Abstract
Primary breast diffuse large B‐cell lymphoma (PB‐DLBCL) is a rare subtype of DLBCL with limited data on patterns of failure. This multicenter study aimed to define the optimum treatment strategy and patterns of failure for PB‐DLBCL patients. We retrospectively reviewed data on 108 PB‐DLBCL patients from 21 Chinese medical centers. Only patients with localized disease (involvement of breast and localized lymph nodes) were included. After a median follow‐up of 3.2 years, 32% of patients developed progression or relapse. A continuous pattern of relapse was observed, characterized by frequent late relapses in the contralateral breast and central nervous system (CNS). Although rituximab significantly reduced the overall cumulative risk of progression or relapse (5‐year cumulative risk 57% vs 24%, P = .029), it had limited effect on the reduction of breast relapse (P = .46). Consolidative radiotherapy significantly decreased the risk of breast relapse, even in the subgroup of patients treated with rituximab (5‐year cumulative risk 21.2% vs 0%, P = .012). A continuous risk of CNS progression or relapse up to 8.2 years from diagnosis was observed (10‐year cumulative risk 28.3%), with a median time to CNS relapse of 3.1 years. Neither rituximab nor prophylactic intrathecal chemotherapy significantly decreased the risk of CNS relapse. In summary, our study indicates that PB‐DLBCL has a continuous pattern of relapse, especially with frequent late relapses in the CNS and contralateral breast. Rituximab and RT confer complementary benefit in the reduction of relapse. However, neither the addition of rituximab nor prophylactic intrathecal chemotherapy could effectively prevent CNS relapse for PB‐DLBCL patients.
Keywords: breast, diffuse large B‐cell lymphoma, radiotherapy, relapse, rituximab
Abbreviations
- CHOP
cyclophosphamide, doxorubicin, vincristine, and prednisone
- CI
confidence interval
- CNS
central nervous system
- CT
chemotherapy
- DLBCL
diffuse large B‐cell lymphoma
- GCB
germinal center B‐cell like
- IPI
International Prognostic Index
- IT
intrathecal chemotherapy
- NHL
non‐Hodgkin's lymphoma
- OS
overall survival
- PB‐DLBCL
primary breast diffuse large B‐cell lymphoma
- PFS
progression‐free survival
- R‐CHOP
rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- RT
radiation therapy
1. INTRODUCTION
Primary breast lymphoma is a rare subtype of NHL representing an estimated 1% of all NHL and less than 3% of extranodal lymphomas.1, 2, 3 Its definition was first proposed in 1972 by Wiseman and Liao as a malignant lymphoma involving predominantly the breast with or without ipsilateral locoregional lymph nodes.4 By this definition, most primary breast lymphoma patients are classified as stage I or II based on the Ann Arbor Staging System, with the exception of bilateral breast lymphoma, which is considered as stage IV by some investigators.5 The most common histology is DLBCL, although diverse lymphoma subtypes have been reported.1, 2, 3
As a rare subentity of DLBCL, PB‐DLBCL has been shown to display characteristic patterns of relapse distinct from those of nodal DLBCL.5 Particularly, a high frequency of extranodal relapses in the breast and CNS has been reported in previous studies.6, 7, 8, 9, 10 However, due to the rarity of this disease, the current published reports on PB‐DLBCL comprise predominantly small retrospective series, with only a few large cohort studies reported so far. Thus, it is unsurprising that the patterns of relapse reported for PB‐DLBCL are not consistent across all studies. One major area of controversy is the risk of CNS relapse, which ranges widely from 5% to 19% in the published work.6, 7, 8, 9, 10 As a result, there is currently no consensus among clinicians with respect to the routine use of prophylactic CNS therapy in PB‐DLBCL patients.
The incorporation of rituximab into CT has led to a paradigm shift in the treatment of DLBCL, with a number of phase III studies reporting significant survival benefit of R‐CHOP vs CHOP as first‐line therapy of DLBCL patients.11, 12 This benefit from rituximab has also challenged the value of consolidative RT in the treatment of limited‐stage DLBCL, as results from one randomized controlled trial have shown that consolidative RT could not offer additional benefit for early stage DLBCL patients treated in the rituximab era.13 However, in the case of PB‐DLBCL, only a few studies have evaluated the role of rituximab, and most of them did not show an advantage in PFS or OS.7, 14, 15 Given this uncertain benefit of rituximab, whether consolidative RT is beneficial for PB‐DLBCL patients who have been treated with rituximab‐containing regimens remains unclear.
At present, most reports of PB‐DLBCL were from Western countries or regions, with a scarcity of data reported for Chinese patients. In light of the unresolved clinical issues mentioned above and the lack of large PB‐DLBCL series reported from China, a multicentric collaborative effort was initiated by the Leukemia and Lymphoma Committee of the Chinese Geriatric Oncology Society, in order to further elucidate the patterns of progression or relapse and the impact of different treatments on the risk of relapse in PB‐DLBCL patients.
