported in Bedlington Terriers in 1975,8 and the genetic basis has been well‐described in this breed.9, 10 However, CAH is now recognized in West Highland White Terriers, Dalmatians, Doberman Pinschers, and Labrador Retrievers.7, 11, 12, 13, 14, 15, 16 The pathogenesis is complex and poorly characterized for these dogs, but given the strong breed predispositions and histologic pattern of copper accumulation, a genetic basis is suspected.7 In partial support of this, a recent study of Labrador Retrievers documented mutations in the copper‐transporting ATP7B and ATP7A genes which were associated with increased or decreased risk, respectively, for development of CAH, but these mutations accounted for only 12% of total heritability.17 Matings of West Highland White Terriers with increased hepatic copper concentrations have been reported to produce offspring with increased hepatic copper concentrations, but a clear pattern of inheritance was not identified.15
Our observations, as well as those of others, suggest CAH has been diagnosed with increasing frequency during the past decade.18 Furthermore, CAH has been identified in various pure‐ and mixed‐breed dogs that are not typically considered predisposed to pathologic copper accumulation.19, 20 These observations raise concern that increased environmental copper exposure could be contributing to disease manifestation. From the frequent use of copper piping in households to changing Association of American Feed Control Officials’ (AAFCO) requirements for copper supplementation in dog food, the possibility for an environmental etiology exists.21, 22 Dietary copper concentrations are associated with quantitative hepatic copper concentrations ([Cu]H) in Labrador Retrievers with CAH.23, 24 The presumed increase in CAH prevalence in Labrador Retrievers could be due to changes in the form of supplemental copper in dog food.18 However, this speculation and the exact timing of potential changes have not been investigated in detail, nor have potential changes been investigated in other at‐risk breeds. Although Labrador Retrievers are the most common purebred dog in the United States, mixed breed or mongrel dogs are thought to represent over 50% of the pet dog population in the Unites States.25, 26 As such, an important knowledge gap also exists in breeds and crosses that are not typically considered to be at‐risk for CAH. The purpose of our study was to investigate [Cu]H over a 34 year period in dogs that are, and are not, considered to be predisposed (based on breed) to CAH. Associations of [Cu]H with the presence or absence of hepatitis also were examined. The primary hypotheses were that [Cu]H have increased over time, and these changes would not be limited to predisposed or at‐risk breeds. Additionally, we hypothesized that [Cu]H would be greater in dogs with hepatitis than those without hepatitis, and this difference would be magnified in recent years.
2. MATERIALS AND METHODS
2.1. Study Design
A 2‐part retrospective investigation of hepatic copper accumulation in dogs was conducted for the study period of January 1, 1982 through December 31, 2015 at the Michigan State University Veterinary Diagnostic Laboratory (MSU‐VDL). For part 1 of the study, 2 populations of dogs were investigated in a historical time period comprising 1982–1988 and a contemporary time period comprising 2009–2015. All Labrador Retrievers, West Highland White Terriers, Doberman Pinschers, and Dalmatians, as well as dogs mixed or crossed with these breeds, were classified as the predisposed breed (PB) population given the well‐documented occurrence of CAH in these breeds.7, 12, 13, 14, 15, 16 Bedlington Terriers were excluded from study as disease etiology has been extensively characterized in this breed,9, 10 and Skye Terriers also were excluded given the uncertain predisposition status and lack of available samples for the periods of interest.27 All other dogs were classified as the non‐predisposed breed (NPB) population. The historical time period of 1982–1988 was selected as it predates widespread recognition of CAH and was the earliest time period in which archived liver specimens were readily available. The 2009–2015 period was selected as CAH occurrence was well‐established in all of the predisposed dog breeds, and it matched the 7‐year time interval of the historical period. Furthermore, this was a time in which reports of CAH surfaced in breeds not classically considered to be predisposed to CAH which also corresponded to our observations at the Michigan State University College of Veterinary Medicine.19, 20
For the second part of the study, Labrador Retrievers were investigated for the entire time period of January 1, 1982 through December 31, 2015. Given the popularity of the breed and known propensity for hepatic copper accumulation, Labrador Retrievers were used as sentinels to aid in more detailed investigations of [Cu]H and potential temporal associations.18
2.2. Case Selection and Review
The electronic database at the MSU‐VDL was searched to identify all submission records for cases in which hepatic histopathology had been performed and for which archived liver specimens were still available. Approximately 90% of specimens were submitted from practices within the State of Michigan. Information retrieved from the submission records included breed, age, sex, neuter status, and date of biopsy or necropsy. Records and liver tissue for all dogs in the PB population as described above were retrieved. Due to the large number of available NPB specimens, a sample size calculation was performed for this population using publicly available software (PS Power and Sample Size Calculations Version 3.0, Vanderbilt University Department of Biostatistics, Nashville, TN). Archived specimens not used in the current study in which [Cu]H had been previously determined were used for the analysis which suggested 86 dogs from both the 1982–1988 and 2009–2015 periods would be needed to detect an approximate 75 μg/g difference in [Cu]H between eras with power of 0.8 and α of 0.05. Cases for the NPB population were randomly selected using a computerized randomized number generator. Dogs ≤6 months of age were excluded from the search, and cases with insufficient archived tissue available for both histopathology and copper quantification were excluded from the study. For cases in which 2 or more histology submissions were available from different dates, only the first submission and tissue were included.
