Abstract
The ball-mill-based mechanochemical activation of metallic copper powder facilitates solvent-free alkyne-azide click reactions (CuAAC). All parameters that affect reaction rate (i.e., milling time, revolutions/min, size and milling ball number) have been optimized. This new, efficient, facile and eco-friendly procedure has been tested on a number of different substrates and in all cases afforded the corresponding 1,4-disubstituted 1,2,3-triazole derivatives in high yields and purities. The final compounds were isolated in almost quantitative overall yields after simple filtration, making this procedure facile and rapid. The optimized CuAAC protocol was efficiently applied even with bulky functionalized β-cyclodextrins (β-CD) and scaled-up to 10 g of isolated product.
Keywords: click reaction, copper catalyzed alkyne-azide cycloaddition, ball mill, solvent-free reaction, mechanochemistry
1. Introduction
In the 1960s, Huisgen and co-workers extensively studied the 1,3-dipolar cycloaddition reaction of azides and alkynes [1,2]. In Denmark, Meldal et al. [3] reported the Cu(I)-catalyzed alkyne-azide click reaction (CuAAC) at the same time as Fokin and Sharpless [4] in the U.S. The use of copper salts as the catalyst exclusively afforded 1,4-disubstituted 1,2,3-triazoles, whereas the uncatalyzed reaction provided mixtures of 1,4- and 1,5-triazole regioisomers, while also necessitating much higher temperatures. The CuAAC is considered to be the “click chemistry” reaction par excellence. In 2001, Sharpless coined this term to describe a set of powerful bond-forming reactions with almost complete orthogonality (i.e., which do not interfere with other chemical functionalities) [5]. CuAAC has been used in many fields, has become a true interdisciplinary reaction and can boast of extremely wide applicability [6,7,8,9]. The required Cu(I) catalyst is usually generated in situ via the reduction of a Cu(II) salt with sodium ascorbate.
Enabling techniques such as microwave heating (MW), ultrasound irradiation (US) and mechanochemical activation are the most reliable energy sources with which to activate catalysts and promote chemical reactions [10,11,12,13]. The study of highly efficient MW- and US-promoted CuAAC protocols has become an important goal [14] and a wide range of compounds has been synthesized under non-conventional conditions [15], while increasing interest in biological applications has lead to MW being used to obtain multimeric peptides and peptidomimetcs [16,17], to modify nucleotides or nucleic acid [18,19] and to synthesize dendrimeric multicarriers [20,21]. MW-assisted CuAAC have been applied to material grafting and polymer functionalization [22,23,24,25], as well as for the preparation of functional polytriazoles [26].
Sonochemical reactions fulfil green chemistry requirements and thus cause a sizeable reduction in energy consumption [27]. In particular, US has found its main domain in heterogeneous catalysis and in metal surface activation [28,29,30]. Metallic copper is one of the cheapest solid catalysts that in a relatively longer reaction time can afford very clean final products after simple removal of copper powder or turnings [31,32,33]. Our experience has shown us that US irradiation smoothly activates the redox process between metallic Cu and Cu2O on the metal surface, generating Cu(I) species. This means that simple copper turnings can be used as the catalyst of choice in Huisgen 1,3-dipolar cycloadditions [34,35]. Despite the different reaction environment, a combination of US and mechanochemical grinding can convert mechanical energy into a chemical outcome [36], and so planetary mills (PM) can be exploited to activate the metallic copper surface. The amount of energy which can be imparted to a system under mechanical activation can be sufficient to break chemical bonds [37]. Although the full extent of the technique applicability still remain uncovered [38,39] the use of PM for metal activation is well known [40], with a number of mechanochemical organic reactions [41], among them oxidations [42], Knovenagel and domino condensation reactions [43], metal-catalyzed cycloadditions [44,45,46]. PM has been exploited as a tool to reduce the energy demand in C-C couplings such as Suzuki, Heck, Sonogashira reactions [47] and possibly at least in part replace palladium catalysts with less expensive transition metals. Few examples of mechanochemical copper-catalysed organic reactions have been reported by Thorwirth et al. (CuAAC with a classic Cu(OAc)2/ascorbate system) [48] and by Friščić et al., (Cu(I)-catalysed C-N couplings) [49]. On the basis of our experience in sonochemistry, Cu(0) powder could also be a good source of Cu(I) catalyst for CuAAC under mechanochemical conditions [30,50].
Green chemistry utilizes a set of principles that favours the reduction or elimination of organic solvents [51], prefers heterogeneous catalysis, minimizes waste production and energy consumption. The advantages of non-conventional techniques such as MW, US and mechanochemistry either in benign reaction media, such as water, or in solventless conditions have been well documented in the literature [52,53]. In this work, we aimed to design a scalable mechanochemical CuAAC protocol using metallic copper powder under solvent-free conditions. The study included the optimization of all parameters that influence reaction rate and product yields.
