Abstract
One new sesquiterpene and six known compounds were isolated from Dryopteris fragrans (L.) Schot. They were identified as 3-O-β-D-glucopyranosylalbicanol-11-O-β-D-glucopyranoside (1), dihydroconiferylalcohol (2), (E)-3-(4-hydroxyphenyl)acrylic acid (3), esculetin (4), 5,7-dihydroxy-2-hydroxymethylchromone (5), eriodictyol (6) and isoorientin (7) by UV, MS, 1D-NMR and 2D-NMR spectroscopy. The antifungal activities of the seven isolated compounds were screened. Compounds 2, 3, 4 and 5 showed obvious activities against Microsporum canis and Epidermophyton floccosum.
Keywords: Dryopteris fragrans (L.) Schott, chemical constituents, activity screen, antifungal activity
1. Introduction
Dryopteris fragrans (L.) Schott, a deciduous perennial herb from the genus Dryopteris (Dryopteridaceae), is mainly distributed in Northeast China, Russia, Japan, Korea and North America. The herb is used for treatment of skin diseases such as psoriasis, rashes, dermatitis, other skin diseases, barbiers and arthritis [1,2,3,4]. Previous research had discovered phloroglucins, terpenes, flavonoids, saponins, essential oils and sterols in this plant, and activity screenings of the related constituents have become popular [2,5,6]. Our research group has reported one new phenolic acid from the herb [7,8]. In this paper, we report the isolation and structural identification of one new sesquiterpene together with six known compounds which were obtained from genus Dryopteris for the first time and the assay of their antifungal activity in order to identify the active compounds.
2. Results and Discussion
2.1. Chemical Structure Identification and Spectroscopic Data
Compound 1, obtained as a light yellow oil, had a molecular formula of C27H46O12 based on the HRESIMS ([M+Na]+ 585.2890), which indicated five degrees of unsaturation. The UVmax absorption at 205.242 nm indicated an isolated chromophore in the structure. Four methyl groups (δH 0.80, 1.17, 1.26, 2.05), one olefinic proton (δH 5.47), one oxygenated methine proton (δH 4.06) and two oxygenated methylenes (δH 4.01) were observed. Furthermore, we deduced the presence of two sugar residues from the signal of two anomeric protons at δH 4.84 (1H, d, J = 7.8 Hz) and δH 4.90 (1H, d, J = 7.8 Hz, Table 1). The acid hydrolysis of 1 with aqueous 2 M HCl yielded D-glucose, which was identified by GC comparison with a sugar standard.
Table 1.
1H- and 13C-NMR data of compound 1.
| No. | H | C | No. | H | C |
|---|---|---|---|---|---|
| 1 | 2.18 (1H, m), 1.82 (1H, m) | 37.8 | 15 | 1.26 (3H, s) | 28.2 |
| 2 | 24.6 | 1' | 4.90 (1H, d, J = 7.8 Hz) | 106.9 | |
| 3 | 4.06 (1H, m) | 89.0 | 2' | 4.01 (m) | 75.8 |
| 4 | 39.4 | 3' | 4.25 (m) | 78.8 | |
| 5 | 1.91 (1H, brs) | 50.1 | 4' | 4.27 (m) | 71.8 |
| 6 | 2.18 (1H, m), 1.82 (1H, m) | 27.9 | 5' | 4.29 (m) | 78.4 |
| 7 | 5.47 (1H, brs) | 122.7 | 6' | 4.61 (1H, dd, J = 12.0, 1.2 Hz) 3.41 (1H, dd, J = 12.0, 3.2 Hz) |
63.1 |
| 8 | 134.4 | 1'' | 4.84 (1H, d, J = 7.8 Hz) | 105.3 | |
| 9 | 54.9 | 2'' | 4.01 (m) | 75.3 | |
| 10 | 35.6 | 3'' | 4.25 (m) | 78.7 | |
| 11 | 4.01 (1H, m) | 69.9 | 4'' | 4.27 (m) | 71.8 |
| 12 | 2.05 (3H, s) | 22.5 | 5'' | 4.29 (m) | 78.7 |
| 13 | 0.80 (3H, s) | 14.8 | 6'' | 4.61 (1H, dd, J = 12.0, 1.2 Hz) 3.63 (1H, dd, J = 12.0, 6.6 Hz) |
62.9 |
| 14 | 1.17 (3H, s) | 16.6 |
In the 13C-NMR spectrum (Table 1) 27 carbon signals were resolved. Besides the carbon signals of the two D-glucoses there were also 15 carbon signals comprising four methyls (δC 22.5, 14.8, 16.6, 28.2), four methylenes (δC 37.8, 27.9, 24.6, 69.9), four methines (δC 89.0, 50.1, 122.7, 54.9), and three quaternary carbons (δC 39.4, 134.4, 35.6) as classified by their chemical shifts and from the HSQC spectrum. All of the signals above suggested the aglycone of compound 1 was a sesquiterpene.
