Abstract
Thymoquinone (TQ, 2‐methyl‐5‐isopropyl‐1,4‐benzoquinone), a bioactive constituent extracted from the seeds of Nigella sativa, has been proved to exert anti‐tumor efficiency in various cancers. Autophagy is a self‐digestion phenomenon, and its role in tumor formation and progression remains controversial. In the present study, we investigated the effects of TQ on renal cell cancer (RCC) cell lines (786‐O and ACHN) using wound healing assay, transwell assay and western blot analysis. We found that TQ effectively inhibited the metastatic capacity of RCC cells in vitro, which was also verified in a xenograft model. Meanwhile, we observed LC3 puncta and detected the expression of LC3 in TQ‐treated RCC cells, and then found that autophagy was induced by TQ in 786‐O and ACHN cell lines. In addition, TQ inhibited the migration and invasion as well as the EMT in RCC cells in an autophagy‐dependent manner. To further explore the underlying mechanism, we detected the AMPK/mTOR signaling pathway. The results indicated that TQ inhibited the metastasis of RCC cells by inducing autophagy via AMPK/mTOR signaling pathway. In conclusion, our findings provide a novel therapeutic strategy that aims at TQ‐induced autophagy in RCC treatment.
Keywords: AMPK/mTOR, autophagy, metastasis, renal cell cancer, thymoquinone
1. INTRODUCTION
Kidney cancer is the 6th most common cancer in men and the 9th most common in women, accounting for 3.7% of newly diagnosed cancer cases in 2017 in the United States.1 Renal cell carcinoma (RCC) is the most common form of kidney cancer and makes up approximately 80% of total cases.2 Thanks to improved early‐detection techniques, the 5‐year survival rate has increased over the past 5 decades. However, the overall prognosis is still poor. More than 14 000 patients die of RCC every year, approximately 30% of RCC patients are diagnosed with local progression or metastasis, and one‐third of local RCC patients will experience disease recurrence after surgical resection.3 RCC is relatively resistant to radiotherapy as well as chemotherapy. Although new therapies such as targeted therapy (e.g. tyrosine kinase inhibitors, vascular endothelial growth factor inhibitors and mTOR inhibitors) and immunotherapy have been developed, the outcomes are far from ideal.4, 5 Moreover, these therapies are usually expensive, with limited efficiency and extended side effects. Therefore, a novel therapeutic substance for the treatment of RCC, which costs less and performs better, is urgently required.
Thymoquinone (TQ, 2‐methyl‐5‐isopropyl‐1,4‐benzoquinone), a bioactive component extracted from the seeds of Nigella sativa, has been proved to exert wide‐ranging pharmacological activities, with anti‐tumor, anti‐inflammatory, anti‐oxidant, anti‐microbial, anti‐convulsant and anti‐diabetic properties.6, 7, 8, 9 Therefore, TQ has been regarded as a promising agent for numerous diseases. Previous studies have revealed that TQ may exhibit high anti‐tumor efficiency against various cancers, such as breast cancer, gastric cancer, cervical cancer, colon cancer, central nervous system cancer, ovarian cancer, prostate cancer, bladder cancer and renal cell cancer.10, 11, 12, 13, 14, 15, 16, 17, 18 It is well known that invasion and metastasis play vital roles in the progression of tumors. Recent research has shown that TQ can inhibit metastasis and EMT in prostate cancer via the transforming growth factor‐beta/Smad2/3 signaling pathway.19 Jun Li and his team also indicated that TQ inhibited the migration and invasion abilities in cervical cancer cells, which might be regulated via the expression of Twist1, Zeb1 and E‐cadherin directly.12 However, there are few studies that consider the effect of TQ on metastasis in renal cell cancer.
Autophagy is a self‐digestion phenomenon, first identified by Christian de Duve in 1963, which is evolutionarily conserved from yeast to mammals. During the autophagy process, a double‐membrane vesicle, named autophagosome or autophagic vacuole, sequesters cytoplasmic proteins and organelles and then delivers them to lysosome, where they are degraded.20 The role of autophagy in tumors constantly changes with the progression of tumors. In the early stages of tumor formation, autophagy suppresses cancer initiation through eliminating inflammation, genome instability and tissue damage. Alternatively, as a tumor grows, autophagy promotes tumor growth through providing substrates for metabolism.21, 22 Emerging observations indicate that there is an intricate relationship between autophagy and metastasis in cancer. On the one hand, autophagy helps to support viability for the potentially metastatic cancer cells. On the other hand, autophagy tends to inhibit early metastasis and to reverse of epithelial‐mesenchymal transition (EMT) in cancer cells.23, 24
Hence, in the present study, we aim to explore the effect of TQ on metastasis in RCC and the role of autophagy in this process. We also elucidate the underlying molecular mechanisms.
2. MATERIALS AND METHODS
2.1. Cell culture
Human renal carcinoma cell lines ACHN and 786‐O were purchased from ATCC (Manassas, VA, USA). The ACHN and 786‐O cells were cultured in RPMI‐1640 medium (Hyclone, UT, USA) supplemented with 10% FBS (Gibco, NY, USA), 1% antibiotic solution (penicillin 100 U/mL and streptomycin 100 g/mL) (Solarbio, Shanghai, China) at 37°C in a humidified incubator with 5% CO2.
