Abstract
Objectives:
Recent studies demonstrate autoantibodies are powerful tools to interrogate molecular events linking cancer and the development of autoimmunity in scleroderma. Investigating cancer risk in these biologically relevant subsets may provide an opportunity to develop personalized cancer screening guidelines. In this study, we examined cancer risk in distinct serologic and phenotypic scleroderma subsets and compared estimates to the general population.
Methods:
Patients in the Johns Hopkins Scleroderma Center observational cohort were studied. Overall and site-specific cancer incidence was calculated in distinct autoantibody and scleroderma phenotypic subsets, and compared with the Surveillance, Epidemiology and End Results (SEER) registry, a representative sample of the US population.
Results:
2383 scleroderma patients contributing 37,686 person-years were studied. 205 patients (8.6%) had a diagnosis of cancer. Within 3 years of scleroderma onset, cancer risk was increased in patients with RNA polymerase III autoantibodies (anti-pol; SIR 2.84, 95% CI 1.89–4.10) and those lacking centromere, topoisomerase-1 and pol antibodies (SIR 1.83, 95% CI 1.10–2.86). Among anti-pol-positive patients, cancer specific risk may vary by scleroderma subtype; those with diffuse scleroderma had an increased breast cancer risk, whereas those with limited scleroderma had high lung cancer risk. In contrast, patients with anti-centromere antibodies had a lower risk of cancer during follow-up (SIR 0.59, 95% CI 0.44–0.76).
Conclusions:
Autoantibody specificity and disease subtype are biologically meaningful filters that may inform cancer risk stratification in patients with scleroderma. Future research testing the value of targeted cancer screening strategies in scleroderma patients is needed.
Keywords: systemic sclerosis, malignancy, autoantibodies
Introduction
Prior investigations have demonstrated an increase in cancer risk in patients with systemic sclerosis (scleroderma) compared to the general population.1–11 In a study of scleroderma patients with cancer, our group showed that patients with RNA polymerase III autoantibodies (anti-pol) had cancer occur within a short interval of scleroderma onset.12 Subsequent studies demonstrated that these patients have genetic alterations (somatic mutations and/or loss of heterozygosity) at the POLR3A locus that encodes for RNA polymerase III in their cancers, with both mutation-specific and cross reactive immune responses seen.13 These data strongly suggest that alterations of autoantigen sequence in cancers may trigger anti-tumor immune responses that spread to the wild type molecule, resulting in autoimmunity.14
Many international scleroderma cohorts have similarly observed that patients with scleroderma and anti-pol have a significantly increased risk of cancer at the time of scleroderma onset compared to scleroderma patients without these antibodies.15–19 In addition, patients lacking antibodies against centromere, topoisomerase-1, and pol (hereafter referred to as “CTP-negative”) also have more cancer diagnosed within a short interval of scleroderma onset, suggesting there may be other serologic subsets of cancer-associated scleroderma.17,20,21 Case reports suggest that therapy of coincident cancer may induce scleroderma remission,22–24 raising the possibility that early cancer detection and therapy in patients with new onset scleroderma might improve scleroderma outcomes.
Our prior work suggests that investigating cancer risk in scleroderma as a group, without differentiating between serologically relevant subsets or using the cancer-scleroderma interval as a filter, may mask important differences in the relationship between cancer and autoimmunity. In the current study, we examined overall and site-specific cancer risk at scleroderma onset in distinct serologic and phenotypic subsets and for the first time compared these estimates to the general population.
Methods
Study population
Patients seen at the Johns Hopkins Scleroderma Center for their first visit between January 1, 2000 and December 31, 2015 were eligible for the study if they consented to participate in our IRB-approved cohort database and had a diagnosis of scleroderma. Scleroderma was defined by 1980 or 2013 ACR/EULAR classification criteria,25–26 at least 3 of 5 CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome criteria, or having definite Raynaud’s, abnormal nailfold capillaries and a scleroderma-specific autoantibody. Clinical and serological data are collected prospectively at baseline and at 6-month intervals. Patients were classified as having limited or diffuse scleroderma by established criteria.27 Four autoantibody categories were assessed: anti-centromere A/B (cenp), anti-topoisomerase-1 (topo), anti-pol, and CTP-negative. Patients were considered positive for an autoantibody if they were ever positive based on clinically obtained assays. Double autoantibody positivity was infrequent: 14 patients were positive for both anti-cenp and anti-pol, 6 for anti-cenp and anti-topo, and 14 for anti-pol and anti-topo. Patients who could not be classified into an autoantibody subset because of missing autoantibody data were only included in the overall scleroderma cohort analyses. For all analyses, the timing of scleroderma onset was defined by the first scleroderma symptom, either Raynaud’s or non-Raynaud’s. Patient reported cancer diagnoses and dates of diagnosis, obtained at the enrollment visit and during follow up, were confirmed by medical record review and pathology reports if available.17 Electronic medical records were comprehensively reviewed to ensure that all cancer cases were captured during follow up.