2.3. Hepatic Histopathology
Hematoxylin and eosin stained slides were reviewed by two veterinary pathologists (RCS and KJO), and cases were classified as hepatitis or non‐hepatitis due to the frequent association of hepatic copper accumulation and inflammation.6, 13, 14, 15 Cases were excluded if any of the following were observed: (1) marked autolysis, (2) a neoplastic infiltrate obscuring the majority of available sections, (3) end‐stage cirrhosis or fibrosis obscuring the majority of available sections, (4) regenerative or hyperplastic nodules expanding the majority of available sections, (5) predominant histologic lesion of submassive centrilobular necrosis or massive necrosis, (6) histologic lesions consistent with infectious causes of acute inflammation (i.e. viral hepatitis, hepatic abscessation, bacterial septicemia, or dissociation of hepatocytes suggestive of Leptospiral infection), or (7) small sample size (less than 14mm2 surface area). Cases with fibrous tissue, regenerative nodules, or neoplasia comprising the majority of the sections were excluded as they were likely to preclude accurate determination of [Cu]H.15, 28 Cases of submassive or massive necrosis, which potentially could include copper toxicosis but were more likely to represent other forms of acute hepatotoxicosis, were excluded because it is unknown if stored copper is maintained in necrotic hepatocytes. Infectious hepatitis cases were excluded because of the known cause of inflammation and unknown effect on copper homeostasis. The inclusion of such cases could potentially skew results if not balanced evenly between study periods. All remaining cases were scored using a previously described classification system for necroinflammatory liver disease and classified into hepatitis or non‐hepatitis categories.29, 30 Briefly, all cases with a necroinflammatory activity score of 1 (mild) or greater for the categories of (1) periportal interface hepatitis or (2) focal (spotty) lytic necrosis, apoptosis and focal inflammation (which included centrilobular hepatitis), were considered to have hepatitis. This included dogs with chronic hepatitis and acute or subacute hepatitis due to an unknown cause.31 The non‐hepatitis group consisted of dogs with histologically normal livers as well as dogs with livers with vacuolar change, bile duct hyperplasia, mild periportal fibrosis, pigmented granulomas, or vascular congestion. Cases with minimal portal mononuclear inflammatory infiltrates not breaching the limiting plate (no interface hepatitis) that had no other inflammatory or necrotizing lesions also were characterized as non‐hepatitis as they were likely to have primary splanchnic bed disease or other extrahepatic illness,31 and they did not meet the above criteria for hepatitis.
2.4. Quantitative Hepatic Copper Concentrations
Hepatic copper concentrations were determined on formalin‐fixed and paraffin embedded tissue, which has been shown to provide similar values to fresh tissue,32 using slight modifications of previously described methodology for deparaffinizaton and analysis (Braselton WE, Slanker MR, Stuart KJ, Verlinde ML. Comparison of element concentrations determined in fresh, formalin fixed and paraffin embedded tissue samples. American Association of Veterinary Laboratory Diagnosticians Proceedings 1997;40:74). More specifically, deparaffinized tissues were digested overnight in a 95 °C oven, using at least 10x the dry tissue mass of nitric acid. The digested samples then were diluted with water to at least 100x the dried tissue mass. Elemental analysis followed the method of Wahlen et al. using an Agilent 7500 Inductively Coupled Plasma – Mass Spectrometer (ICP/MS; Agilent 7500ce ICP‐MS, Agilent Technologies, Santa Clara, California USA).33 A fraction of each diluted tissue digest and calibration standard was diluted 40‐fold with a solution containing 0.5% EDTA and Triton X‐100, 1% ammonium hydroxide, 2% propanol and 5 ppb of scandium and 7.5 ppb of germanium, rhodium, indium, and bismuth as internal standards. The ICP/MS was tuned to yield a minimum of 7500 counts per second sensitivity for 1 ppb yttrium (mass 89), less than 1.0% oxide level as determined by the 156/140 mass ratio and less than 2.0% double charged ions as determined by the 70/140 mass ratio. Elemental concentrations were calibrated using a 5‐point linear curve of the analyte‐internal standard response ratio. Commercially available standards (Inorganic Ventures, Christiansburg, VA 24073) and controls (bovine liver and mussel standards, National Institute of Standards and Technology, Gaithersburg MD 20899) were used. The diagnostic reference interval in our laboratory for canine [Cu]H is 137 to 400 μg/g dry weight basis. Additional descriptions of the ICP‐MS methodology and in‐house, single laboratory validation statements can be found on file at the MSU‐VDL. If hyperplastic nodules, regenerative nodules, or a neoplastic infiltrate were present in some portions of the archived sample as determined by histologic assessment, careful sampling of the adjacent liver tissue was performed to avoid inclusion of such lesions in the tissue used for [Cu]H determinations.