2. MATERIALS AND METHODS
Data were collected on patients with primary breast DLBCL diagnosed between January 2000 and December 2015 from 21 Chinese medical centers. Institutional review board approval was obtained at each site. Eligibility criteria required confirmed a pathological diagnosis of DLBCL according to the 2001 or 2008 WHO classification of lymphoid neoplasms and disease localized to one or both breasts with or without ipsilateral regional (axillary and/or supraclavicular) lymph node involvement. Patients with systemic disease with breast involvement or transformed DLBCL from low‐grade lymphoma and patients who did not receive any treatment after diagnosis were excluded. A study‐specific data collection form was completed for each case. Collected data included patient and tumor characteristics, results of diagnostic tests, staging workup, treatments, time and sites of disease progression or relapse, and clinical outcomes. Available immunohistochemistry data obtained from the diagnostic specimens were reported from each of the participating centers. Staging workup included computed tomography scans of the neck, chest, abdomen, and pelvis and bone marrow smear and biopsy. In addition, whole‐body PET computed tomography, cerebrospinal fluid cytology test, and brain MRI were carried out in 18.5%, 22%, and 3% of patients at diagnosis, respectively. Patients were staged according to the Ann Arbor system.16 Patients with bilateral breast involvement were classified as stage IV in this study. The IPI was determined using all the available information.
Time to progression or relapse was measured from the date of diagnosis to the date of first progression or relapse. Progression‐free survival was measured from the date of diagnosis until the date of disease progression or death from any cause. Overall survival was measured from the date of diagnosis to the date of death or last follow‐up. The median follow‐up time was calculated by the reverse Kaplan‐Meier method.17 Cumulative incidence of progression or relapse at each site (eg nodal sites, breast, and CNS) was calculated by competing risk analysis using Fine and Gray's proportional hazard model.18 In this analysis, death without the prespecified site of relapse was defined as the competing risk. Both PFS and OS were estimated according to the Kaplan‐Meier method. Log‐rank test and Cox regression methods were used to analyze time‐to‐event data. A two‐tailed P value <0.05 was considered statistically significant. Analyses were carried out using the SPSS 19.0 package (IBM, Chicago, IL, USA) and the R language version 3.4.3.
3. RESULTS
3.1. Patients’ characteristics
One‐hundred and eight patients who met the eligibility criteria were included. The baseline characteristics are shown in Table 1. All patients were female, with a median age of 47 years (range, 16‐85 years). The most common presentation was an ipsilateral breast painless palpable mass, with right breast involvement more common than left (59% vs 35%). Bilateral breast involvement was present in 5.6% of patients at diagnosis. The median tumor size was 3.7 cm (range, 1.2‐12.8 cm), and regional nodal involvement was present in 39% of patients. Among the 91 patients with available information on IPI, 78 patients (86%) had a low IPI score of 0 or 1, with 48 patients (53%) in the very good risk group (IPI 0). Among the 89 patients in whom immunohistochemistry was available, CD10 was positive in 31%, BCL6 was positive in 74%, and MUM1 was positive in 82%. Fifty‐nine patients (66%) were classified as non‐GCB phenotype and 30 patients (34%) as GCB type according to the algorithms described by Hans et al.19 CD5 was positive in 9 patients (10%), and the median Ki‐67 index was 80%.
Table 1.
Clinical characteristics of 108 Chinese patients with primary breast diffuse large B‐cell lymphoma, at presentation
| Parameters | No. (%) | |
|---|---|---|
| Age | Median | 47 |
| Range | 16‐85 | |
| Sex | Female | 108 (100) |
| ECOG‐PS | 0 | 76 (70.4) |
| 1 | 26 (24.1) | |
| 2 | 3 (2.8) | |
| Unknown | 3 (2.8) | |
| Presence of B symptoms | 5 (4.6) | |
| Primary site of lymphoma | Left breast | 38 (35.2) |
| Right breast | 64 (59.3) | |
| Bilateral | 6 (5.6) | |
| Tumor size | Median | 3.7 cm |
| Range | 1.2‐12.8 cm | |
| Nodal sites involvement | None | 66 (61.1) |
| Axillary only | 32 (29.6) | |
| Supraclavicular ± axillary | 10 (9.3) | |
| Ann Arbor stage | IE | 62 (57.4) |
| IIE | 40 (37.0) | |
| IV | 6 (5.6) | |
| LDH | Normal | 74 (68.5) |
| Elevated | 18 (16.7) | |
| Unknown | 16 (14.8) | |
| IPI | 0 | 48 (44.4) |
| 1 | 30 (27.8) | |
| 2 | 11 (10.2) | |
| 3 | 2 (1.9) | |
| Unknown | 17 (15.7) | |
IPI, International Prognostic Index; LDH, lactate dehydrogenase; PS, performance status.
3.2. Treatment
The front‐line therapy is summarized in Table 2. All patients received anthracycline‐containing CT, with CHOP or CHOP‐like regimens being the most common (77%). A minority of patients (23%) with high‐risk features (eg IPI ≥2, activated B‐cell type) were treated with cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone, or etoposide, doxorubicin, and vincristine by 96‐hour continuous infusion, cyclophosphamide, and prednisone. Epidoxorubicin or liposomal doxorubicin was used instead of doxorubicin in some patients. The median number of cycles of chemotherapy was 6 (range, 1‐8), with 92% of patients receiving at least 4 cycles of chemotherapy. Rituximab was routinely recommended to all PB‐DLBCL patients in this study. However, due to patients’ varying economic status (eg a proportion of patients with low income could not afford rituximab), only 61% of cases received rituximab in addition to chemotherapy. Consolidative radiation therapy to the involved breast with or without ipsilateral regional nodes was used in 36% of patients with a median dose of 45 Gy (range, 30‐50 Gy), including 2 patients who also received additional prophylactic RT to the contralateral breast. Prophylactic CNS CT was given to 48 (44%) patients; 44 patients received IT and the other 4 patients received systemic high‐dose methotrexate in combination with IT. Of the 6 patients with stage IV disease, 3 received prophylactic IT. Regimens for IT consisted of methotrexate 10‐15 mg plus dexamethasone 10 mg, or cytarabine 50 mg plus dexamethasone 10 mg. The median number of doses of IT was 4 (range, 1‐8). There was no significant difference in baseline clinical characteristics between patients treated with and without rituximab (Table S1) or between patients treated with and without prophylactic IT (Table S2). Mastectomy was carried out in 19% of patients.