2.2. Reagents, antibodies and plasmid
Thymoquinone was obtained from Sigma‐Aldrich (St. Louis, MO, USA) and dissolved in DMSO at a final concentration of 40 μmol/L as a stock solution. Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Protease inhibitor and phosphatase inhibitor were obtained from Roche (Basel, Switzerland). The following primary antibodies were used in this study: anti‐β‐actin, anti‐E‐cadherin, anti‐N‐cadherin, anti‐vimentin, anti‐LC3‐I/II, anti‐AMPK, anti‐p‐AMPK (Thr172), anti‐mTOR, anti‐p‐mTOR (Ser2448) and anti‐p‐S6K (Thr389), purchased from Cell Signal Technology (Boston, MA, USA). Anti‐mouse (CWBIO, Beijing, China) and anti‐rabbit antibodies (CWBIO, Beijing, China) were used as secondary antibodies. The mRFP‐EGFP‐LC3 reporter plasmid was a gift from Tamotsu Yoshimori (Addgene plasmid # 21074).
2.3. Cell viability assay
786‐O and ACHN cells were plated into 96‐well culture dishes (Corning, NY, USA) at a density of 5 × 103 cells/well with complete culture medium. After being incubated overnight, the medium was removed and replaced with fresh medium containing different concentrations of TQ (0, 10, 20, 40, 60, 80 and 100 μmol/L) for 24 or 48 hours, respectively. Then the medium was removed and 20 μL CCK8 was added into each well and incubated for 4 hours at 37°C. The optical density (OD) of each well was measured at 490 nm by microplate reader.
2.4. Transwell migration and invasion assay
We used 24‐well plates with chambers of 8‐μm pore size (Millipore, Germany) in the transwell analysis. In the migration assay, 1 × 104 RCC cells were seeded in the upper chamber. In the invasion assay, 2 × 104 cells were seeded in the upper chamber pretreated with Matrigel. Cells in the upper chamber were resuspended in 200 μL serum‐free medium with different concentrations of TQ. The lower chamber was filled with 800 μL complete medium containing the same concentration of TQ as in the upper chamber. After incubation for 24 hours, the non‐migrated or non‐invaded cells in the upper chamber were removed by cotton swab, and the migrated or invaded cells were fixed with 4% paraformaldehyde for 30 minutes and then stained with .1% crystal violet for another 30 minutes. Then, the cells were counted under a microscope and 4 random fields for each chamber were selected for statistical analysis.
2.5. Wound healing assay
Renal cell cancer cells were seeded into 6‐well plates with complete medium. We used 200‐μL pipet tips to scratch wounds when the cells had grown to 90%. The plates were washed 3 times to remove the floated cells and then filled with serum‐free medium containing different concentrations of TQ for 24 hours. The cells were observed at different time points and the wound closure was determined by inverted microscope.
2.6. Tandem fluorescence microscopy
Renal cell cancer cells were plated into 6‐well dishes with complete medium. When cells grew to 70%, the medium was discarded and replaced with serum‐free medium. Then, the cells were transfected with tandem fluorescent mRFP‐EGFP‐tagged LC3 expressing plasmids using Lipofectamine 2000. After 6 hours, the medium containing Lipofectamine 2000 was abandoned and new complete medium was added to the dishes to maintain cells for 18 hours. Next, cells were treated with TQ for 24 hours and then fixed with 4% paraformaldehyde. Then, the expression of enhanced green fluorescent protein (EGFP) and mRFP was observed by fluorescence microscopy (Olympus, Tokyo, Japan).
2.7. Western blot
Renal cell cancer cells were lysed by RIPA buffer containing protease inhibitor or phosphatase inhibitor and PMSF for 10 minutes on ice. Then, the cell debris was collected and centrifuged at 12 400 g for 15 minutes. The supernatants were transferred into new tubes, and protein concentrations were detected by BCA Protein Assay Kit (Heart, Xi'an, China). The supernatants were mixed with 1/5 volume of 6X buffer and then boiled for 5 minutes. The whole proteins were subjected to 10%‐15% SDS‐PAGE and then transferred to PVDF membranes. The membranes were first sealed with 10% skim milk for 2 hours at room temperature and then incubated with primary antibodies overnight at 4°C. Subsequently, the membranes were washed with TBST 3 times and incubated with secondary antibodies for 1 hour. The membranes were visualized and measured using an ECL system (Bio‐Rad, CA, USA).
2.8. In vivo experiments
All animal experiments were performed in accordance with standard ethical guidelines and approved by the Ethics Committee of Xi'an Jiaotong University. Ten male nude mice were included in the study. All mice were 5 weeks old and each weighted 19‐22 g. Harvested 786‐O cells (1 × 106 cells) were suspended in 200 μL serum‐free medium containing 100 μL Matrigel, and subcutaneously injected into the right flank of every mouse. When the tumors had grown to approximately 100‐150 mm3 in size, the mice were randomly divided into 2 groups (5 in each group) and intraperitoneal injected with DMSO (control group) or TQ 20 mg/kg, respectively, every 3 days. During the treatment, the tumor volumes were calculated and the mice were weighted with the same frequency. After 30 days, tumors were harvested, weighted and analyzed. The volume was calculated using the following formula: tumor volume = (length × width2) ×.5. To establish the metastatic tumor model, luciferase‐tagged 786‐O cells were injected into mice via tail veins. Then, the mice were divided into 2 groups and received the same treatment as above. After 30 days, the mice were intraperitoneal injected with D‐luciferin (150 mg/kg). Ten minutes later, mice were anesthetized with 10% chloral hydrate (.004 mL/g) and imaged using the IVIS Lumina II with Living Image Software.