2.5. Data and Statistical Analysis
Copper concentration data did not follow a normal distribution as assessed by boxplot analysis and Kolmogorov–Smirnov testing and were reported as medians and interquartile ranges (IQR). Statistical analyses were performed to investigate potential changes in [Cu]H over time. For the first set of analyses, [Cu]H were compared within and across time periods based on breed classification. For the second set of analyses, [Cu]H were compared between time periods based on the presence or absence of hepatitis in both the PB and NPB populations. Mann–Whitney U testing was used for the aforementioned analyses. As data were available for Labrador Retrievers for the continuous time period of 1982–2015, a scatterplot was used to further evaluate changes in [Cu]H over time in this breed. The proportionate differences in the number of dogs with [Cu]H > 300 μg/g, > 400 μg/g, and > 1000 μg/g (dry weight) between time periods were compared using Fisher exact testing. While normal [Cu]H are not clearly established in dogs, the cut‐point of 300 μg/g was selected as it was the approximate upper‐end of reference intervals for normal dogs in the historical time period, and it also represents the approximate midpoint of several commonly reported current reference intervals for dogs.15, 34, 35, 36 The cut‐point of 400 μg/g was selected as it represented the upper‐end of most currently reported reference intervals.33, 37 The cut‐point of 1000 μg/g was selected as it is a concentration frequently associated with histologic changes including inflammation and hepatocyte necrosis although the exact point at which copper accumulation induces hepatocyte injury is variable and not clearly established.18, 37 For the comparisons of median [Cu]H, a Bonferroni post‐test correction adjusted the significance level to P ≤ .005 in order to maintain the overall type‐1 error rate at ≤ .05. For other analyses, P ≤ .05 was considered significant. All statistical analyses were performed with commercially available software (Statistica, Dell StatSoft Inc, Austin, TX).
2.6. Results
Five hundred and forty six dogs, including 349 PB dogs and 197 NPB dogs, were included in this study (Table 1). The PB population consisted of 258 Labrador Retrievers, 61 Doberman Pinschers, 22 West Highland White Terriers, and 8 Dalmatians. The NPB population consisted of 43 mixed breed dogs, 13 German Shepherds, 9 Beagles, 9 Golden Retrievers, 7 Cocker Spaniels, 7 Siberian Huskies, 7 Yorkshire Terriers, 6 Great Danes, and 6 Shetland Sheepdogs. All other breeds were represented by 5 or fewer samples (Supplementary Table 1). Overall, there were 225 female dogs (133 spayed, 92 intact), 236 males (139 castrated, 97 intact) and 85 unknown. The median age of dogs at the time of sample collection was 7.8 years (IQR, 4.3–10.0 years). In the 1982–1988 period, 68% of specimens were obtained via biopsy and 32% were obtained during necropsy. In the 2009–2015 period, 72% of specimens were obtained via biopsy and 28% were obtained during necropsy.
Table 1.
Demographics of the 546 dogs included in this study of hepatic copper concentrations
| N | Age (IQR) | Sex | |||
|---|---|---|---|---|---|
| Male | Female | Unknown | |||
| NPB | 197 | 8.0 | 87 | 83 | 27 |
| (4.0–10.0) | |||||
| PB | 349 | 7.5 | 149 | 142 | 58 |
| (5.0–10.0) | |||||
| LR | 258 | 8.0 | 121 | 95 | 42 |
| (5.0–10.0) | |||||
Overall, the age and gender distribution were similar in both the PB and NPB populations. The PB population represents all Labrador Retrievers, West highland White Terriers, Doberman Pinschers, and Dalmatians included in this study. Labrador Retrievers also were presented as an individual breed given the further analyses within this group. Sex status was not available for all dogs due to omission of this information from biopsy submission forms. IQR, interquartile range; LR, Labrador Retrievers; NPB, non‐predisposed breeds; PB, predisposed breeds.
Hepatic copper concentrations increased over time in both PB and NPB dogs. In the PB population, the median [Cu]H increased from 291.4 μg/g (IQR, 190.62–485.11 μg/g) in the 1982–1988 period to 604.0 μg/g (IQR, 281.2–1145.0 μg/g) in the 2009–2015 period (P < .001). Within the PB population, Labrador Retrievers had a median increase of 324.9 μg/g and the combined group of Doberman Pinschers, West Highland White Terriers, and Dalmatians had a median increase of 285.6 μg/g. The [Cu]H also increased in the NPB population from a median of 177.1 μg/g (IQR, 108.0–310.6 μg/g) in the 1982–1988 period to a median of 278.0 μg/g (IQR, 177.5–441.9 μg/g) in the 2009–2015 period (P < .001). The median increases in [Cu]H between time periods were 312.6 μg/g and 100.9 μg/g in PB and NPB dogs, respectively. The proportion of dogs with [Cu]H exceeding 300 μg/g increased over time from 48.2% to 71.4% in PB dogs (P = .002) and from 27.5% to 49.1% in NPB dogs (Table 2; P = .002). Hepatic copper concentrations in PB dogs were greater than in NPB dogs in both time periods (P < .001 for both comparisons), although the observed differences between populations were of greater magnitude in the 2009–2015 period (326.0 μg/g) as compared to the OTP (114.3 μg/g).
Table 2.