Table 2.
Primary therapy of Chinese patients with primary breast diffuse large B‐cell lymphoma (n = 108)
| N. | % | ||
|---|---|---|---|
| Chemotherapy | CHOP | 83 | 76.9 |
| CHOEP | 14 | 13.0 | |
| EPOCH | 10 | 9.3 | |
| BACOP | 1 | 0.9 | |
| Rituximab | Yes | 66 | 61.1 |
| No | 42 | 38.9 | |
| RT | Yes | 39 | 36.1 |
| No | 69 | 63.9 | |
| Mastectomya | Yes | 21 | 19.4 |
| No | 87 | 80.6 | |
| CNS prophylaxis | IT only | 44 | 40.0 |
| IT+HD MTX | 4 | 4.4 | |
| None | 60 | 55.6 |
Including modified/radical mastectomy and simple mastectomy, excluding lumpectomy or excisional biopsy.
BACOP, bleomycin, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CNS, central nervous system; EPOCH, etoposide, doxorubicin, and vincristine (96‐hour continuous infusion), cyclophosphamide, and prednisone; HD MTX, high‐dose methotrexate; IT, intrathecal chemotherapy; RT, radiotherapy.
3.3. Patterns of progression or relapse
At a median follow‐up of 3.2 years (range, 0.1‐11.5 years), 35 (32%) patients developed disease progression or relapse, with 18 patients having 2 or more sites of involvement at first relapse. The median time to first progression or relapse was 1.5 years (range, 0.2‐8.2 years). The time to first progression or relapse was more than 3 years in 10 patients and more than 5 years in 6 patients. The estimated 2‐, 5‐, and 10‐year cumulative risks of all progression or relapse were 20.0%, 37.0%, and 61.6%, respectively (Figure 1A). Information regarding relapse sites is shown in Table 3. The most common site of first progression or relapse was the lymph nodes (43%), followed by the breast (41%) and CNS (24%). Extranodal relapse (n = 25) was more common than relapse confined to only nodal sites (n = 10). There was no significant difference in the cumulative risk of nodal relapse between patients with and without nodal involvement at initial diagnosis (5‐year cumulative risk of nodal relapse, 15.3% vs 21.2%, P = .69; Figure S1A).
Figure 1.
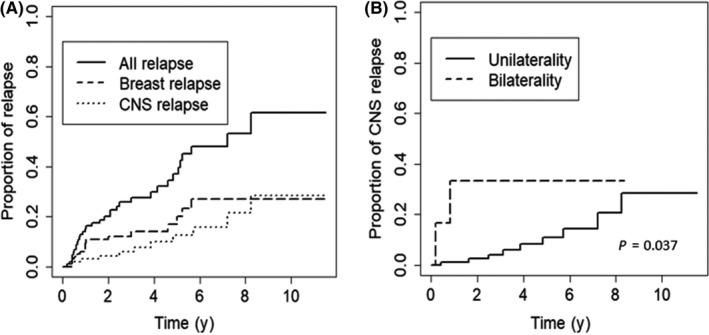
Risk of relapse among 108 Chinese women with primary breast diffuse large B‐cell lymphoma. A, Cumulative risk of relapse at all sites, breast, and central nervous system (CNS). B, Cumulative risk of CNS relapse in patients with unilateral and bilateral breast involvement at initial diagnosis
Table 3.
Anatomic sites of first relapse in 35 Chinese patients with primary breast diffuse large B‐cell lymphoma
| Relapse sites | Overall | Relapse ≤3 years from diagnosis | Relapse >3 years from diagnosis |
|---|---|---|---|
| Patients | 35 | 25 | 10 |
| Extranodal relapse | 25 | 17 | 8 |
| Total CNS relapse | 8 | 4 | 4 |
| Brain relapse | 6 | 2 | 4 |
| Leptomeningeal relapse | 1 | 1 | 0 |
| Unknown | 1 | 1 | 0 |
| Total breast relapse | 14 | 10 | 4 |
| Ipsilateral breast relapse | 8 | 8 | 0 |
| Contralateral breast relapse | 6 | 2 | 4 |
| Extranodal sites other than CNS and breast | 7 | 7 | 0 |
| Bone marrow | 2 | 2 | 0 |
| Bone | 2 | 2 | 0 |
| Other sitesa | 3 | 3 | 0 |
| Nodal relapseb | 15 | 12 | 3 |
| Regional nodal relapse | 8 | 6 | 2 |
| Distant nodal relapse | 13 | 10 | 3 |
Including kidney, adnexa of uterus, and soft tissue.
Nodal‐only relapse was observed in 10 patients.
CNS, central nervous system.
Breast progression or relapse was observed in a total of 16 patients, including 2 patients with breast relapses occurring during salvage therapy. The estimated 5‐ and 10‐year cumulative risks of all breast progression or relapse were 20.0% and 26.9%, respectively (Figure 1A). All ipsilateral breast relapses occurred within the first 3 years following diagnosis, whereas contralateral breast relapses tended to occur later with a median time to relapse of 4.6 years (range, 0.4‐5.6 years).