The lung metastatic tumors were then harvested and stained with H&E.
2.9. Immunohistochemical assay
Renal tumors were separated from xenograft mice and fixed with 10% formaldehyde for 24 hours. Then, they were embedded in paraffin and cut into 5‐μm‐thick sections. After that, the tissue sections were subjected to deparaffinization, rehydration, endogenous peroxidase blocking and antigen retrieval. Next, the sections were blocked with 1% BSA for 10 minutes. Subsequently, they were incubated with primary antibodies overnight and appropriate secondary antibodies for 1 hour. The sections were then visualized using a DBA kit following the manufacturer's instructions.
2.10. Statistical analysis
All data were presented as mean ± SD of 3 independent experiments and analyzed using GraphPad Prism 5.2 software. In all cases, differences were considered statistically significant when P‐value <.05.
3. RESULTS
3.1. Thymoquinone suppresses migration, invasion and epithelial‐mesenchymal transition in renal cell cancer cells
To choose proper concentrations of TQ in the present study, first we observed the cell viability in TQ‐treated RCC cell lines 786‐O and ACHN using the CCK8 assay. Cells were incubated with TQ at different concentrations (0, 10, 20, 40, 60, 80, 100 μmol/L) for 24 hours or 48 hours, respectively. The results showed that TQ exhibited concentration‐dependent inhibition on cell growth in RCC cells, with the IC50 value of 55 μmol/L in 786‐O and 72 μmol/L in ACHN at 24 hours (Table S1). As shown in Figure 1A, 40 μmol/L TQ exhibited a less than 20% inhibitory rate of cell proliferation in both cell lines. Furthermore, we observed the effect of TQ on normal renal tubular epithelial cell HK‐2. The results demonstrated that there was no significant decrease in cell growth in HK‐2 under low doses of TQ (less than 60 μmol/L) (Figure S1). Therefore, the concentration of 40 μmol/L at 24 hours was used in subsequent experiments. To investigate the effects of TQ on cancer cell migration and invasion, we conducted wound healing and transwell assays. The wound healing and transwell migration assays showed that TQ attenuated cancer cell migration in a time‐dependent and concentration‐dependent manner. The invasion assay results revealed that the number of invaded cells decreased with the increase of TQ concentration, which was consistent with the result of migration assay (Figure 1B,C). To determine whether TQ participated in the EMT procedure in renal cancer cells, we also detected epithelial‐mesenchymal transition (EMT)‐related proteins by western blot. Cancer cells were treated with different concentrations of TQ for 24 hours or 40 μmol/L TQ for different periods of time. The results demonstrated that TQ upregulated epithelial markers (E‐cadherin), while downregulating mesenchymal markers (N‐cadherin, vimentin) in 786‐O cells in a concentration‐dependent and time‐dependent manner, suggesting that TQ induced mesenchymal‐epithelial transition (MET) in 786‐O cells. Similar results were observed in ACHN cells (Figure 1D). In addition, we observed EMT‐related markers (E‐cadherin and vimentin) in TQ‐treated ACHN by fluorescent immunostaining and found that the results were consistent with those for western blot analysis (Figure S2). These results suggest that TQ effectively inhibits migration, invasion and the EMT process in RCC cells in vitro.
Figure 1.
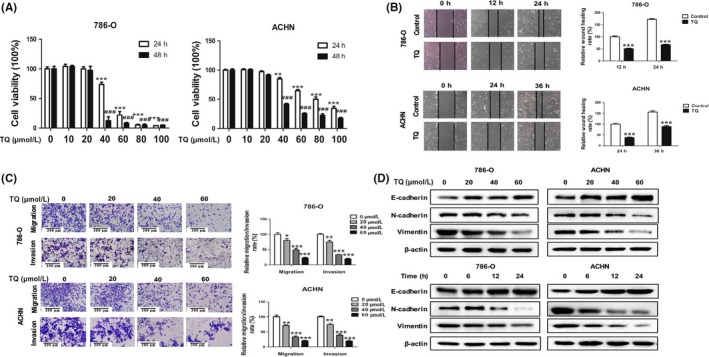
Thymoquinone (TQ) suppresses migration, invasion and epithelial‐mesenchymal transition (EMT) in renal cell carcinoma (RCC) cells. A, Cell viability in 786‐O and ACHN cells treated with TQ. Cells were cultured with different concentrations (0, 10, 20, 40, 60, 80 and 100 μmol/L) of TQ for 24 or 48 hours. Cell viability was tested using CCK8 assay. ** P < .01, *** P < .001 and ### P < .001 vs non‐treated group (0 μmol/L). B, Wound healing assay was conducted in 786‐O and ACHN cells. Cells were cultured with TQ (40 μmol/L); then, the wound healing assay was observed at 0, 12 and 24 hours in 786‐O cells and 0, 24 and 36 hours in ACHN cells. *** P < .001 vs control group. C, Transwell migration and invasion assay was observed in 786‐O and ACHN cells. Cells were treated with TQ at different concentrations (0, 20, 40 and 60 μmol/L). The migration assay was observed at 24 hours and the invasion assay was observed at 48 hours in both 786‐O and ACHN cells. *P < .05, **P < .01 and ***P < .001 vs non‐treated group (0 μmol/L). D, The expressions of EMT‐related proteins in TQ‐treated RCC cells. Epithelial markers (E‐cadherin) and mesenchymal markers (N‐cadherin, vimentin) were detected by western blot in TQ‐treated 786‐O and ACHN cells at indicated concentrations and times. All data are presented as mean ± SD of 3 independent experiments