Examination of cancer risk in scleroderma compared to the general population
Cancer risk was determined by comparing cancer incidence in our cohort with the Surveillance, Epidemiology and End Results (SEER) registry, a nationally representative sample of the US population. Cancer incidence was examined in the overall scleroderma cohort, and in autoantibody and cutaneous subsets. We computed standardized incidence ratios (SIR) for cancer overall and individual cancer types. Our cancer subtype analyses focused on breast and lung cancers as these are the most prevalent cancers in scleroderma, but other cancer sites were also examined (Appendix 1). The observed number of cancers in our cohort was compared to the expected number of cancer cases for the US population by identifying the crude rate of incident cancers corresponding to each patient’s age (within 5 year intervals), gender, race, ethnicity and the calendar year of exposure in SEER.28 Person time prior to 1973 was not examined as SEER data begins in 1973. At the time of analysis, SEER data were complete through 2014. SEER crude rates for 2014 were used as a surrogate for person time after 2014. The sum of the crude rates for all years of exposure for all patients yielded the expected number of cancer cases. To find the 95% confidence limits, we followed standard procedure.28–29
Because we are interested in cancer diagnosed close to the time of scleroderma onset (defined as time zero) that may be suggestive of cancer-induced autoimmunity, we examined two time windows for our primary analyses: (i) 3 years before scleroderma onset until cancer diagnosis date or the last visit date (termed “overall cancer risk” during follow up) and (ii) 3 years before scleroderma onset until 3 years after scleroderma onset (±3 years, “cancer-associated scleroderma”). Patients with cancers preceding these time windows were excluded from our analysis. Administrative censoring occurred at the cancer diagnosis date or last visit date, whichever came first. The study population for our primary analyses comprised 2383 scleroderma patients.
Since including individuals with cancers diagnosed a few years before joining the cohort may introduce a form of immortal person time bias, we performed two additional analyses restricting our study population to patients who presented to our Center within 5 years of their first scleroderma symptom (“recent onset scleroderma”). In the first analysis, we only included cancer diagnoses that occurred after the first visit to our center. As referral to a tertiary center is often delayed, we also performed an analysis involving patients with recent onset scleroderma and examined cancer diagnoses after scleroderma symptom onset. This time point better reflects presentation to a community rheumatology practice.
Finally, we graphically examined cancer risk over time (starting 3 years before scleroderma onset) in scleroderma patients compared to the general population. The expected cancer incidence was computed using SEER data for each patient-year of exposure. Observed and expected numbers of cancer cases, and the corresponding SIR, were plotted in 6-year time windows (i.e. ±3 year increments with time zero denoting scleroderma onset). For each patient, cancer risk exposure ended on the date of cancer diagnosis, last visit or at the end of the six-year window. The cumulative incidence of cancer was also plotted over time for scleroderma patients overall and in each autoantibody subgroup.
Analyses were performed using MATLAB R2016b (MathWorks, Natick, Massachusetts) and R version 3.4.0 (R Foundation, Vienna, Austria). Bonferroni adjustment for multiple (10) comparisons was performed, as each time window and tumor type had 10 autoantibody-subtype comparisons. Therefore, p≤0.05/10 or p≤0.005 was considered statistically significant.
Results
The study population for our primary analyses consisted of 2383 scleroderma patients contributing 37,686 person-years (Table 1). The mean age at scleroderma onset was 42.4±15.1 years. Sixty percent of patients had limited scleroderma, 83% were female, and 76% self-identified as white race. Among the 1712 patients with autoantibody data, 608 (35.5%) were positive for anti-cenp, 481 (28.1%) for anti-topo, and 278 (16.2%) for anti-pol; 379 patients (22.1%) were CTP-negative. An additional 671 patients could not be classified into an antibody subset because of missing data. Approximately 9% of patients (205/2383) had a history of cancer (see Appendix Figure 1 for tumor sites). Additional scleroderma characteristics of this population are detailed in Appendix Table 1.
Table 1.
Risk for all cancers.*
| Analysis time | Antibody | Subtype | Sample size | Person-years | No. observed | No. expected | SIR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Overall risk | All | limited | 1470 | 26,624 | 128 | 182.0 | 0.70 (0.59–0.84) | <0.001** |
| diffuse | 913 | 11,062 | 77 | 69.0 | 1.12 (0.88–1.39) | 0.36 | ||
| Cenp | limited | 570 | 11,857 | 53 | 90.0 | 0.59 (0.44–0.77) | <0.001** | |
| diffuse | 38 | 754 | 3 | 5.5 | 0.55 (0.11–1.60) | 0.41 | ||
| Topo | limited | 241 | 4,035 | 20 | 25.5 | 0.78 (0.48–1.21) | 0.32 | |
| diffuse | 240 | 3,134 | 17 | 17.7 | 0.96 (0.56–1.53) | 0.99 | ||
| Pol III | limited | 59 | 962 | 9 | 6.8 | 1.33 (0.61–2.53) | 0.48 | |
| diffuse | 219 | 2,509 | 36 | 17.6 | 2.05 (1.44–2.84) | <0.001** | ||
| CTP-Negative | limited | 242 | 4,065 | 31 | 25.5 | 1.21 (0.82–1.72) | 0.33 | |
| diffuse | 137 | 1,709 | 8 | 10.6 | 0.75 (0.32–1.48) | 0.53 | ||
| ± 3 years | All | limited | 1470 | 7,935 | 35 | 41.4 | 0.84 (0.59–1.18) | 0.36 |
| diffuse | 913 | 5,210 | 44 | 28.2 | 1.56 (1.13–2.10) | 0.007 | ||
| Cenp | limited | 570 | 3,003 | 10 | 16.7 | 0.60 (0.29–1.10) | 0.111 | |
| diffuse | 38 | 212 | 0 | 1.1 | 0.00 (0.00–3.34) | 0.66 | ||
| Topo | limited | 241 | 1,353 | 4 | 6.6 | 0.60 (0.16–1.54) | 0.42 | |
| diffuse | 240 | 1,393 | 10 | 6.4 | 1.55 (0.75–2.86) | 0.23 | ||
| Pol III | limited | 59 | 305 | 3 | 1.9 | 1.59 (0.33–4.66) | 0.58 | |
| diffuse | 219 | 1,209 | 25 | 8.0 | 3.13 (2.03–4.62) | <0.001** | ||
| CTP-Negative | limited | 242 | 1,335 | 15 | 6.2 | 2.43 (1.36–4.00) | 0.004** | |
| diffuse | 137 | 808 | 4 | 4.2 | 0.95 (0.26–2.44) | 0.99 |
Excluding non-melanoma skin cancers.