Central nervous system progression or relapse developed in a total of 11 patients, including 3 patients whose CNS relapse occurred during salvage treatment. The median time to CNS progression or relapse was 3.1 years (range, 0.2‐8.2 years). The estimated 2‐year, 5‐year, and 10‐year cumulative risks of all CNS progression or relapse were 4.3%, 12.5%, and 28.3%, respectively (Figure 1A). Central nervous system relapse was reported in 8 stage I patients, 1 stage II, and 2 stage IV patients. Brain parenchymal relapse was observed in 8 patients, leptomeningeal relapse in 1 patient, and the specific sites of CNS relapse in the other 2 patients were unknown. Six patients developed isolated CNS relapse without concurrent systemic disease, whereas the remaining 5 patients had CNS disease accompanied by relapse or progression in other sites. Bilateral breast involvement at initial diagnosis was associated with a significantly increased risk of early CNS progression (1‐year cumulative risk 33.3% vs 1.0%, P = .037; Figure 1B), whereas neither regional nodal involvement nor tumor size larger than 5 cm was associated with a higher risk of CNS relapse (Figure S1B,C). Notably, of the 9 patients with CD5 positivity, only 1 patient developed CNS relapse.
When patterns of progression or relapse were analyzed according to the cell‐of‐origin classification, there was no significant difference in the cumulative risk of overall progression or relapse or site‐specific progression or relapse (eg nodal, breast, and CNS relapse) between GCB and non‐GCB subtypes, although a trend towards higher risk of nodal relapse was observed for the non‐GCB subtype (P = .057; Figure S2). When relapse sites were analyzed according to the time to relapse, there is a major difference in the distribution of relapse sites between early relapse and late relapse (Table 3). In the patients (n = 25) whose first relapse occurred within 3 years from initial diagnosis, the most common sites of relapse were the lymph nodes and ipsilateral breast. In the patients (n = 10) whose first relapse occurred more than 3 years from diagnosis, the predominant sites of relapse were the contralateral breast and CNS.
3.4. Impact of treatment methods on patterns of progression or relapse
3.4.1. Rituximab
The addition of rituximab significantly reduced the overall cumulative risk of progression or relapse in patients with PB‐DLBCL (P = .029; Figure 2A). The estimated 5‐year cumulative risk of progression or relapse in patients treated with rituximab and without rituximab was 24% and 57%, respectively. In relapse site‐specific analyses, rituximab was associated with significantly decreased risk of nodal relapse (5‐year cumulative risk, 35.5% vs 10.0%, P = .012; Figure 2B) but not a significant reduction in the risk of extranodal relapse (P = .117; Figure 2C). In particular, rituximab did not mitigate the cumulative risk of relapse in the breast (P = .46; Figure 2D) or in the CNS (P = .72; Figure 2E).
Figure 2.
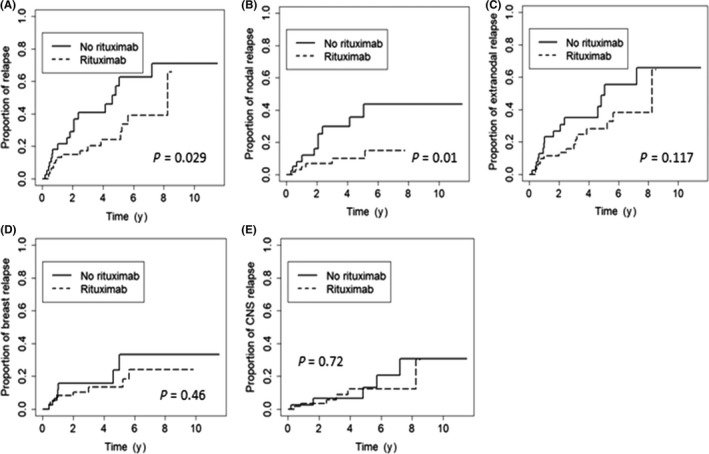
Risk of relapse among 108 Chinese women with primary breast diffuse large B‐cell lymphoma. Cumulative risk at all sites (A), nodal sites (B), extranodal sites (C), breast (D), and CNS (E) according to the use of rituximab
3.4.2. Consolidative RT
Consolidative RT was associated with a significant reduction in the overall cumulative risk of relapse (P = .042; Figure 3A). In site‐specific analyses, RT significantly reduced the cumulative risk of relapse in both the ipsilateral and the contralateral breast (5‐year cumulative risk, 32.7% vs 0.0%, P < .001; Figure 3B). Notably, in the subgroup of patients treated with rituximab, RT was still associated with a significant reduction in the risk of breast relapse (5‐year cumulative risk, 21.2% vs 0.0%, P = .012; Figure 3C). No significant associations between RT and the risk of nodal relapse (P = .146) or CNS relapse (P = .714) were observed (Figure S3). Secondary malignancy was observed in 1 patient receiving consolidative RT who developed breast carcinoma with squamous and chondroid metaplasia on the involved side of breast 5 years later.
Figure 3.