3.2. Thymoquinone induces autophagy in renal cell cancer cells
Studies have shown that TQ could induce autophagy in squamous carcinoma cells and in drug‐resistant colon cancer cells, while the effect of TQ on autophagy in RCC cells has not been reported. To determine whether TQ could induce autophagy in RCC cells, we cultured ACHN and 786‐O cells with 40 μmol/L TQ for 24 hours and used an inverted microscope to observe the morphological changes in RCC cells. As shown in Figure 2A, cytoplasmic vacuoles were remarkably accumulated in RCC cells treated with TQ. Because the amount of LC3‐II is strongly correlated with the number of autophagosomes and autophagic structures, LC3 is widely used as an autophagosome marker. To confirm that the vacuoles induced by TQ were relevant to autophagy, we transiently transfected the RCC cells with mRFP‐EGFP‐LC3 reporter plasmids to observe LC3 puncta. As a result of differential sensitivity to acidity, tandem fluorescent‐tagged LC3 plasmid emits different fluorescence signals in autophagy. Yellow LC3 puncta (mRFP‐positive/EGFP‐positive) can be seen when autophagosomes form and red LC3 puncta (mRFP‐positive/EGFP‐negative) can be found when autophagosomes fuse with lysosomes, which forms a low PH environment. As shown in Figure 2B, the number of both yellow and red puncta increased dramatically in TQ‐treated RCC cells when compared with the control group. In addition, we treated ACHN and 786‐O cells with different concentrations of TQ and detected the expression of LC3 by western blot. The results revealed that TQ could upregulate LC3‐II in a concentration‐dependent manner (Figure 2D). Collectively, these data indicate that TQ can induce autophagy in RCC cells.
Figure 2.
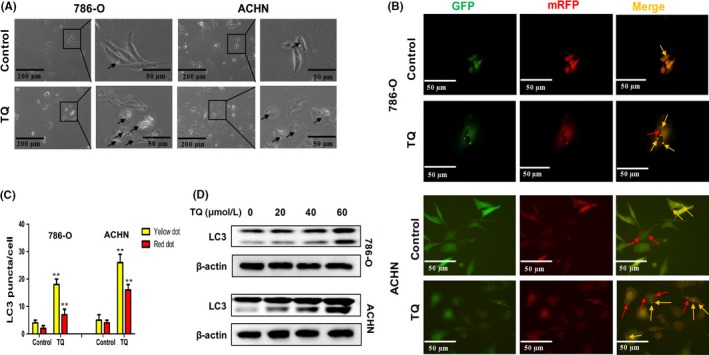
Thymoquinone (TQ) induces autophagy in renal cell carcinoma (RCC) cells. A, Morphological changes in 786‐O and ACHN cells. RCC cells were treated with 40 μmol/L TQ for 24 hours; then, an inverted microscope was used to observe the morphological changes (×40 and ×100). Arrows point to cytoplasmic vacuoles. B, Autophagosomes and autolysosomes induced by TQ in RCC cells. Cells were transfected with mRFP‐EGFP‐LC3 reporter plasmids and treated with 40 μmol/L TQ for 24 hours. The LC3 puncta was observed by fluorescence microscopy (×200). Yellow arrows point to autophagosomes (mRFP‐positive/EGFP‐positive) and red arrows point to autolysosomes (mRFP‐positive/EGFP‐negative). C, Quantification of the number of autophagosomes and autolysosomes per cell. D, The level of LC3 was analyzed by western blot in 786‐O and ACHN cells after treatment with different concentrations of TQ. All data are presented as mean ± SD of 3 independent experiments
3.3. Inhibition of autophagy attenuates thymoquinone‐induced anti‐tumor effects in renal cell cancer cells
Accumulated evidence has suggested that there is an association between autophagy and metastasis. We hypothesized that autophagy induced by TQ may affect the migration and invasion in RCC cells. To verify the hypothesis, 3‐methyladenine (3‐MA), an inhibitor of autophagy, was used in the study. On the basis of different treatments, 786‐O and ACHN cells were divided into 4 groups: control, TQ, 3‐MA, and TQ together with 3‐MA. As shown in Figure 3A, the anti‐migratory ability of TQ was remarkably attenuated in 786‐O or ACHN cells when autophagy was inhibited by 3‐MA. Consistent results could be seen in the invasion experiments. Furthermore, to investigate whether autophagy inhibitor affects TQ‐induced MET in RCC cells, we detected EMT‐related proteins by western blot and found that 3‐MA could abate TQ‐induced MET (Figure 3B). Taken together, these data show that inhibition of autophagy attenuates TQ‐induced anti‐tumor effects in RCC cells.
Figure 3.