Statistically significant p-value after adjustment for multiple (10) comparisons per analysis
Determination of cancer risk relative to the general population: All cancers
Patients with diffuse scleroderma did not have an increased risk of cancer (SIR 1.12, 95% CI 0.88–1.39; Table 1, overall cancer risk). In contrast, an increased risk of cancer was observed among anti-pol patients with diffuse disease (SIR 2.05, 95% CI 1.44–2.84). Patients with limited scleroderma had a 30% lower risk of cancer (SIR 0.70, 95% CI 0.59–0.84), and this was notable in anti-centromere positive patients (SIR 0.59, 95% CI 0.44–0.77).
Next we sought to determine the risk of cancer within 3 years of scleroderma onset (“cancer-associated scleroderma”) compared to individuals in the general population. While patients with limited scleroderma did not have an increased risk of cancer-associated scleroderma (Table 1, ±3 years), patients with diffuse scleroderma had a 56% increased risk compared to the general population (SIR 1.56, 95% CI 1.13–2.10). This risk increase was notable among anti-pol patients with diffuse disease (SIR 3.13, 95% CI 2.03–4.62). Additionally, CTP-negative patients with limited scleroderma had an increased risk of cancer-associated scleroderma (SIR 2.43, 95% CI 1.36–4.00).
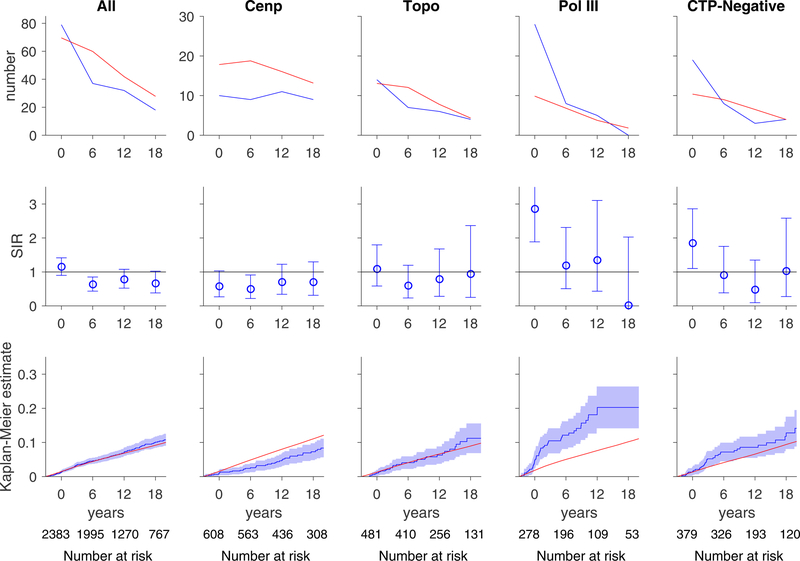
The increased risk of cancer at scleroderma onset among anti-pol-positive and CTP-negative patients is illustrated in Figure 1. The number of cancer cases observed around the time of scleroderma onset (top row, blue curve) is greater than the number of expected cancer cases based on SEER data (red curve) in these two autoantibody subsets. The relative risk of cancer compared to the general population is presented in time-dependent SIRs (middle row) and was increased for anti-pol-positive and CTP-negative groups close to scleroderma onset. The cumulative incidence of cancer was significantly higher among anti-pol patients (blue lines, blue dashed lines 95% CI) compared to that expected in the general population (red line) (bottom row, Figure 1). In contrast, the cumulative incidence of cancer was lower than expected in the anti-centromere group.
Figure 1. Risk of all cancers over time.
In each graph, the x-axis reflects time from scleroderma onset (defined as time zero). Top and middle rows, each time window represents a 6-year period (±3 years); for example, data plotted at time zero reflects cancer risk within ±3 years of scleroderma onset. The number at risk for each time window is denoted at the bottom of the graph. Top row, the observed number of cancer cases (blue) is presented in comparison with the number of cancer cases that are expected based on SEER data (red). Middle row, the ratio between the observed and expected cancer cases is presented as a standardized incidence ratio (SIR) along with its 95% confidence interval. Values of 1 denote a cancer risk equivalent to that of the background population. Bottom row, the cumulative incidence of cancer among scleroderma patients (solid blue line) starting at 3 years before scleroderma onset is presented with 95% confidence intervals (shaded blue region). Red lines represent the expected cumulative incidence of cancer based on SEER data for the general population. Scleroderma patients with anti-centromere antibodies appear to have a decreased risk of cancer over time. Scleroderma patients with pol III antibodies and the CTP-Negative group have an increased risk of cancer that is prominent at scleroderma onset. The cumulative incidence of cancer is significantly higher than that observed in the general population among patients with pol III autoantibodies.