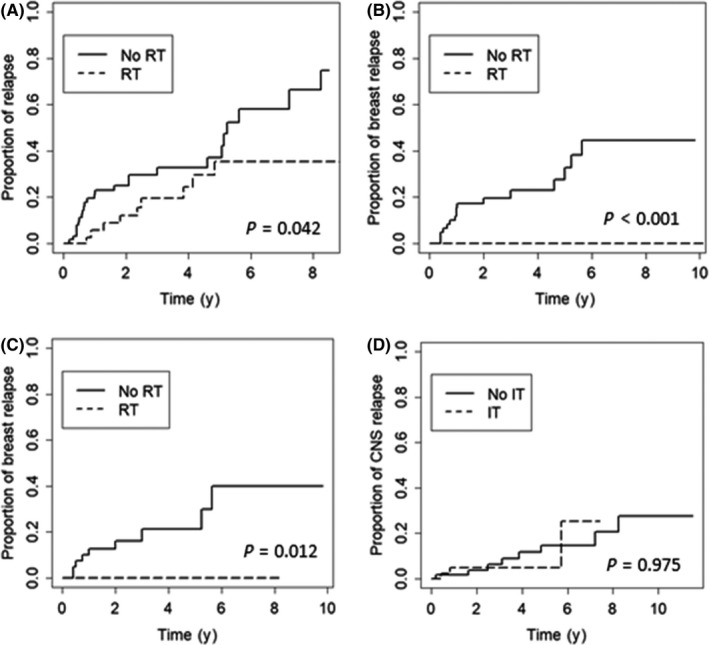
A, Cumulative risk of relapse at all sites in 108 Chinese women with primary breast diffuse large B‐cell lymphoma, according to the use of consolidative radiotherapy (RT). B,C, Cumulative risk of breast relapse according to the use of consolidative RT in the entire cohort (B) and in the subgroup of patients treated with rituximab (C). D, Cumulative risk of central nervous system (CNS) relapse according to the use of prophylactic intrathecal chemotherapy (IT)
3.4.3. Prophylactic IT
Overall, CNS progression or relapse occurred in 3 of 48 patients who received prophylactic IT and 8 of 60 patients without IT prophylaxis, with no significant difference in the rates of relapse between the two groups (6.3% vs 13.3%, P = .374). In the univariate analysis, prophylactic IT was not associated with a significant reduction in the cumulative risk of CNS relapse (P = .975; Figure 3D).
3.4.4. Mastectomy
Mastectomy was not associated with a significant reduction in the overall cumulative risk of progression or relapse (P = .48) compared with biopsy or lumpectomy only, nor did mastectomy decrease the risks of relapse in the breast (P = .94) or CNS (P = .21) (Figure S4).
3.5. Survival and prognostic factors
The median PFS for the entire cohort was 6.3 years (95% CI, 4.2‐8.4 years), and the median OS was not reached. The 5‐year PFS and 5‐year OS were 61.2% (95% CI, 49.0‐73.4%) and 77.3% (95% CI, 66.1‐88.5%), respectively (Figure 4).
Figure 4.
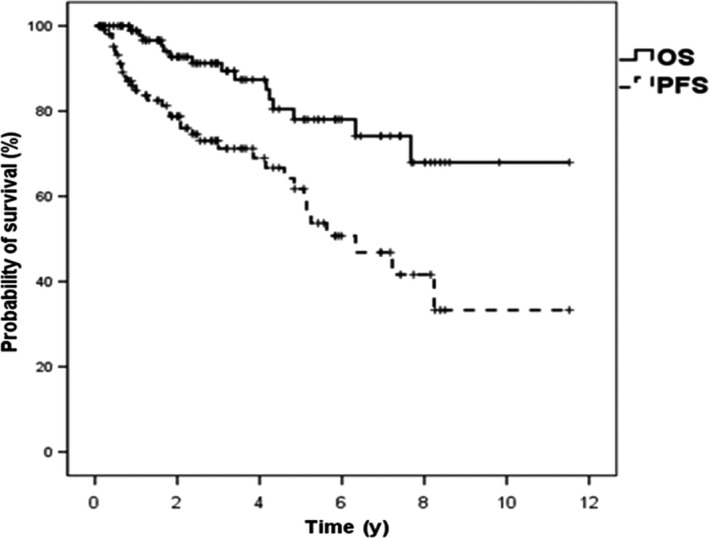
Progression‐free survival (PFS) and overall survival (OS) among Chinese women with primary breast diffuse large B‐cell lymphoma (n = 108)
Results of prognostic factor analyses are detailed in Table 4. In multivariate analyses, IPI, rituximab, and RT were significantly associated with PFS. International Prognostic Index was the only significant prognostic factor associated with OS. Regional nodal involvement and tumor size larger than 5 cm were not associated with significant changes in PFS or OS.
Table 4.
Multivariate analyses of predictive factors for progression‐free survival (PFS) and overall survival (OS) in Chinese patients with primary breast diffuse large B‐cell lymphoma
| Factor | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| IPI | 2.06 | 1.18‐3.60 | .012 | 4.52 | 1.64‐12.79 | .008 |
| Regional nodal involvement | 1.23 | 0.50‐3.07 | .655 | 0.57 | 0.09‐3.66 | .550 |
| Rituximab | 0.33 | 0.12‐0.92 | .028 | 1.04 | 0.19‐5.72 | .969 |
| RT | 0.29 | 0.10‐0.80 | .034 | 0.50 | 0.09‐2.88 | .439 |
| IT | 0.71 | 0.27‐1.91 | .502 | 0.41 | 0.04‐3.94 | .439 |
| Mastectomy | 0.45 | 0.15‐1.30 | .141 | 0.44 | 0.07‐2.83 | .389 |
Bold values indicate statistically significant factors.
CI, confidence interval; HR, hazard ratio; IPI, International Prognostic Index; IT, intrathecal chemotherapy; RT, radiotherapy.