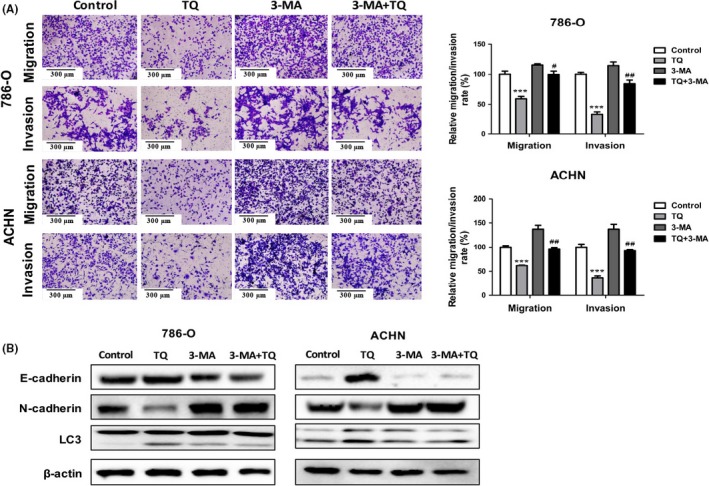
Inhibition of autophagy attenuates thymoquinone (TQ)‐induced anti‐tumor effects in renal cell carcinoma (RCC) cells. 786‐O and ACHN cells were mock‐treated or treated with TQ (40 μmol/L) in the presence or absence of 3‐MA (5 mmol/L); then, the migratory and invasive abilities were detected by transwell assay (A), and the expression of epithelial‐mesenchymal transition (EMT) markers and LC3 was determined by western blot (B). All data are presented as mean ± SD of 3 independent experiments
3.4. AMPK/mTOR pathway is involved in anti‐tumor effects of autophagy induction by thymoquinone in renal cell cancer cells
Numerous studies have indicated that autophagy can be mediated by various pathways, including PI3K/AKT/mTOR, AMPK/mTOR, MAPK/ERK1/2, P53/DRAM and HGF/MET.25 Among these pathways, the AMPK/mTOR signaling pathway plays a vital role in the process of autophagy. It positively regulates autophagy through increasing AMPK phosphorylation and decreasing mTOR phosphorylation. To confirm whether the AMPK/mTOR signaling pathway is involved in the induction of autophagy by TQ in RCC cells, the protein levels of AMPK, p‐AMPK (Thr172), mTOR, p‐mTOR (Ser2448) and p‐S6K (Thr389) were detected after treatment with different concentrations of TQ. As expected, the expression of p‐AMPK was elevated by TQ, while expressions of p‐mTOR and p‐S6K were reduced, which indicated the activation of the AMPK/mTOR pathway (Figure 4A). Then, we pretreated the RCC cells with AICAR (an activator of AMPK) or compound C (an inhibitor of AMPK) and detected the expression of p‐AMPK, p‐mTOR and LC3. As shown in Figure 4B, the effects of TQ on LC3 could be receded when the AMPK/mTOR signaling pathway was inhibited by compound C, which suggested that autophagy was induced by TQ through the AMPK/mTOR pathway. To further elucidate the role of AMPK/mTOR in the anti‐tumor effects of autophagy induced by TQ, we conducted the transwell experiments and analyzed the EMT‐related proteins using western blot analysis. Results showed that compound C attenuated the migratory and invasive capacities of autophagy induced by TQ. Furthermore, it also weakened TQ‐induced MET (Figure 4C,D). These data reveal that TQ inhibits the metastasis characteristics of RCC cells by inducing autophagy via the AMPK/mTOR signaling pathway. Because evidence has shown that the inhibition of mTOR is as an attractive strategy to treat RCC, in our study, we further compared the effects of TQ with everolimus, an inhibitor of mTOR. The results showed that everolimus was significantly more potent than TQ in inhibiting migration and invasion, inducing autophagy and MET in RCC cells (Figure S3), suggesting that the mTOR inhibitor showed more anti‐tumor effects when compared with TQ in RCC treatment. However, despite the promise of mTOR inhibition as an anti‐tumor strategy, the clinical performance of mTOR inhibitors in relation to avoiding drug resistance is still less than satisfactory at present. Therefore, drugs that target multiple components of pathways, such as autophagy/AMPK/mTOR, are urgently needed.
Figure 4.