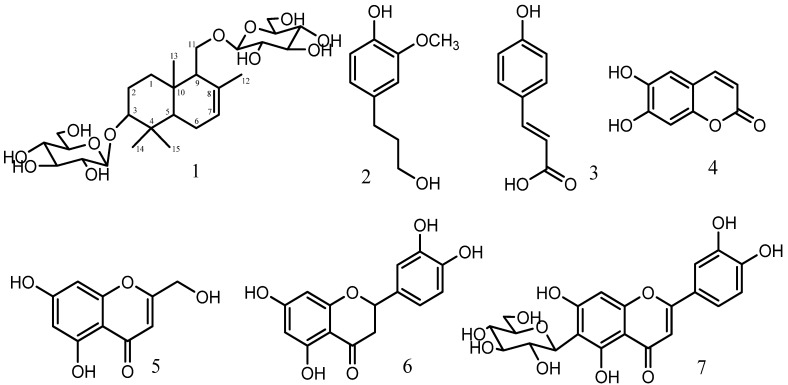
The two sugar residues in compound 1 were linked at C-3 and C-11, as determined by the HMBC correlations from δH 4.90 (H-1') to δC 89.0 (C-3) and from δH 4.84 (H-1'') to δC 69.9 (C-11). Furthermore HMBC correlations between δH 1.17 (H-14) and 1.25 (H-15) and δC 39.4 (C-4), between δH 2.05 (H-12) and δC 134.4 (C-8) and between δH 0.80 (H-11) and δC 35.6 (C-10), suggested four methyl groups were attached to C-4, C-8 and C-10 respectively. Therefore, the structure of compound 1 was established as shown in Figure 1. The known compounds 2–7 were identified by comparison of the spectral data (1H-NMR, 13C-NMR) with the literature data.
Figure 1.
Chemical strctures of compounds 1–7.
2.2. Screening for In Vitro Antifungal Activities [9,10,11]
Compounds 1–7 were screened for antifungal acitvities against Microsporum canis and Epidermophyton floccosum. The corresponding Minimum Inhibitory Concentration (MIC, μg/mL) values are listed in Table 2. Compounds 2–5 showed big differences compared with the reference standard griseofulvin (MIC value 1.0 μg/mL–0.03125 μg/mL. Table 2). The new compound 1 was inactive.
Table 2.
Minimum inhibitory concentration (MIC) distribution of the seven isolated compounds against M. canis and E. floccosum.
| Compounds No. | Minimum Inhibitory Concentration (MIC Values, μg/mL) | |
|---|---|---|
| Microsporum canis | Epidermophyton floccosum | |
| 1 | na | na |
| 2 | 0.0625 | <0.015625 |
| 3 | <0.015625 | 0.03125 |
| 4 | <0.015625 | <0.015625 |
| 5 | <0.015625 | 0.03125 |
| 6 | 8 | 4 |
| 7 | >32 | 0.5 |
| Griseofulvin | 1 | 0.03125 |
na = inactive.
3. Experimental
3.1. General
1H- and 13C-NMR spectra were recorded on a Bruker AM-400 (Bruker Corporation, Fällanden, Switzerland) with TMS as an internal standard. ESIMS were recorded on API QSTAR Pulsari (Applied Biosystems, MDS Sciex, Framingham, MA, USA) and VG-Autospec-3000 mass spectrometers (AB SCIEX mass spectrometers, Framingham, MA, USA). UV spectra were obtained on a Shimadzu UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan). Optical rotations were measured on a SEPA-300 polarimeter (Horiba, Kyoto, Japan). The GC was performed on HP6890 N gas chromatograph (Agilent, Milton-Freewater, OR, USA) equipped with a flame ionization detector and a HP-5 capillary column (30 m × 0.32 mm × 0.25 μm), injector temperature: 230 °C, detector temperature: 250 °C, column temperature ramp: 150–280 °C at a rate of 5 °C/min. Silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Co. Ltd., Qingdao, China), AB-8 Macroporous adsorption resin (Nankai Chemical Co. Ltd., Tianjin, China), MCI gel (75–150 μm (Mitsubishi Chemical Corporation, Kyoto, Japan)), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used for column chromatography (CC) Semi-preparative HPLC was performed on an Agilent 1100 liquid chromatography (Agilent Corporation, Waldbronn, Germany) with a Zorbax SB-C18 (9.4 mm × 25 cm) column (Agilent Corporation). Silica gel GF254 (Qingdao Marine Chemical Inc.) were employed for thin-layer chromatography (TLC).