4. DISCUSSION
As the largest case series of PB‐DLBCL in the rituximab era to date, our study revealed a continuous pattern of relapse for PB‐DLBCL, which is largely attributable to frequent late relapses in the CNS and contralateral breast. In addition, our study revealed for the first time the differential effect of rituximab on nodal and extranodal relapse and the complementary benefit of rituximab and RT in the management of PB‐DLBCL. The underlying biology and clinical implications of these findings are discussed below.
The correlation between pathological characteristics and patterns of failure in PB‐DLBCL has not been extensively studied. Previous reports have shown the predominance of non‐GCB type in PB‐DLBCL, yet reported treatment outcomes were similar between the GCB and non‐GCB patients.20, 21 Consistent with these reports, our analysis found no significant difference in patterns of failure between GCB and non‐GCB subgroups, suggesting that cell‐of‐origin classification has limited prognostic value for PB‐DLBCL. CD5 positivity was observed in 10% of our cases, similar to the prevalence of CD5 positivity reported in the general DLBCL population (5%‐10%).22 Furthermore, the incidence of CNS relapse was similar between the CD5‐positive cases and the remainder of the cohort, indicating that CD5 positivity might not be the main cause of the high CNS relapse rate in PB‐DLBCL.
The benefit of rituximab in the treatment of PB‐DLBCL has been controversial. Two multicenter studies found no significant improvement in PFS or OS from the addition of rituximab,7, 14 whereas another small retrospective series reported significantly better 5‐year OS for PB‐DLBCL patients treated with R‐CHOP vs CHOP alone.23 Interestingly, our analysis revealed a differential effect of rituximab against nodal and extranodal (mainly breast and CNS) relapse. Whereas rituximab significantly decreased the risk of systemic nodal relapse, it showed limited benefit against breast and CNS relapse. A possible reason why rituximab could not effectively reduce CNS relapse might be related to its poor penetration across the blood‐brain barrier. Previous pharmacokinetic studies revealed that levels of rituximab in the cerebrospinal fluid are only 0.1% of matched serum levels following an i.v. dose.24 In published clinical studies, the impact of rituximab on CNS relapse of DLBCL was also controversial.25, 26 Villa et al26 suggested that the beneficial effect of rituximab against CNS relapse might be through superior eradication of systemic disease, which secondarily contributes to a reduction of late CNS relapse, rather than a direct CNS prophylactic effect. As none of our patients had systemic disease at initial diagnosis, and the majority of patients (6 of 11) developed isolated CNS relapse with no concurrent systemic disease, the CNS prophylactic effect of rituximab in this setting might be limited. In terms of breast relapse, there is currently no evidence of a “blood‐breast barrier” restricting the passage of i.v. rituximab into the breast tissue. However, as breast relapse can be considered as “local relapse” in PB‐DLBCL, this observation suggests systemic immunochemotherapy alone might be insufficient to achieve optimal local tumor control. Given these considerations, additional therapies to reduce local breast relapse and CNS relapse should be considered in the management of PB‐DLBCL patients who received rituximab‐containing regimens.
The benefit of consolidative RT for the treatment of PB‐DLBCL has been confirmed by randomized controlled trial in the prerituximab era,8 yet the role of RT for PB‐DLBCL patients receiving rituximab‐based regimens remains largely unknown. Our study showed that consolidative RT significantly reduced the risk of breast relapse, even in those who had received rituximab, suggesting the beneficial effect of RT in PB‐DLBCL patients treated with rituximab‐containing regimens. This finding is in line with the abovementioned assumption that immunochemotherapy alone is insufficient to achieve optimal local tumor control, and suggests a complementary benefit between rituximab and RT in the management of PB‐DLBCL. However, our finding seems to be at odds with results of a recently published phase III study that reported no significant difference in event‐free survival and OS between limited‐stage DLBCL patients treated with R‐CHOP with and without RT.13 This discrepancy could be explained by the different patterns of relapse between PB‐DLBCL and DLBCL in general. Relapses in this phase III study were predominantly systemic, and the incidence of local relapse (1.5%) was much lower compared with that of our cohort. Therefore, the high incidence of local breast relapse and the limited effect of rituximab on the reduction of breast recurrence might contribute to the benefit of RT observed in our PB‐DLBCL patients. Another interesting observation is that RT to the involved breast seemed to reduce the risk of contralateral relapse, given that only a few cases received prophylactic RT to contralateral breast in our study. This finding suggests that the risk of contralateral breast relapse is to a large extent associated with local tumor control. Studies into the lymphatic system of breast have revealed the existence of lymphatic channels cross‐linking bilateral breast, which might allow direct lymphatic drainage from one breast to the other.27 Therefore, it is reasonable to postulate that by improving local tumor control, consolidative RT could decrease the likelihood of late lymphatic metastasis of residue disease to the contralateral breast. Although these hypotheses should be regarded as only suggestive due to the lack of sound evidence, they are worthy of further exploration.