Cancer risk in patients with recent onset scleroderma
We performed two additional analyses restricting our study population to patients who presented to our scleroderma center within 5 years of their first scleroderma symptom and examined cancer diagnoses (i) after first visit to our tertiary referral center or (ii) after the first scleroderma symptom. Our findings of an increased risk of cancer among anti-pol-positive patients with diffuse scleroderma remained unchanged in both analyses, although in these restricted analyses this was statistically significant only after first symptom when adjusting for multiple comparisons (Appendix Table 2).
Breast cancer
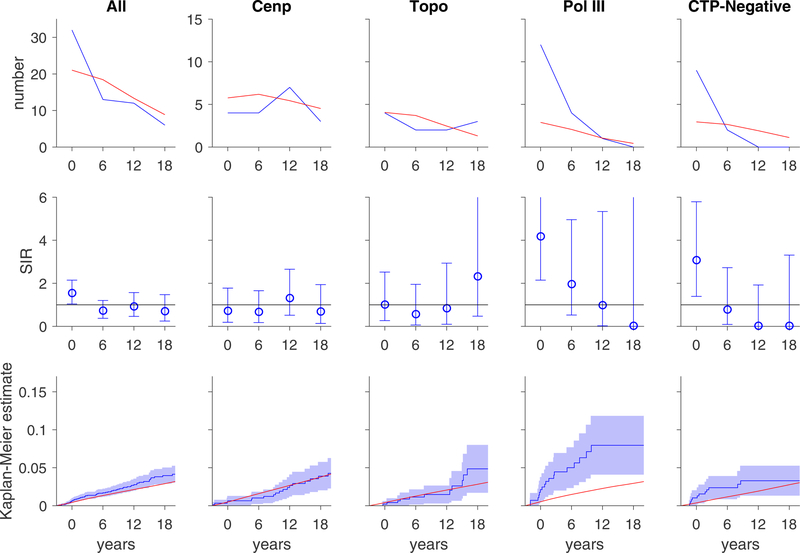
Anti-pol positive patients with diffuse scleroderma had an increased risk of breast cancer overall (SIR 3.06, 95% CI 1.75–4.98) and within 3 years of scleroderma onset (SIR 5.14, 95% CI 2.66–8.98; Table 2). Within 3 years of scleroderma onset, CTP-negative patients with limited disease also have an increased risk of breast cancer (SIR 4.44, 95% CI 1.92–8.74). The marked increased risk of breast cancer at scleroderma onset in these two autoantibody subsets is illustrated in Figure 2 (top and middle rows). The cumulative incidence of breast cancer is significantly higher among anti-pol patients compared to the general population (bottom row, Figure 2).
Table 2.
Risk for breast cancer.
| Analysis time | Antibody | Subtype | Person-years | No. observed | No. expected | SIR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Overall risk | All | limited | 26624 | 42 | 57.6 | 0.73 (0.53–0.99) | 0.039 |
| diffuse | 11062 | 28 | 20.3 | 1.38 (0.91–1.99) | 0.123 | ||
| Cenp | limited | 11857 | 18 | 29.9 | 0.60 (0.36–0.95) | 0.027 | |
| diffuse | 754 | 3 | 1.6 | 1.84 (0.38–5.39) | 0.45 | ||
| Topo | limited | 4035 | 8 | 8.2 | 0.97 (0.42–1.92) | 0.99 | |
| diffuse | 3134 | 5 | 5.3 | 0.95 (0.31–2.21) | 0.99 | ||
| Pol III | limited | 962 | 1 | 1.9 | 0.52 (0.01–2.91) | 0.86 | |
| diffuse | 2509 | 16 | 5.2 | 3.06 (1.75–4.98) | <0.001** | ||
| CTP-Negative | limited | 4065 | 12 | 7.2 | 1.66 (0.86–2.89) | 0.130 | |
| diffuse | 1709 | 1 | 3 | 0.33 (0.01–1.83) | 0.39 | ||
| ± 3 years | All | limited | 7935 | 15 | 12.8 | 1.17 (0.66–1.94) | 0.60 |
| diffuse | 5210 | 17 | 8.3 | 2.06 (1.20–3.29) | 0.010 | ||
| Cenp | limited | 3003 | 4 | 5.4 | 0.75 (0.20–1.91) | 0.76 | |
| diffuse | 212 | 0 | 0.4 | 0.00 (0.00–9.33) | 0.99 | ||
| Topo | limited | 1353 | 1 | 2.1 | 0.48 (0.01–2.69) | 0.78 | |
| diffuse | 1393 | 3 | 2 | 1.51 (0.31–4.41) | 0.64 | ||
| Pol III | limited | 305 | 0 | 0.6 | 0.00 (0.00–6.66) | 0.99 | |
| diffuse | 1209 | 12 | 2.3 | 5.14 (2.66–8.98) | <0.001** | ||
| CTP-Negative | limited | 1335 | 8 | 1.8 | 4.44 (1.92–8.74) | 0.001** | |
| diffuse | 808 | 1 | 1.1 | 0.87 (0.02–4.86) | 0.99 |
Statistically significant p-value after adjustment for multiple (10) comparisons per analysis
Figure 2. Risk of breast cancers over time.