3.2. Plant Material
The whole plant of Dryopteris fragrans (L.) Schott were collected in Wu-da-lian-chi, Heilongjiang Province, China in August 2009, and identified by Prof. Zhen-Yue Wang, Heilongjiang University of Chinese Medicine. The voucher specimen (registration number: XLMJ-20110812) of this plant was deposited in the Herbarium of Heilongjiang University of Chinese Medicine, Harbin, China.
3.3. Extraction and Isolation
Air-dried, powdered whole plants of D. fragrans (L.) Schott (20 kg) were extracted three times at room temperature for 2.0 h with 95% ethanol (200 L, 160 L and 120 L). The combined 95% EtOH extracts were evaporated to near dryness and the dry residue (1.0 kg) was suspended in H2O and successively eluted from an AB-8 macroporous adsorption resin column with H2O (3 × 4 L), 30% EtOH (3 × 5.0 L), 60% EtOH (3 × 5.0 L) and 95% EtOH (3 × 5.0 L). The 30% EtOH fraction (100 g) was subjected to silica gel column chromatography with a CHCl3/MeOH (100:0→1:1, v/v) gradient as eluent to give fractions D1→D5. Repeated silica gel chromatography of fraction D2 (20 g) eluting with CHCl3/MeOH (30:0→10:1, v/v) yielded compounds 3 (75 mg) and 5 (135 mg). Compounds 2 (55 mg) and 4 (5.6 mg) were isolated from D1 (10 g) by silica gel column chromatography elutin g with CHCl3. D3 (3.0 g) was subjected to ODS column chromatography with MeOH/H2O (35:65, v/v) as eluent to yield compound 6, D4 (3.5g) was purified by preparative HPLC on a ODS column (10 μm, 20 × 300 mm, flow rate 8 mL/min) with MeOH/H2O (35:65) and MeOH/H2O (45:55) as eluents to give 1 (65 mg), and 7 (95 mg).
3.4. Characterization of Isolated Compounds
3-O-β-D-Glucopyranosylalbicanol-11-O-β-D-glucopyranoside (1). A light yellow oil. 1H-NMR (MeOD) δH: 5.47 (brs, 1H, H-7), 1.91 (brs, 1H, H-5), 2.05 (s, 3H, -CH3), 1.26 (s, 3H, -CH3), 1.17 (s, 3H, -CH3), 0.80 (s, 3H, -CH3), 4.90 (d, J = 7.8 Hz, 1H, H-1'), 4.84 (d, J = 7.8 Hz, 1H, H-1''). 13C-NMR (MeOD) δC: 37.8 (C-1), 24.6 (C-2), 89.0 (C-3), 39.4 (C-4), 50.1 (C-5), 27.9 (C-6), 122.7 (C-7), 134.4 (C-8), 54.9 (C-9), 35.6 (C-10), 69.9 (C-11), 22.5 (C-12), 14.9 (C-13), 16.6 (C-14), 28.2 (C-15), 106.9 (C-1'), 75.8 (C-2'), 78.8 (C-3'), 71.8 (C-4'), 78.4 (C-5'), 63.1 (C-6'), 105.3 (C-1''), 75.3 (C-2''), 78.7 (C-3''), 71.8 (C-4''), 78.7 (C-5''), 62.9 (C-6'').
Dihydroconiferylalcohol (2). Colorless crystals. 1H-NMR (MeOD) δH: 7.28 (s, 1H), 6.77 (d, J = 1.8 Hz, 1H, H-2), 6.70 (d, J = 8.0 Hz, 1H, H-5), 6.62 (dd, J = 8.0, 1.8 Hz, 1H, H-6), 2.59 (t, J = 7.6 Hz, 2H, H-7), 1.80 (m, 2H, H-8), 3.56 (t, J = 6.5 Hz, 2H, H-9), 3.82 (s, 3H, -OCH3). 13C-NMR (MeOD) δC: 134.9 (s, C-1), 113.1 (d, C-2), 148.8 (s, C-3), 145.5 (s, C-4), 116.1 (d, C-5), 121.8 (d, C-6), 32.7 (t, C-7), 35.7 (t, C-8), 62.3 (t, C-9), 56.3 (q, C-10).