Proportion of dogs with [Cu]H exceeding 300 μg/g, 400 μg/g, and 1000 μg/g
| 1982–1988 | 1989–1996 | 1997–2007 | 2009‐2015a | ||
|---|---|---|---|---|---|
| NPB | >300 μg/g | 27.5% (25/91)** | N/A | N/A | 49.1% (52/106)** |
| >400 μg/g | 19.8% (18/91) | N/A | N/A | 30.2% (32/106) | |
| >1,000 μg/g | 4.4% (4/91) | N/A | N/A | 5.7% (6/106) | |
| PB | >300 μg/g | 48.2% (41/85)** | N/A | N/A | 71.4% (75/105)** |
| >400 μg/g | 38.8% (33/85)** | N/A | N/A | 61.0% (64/105)** | |
| >1,000 μg/g | 10.6% (9/85)*** | N/A | N/A | 31.4% (33/105)*** | |
| LR | >300 μg/g | 34.6% (9/26) | 36.7% (11/30)*** | 73.5% (75/102)*** | 71.0% (71/100) |
| >400 μg/g | 23.1% (6/26) | 29.4% (10/30)* | 56.9% (58/102)* | 60.0% (60/100) | |
| >1,000 μg/g | 0% (0/26) | 3.3% (1/30)* | 19.6% (20/102)* | 30.0% (30/100) |
The percentage of dogs with hepatic copper concentrations >300 μg/g, > 400 μg/g, and > 1000 μg/g for the various breed groupings are presented. The actual number of dogs are in parentheses. LR, Labrador Retrievers; N/A, not available; NPB, non‐predisposed breeds; PB, predisposed breeds.
For Labrador Retrievers only, this grouping consisted of the years 2008–2015, not 2009–2015, to allow a more even distribution of cases in the latter 2 periods.
Statistical comparisons were made within each row of data using Fisher exact testing.
P < .05
P < .01
P < .001
Additional comparisons of [Cu]H were made when dogs were categorized by the presence or absence of hepatitis (Table 3). Overall, dogs with hepatitis (median of 563.0 μg/g, IQR, 257.9–1233.0 μg/g) had greater [Cu]H than dogs without hepatitis (median of 287.3 μg/g, IQR, 175.0–511.4 μg/g; P < .001). In the 2009–2015 period, 44.3% of dogs (27 of 61) with hepatitis had [Cu]H > 1000 μg/g whereas only 16.7% of dogs (12 of 72) with hepatitis had [Cu]H > 1000 μg/g in the 1982–1988 period (P < .001).
Table 3.
Hepatic copper concentrations in dogs with and without hepatitis
| 1982–1988 | 2009–2015 | P‐value | ||
|---|---|---|---|---|
| PB dogs | Non‐hepatitis | 249.2 μg/g (154.8–429.0) | 381.6 μg/g (250.3–763.5) | .004 |
| n = 40 | n = 66 | |||
| Hepatitis | 404.2 μg/g (191.4–821.1) | 1274.0 μg/g (563.0–1773.0) | < .001 | |
| n = 45 | n = 39 | |||
| NPB dogs | Non‐hepatitis | 170.0 μg/g (104.3–310.3) | 262.5 μg/g (166.0–398.8) | .013 |
| n = 64 | n = 84 | |||
| Hepatitis | 181.1 μg/g (129.8–346.1) | 542.2 μg/g (270.3–862.3) | .004 | |
| n = 27 | n = 22 |
Median (IQR) hepatic copper concentrations (μg/g) for both predisposed breed (PB) and non‐predisposed breed (NPB) dogs are presented when further stratified by the presence or absence of hepatitis. In the PB population, hepatic copper concentrations were greater in the 2009–2015 period as compared to the 1982–1988 period in both dogs with and without hepatitis. In the NPB population, dogs with hepatitis in the 2009–2015 period also had greater concentrations than dogs with hepatitis in the 1982–1988. The difference in copper concentrations in the 1982–1988 and 2009–2015 periods in NPB dogs without hepatitis was not significant when corrected for multiple comparisons (P = 0.013).
Hepatic copper concentrations of Labrador Retrievers were analyzed for the continuous period of 1982–2015 (Fig 1). The median [Cu]H of 512.2.0 μg/g (IQR, 271.8–995.2 μg/g) in the years 1997–2015 was greater than the median [Cu]H of 243.1 μg/g (IQR, 181.3–440.6 μg/g) in the years 1982–1996 (P < .001). Labrador Retrievers then were stratified into 4 time periods: 1982–1988, 1989–1996, 1997–2007, and 2008–2015 to evaluate the proportion of dogs with [Cu]H exceeding selected cut‐points (Table 2). No differences in the proportion of dogs with [Cu]H > 300 μg/g were observed between the 1982–1988 and 1989–1996 periods (34.6% and 36.7%, respectively; P = .99). Likewise, no differences in the proportion of dogs with [Cu]H > 300 μg/g were observed between the 1997–2007 and 2008–2015 periods (73.5% and 71.0%, respectively; P = .64). However, the proportion of Labrador Retrievers in the 1989–1996 time with [Cu]H > 300 μg/g was different than in the 1997–2007 period (36.7% and 73.5%, respectively; P < .001). Differences in the proportion of Labrador Retrievers with [Cu]H > 1000 μg/g were also observed between the 1989–1996 and 1997–2006 time periods (3.3% and 19.6%, respectively; P = .044).