In each graph, the x-axis reflects time from scleroderma onset (defined as time zero). Top and middle rows, each time window represents a 6-year period (±3 years); for example, data plotted at time zero reflects breast cancer risk within ±3 years of scleroderma onset. Top row, the observed number of breast cancer cases (blue) is presented in comparison with the number of breast cancer cases that are expected based on SEER data (red). Middle row, the ratio between the observed and expected breast cancer cases is presented as a standardized incidence ratio (SIR) along with its 95% confidence interval. Values of 1 denote a breast cancer risk equivalent to that of the background population. Bottom row, the cumulative incidence of breast cancer among scleroderma patients (solid blue line) starting at 3 years before scleroderma onset is presented with 95% confidence intervals (shaded blue region). Red lines represent the expected cumulative incidence of breast cancer based on SEER data for the general population. Patients with topo and cenp antibodies do not have an increased risk of breast cancer. Scleroderma patients with pol III antibodies and the CTP-Negative group have an increased risk of breast cancer that is prominent at scleroderma onset. The cumulative incidence of breast cancer is significantly higher than that observed in the general population among patients with pol III autoantibodies.
Lung cancer
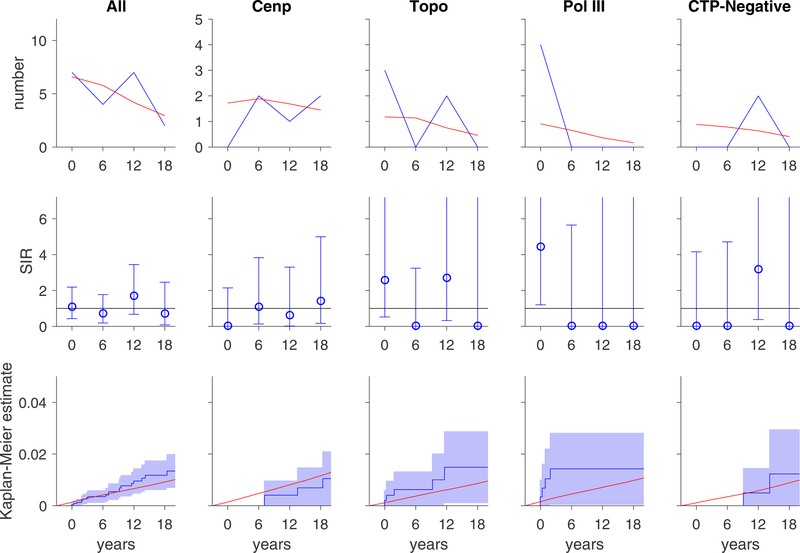
The number of lung cancer cases was small (N=30 overall). However, in an exploratory analysis, an increased risk of lung cancer was seen in anti-pol patients with limited disease within 3 years of scleroderma onset (SIR 10.4, 95% CI 1.26–37.7; Table 3; Figure 3).
Table 3.
Risk for lung cancer.
| Analysis time | Antibody | Subtype | Person-years | No. observed | No. expected | SIR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Overall risk | All | limited | 26624 | 24 | 18.9 | 1.27 (0.81–1.89) | 0.29 |
| diffuse | 11062 | 6 | 6.6 | 0.91 (0.34–1.99) | 0.99 | ||
| Cenp | limited | 11857 | 8 | 9.6 | 0.83 (0.36–1.63) | 0.75 | |
| diffuse | 754 | 0 | 0.6 | 0.00 (0.00–6.27) | 0.99 | ||
| Topo | limited | 4035 | 6 | 2.5 | 2.40 (0.88–5.23) | 0.084 | |
| diffuse | 3134 | 2 | 1.7 | 1.19 (0.14–4.31) | 0.99 | ||
| Pol III | limited | 962 | 3 | 0.7 | 4.31 (0.89–12.61) | 0.067 | |
| diffuse | 2509 | 2 | 1.6 | 1.28 (0.15–4.62) | 0.93 | ||
| CTP-Negative | limited | 4065 | 2 | 2.5 | 0.81 (0.10–2.91) | 0.99 | |
| diffuse | 1709 | 0 | 0.9 | 0.00 (0.00–3.91) | 0.78 | ||
| ± 3 years | All | limited | 7935 | 5 | 3.9 | 1.27 (0.41–2.96) | 0.72 |
| diffuse | 5210 | 2 | 2.7 | 0.75 (0.09–2.73) | 0.99 | ||
| Cenp | limited | 3003 | 0 | 1.6 | 0.00 (0.00–2.28) | 0.40 | |
| diffuse | 212 | 0 | 0.1 | 0.00 (0.00–35.12) | 0.99 | ||
| Topo | limited | 1353 | 2 | 0.6 | 3.42 (0.41–12.34) | 0.23 | |
| diffuse | 1393 | 1 | 0.6 | 1.69 (0.04–9.41) | 0.89 | ||
| Pol III | limited | 305 | 2 | 0.2 | 10.43 (1.26–37.67) | 0.032 | |
| diffuse | 1209 | 2 | 0.7 | 2.80 (0.34–10.11) | 0.32 | ||
| CTP-Negative | limited | 1335 | 0 | 0.5 | 0.00 (0.00–6.88) | 0.99 | |
| diffuse | 808 | 0 | 0.4 | 0.00 (0.00–10.48) | 0.99 |
Statistically significant p-value after adjustment for multiple (10) comparisons per analysis
Figure 3. Risk of lung cancers over time.