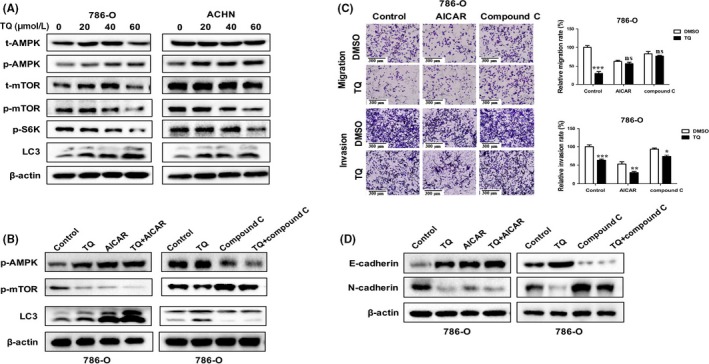
AMPK/mTOR pathway is involved in anti‐tumor effects of autophagy induction by thymoquinone (TQ) in renal cell carcinoma (RCC) cells. A, 786‐O and ACHN cells were treated with different concentrations (0, 20, 40, 60 μmol/L) of TQ for 24 hours; the levels of t‐AMPK, p‐AMPK, t‐mTOR, p‐mTOR, p‐S6K and LC3 were evaluated by western bolt. B, RCC cells were pretreated with AICAR (1 mmol/L) or compound C (50 μmol/L) for 4 hours, and then incubated with TQ (40 μmol/L) for 24 hours. The expression of p‐AMPK, p‐mTOR and LC3 was detected by western bolt. C, D, Then, the migratory and invasive abilities were detected by transwell assay and epithelial‐mesenchymal transition‐related proteins were measured by western blot. All data are presented as mean ± SD of 3 independent experiments
3.5. Anti‐tumor effects of thymoquinone on renal cell cancer cells in vivo
We further investigated the effects of TQ on RCC cell growth in a xenograft tumor model. As shown in Figure 5A, tumors in the group treated with TQ grew more slowly than those in the control group. After 4 weeks, tumors had reached approximately 500 mm3 in the control group, and 270 mm3 in the TQ group. Finally, we harvested and weighed all of the tumors. The weight of tumors in the control group was heavier than that in the TQ group, suggesting that TQ inhibited RCC cell growth in vivo (Figure 5B,C). Furthermore, we detected the expression of EMT‐related protein using immunohistochemistry and western blot analysis. The results showed an increased expression of E‐cadherin and decreased expression of N‐cadherin in the TQ group, which was consistent with the finding in vitro (Figure 5D,E). To determine the anti‐tumor effect of TQ on metastasis formation, we further established a metastatic tumor model using luciferase‐tagged 786‐O cells. As shown in Figure 5F, TQ significantly inhibited lung metastasis compared with the control group. H&E staining of lungs revealed a significant decrease in the number of metastatic nodules in the lungs of TQ‐treated mice compared to the control group (Figure S4). These data indicate that TQ inhibits RCC cell growth and metastasis in vivo.
Figure 5.
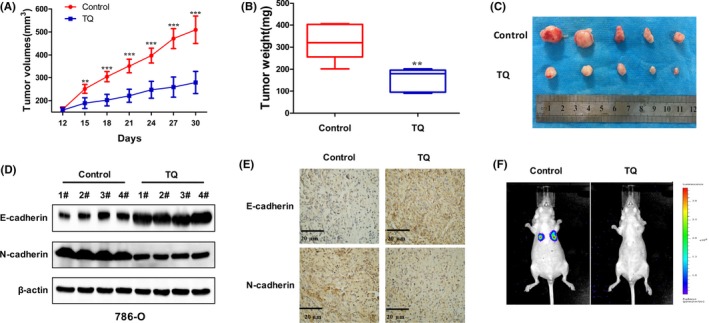
Anti‐tumor effects of thymoquinone (TQ) on renal cell carcinoma (RCC) cells in vivo. A, The tumor volumes were calculated every 3 days in the control or the TQ‐treated group. B, C, Xenograft tumors were harvested and the tumor weight was measured at the end of the experiment. D, E, The expression of E‐cadherin and N‐cadherin in xenograft tumors were detected by western blot and immunohistochemistry (×200). F, Bioluminescence images of mice in the control and the TQ‐treated group. Data are presented as mean ± SD. (n = 5)
4. DISCUSSION
Over the past 30 years, plant‐derived drugs have gained increasing attention as they are relatively nontoxic and inexpensive when compared to modern medicines. Medicinal plants and their extracted ingredients are extensively used for their biological properties, such as anti‐inflammatory, anti‐oxidant, anti‐diabetic and anti‐cancer effects. Numerous studies have indicated the benefits of many medicinal plants occurring around the world agents against cancers.26 Among them, a bioactive constituent of black seed oil called TQ that mainly grows in the Mediterranean region and Western Asia, including Afghanistan, India and Pakistan, has been proved to exert anti‐cancer effects both in cell culture systems and animal experiments.27, 28 RCC, the most common cancer among kidney‐related tumors, is insensitive to many conventional treatments. Therefore, candidates originated from medicinal plants have been studied extensively for application in this disease. Although the anti‐cancer effects of TQ have been widely reported in various cancers, few studies have explored its efficacy in renal cell carcinoma.
In this study, we explored the effects of TQ on RCC cell lines 786‐O and ACHN, and found that TQ exhibited cytotoxic effects on RCC cells in a concentration‐dependent and time‐dependent manner. Moreover, TQ remarkably inhibited the migration and invasion of RCC cells with the prolongation of time and increase of dose. It is well documented in the literature that cells undergoing EMT can transform the epithelial phenotype into mesenchymal phenotype, leading to an increase in cell motility and invasiveness.29 Accumulating research has suggested that EMT has a close correlation with the migratory and invasive abilities of cancer cells. Then, we detected EMT‐related proteins in our study. The results showed that TQ could upregulate epithelial markers and downregulate mesenchymal markers, suggesting that TQ could inhibit EMT in RCC cells. To observe the effects of TQ on tumor growth in vivo, we calculated the volume of the tumors and found that tumors in the group treated with TQ grew more slowly when compared to those in the control group. Moreover, the tumors in the TQ‐treated group showed higher expression of E‐cadherin and lower expression of N‐cadherin, which concurred with our findings in vitro. We further established a metastatic tumor model and the results indicated that TQ significantly inhibited metastasis compared with the control group, which was consistent with our expectations.