3. DISCUSSION
This study of [Cu]H in dogs was unique in its scale (n = 546) and inclusion of both dogs that are, and are not, considered to be at‐risk for CAH. Both overall [Cu]H and the proportion of dogs with [Cu]H > 300 μg/g in the NPB population were increased in recent years as compared to a historical time period. The exact clinical relevance of the 55% increase in [Cu]H requires further investigation as the proportion of NPB dogs with [Cu] > 1000 μg/g did not differ between time periods. A hepatic copper concentration of 1000 μg/g is speculated to be a concentration at which hepatic injury is likely, but this does not exclude the possibility that lower level accumulation is also pathologic.18, 37 Indeed, some clinicians institute therapy for CAH when [Cu]H exceed 600 to 800 μg/g.37, 38 In some cases, hepatitic injury is clearly present despite only modest increases in [Cu]H.38 Dog breeds considered to be at‐risk for CAH had greater increases in [Cu]H over time as compared to NPB dogs, and these changes are certainly of clinical consequence. Median [Cu]H have doubled over time, and the proportion of PB dogs in the 2009–2015 period with [Cu]H > 1000 μg/g was 31.4% whereas only 10.6% of dogs in the 1982–1988 period exceeded this cut‐point. Although CAH in Labrador Retrievers has garnered considerable attention in recent years,12, 14, 17, 18 less attention has been devoted to other at‐risk breeds. The decision to include Doberman Pinschers, West Highland White Terriers, and Dalmatians in the same group as Labrador Retrievers was due to the well‐documented occurrence of CAH in these breeds.13, 15, 16 In our report, [Cu]H were alike in both Labrador Retrievers and the other at‐risk breeds as were the changes in [Cu]H between time periods. Collectively, the results of our investigation suggest that substantial changes have occurred in [Cu]H in all of the at‐risk breeds reported herein.
Several interesting findings emerged when dogs were further classified by the presence or absence of hepatitis. Although [Cu]H in NPB dogs with and without hepatitis in the 1982–1988 period were similar (median concentrations of 181 μg/g and 170 μg/g, respectively), there was a 3‐fold increase in [Cu]H in the hepatitis group in the 2009–2015 period as compared to the 1982–1988 period (Table 3). This would suggest that most historical hepatitis cases in the NPB population were not due to copper accumulation. Conversely, the majority of hepatitis cases in NPB dogs in recent years had [Cu]H that would be considered abnormal. Similar, but more pronounced changes were observed in the PB population. Even more convincing, 44.3% of all dogs (PB and NPB dogs) with hepatitis had [Cu]H > 1000 μg/g in recent years whereas only 16.7% of dogs with hepatitis had [Cu]H > 1000 μg/g in the historical period. Taken together, these findings suggest that CAH is a more common cause of hepatitis in recent years as compared to historical periods.
Many liver specimens from Labrador Retrievers were available for our study, likely due to breed popularity and risk of liver disease. It also has been suggested that Labrador Retrievers could serve as sentinels for environmental copper exposure.18 As such, this breed was investigated for the continuous time period of 1982–2015 to allow a more detailed investigation of the timing of potential changes in [Cu]H. Based on visual inspection of the scatterplot (Fig 1), it is apparent that median [Cu]H, as well as the percentage of dogs with markedly increased copper concentrations, increased in the mid to late 1990s. However, the exact timing of changes is unknown. The analyses of Labrador Retrievers in this report share some similarities with another study of Labrador Retrievers by Johnston et al. as both document an increase in [Cu]H in recent years as compared to a more historical time period.18 However, results from the other report suggest that [Cu]H are continuing to climb, potentially at an exponential rate.18 Our data do not support this conclusion as it appears there was a rather rapid and marked change in [Cu]H over several years which then remained relatively static for most of the 2000s. There were several methodologic differences as authors of the previous report selected cases for inclusion in each study period after slide review rather than using a randomization strategy. As suspected copper granules often can be appreciated in dogs with increased [Cu]H on routine H&E stained liver sections, there was a potential for bias in case selection. Similarly, the investigators were not blinded to study period when qualitatively scoring copper accumulation. It is unknown if these or other factors could have influenced the slight discrepancies between reports. Regardless of differences, it is clear that [Cu]H in Labrador Retrievers are substantially greater in recent years as compared to historical time periods, and this change began in the middle to late 1990s. The timing of changes in [Cu]H in other at‐risk breeds is likely similar to Labrador Retrievers, but this speculation requires additional investigation.
Figure 1.