In each graph, the x-axis reflects time from scleroderma onset (defined as time zero). Top and middle rows, each time window represents a 6-year period (±3 years); for example, data plotted at time zero reflects lung cancer risk within ±3 years of scleroderma onset. Top row, the observed number of lung cancer cases (blue) is presented in comparison with the number of lung cancer cases that are expected based on SEER data (red). Middle row, the ratio between the observed and expected lung cancer cases is presented as a standardized incidence ratio (SIR) along with its 95% confidence interval. Values of 1 denote a lung cancer risk equivalent to that of the background population. Bottom row, the cumulative incidence of lung cancer among scleroderma patients (solid blue line) starting at 3 years before scleroderma onset is presented with 95% confidence intervals (shaded blue region). Red lines represent the expected cumulative incidence of lung cancer based on SEER data for the general population. Scleroderma patients with pol III antibodies may have an increased risk of lung cancer at the time of scleroderma onset.
Conclusions
In this investigation, we utilized autoantibodies, cutaneous subtype, and temporal clustering as biologically relevant filters to investigate cancer risk and type in scleroderma patients compared to the general population. We made several novel findings that, if confirmed by others, will inform our approach to early cancer detection in scleroderma, and also provide additional insights into mechanistic connections between cancer and scleroderma. First, while patients with scleroderma did not have an increased overall risk of cancer compared to the general population, anti-pol positive patients with diffuse scleroderma and CTP-negative patients with limited scleroderma are at increased risk for cancer at scleroderma onset. Second, scleroderma patients with anti-pol antibodies may have increased risk of different types of cancers depending on whether they have limited or diffuse cutaneous disease. Third, patients with anti-centromere antibodies may have a decreased risk of cancer. Overall these data suggest that autoantibodies could be useful tools for cancer risk stratification to maximize detection of cancer through enhanced screening of high risk groups, while minimizing the harms and costs from overscreening.
Prior studies investigating cancer incidence in patients with scleroderma relative to the general population have utilized study populations from national centralized registries or cohorts similar to ours.1–6,8–10,30 However, most of these studies excluded patients with cancer diagnoses shortly before scleroderma onset, and many lacked data on autoantibody status or phenotypic subtype. In contrast, we studied cancer risk within (±) 3 years of scleroderma onset, and investigated whether this varied by autoantibody specificity, cutaneous subtype, and cancer type. We included the time before scleroderma diagnosis because our recent data demonstrated that POLR3A is genetically altered in short-interval cancers associated with an immune response to that protein, where both mutation-specific and cross-reactive immune responses were seen.13 These data strongly support a biological model in which cancer precedes scleroderma, and initiates a scleroderma immune response and clinical disease.13–14 The fact that 27% of scleroderma patients with anti-pol antibodies and cancer have cancer shortly preceding scleroderma onset highlights the frequency of this subgroup, and the importance to include them. Our current study demonstrates that the risk of cancer around the time of scleroderma onset in anti-pol-positive patients is manyfold higher than that expected in the general population, supporting the idea that these patients may require more aggressive cancer screening at disease onset.
Interestingly, our data suggest that cancer risk may differ among anti-pol patients depending on their cutaneous subtype, as those with diffuse scleroderma had a higher risk of breast cancer and those with limited scleroderma may have an increased risk of lung cancer. These findings, particularly for lung cancer, require validation in other scleroderma cohorts given the small numbers of lung cancer cases in each autoantibody-subtype stratum. While prior studies have identified an increased risk of breast cancer concomitant with scleroderma onset in anti-pol-positive patients, these studies included scleroderma patients without anti-pol as comparator groups, limiting the ability to determine excess risk compared to the general population.15,31 Our data suggest that enhanced breast cancer screening, incorporating sensitive measures such as MRI, may be warranted in anti-pol-positive women with diffuse scleroderma, but this needs further evaluation. Our exploratory analyses, if confirmed in other cohorts, suggest that anti-pol-positive patients may also require increased vigilance in monitoring for lung, tongue and prostate malignancies (Appendix 1).
Our prior work demonstrated that CTP-negative patients may also be at risk of cancer-associated scleroderma.17,20,21 In this study, CTP-negative patients with limited scleroderma had an increased risk of breast cancer and melanoma (Appendix 1) at scleroderma onset, suggesting that vigilance for breast cancer and comprehensive skin examination is most important. Of note, the CTP-negative group is likely heterogeneous, with several novel unrecognized immune responses.17,20,21 Identifying distinct autoantibodies in this subgroup (e.g. anti-RNPC3)20–21 associated with an increased risk of cancer could facilitate development of a cancer risk prediction model in scleroderma.
Our study showed for the first time that scleroderma patients with anti-centromere antibodies may have a substantially decreased risk of cancer compared to the general population. This unexpected finding possibly explains the different cancer risks observed in scleroderma cohorts internationally because the ratios of anti-centromere-positive to anti-pol-positive patients in cohorts dramatically impacts the blended cancer risk. The finding that distinct serologic subgroups have different cancer risks suggests that cancer immunity may be a common principle across the scleroderma spectrum, with cancer emergence influenced by the different immune responses such that for anti-cenp, cancer emergence may be inhibited, while inhibition is only partial for anti-pol. Prior studies in small cohorts of breast cancer patients have demonstrated that anti-centromere antibodies may be present and associate with improved disease-free and overall survival.32–34 Intriguing recent data also suggest that anti-DNA antibodies can have direct anti-cancer effects in cells with DNA repair defects,35 possibly explaining the decreased risk of breast and other cancers among patients with SLE.36 While it is possible that anti-centromere immune responses exert a similar anti-cancer effect in scleroderma, other possibilities exist, and mechanistic studies are needed.