(E)-3-(4-Hydroxyphenyl)acrylic acid (3). Pale yellow powder. 1H-NMR (acetone-d6) δH: 7.54 (d, J = 8.6 Hz, 2H, H-2, H-6), 6.89 (d, J = 8.6 Hz, 2H, H-3, H-5), 7.63 (d, J = 16.0 Hz, 1H, H-7), 6.35 (d, J = 16.0 Hz, 1H, H-8). 13C-NMR (acetone-d6) δ: 127.2 (s, C-1), 131.3 (d, C-2, C-6), 116.2 (d, C-3, C-5), 161.1 (s, C-4), 146.1 (d, C-7), 117.1 (d, C-8), 169.9 (s, C-9).
Esculetin (4). Green amorphous powder. 1H-NMR (MeOD) δH: 6.18 (d, J = 9.0 Hz, 1H, H-3), 7.79 (d, J = 9.0 Hz, 1H, H-4), 6.94 (s, 1H, H-5), 6.75 (brs, 1H, H-8). 13C-NMR (MeOD) δC: 164.3 (s, C-2), 112.8 (d, C-3), 146.1 (d, C-4), 113.0 (d, C-5), 144.6 (s, C-6), 150.5 (s, C-7), 103.6 (d, C-8), 152.0 (s, C-9), 112.5 (s, C-10).
5,7-Dihydroxy-2-hydroxymethylchromone (5). Pale yellow crystals. 1H-NMR (MeOD) δH: 6.22 (s, H, H-3), 6.27 (brs, 1H, H-6), 6.34 (brs, 1H, H-8), 4.52 (s, 2H, H-11). 13C-NMR (MeOD) δC: 164.3 (s, C-2), 112.8 (d, C-3), 146.1 (d, C-4), 113.0 (d, C-5), 144.6 (s, C-6), 150.5 (s, C-7), 103.6 (d, C-8), 152.0 (s, C-9), 112.5 (s, C-10).
Eriodictyol (6). Pale yellow crystals. 1H-NMR (MeOD) δH: 5.36 (dd, J = 2.9, 12.9 Hz, 1H, H-2), 2.69 (dd, J = 2.9, 17.4 Hz, 2H, H-3α, H-3β), 5.91 (d, J = 2.1 Hz, 1H, H-6), 5.93 (d, J = 2.1 Hz, 1H, H-8), 7.01 (s, 1H, H-2'), 6.84 (s, 2H, H-5', H-6'). 13C-NMR (MeOD) δC: 79.9 (d, C-2), 43.4 (t, C-3), 197.3 (s, C-4), 165.1 (s, C-5), 96.7 (d, C-6), 167.7 (s, C-7), 95.8 (d, C-8), 164.8 (s, C-9), 103.8 (s, C-10), 131.2 (s, C-1'), 114.6 (d, C-2'), 146.1 (s, C-3'), 146.5 (s, C-4'), 115.9 (d, C-5'), 119.0 (d, C-6').
Isoorientin (7). Pale yellow powder. 1H-NMR (MeOD) δH: 13.96 (s, 1H, 5-OH), 6.73 (s, 1H, H-3), 6.77 (s, 1H, H-8), 7.56 (brs, 1H, H-2'), 7.29 (d, J = 8.5 Hz, 1H, H-5'), 7.85 (d, J = 8.5 Hz, 1H, H-6'), 5.97(d, J = 9.8 Hz, 1H, H-1''), 4.23–5.10 (m, 5H, H-2'', H-3'', H-4'', H-5'', H-6''). 13C-NMR (MeOD) δC: 165.1 (s, C-2), 103.4 (d, C-3), 183.1 (s, C-4), 157.4 (s, C-5), 106.1 (s, C-6), 164.6 (s, C-7), 99.2 (d, C-8), 162.3 (s, C-9), 105.4 (s, C-10), 123.4 (s, C-1'), 115.8 (d, C-2'), 147.6 (s, C-3'), 151.6 (s, C-4'), 117.1 (d, C-5'), 120.3 (d, C-6'), 75.8 (d, C-1''), 72.3 (d, C-2''), 81.1 (d, C-3''), 73.1 (d, C-4''), 83.6 (d, C-5''), 63.1 (t, C-6'').