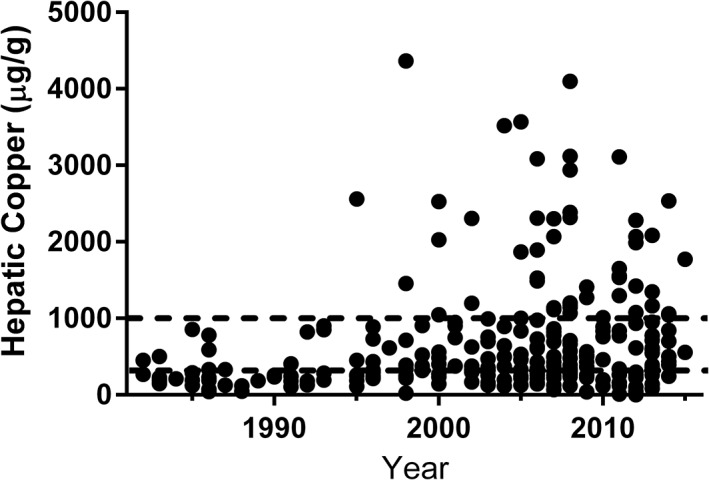
Scatterplot of hepatic copper concentrations (μg/g) in Labrador Retrievers from 1982–2015. Black circles represent individual data points. Dashed lines represent hepatic copper concentrations of 300 μg/g and 1000 μg/g. It should be noted that copper concentrations exceeding 1000 μg/g were observed in approximately 25% of Labrador Retrievers undergoing hepatic tissue sampling beginning in the mid to late 1990s
The exact reasons for the increases in [Cu]H over time in both PB and NPB dogs are unknown. Much debate is currently centered on the role of genetics versus the role of environment in disease etiology.17, 18, 37 The strong breed associations are suggestive of a hereditary component.34 Even in the 1982–1988 period, [Cu]H were greater in PB dogs as compared to NPB dogs which could be consistent with an underlying genetic susceptibility in these breeds. A recent study of Labrador Retrievers documented mutations in copper transport proteins that were associated with disease status; however, these mutations accounted for only a small portion of total heritability.17 The potential role for environmental copper exposure in causing or contributing to CAH manifestation has been a subject of growing interest in recent years.18 Since the 1970s, most homes built in the United States contain copper piping which has the potential to corrode and leach over time.39 Copper is also used in a variety of agricultural applications which could potentially result in soil or water contamination.40 However, most focus has centered around dietary copper supplementation practices in the commercial dog food industry. Beginning in the 1970s, the Association of American Feed Control Officials (AAFCO) nutrient profiles, which were based on National Research Council recommendations, required a minimum amount of copper in pet dog food.41 Many of these trace mineral requirements were extrapolated from non‐species specific data.42, 43 The published AAFCO profiles in 1997 required the use of copper sulfates or chelates in these premixes which were far more bioavailable than the previously utilized copper oxide.21, 22 This was done despite there being no evidence to suggest clinical copper deficiency was a problem at the time. The nutrient profiles published in 2015 have increased copper requirements for growing and lactating dogs, and maximum thresholds have been removed for all dogs.44 Since the original minimum requirements were established in the 1970s, it has been common practice in the commercial pet food industry to formulate mineral premixes to meet or exceed minimum requirements. These premixes are often added to the food without consideration for the copper already present in the ingredients.18 Collectively, these practices could have led to increased dietary copper exposure in dogs. It should be noted that the sharp uptick in the proportion of Labrador Retrievers with [Cu]H > 1000 μg/g was near the time of the above noted changes in AAFCO copper requirements in 1997. Given that copper deficiency is exceedingly rare and [Cu]H have increased in PB and NPB dogs, both the National Research Council and AAFCO should consider a more detailed investigation of their recommendations regarding dietary copper requirements in dogs.
Interestingly, there appear to be geographic differences in CAH prevalence as pathologic copper accumulation was documented to be an uncommon cause of hepatitis in United Kingdom dogs.45 Slight differences in dietary copper supplementation practices between the United States and Europe could contribute to geographic differences,44, 46 but populations of CAH‐affected Labrador Retrievers are well‐described in other parts of Europe.7, 38 It is unknown if differing genetic backgrounds or other factors such as soil or water copper contamination contribute to these geographic differences. These speculations should be clarified with continued improvements in understanding of CAH etiology.
Our results document that [Cu]H have increased over time, but we are unable to determine the exact prevalence of increased [Cu]H or CAH. Dogs undergoing hepatic tissue sampling were likely to have hepatic disease which is not reflective of the general population, and the design of our study did not allow definitive conclusions regarding the presence or absence of CAH in individual dogs. Nonetheless, it is apparent that increased [Cu]H are common in dogs undergoing histologic assessment of liver tissue. One potential limitation of this study is that rhodanine or rubeanic acid stained tissue sections were not evaluated, which would have permitted qualitative assessment of copper concentrations as well as assessment of lobular distribution.34, 47 While some discrepancies are observed between qualitative and quantitative methods of determining [Cu]H, results are positively correlated and generally in agreement.37, 47 Furthermore, the precise localization of copper was not essential to the current study which focused on quantitative changes over time independent of the specific hepatic disease or lobular localization of copper. Additional limitations to this study include the unknown sex status for some dogs and lack of diet histories.
In summary, we document that [Cu]H have increased over time in dogs, and this observation was not limited to breeds considered to be at‐risk for CAH. As both PB and NPB dogs were affected, it is likely that changing environmental copper exposures have influenced [Cu]H in dogs. It was also evident that the more pronounced changes in [Cu]H in Labrador Retrievers over time were similar to the changes observed in other at‐risk breeds including Doberman Pinschers, West Highland White Terriers, and Dalmatians. While causation cannot be determined, the close temporal association of changes in [Cu]H with changes in AAFCO copper supplementation recommendations are concerning.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Supporting information
Supplementary Table 1. Other breeds represented in the non‐predisposed (NPB) population.