Autophagy is a catabolic cellular process in which the dysfunctional cytoplasmic constituents are removed to maintain metabolic balance. It occurs not only in normal cells but also in cancer cells.30 It is widely accepted that autophagy plays a complex role in oncogenesis: it represses tumor growth in the early stages on the one hand but is required for progression to advanced or invasive tumors on the other hand.24 During the past 5 years, autophagy induced by plant‐derived drugs has been widely studied in various tumors. In the present study, we first demonstrated that TQ‐induced autophagy in a concentration‐dependent manner in RCC cells, which was verified by inverted microscope, LC3 turnover assay and western blot. There is an intricate connection between autophagy and metastasis. Autophagy is usually induced during the progression of various cancers to metastasis and it can promote tumor metastasis through numerous mechanisms, including promoting a stem cell phenotype, production of secreted factors and modulating the Rho signaling pathway.24 In contrast, autophagic cells can act as a barrier against tumor progression through inhibiting the tumor transfer from primary sites to secondary sites.31 A study has shown halofuginone‐induced autophagy suppresses breast cancer cell migration and invasion.32 Meanwhile, in osteosarcoma cells, the activation of autophagy is associated with the suppression of EMT.33 In the study, we used 3‐MA to inhibit autophagy and observed the effects of TQ on migration, invasion and EMT in RCC cell lines 786‐O and ACHN. We, therefore, found that inhibition of autophagy could attenuate TQ‐induced anti‐metastasis effects in RCC cells.
The mTOR signaling pathway has been reported to be activated in nearly 100% of RCC, and inhibitors targeting the mTOR signaling seem to offer a promising therapy against RCC. In our supplementary experiments, we compared TQ with everolimus. The results indicated that everolimus showed more anti‐tumor effects as compared to TQ. However, the emergency of drug resistance has limited the application of mTOR inhibitors in clinical treatment and agents targeting multiple pathways have been widely studied. Because the mTOR signaling pathway plays a vital role in the process of autophagy, it is important to study the relationship between the mTOR pathway and autophagy. The mTOR includes 2 functionally and biochemically distinct complexes, mTORC1 and mTORC2.34 Evidence has shown that mTORC1, but not mTORC2, plays a vital role in the regulation of autophagy. The mTORC1 inhibition increases autophagy, while activation of mTORC1 represses this process.34 AMPK is a cellular energy sensor that regulates homeostatic balance. It positively regulates autophagy by inhibiting the activity of mTORC1 through direct phosphorylation of its receptor, a regulatory‐associated protein of mTOR.25
To verify whether TQ induced autophagy through the AMPK/mTOR signaling pathway, we detected the main molecules in this pathway and found that the expression of p‐AMPK was elevated by TQ, while expressions of p‐mTOR and p‐S6K were reduced, accompanied by an increase in LC3. Moreover, we observed an upregulation of LC3 in TQ‐treated RCC cells when activating the AMPK/mTOR pathway, while a downregulation when inhibiting this pathway; taken together, we can conclude that TQ could induce autophagy via the AMPK/mTOR pathway. Then, the migration and invasion experiments showed that the AMPK inhibitor attenuated the anti‐metastatic effects of autophagy induced by TQ, which suggested that TQ could inhibit the metastasis of RCC cells by inducing autophagy through the AMPK/mTOR pathway.
In conclusion, our study demonstrated that TQ exerted anti‐metastatic properties in RCC cell lines 786‐O and ACHN. In addition, these characteristics, including inhibiting migration, invasion and EMT, were mainly dependent on TQ‐induced autophagy. Furthermore, we revealed that the mechanism by which TQ‐induced autophagy inhibited the metastasis of RCC cells was regulated through the AMPK/mTOR pathway. These findings indicated the potential role of TQ‐induced autophagy in cancer treatment and suggested that combining TQ with autophagy inhibitors might enhance its cancer suppression effects.
DISCLOSURE
The authors have no conflict of interests.
Supporting information
Zhang Y, Fan Y, Huang S, et al. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018;109:3865–3873. 10.1111/cas.13808
Contributor Information
Shanzhi Gu, Email: gushanzhi@mail.xjtu.edu.cn.