This was a prospective study utilizing a large, well-defined scleroderma cohort to investigate whether scleroderma-specific immune responses and clinical phenotypes associate with a higher risk of certain cancer types. These findings require validation in other scleroderma cohorts given the observational study design and smaller sample sizes in each subgroup as patients are divided into finer classification schemes. Our primary analyses focused on cancers that were detected up to 3 years before the clinical onset of scleroderma, as we were interested in cancer-induced autoimmunity. We recognize that including person time prior to the first visit to our center raises concerns about immortal person time biases due to mortality from cancer diagnosis prior to presentation. To address this, we performed sensitivity analyses only including patients with recent onset scleroderma and examined cancer diagnoses after first visit to our Center. Our primary findings for anti-pol were similar. Our findings in the other autoantibody subsets were attenuated, likely due to decreased statistical power. Several patients were missing sufficient autoantibody data to be classified into a serologic subset; on average, these patients presented for their first visit 3 years before those who could be classified into an autoantibody category, suggesting a period effect due to limited availability of certain commercial autoantibody assays in earlier years. These differences may affect the generalizability of our findings. We do not think surveillance bias plays a major role in our findings, as historically all clinical cancer screening in our Center has been based on age and gender and was not influenced by scleroderma diagnosis or features. However, we recognize that incidental malignancies may be detected during testing performed for scleroderma; conversely, patients with early cancer or scleroderma may face a competing risk of death from either process before diagnosis of the other disease, resulting in an underestimation of cancer cases at the time of scleroderma onset. Stratified analyses suggested that smoking and interstitial lung disease were effect modifiers for lung cancer risk (data not shown). Unfortunately, smoking information was unavailable in the SEER registry, limiting our ability to fully adjust for this risk factor. Lastly, although our prior biologic studies suggest that certain subsets of scleroderma patients may have cancer-induced autoimmunity, we acknowledge that these data do not prove causality. The relationship between cancer and autoimmunity in scleroderma is likely complex and bidirectional, with many potential links between the two diseases including immunosuppressive therapies or damage from the disease triggering malignancy, or a shared genetic or environmental exposure.
These data suggest that segregation by clinical features and autoantibody response identify scleroderma subgroups with distinct risks of both overall cancer, and specific types of cancer. Application of these simple filters may be useful in designing studies that define guidelines for cancer detection in patients with scleroderma. Investigating the mechanistic basis for differences in cancer risk across scleroderma subgroups is likely to enhance our understanding of scleroderma, autoimmunity and cancer immunity.
Supplementary Material
Acknowledgements, Affiliations and Competing Interests
The authors would like to thank Adrianne Woods and Margaret Sampedro for their excellent support in database management and quality control. Takeru Igusa, Livia Casciola-Rosen, Antony Rosen and Ami Shah have recently submitted a patent application entitled “Materials and Methods for Assessing Cancer Risk and Treating Cancer.”
Financial support information
This study was supported by the NIH (K23-AR061439, P30-AR053503, P30-AR070254, R01-DE12354–15A1, T32-AR048522), the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Stauralakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Scleroderma Research Foundation.
References
- 1.Abu-Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis rheum 1993;36(4):460–4. [published Online First: 1993/04/01] [DOI] [PubMed] [Google Scholar]
- 2.Derk CT, Rasheed M, Artlett CM, et al. A cohort study of cancer incidence in systemic sclerosis. J Rheumatol 2006;33(6):1113–6. [published Online First: 2006/04/20] [PubMed] [Google Scholar]
- 3.Hill CL, Nguyen AM, Roder D, et al. Risk of cancer in patients with scleroderma: a population based cohort study. Ann Rheum Dis 2003;62(8):728–31. [published Online First: 2003/07/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang KY, Yim HW, Kim IJ, et al. Incidence of cancer among patients with systemic sclerosis in Korea: results from a single centre. Scand J Rheumatol 2009;38(4):299–303. doi: 10.1080/03009740802642062 [DOI] [PubMed] [Google Scholar]
- 5.Kuo CF, Luo SF, Yu KH, et al. Cancer risk among patients with systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol 2012;41(1):44–9. doi: 10.3109/03009742.2011.618145 [published Online First: 2011/12/14] [DOI] [PubMed] [Google Scholar]
- 6.Olesen AB, Svaerke C, Farkas DK, et al. Systemic sclerosis and the risk of cancer: a nationwide population-based cohort study. Br J Dermatol 2010;163(4):800–6. doi: 10.1111/j.1365-2133.2010.09861.x [published Online First: 2010/09/22] [DOI] [PubMed] [Google Scholar]