ACKNOWLEDGMENTS
The study was conducted at the Michigan State University College of Veterinary Medicine. This work was supported by Nestlé Purina PetCare, Inc., and the Michigan State University College of Veterinary Medicine Endowed Research Funds. Portions of this work have been presented in poster form at the 2016 American College of Veterinary Internal Medicine Forum, Denver, CO.
Strickland JM, Buchweitz JP, Smedley RC, et al. Hepatic copper concentrations in 546 dogs (1982–2015). J Vet Intern Med. 2018;32:1943–1950. 10.1111/jvim.15308
Funding information Michigan State University College of Veterinary Medicine Endowed Research Funds; Nestlé Purina PetCare
REFERENCES
- 1. Boal AK, Rosenzweig AC. Structural biology of copper trafficking. Chem Rev 2009;32:1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferenci P, Zollner G, Trauner M. Hepatic transport systems. J Gastroenterol Hepatol 2002;17 Suppl:S105–112. [DOI] [PubMed] [Google Scholar]
- 3. Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr 2000;20:291–310. [DOI] [PubMed] [Google Scholar]
- 4. Sharp PA. Ctr1 and its role in body copper homeostasis. Int J Biochem Cell Bio 2003;35:288–291. [DOI] [PubMed] [Google Scholar]
- 5. Wijmenga C, Klomp LW. Molecular regulation of copper excretion in the liver. Proc Nutr Soc 2004;63:31–39. [DOI] [PubMed] [Google Scholar]
- 6. Pereira TC, Campos MM, Bogo MR. Copper toxicology, oxidative stress, and inflammation using zebrafish as experimental model. J App Toxicol 2016;36:876–885. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann G, van den Ingh TS, Bode P, et al. Copper‐associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med 2006;20:856–861. [DOI] [PubMed] [Google Scholar]
- 8. Hardy RM, Stevens JB, Stowe CM. Chronic progressive hepatitis in Bedlington Terriers associated with elevated liver copper concentrations. Minnesota Veterinarian 1975;15:13–24. [Google Scholar]
- 9. van De Sluis B, Rothuizen J, Pearson PL, et al. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Gen 2002;11:165–173. [DOI] [PubMed] [Google Scholar]
- 10. Yuzbasiyan‐Gurkan V, Blanton SH, Cao Y, et al. Linkage of a microsatellite marker to the canine copper toxicosis locus in Bedlington terriers. Am J Vet Res 1997;58:23–27. [PubMed] [Google Scholar]
- 11. Crawford MA, Schall WD, Jensen RK, et al. Chronic active hepatitis in 26 Doberman pinschers. J Am Vet Med Ass 1985;187:1343–1350. [PubMed] [Google Scholar]
- 12. Langlois DK, Smedley RC, Schall WD, Kruger JM. Acquired proximal renal tubular dysfunction in 9 Labrador Retrievers with copper‐associated hepatitis (2006‐2012). J Vet Intern Med 2013;27:491–499. [DOI] [PubMed] [Google Scholar]
- 13. Mandigers PJ, van den Ingh TS, Bode P, et al. Association between liver copper concentration and subclinical hepatitis in Doberman Pinschers. J Vet Intern Med 2004;18:647–650. [DOI] [PubMed] [Google Scholar]
- 14. Smedley R, Mullaney T, Rumbeiha W. Copper‐associated hepatitis in Labrador Retrievers. Vet Path 2009;46:484–490. [DOI] [PubMed] [Google Scholar]
- 15. Thornburg LP, Shaw D, Dolan M, et al. Hereditary copper toxicosis in West Highland White Terriers. Vet Path 1986;23:148–154. [DOI] [PubMed] [Google Scholar]
- 16. Webb CB, Twedt DC, Meyer DJ. Copper‐associated liver disease in Dalmatians: a review of 10 dogs (1998‐2001). J Vet Intern Med 2002;16:665–668. [DOI] [PubMed] [Google Scholar]
- 17. Fieten H, Gill Y, Martin AJ, et al. The Menkes and Wilson disease genes counteract in copper toxicosis in Labrador retrievers: a new canine model for copper‐metabolism disorders. Dis Models Mech 2016;9:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnston AN, Center SA, McDonough SP, et al. Hepatic copper concentrations in Labrador Retrievers with and without chronic hepatitis: 72 cases (1980‐2010). J Amer Vet Med Ass 2013;242:372–380. [DOI] [PubMed] [Google Scholar]
- 19. Appleman EH, Cianciolo R, Mosenco AS, et al. Transient acquired fanconi syndrome associated with copper storage hepatopathy in 3 dogs. J Vet Intern Med 2008;22:1038–1042. [DOI] [PubMed] [Google Scholar]
- 20. Rifkin J, Miller MD. Copper‐associated hepatitis in a Pembroke Welsh corgi. Can Vet J 2014;55:573–576. [PMC free article] [PubMed] [Google Scholar]
- 21. Association of American Feed Control Officials . Official publication. Oxford, Ind: Association of Feed Control Officials, 1997. [Google Scholar]
- 22. Czarnecki‐Maulden G RR, Chausow D. Copper Bioavailability and requirement in the dog: comparison of copper oxide and copper sulfate. FASEB J 1993;7. [Google Scholar]
- 23. Fieten H, Hooijer‐Nouwens BD, Biourge VC, et al. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J Vet Intern Med 2012;26:1274–1280. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann G, Jones PG, Biourge V, et al. Dietary management of hepatic copper accumulation in Labrador Retrievers. J Vet Intern Med 2009;23:957–963. [DOI] [PubMed] [Google Scholar]
- 25. Staff A. Most Popular Dog Breeds ‐ Full Ranking List. In: AKC News. http://www.akc.org: 2017.