Xinhan Zhao, Email: zhaoxinhanprof@163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Znaor A, Lortet‐Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67(3):519‐530. [DOI] [PubMed] [Google Scholar]
- 3. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v58‐v68. [DOI] [PubMed] [Google Scholar]
- 4. Maia MC, Adashek J, Bergerot P, Almeida L, Dos SS, Pal SK. Current systemic therapies for metastatic renal cell carcinoma in older adults: a comprehensive review. J Geriatr Oncol. 2018;9(3):265‐274. [DOI] [PubMed] [Google Scholar]
- 5. Subramanian P, Haas NB. Recent advances in localized RCC: a focus on VEGF and immuno‐oncology therapies. Urol Oncol. 2018;36(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad A, Husain A, Mujeeb M, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ravindran J, Nair HB, Sung B, Prasad S, Tekmal RR, Aggarwal BB. Thymoquinone poly (lactide‐co‐glycolide) nanoparticles exhibit enhanced anti‐proliferative, anti‐inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79(11):1640‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Inci M, Davarci M, Inci M, et al. Anti‐inflammatory and antioxidant activity of thymoquinone in a rat model of acute bacterial prostatitis. Hum Exp Toxicol. 2013;32(4):354‐361. [DOI] [PubMed] [Google Scholar]
- 9. Negi P, Rathore C, Sharma G, Bhoop BS, Katare OP. Thymoquinone (TQ) a potential therapeutic molecule: role of colloidal carriers in its effective delivery. Recent Pat Drug Deliv Formul. 2018;12(1):3‐22. [DOI] [PubMed] [Google Scholar]
- 10. Alobaedi OH, Talib WH, Basheti IA. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac J Trop Med. 2017;10(4):400‐408. [DOI] [PubMed] [Google Scholar]
- 11. Feng LM, Wang XF, Huang QX. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J Biosci. 2017;42(4):547‐554. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Khan MA, Wei C, et al. Thymoquinone inhibits the migration and invasive characteristics of cervical cancer cells SiHa and CaSki in vitro by targeting epithelial to mesenchymal transition associated transcription factors Twist1 and Zeb1. Molecules. 2017;22(12):2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen MC, Lee NH, Hsu HH, et al. Inhibition of NF‐kappaB and metastasis in irinotecan (CPT‐11)‐resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ Toxicol. 2017;32(2):669‐678. [DOI] [PubMed] [Google Scholar]
- 14. Farkhondeh T, Samarghandian S, Hozeifi S, Azimi‐Nezhad M. Therapeutic effects of thymoquinone for the treatment of central nervous system tumors: a review. Biomed Pharmacother. 2017;96:1440‐1444. [DOI] [PubMed] [Google Scholar]
- 15. Liu X, Dong J, Cai W, Pan Y, Li R, Li B. The effect of thymoquinone on apoptosis of SK‐OV‐3 ovarian cancer cell by regulation of Bcl‐2 and Bax. Int J Gynecol Cancer. 2017;27(8):1596‐1601. [DOI] [PubMed] [Google Scholar]
- 16. Koka PS, Mondal D, Schultz M, Abdel‐Mageed AB, Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: role of reactive oxygen species. Exp Biol Med (Maywood). 2010;235(6):751‐760. [DOI] [PubMed] [Google Scholar]
- 17. Iskender B, Izgi K, Hizar E, et al. Inhibition of epithelial‐mesenchymal transition in bladder cancer cells via modulation of mTOR signalling. Tumour Biol. 2016;37(6):8281‐8291. [DOI] [PubMed] [Google Scholar]
- 18. Park EJ, Chauhan AK, Min KJ, Park DC, Kwon TK. Thymoquinone induces apoptosis through downregulation of c‐FLIP and Bcl‐2 in renal carcinoma Caki cells. Oncol Rep. 2016;36(4):2261‐2267. [DOI] [PubMed] [Google Scholar]
- 19. Kou B, Liu W, Zhao W, et al. Thymoquinone inhibits epithelial‐mesenchymal transition in prostate cancer cells by negatively regulating the TGF‐beta/Smad2/3 signaling pathway. Oncol Rep. 2017;38(6):3592‐3598. [DOI] [PubMed] [Google Scholar]
- 20. Helgason GV, Holyoake TL, Ryan KM. Role of autophagy in cancer prevention, development and therapy. Essays Biochem. 2013;55:133‐151. [DOI] [PubMed] [Google Scholar]
- 21. White E. Deconvoluting the context‐dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shintani T, Klionsky DJ. Autophagy in health and disease: a double‐edged sword. Science. 2004;306(5698):990‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gugnoni M, Sancisi V, Manzotti G, Gandolfi G, Ciarrocchi A. Autophagy and epithelial‐mesenchymal transition: an intricate interplay in cancer. Cell Death Dis. 2016;7(12):e2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2017;36(12):1619‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu C, Li WB, Liu JB, Lu JW, Feng JF. Autophagy: novel applications of nonsteroidal anti‐inflammatory drugs for primary cancer. Cancer Med. 2018;7(2):471‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 27. Banerjee S, Padhye S, Azmi A, et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62(7):938‐946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gali‐Muhtasib H, Roessner A, Schneider‐Stock R. Thymoquinone: a promising anti‐cancer drug from natural sources. Int J Biochem Cell Biol. 2006;38(8):1249‐1253. [DOI] [PubMed] [Google Scholar]
- 29. Kotiyal S, Bhattacharya S. Events of molecular changes in epithelial‐mesenchymal transition. Crit Rev Eukaryot Gene Expr. 2016;26(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 30. Law BY, Mok SW, Wu AG, Lam CW, Yu MX, Wong VK. New potential pharmacological functions of chinese herbal medicines via regulation of autophagy. Molecules. 2016;21(3):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double‐edged sword. Curr Opin Cell Biol. 2010;22(2):241‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia X, Wang L, Zhang X, et al. Halofuginone‐induced autophagy suppresses the migration and invasion of MCF‐7 cells via regulation of STMN1 and p53. J Cell Biochem. 2017;119:4009‐4020. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Zhang Y, Zou J, et al. Andrographolide induces autophagic cell death and inhibits invasion and metastasis of human osteosarcoma cells in an autophagy‐dependent manner. Cell Physiol Biochem. 2017;44(4):1396‐1410. [DOI] [PubMed] [Google Scholar]
- 34. Paquette M, El‐Houjeiri L, Pause A. mTOR pathways in cancer and autophagy. Cancers (Basel). 2018;10(1):18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