- 7.Onishi A, Sugiyama D, Kumagai S, et al. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum 2013;65(7):1913–21. doi: 10.1002/art.37969 [published Online First: 2013/04/12] [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal AK, McLaughlin JK, Gridley G, et al. Incidence of cancer among patients with systemic sclerosis. Cancer 1995;76(5):910–4. [published Online First: 1995/09/01] [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal AK, McLaughlin JK, Linet MS, et al. Scleroderma and malignancy: an epidemiological study. Ann Rheum Dis 1993;52(7):531–3. [published Online First: 1993/07/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siau K, Laversuch CJ, Creamer P, et al. Malignancy in scleroderma patients from south west England: a population-based cohort study. Rheumatology Int 2011;31(5):641–5. doi: 10.1007/s00296-009-1348-y [published Online First: 2010/01/09] [DOI] [PubMed] [Google Scholar]
- 11.Bonifazi M, Tramacere I, Pomponio G, et al. Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies. Rheumatology 2013;52(1):143–54. doi: 10.1093/rheumatology/kes303 [published Online First: 2012/11/24] [DOI] [PubMed] [Google Scholar]
- 12.Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum 2010;62(9):2787–95. doi: 10.1002/art.27549 [published Online First: 2010/05/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014;343(6167):152–7. doi: 10.1126/science.1246886 [published Online First: 2013/12/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AA, Casciola-Rosen L, Rosen A. Review: cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol 2015;67(2):317–26. doi: 10.1002/art.38928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moinzadeh P, Fonseca C, Hellmich M, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther 2014;16(1):R53. doi: 10.1186/ar4486 [published Online First: 2014/02/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikpour M, Hissaria P, Byron J, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther 2011;13(6):R211. doi: 10.1186/ar3544 [published Online First: 2011/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah AA, Hummers LK, Casciola-Rosen L, et al. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis Rheumatol 2015;67(4):1053–61. doi: 10.1002/art.39022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Airo P, Ceribelli A, Cavazzana I, et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J Rheumatol 2011;38(7):1329–34. doi: 10.3899/jrheum.101144 [published Online First: 2011/04/05] [DOI] [PubMed] [Google Scholar]
- 19.Saigusa R, Asano Y, Nakamura K, et al. Association of anti-RNA polymerase III antibody and malignancy in Japanese patients with systemic sclerosis. J Dermatol 2015;42(5):524–7. doi: 10.1111/1346-8138.12827 [DOI] [PubMed] [Google Scholar]
- 20.Shah AA, Xu G, Rosen A, et al. Brief Report: Anti-RNPC-3 Antibodies As a Marker of Cancer-Associated Scleroderma. Arthritis Rheumatol 2017;69(6):1306–12. doi: 10.1002/art.40065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu GJ, Shah AA, Li MZ, et al. Systematic autoantigen analysis identifies a distinct subtype of scleroderma with coincident cancer. Proc Natl Acad Sci U S A 2016;113(47):E7526–E34. doi: 10.1073/pnas.1615990113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa M, Sato S, Sakai H, et al. Systemic sclerosis revealing T-cell lymphoma. Dermatology 1999;198(1):75–8. doi: 18070 [published Online First: 1999/02/23] [DOI] [PubMed] [Google Scholar]
- 23.Juarez M, Marshall R, Denton C, et al. Paraneoplastic scleroderma secondary to hairy cell leukaemia successfully treated with cladribine. Rheumatology 2008;47(11):1734–5. doi: 10.1093/rheumatology/ken367 [published Online First: 2008/09/25] [DOI] [PubMed] [Google Scholar]
- 24.Bruni C, Lages A, Patel H, et al. Resolution of paraneoplastic PM/Scl-positive systemic sclerosis after curative resection of a pancreatic tumour. Rheumatology 2017;56(2):317–18. doi: 10.1093/rheumatology/kew382 [DOI] [PubMed] [Google Scholar]
- 25.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23(5):581–90. [published Online First: 1980/05/01] [DOI] [PubMed] [Google Scholar]
- 26.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65(11):2737–47. doi: 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15(2):202–5. [published Online First: 1988/02/01] [PubMed] [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results (SEER) Program (https://www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2016 Sub (1973–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
- 29.Liddell FD. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health 1984;38(1):85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S, Dombi GW, Severson RK, et al. Risk of malignancy in scleroderma: a population-based cohort study. Arthritis Rheum 2005;52(8):2415–24. doi: 10.1002/art.21225 [published Online First: 2005/07/30] [DOI] [PubMed] [Google Scholar]
- 31.Lazzaroni MG, Cavazzana I, Colombo E, et al. Malignancies in Patients with Anti-RNA Polymerase III Antibodies and Systemic Sclerosis: Analysis of the EULAR Scleroderma Trials and Research Cohort and Possible Recommendations for Screening. J Rheumatol 2017;44(5):639–47. doi: 10.3899/jrheum.160817 [DOI] [PubMed] [Google Scholar]
- 32.Atalay C, Atalay G, Yilmaz KB, et al. The role of anti-CENP-B and anti-SS-B antibodies in breast cancer. Neoplasma 2005;52(1):32–5. [PubMed] [Google Scholar]
- 33.Atalay C, Dogan L, Atalay G. Anti-CENP-B antibodies are associated with prolonged survival in breast cancer. Future Oncol 2010;6(3):471–7. doi: 10.2217/fon.10.6 [DOI] [PubMed] [Google Scholar]
- 34.Fritzler MJ, Rattner JB, Luft LM, et al. Historical perspectives on the discovery and elucidation of autoantibodies to centromere proteins (CENP) and the emerging importance of antibodies to CENP-F. Autoimmun Rev 2011;10(4):194–200. doi: 10.1016/j.autrev.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 35.Hansen JE, Chan G, Liu Y, et al. Targeting cancer with a lupus autoantibody. Sci Transl Med 2012;4(157):157ra42. doi: 10.1126/scitranslmed.3004385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernatsky S, Ramsey-Goldman R, Labrecque J, et al. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun 2013;42:130–5. doi: 10.1016/j.jaut.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.