- 26. Littlefield S‐E. Meet Frank and Amelia's Mutts. In: WCCO , ed. MN: CBS Minnesota; 2017. [Google Scholar]
- 27. Haywood S, Rutgers HC, Christian MK. Hepatitis and copper accumulation in Skye terriers. Vet Path 1988;25:408–414. [DOI] [PubMed] [Google Scholar]
- 28. Thornburg LP. A perspective on copper and liver disease in the dog. J Vet Diagn Invest 2000;12:101–110. [DOI] [PubMed] [Google Scholar]
- 29. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–699. [DOI] [PubMed] [Google Scholar]
- 30. Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol 2000;113:40–55. [DOI] [PubMed] [Google Scholar]
- 31. Van den Ingh TSGAM, Van Winkle TJ, Cullen JM, et al. Morphological classification of parenchymal disorders of the canine and feline liver In: Rothuizen J. and Cullen JM, eds. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases, 1st ed. Philadelphia: Saunders Elsevier; 2006:85–101. [Google Scholar]
- 32. Johnston AN, Center SA, McDonough SP, Warner KL. Influence of biopsy specimen size, tissue fixation, and assay variation on copper, iron, and zinc concentrations in canine livers. Am J Vet Res 2009;70:1502–1511. [DOI] [PubMed] [Google Scholar]
- 33. Wahlen R, Evans L, Turner J, Hearn R. The use of collision/reaction cell ICP‐MS for the determination of elements in blood and serum samples. Spectroscopy 2005;20:84–89. [Google Scholar]
- 34. Hoffmann G. Copper‐associated liver diseases. Vet Clin North Am Small Anim Pract 2009;39:489–511. [DOI] [PubMed] [Google Scholar]
- 35. Thornburg LP, Rottinghaus G, McGowan M, Kupka K, Crawford S, Forbes S. Hepatic copper concentrations in purebred and mixed‐breed dogs. Vet Pathol 1990;27:81–88. [DOI] [PubMed] [Google Scholar]
- 36. Twedt DC, Sternlieb I, Gilbertson SR. Clinical, morphologic, and chemical studies on copper toxicosis of Bedlington Terriers. J Am Vet Med Assoc 1979;175:269–275. [PubMed] [Google Scholar]
- 37. Dirksen F, Fieten H. Canine copper‐associated hepatitis. Vet Clin North Am Small Anim Pract 2017;47:631–644. [DOI] [PubMed] [Google Scholar]
- 38. Fieten H, Dirksen K, van den Ingh TS et al. D‐penicillamine treatment of copper‐associated hepatitis in Labrador retrievers. Vet J 2013;196:522–527. [DOI] [PubMed] [Google Scholar]
- 39. Hong PKA, MacAuley YY. Corrosion and leaching of copper tubing exposed to chlorinated drinking water. Water Air Soil Pollut 1998;108:457–471. [Google Scholar]
- 40. Song LY, Wang YQ. Investigation of microbial community structure of a shallow lake after one season copper sulfate algaecide treatment. Microbiol Res 2015;170:105–113. [DOI] [PubMed] [Google Scholar]
- 41. National Research Council of the National Academy of Sciences . Nutrient requirements of dogs In: Nutrient requirements of domestic animals. Washington, DC: National Academy Press, 1974. [Google Scholar]
- 42. Cromwell GL, Stahly TS, Monegue HJ. Effects of source and level of copper on performance and liver copper stores in weanling pigs. J Anim Sci 1989;67:2996–3002. [DOI] [PubMed] [Google Scholar]
- 43. Ledoux DR, Miles RD, Ammerman CB, et al. Interaction of dietary nutrient concentration and supplemental copper on chick performance and tissue copper concentrations. Poult Sci 1987;66:1379–1384. [DOI] [PubMed] [Google Scholar]
- 44. Association of American Feed Control Officials . Official publication. Oxford, Ind: Association of Feed Control Officials, 2015. [Google Scholar]
- 45. Bexfield NH, Buxton RJ, Vicek TJ, et al. Breed, age and gender distribution of dogs with chronic hepatitis in the United Kingdom. Vet J 2012;193:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. European Food Safety Authority FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) . Scientific opinion on the revision of the currently authorised maximum copper content in complete feed. EFSA Journal 2016;14:e04563 [Google Scholar]
- 47. Center SA, McDonough SP, Bogdanovic L. Digital image analysis of rhodanine‐stained liver biopsy specimens for calculation of hepatic copper concentrations in dogs. Am J Vet Res 2013;74:1474–1480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Other breeds represented in the non‐predisposed (NPB) population.