The risk of CNS relapse in PB‐DLBCL remains controversial with the reported rates of CNS relapse ranging from 5% to 19.6% (see Table S3).1, 6, 7, 8, 9, 10, 14 In our study, we observed a rate of CNS relapse of 10% with an estimated 10‐year cumulative risk of 28.3%, which is comparable to that of primary testicular DLBCL.28, 29 Notably, in contrast to the general observation that CNS relapse in DLBCL occurred early (median, 6.7‐8.1 months),30, 31, 32, 33 the PB‐DLBCL patients in our study showed a persistent risk of CNS relapse occurring up to 8.2 years after initial diagnosis (median, 3.1 years). In addition, our study showed for the first time that synchronous bilateral breast involvement was significantly associated with a higher risk of early CNS progression compared with patients with unilateral breast disease. However, it is noteworthy that, although patients with unilateral breast lymphoma had a low incidence of CNS progression in the first few years, this risk accumulated gradually, reaching up to 28% at 10 years, suggesting that CNS relapse is still a major cause of treatment failure for these patients. Based on these findings, more effective strategies for CNS prophylaxis against PB‐DLBCL need to be explored.
The optimum strategy of CNS prophylaxis in PB‐DLBCL has not been established. Rituximab seems to be ineffective against CNS relapse, as discussed above. Furthermore, we found no significant difference in the risk of CNS relapse between patients with and without IT prophylaxis. This finding is in line with several other studies of DLBCL that suggested the inadequate efficacy of intrathecal prophylactic chemotherapy in preventing CNS disease.30, 32, 33 As brain parenchymal relapse was more common than leptomeningeal relapse in PB‐DLBCL, a more promising option would be systemic CNS chemotherapy prophylaxis such as high‐dose i.v. methotrexate. However, because only 4 patients received high‐dose i.v. methotrexate in our cohort, the efficacy of this approach could not be determined.
As the largest study of PB‐DLBCL in a Chinese population, our cohort shares some common clinical characteristics with PB‐DLBCL reports from other East Asian countries or regions (eg South Korea, Japan, and Taiwan),14, 34, 35, 36 yet these clinical characteristics seem to be distinct from non‐Asian PB‐DLBCL reports. These differences include an earlier age of disease onset (median age, 47‐57 years vs 62‐64 years),6, 7 the different prognostic impact of regional nodal involvement (eg regional nodal involvement is associated with worse prognosis in non‐Asian reports but not East Asian ones),5 and different patterns of CNS relapse (eg continuous relapse vs early relapse).7, 14 Taken together, these findings suggest a possibility of racial difference in the biology of PB‐DLBCL between the East Asian and non‐Asian populations. Although these findings need to be validated by further evidence, they are hypothesis‐generating and warrant further investigation.
Although both rituximab and RT decreased disease relapse and improved PFS in our study, they were not associated with significant changes in OS. This result might be due to the limited duration of follow‐up in the present study. Given the favorable prognosis of PB‐DLBCL (median OS, 8‐14 years)6, 7 and the pattern of frequent late relapse, it is possible that a 3.2‐year follow‐up might be insufficient to detect a significant survival difference between different treatment groups. In addition, the majority of patients who had disease progression received multiple lines of salvage therapy, including the use of rituximab and autologous/allogeneic stem cell transplant, which might also impact the survival outcome.
Several limitations need to be noted in this study. First, evaluation of CNS involvement was not carried out in all patients at diagnosis, which could have resulted in understaging and inclusion of some patients with occult CNS disease. However, patients with occult CNS involvement at diagnosis typically develop clinical CNS progression early (usually earlier than 1 year from diagnosis), whereas our cohort was characterized by frequent late CNS relapse, thus the likelihood of initial CNS involvement for the patients who developed CNS relapse in our series is not very high. Second, the relatively limited follow‐up duration could mask the long‐term survival difference of patients receiving different treatments. Finally, a central pathology review was not undertaken, which might have an undefined impact given the possible interobserver variability among pathologists. However, all pathologists worked at centers with expertise in the management of lymphoma in our study.
In conclusion, our study provides a comprehensive analysis of clinical features, patterns of failure, and the effect of various therapeutic strategies on the pattern of relapse in a large cohort of Chinese PB‐DLBCL patients. Based on our results, a combination of immunochemotherapy with consolidative RT and CNS prophylaxis could be the optimum treatment choice for patients with newly diagnosed PB‐DLBCL. One major challenge is to explore effective strategies of CNS prophylaxis because neither rituximab nor prophylactic IT decreased the risk of CNS relapse in PB‐DLBCL patients.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
The authors acknowledge the patients, medical staff, and physicians who participated in this study. The authors acknowledge members of the Leukemia and Lymphoma Committee of the Chinese Geriatric Oncology Society (LLC‐CGOS) for providing assistance in the initiation and management of this collaborative study.
Hu S, Song Y, Sun X, et al. Primary breast diffuse large B‐cell lymphoma in the rituximab era: Therapeutic strategies and patterns of failure. Cancer Sci. 2018;109:3943–3952. 10.1111/cas.13828
Shaoxuan Hu and Yuqin Song contributed equally to this work.
Contributor Information
Yufu Li, Email: yufu_li85@163.com.
Xiaohui He, Email: xiaohuih2008@163.com.