3.5. Acid Hydrolysis
Compound 1 (5 mg) was hydrolyzed with 2 mol/L HCl (5 mL) for 5 h at 90 °C. After cooling to room temperature, the reaction mixture was extracted with EtOAc (5 mL) three times. Each remaining aqueous layer was neutralized with 0.5 N NaOH and then freeze-dried to give a residue. The residue was dissolved in pyridine (2 mL) and L-cysteine methyl ester hydrochloride (3 mg) was added to the solution. The solution was kept at 60 °C for 1 h. Then trimethylchlorosilane (0.5 mL) was added to the reaction mixture and heated at 60 °C for another 30 min. After centrifugation, the supernatant was analyzed by GC. The sugar derivatives obtained from compounds 1 showed a single peak at 32.3 min. The retention time was similar to that of a D-glucose derivative, so the sugar was identified as D-glucose.
3.6. Microsporum Canis and Epidermophyton floccosum Strains [8,9,10]
M. canis and E. floccosum were obtained from the fungus preservation center in the China Academy of Sciences and the Institute of Medicine of Dermatology, respectively. Antifungal tests were performed using the method of Dilution Antifungal Susceptibility Testing of Filamentous Fungi as described by the National Committee for Clinical Laboratory Standards [9].
4. Conclusions
A new sesquiterpene 1 and known compounds 2–7 were isolated from the genus Dryopteris for the first time. The antifungal activity screening results with Microsporum canis and Epidermophyton floccosum showed that compounds 2, 3, 4 and 5 have remarkable activities against both species.
Acknowledgments
The authors are thankful to the financial support provided by Natural Science Foundation Project (No. D201023) funded by Heilongjiang Province and Science and Technology Research Project (No. 11551384) funded by the Heilongjiang Provincial Department of Education.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–7 are available from the authors.
References
- 1.Shen Z.B., Luo W.Y., Yan Y.S., Zhu J.F. Study on terpenes of Dryopteris fragrans L. Zhong Yao Cai. 2006;29:334–335. [PubMed] [Google Scholar]
- 2.Li X.J., Fu Y.J., Luo M., Wang W., Zhang L., Zhao C.J., Zu Y.G. Preparative separation of dryofragin and aspidin BB from Dryopteris fragrans extracts by macroporous resin column chromatography. J. Pharm. Biomed. Anal. 2012;61:199–206. doi: 10.1016/j.jpba.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Li B., Zhu J.F., Zou Z.J., Yin Y.Q., Shen Z.B. Studies on the chemical constituents of Dryopteris fragrans. Zhong Yao Cai. 2009;32:1232–1233. [PubMed] [Google Scholar]
- 4.Kuang H., Sun C., Zhang Y., Chen D., Yang B., Xia Y. Three drimane sesquiterpene glucoside from the aerial parts of Dryopteris fragrans (L.) Schott. Fitoterapia. 2009;80:134–137. doi: 10.1016/j.fitote.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Fan H.Q., Shen Z.B., Chen Y.F., Wu J.Y., Yang C.Y., Liang W.N., Tang C.P. Study on antifungal susceptibility of different extract of Dryopteris fragrans. Zhong Yao Cai. 2012;35:1981–1985. [PubMed] [Google Scholar]
- 6.Oller-Lopez J.L., Iranzo M., Mormeneo S., Oliver E., Cuerva J.M., Oltra J.E. Bassianolone: An antimicrobial precursor of cephalosporolides E and F from the entomoparasitic fungus Beauveria bassiana. Org. Biomol. Chem. 2005;3:1172–1173. doi: 10.1039/b417534d. [DOI] [PubMed] [Google Scholar]
- 7.Kuang H., Zhang Y., Li G., Zeng W., Wang H., Song Q. A new phenolic glycoside from the aerial parts of Dryopteris fragrans. Fitoterapia. 2008;79:319–320. doi: 10.1016/j.fitote.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Ito H., Muranaka T., Mori K., Jin Z.X., Tokuda H., Nishino H., Yoshida T. Ichthyotoxic phloroglucinol derivatives from Dryopteris fragrans and their anti-tumor promoting activity. Chem. Pharm. Bull. Tokyo. 2000;48:1190–1195. doi: 10.1248/cpb.48.1190. [DOI] [PubMed] [Google Scholar]
- 9.Ali I., Khan F.G., Suri K.A., Gupta B.D., Satti N.K., Dutt P., Afrin F., Qazi G.N., Khan I.A. In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann. Clin. Microbiol. Antimicrob. 2010;9:7–15. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mares D. Antimicrobial activity of protoanemonin, a lactone from ranunculaceous plants. Mycopathologia. 1987;98:133–140. doi: 10.1007/BF00437648. [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Zhang J., Yi J., Zhang R., Huang H. Susceptibility test of dermatophyte to four antifungal drugs using a modified M38-A protocol. Chin. J. Mycol. 2009;4:214–217. [Google Scholar]