REFERENCES
- 1. Validire P, Capovilla M, Asselain B, et al. Primary breast non‐Hodgkin's lymphoma: a large single center study of initial characteristics, natural history, and prognostic factors. Am J Hematol. 2009;84:133‐139. [DOI] [PubMed] [Google Scholar]
- 2. Thomas A, Link BK, Altekruse S, Romitti PA, Schroeder MC. Primary Breast Lymphoma in the United States: 1975–2013. J Natl Cancer Inst 2017;109:djw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev. 2014;4:900‐908. [DOI] [PubMed] [Google Scholar]
- 4. Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972;29:1705‐1712. [DOI] [PubMed] [Google Scholar]
- 5. Aviv A, Tadmor T, Polliack A. Primary diffuse large B‐cell lymphoma of the breast: looking at pathogenesis, clinical issues and therapeutic options. Ann Oncol. 2013;24:2236‐2244. [DOI] [PubMed] [Google Scholar]
- 6. Ryan G, Martinelli G, Kuper‐Hommel M, et al. Primary diffuse large B‐cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol. 2008;19:233‐241. [DOI] [PubMed] [Google Scholar]
- 7. Hosein PJ, Maragulia JC, Salzberg MP, et al. A multicentre study of primary breast diffuse large B‐cell lymphoma in the rituximab era. Br J Haematol. 2014;165:358‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avilés A, Delgado S, Nambo MJ, et al. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005;69:256‐260. [DOI] [PubMed] [Google Scholar]
- 9. Fukuhara S, Watanabe T, Munakata W, et al. Bulky disease has an impact on outcomes in primary diffuse large B‐cell lymphoma of the breast: a retrospective analysis at a single institution. Eur J Haematol. 2011;87:434‐440. [DOI] [PubMed] [Google Scholar]
- 10. Jeanneret‐Sozzi W, Taghian A, Epelbaum R, et al. Primary breast lymphoma: patient profile, outcome and prognostic factors. A multicentre rare cancer network study. BMC Cancer 2008;8:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013‐1022. [DOI] [PubMed] [Google Scholar]
- 12. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116: 2040‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamy T, Damaj G, Soubeyran P, et al. R‐CHOP 14 with or without radiotherapy in nonbulky limited‐stage diffuse large B‐cell lymphoma. Blood. 2018;131:174‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yhim HY, Kang HJ, Choi YH, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma; Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer. 2010;10:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avilés A, Castaneda C, Neri N, Cleto S, Nambo MJ. Rituximab and dose dense chemotherapy in primary breast lymphoma. Haematologica. 2007;92:1147‐1148. [DOI] [PubMed] [Google Scholar]
- 16. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860‐1861. [PubMed] [Google Scholar]
- 17. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials. 1996;17:343‐346. [DOI] [PubMed] [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 19. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275‐282. [DOI] [PubMed] [Google Scholar]
- 20. Aviles A, Neri N, Nambo MJ. The role of genotype in 104 cases of diffuse large B‐cell lymphoma primary of breast. Am J Clin Oncol. 2012;35:126‐129. [DOI] [PubMed] [Google Scholar]
- 21. Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299‐1309. [DOI] [PubMed] [Google Scholar]
- 22. Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5‐positive diffuse large B‐cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22:1601‐1607. [DOI] [PubMed] [Google Scholar]
- 23. Zhao S, Zhang QY, Ma WJ, et al. Analysis of 31 cases of primary breast lymphoma: the effect of nodal involvement and microvascular density. Clin Lymphoma Myeloma Leuk. 2011;11:33‐37. [DOI] [PubMed] [Google Scholar]
- 24. Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466‐468. [DOI] [PubMed] [Google Scholar]
- 25. Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large‐B‐cell lymphoma: influence of rituximab. Ann Oncol. 2004;15:129‐133. [DOI] [PubMed] [Google Scholar]
- 26. Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B‐cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21:1046‐1052. [DOI] [PubMed] [Google Scholar]
- 27. Tanis PJ, Nieweg OE, Valdés Olmos RA, et al. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001;192:399‐409. [DOI] [PubMed] [Google Scholar]
- 28. Deng L, Xu‐Monette ZY, Loghavi S, et al. Primary testicular diffuse large B‐cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30:361‐372. [DOI] [PubMed] [Google Scholar]
- 29. Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large‐cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20‐27. [DOI] [PubMed] [Google Scholar]
- 30. Schmitz N, Zeynalova S, Glass B, et al. CNS disease in younger patients with aggressive B‐cell lymphoma: an analysis of patients treated on the MabThera International Trial and trials of the German High‐Grade Non‐Hodgkin Lymphoma Study Group. Ann Oncol. 2012;23:1267‐1273. [DOI] [PubMed] [Google Scholar]
- 31. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B‐Cell lymphoma treated With R‐CHOP. J Clin Oncol. 2016;34:3150‐3156. [DOI] [PubMed] [Google Scholar]
- 32. Gleeson M, Counsell N, Cunningham D, et al. Central nervous system relapse of diffuse large B‐cell lymphoma in the rituximab era: results of the UK NCRI R‐CHOP‐14 versus 21 trial. Ann Oncol. 2017;28:2511‐2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP‐14) with or without rituximab: an analysis of patients treated in the RICOVER‐60 trial of the German High‐Grade Non‐Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113:3896‐3902. [DOI] [PubMed] [Google Scholar]
- 34. Niitsu N, Okamoto M, Nakamine H, Hirano M. Clinicopathologic features and treatment outcome of primary breast diffuse large B‐cell lymphoma. Leuk Res. 2008;32:1837‐1841. [DOI] [PubMed] [Google Scholar]
- 35. Ou CW, Shih LY, Wang PN, et al. Primary breast lymphoma: a single‐institute experience in Taiwan. Biomed J. 2014;37:321‐325. [DOI] [PubMed] [Google Scholar]
- 36. Yhim HY, Kim JS, Kang HJ, et al. Matched‐pair analysis comparing the outcomes of primary breast and nodal diffuse large B‐cell lymphoma in patients treated with rituximab plus chemotherapy. Int J Cancer. 2012;131:235‐243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