2. Results and Discussion
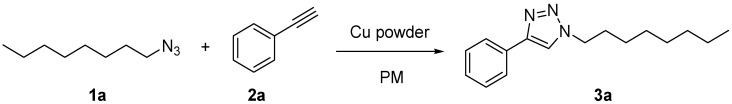
As depicted in Scheme 1, the CuAAC with octyl azide (1a) and phenylacetylene (2a) has been selected as our model reaction. It was chosen because of its resistance to mechanical shock. The reaction was first studied under conventional conditions: the reagents were dissolved in t-BuOH:H2O 1:1 and the reaction was stirred at 70 °C for 20 h (full conversion by GC-MS analysis).
Scheme 1.
CuAAC of octyl azide (1a) with phenylacetylene (2a) forming 1-octyl-4-phenyl-1H-1,2,3-triazole (3a).
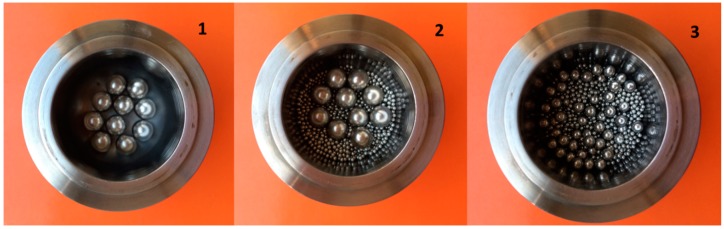
The first attempt to mechanochemicalCuAAC was carried out in a stainless steel jar (50 mL) with 10 balls (10 mm Ø), octyl azide (1 mmol) and phenylacetylene (1 mmol) Cu powder (1 mmol), and SiO2 (5 g) used as grinding auxiliary. After 30 min at 650 rpm the triazole derivative was obtained in a 67% yield. As already reported in the literature, stress energy can be considered an energy distribution which sums all single stress events [38]. The number of stress events and the stress frequency, which are correlated to ball diameter and the number of balls, influence reaction outcome. Ball dimension and number were optimized while other parameters were fixed (time = 30 min, rotation speed = 650 rpm, auxiliary = 5 g of SiO2) (See Table 1 and Figure 1).
Table 1.
Screening of ball features.
| Entry | Ball Number | Active Surface Area b (mm2) | Yield (%) a | ||
|---|---|---|---|---|---|
| Small (Ø = 2 mm) | Medium (Ø = 5 mm) | Big (Ø = 10 mm) | |||
| 1 | none | none | 10 | 10,666 | 67 |
| 2 | 625 | none | 10 | 18,520 | 80 |
| 3 | 1500 | 48 | none | 30,144 | 99 |
a: Isolated yield; b: Active surface area = surfaceballs + surfacejar.
Figure 1.
Jar and balls (Entries 1–3 Table 1).
Three different reaction conditions were compared. Small balls were added to the large 10 mm balls to fill empty space and increase the active surface area (Entry 2, Table 1). As shown in Table 1, the increase in effective energy transfer to the mill boosted the 3a yield from 67% to 80%. A further improvement was achieved in a third experiment: the overall mass of the milling balls was kept constant and ball size and ball number was varied. The yield reached 99% as a result of the increase in grinding material active surface area.
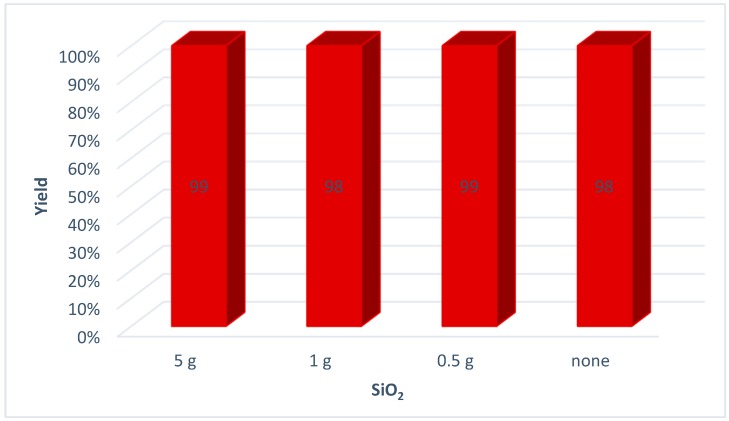
The grinding of liquids in PM can be performed in two ways: either by pre-freezing the reaction mixture below the eutectic melting point or by using grinding auxiliaries. The first technique is disadvantageous for economic reasons and because the compounds are rapidly heated during the milling process. The various amounts of silica (5 g, 1 g, 0.5 g) that were added to the reaction mixture did not affect the reaction outcome. The same yield was obtained in the absence of a grinding auxiliary (Figure 2).
Figure 2.
Tests with different grinding auxiliary amounts. Reaction conditions: Octyl azide (1a) (1 mmol), phenylacetylene (2a) (1 mmol), Cu powder (1 mmol), SiO2; stainless steel jar (50 mL), 1500 small balls (Ø = 2 mm) and 48 medium balls (Ø = 5 mm).
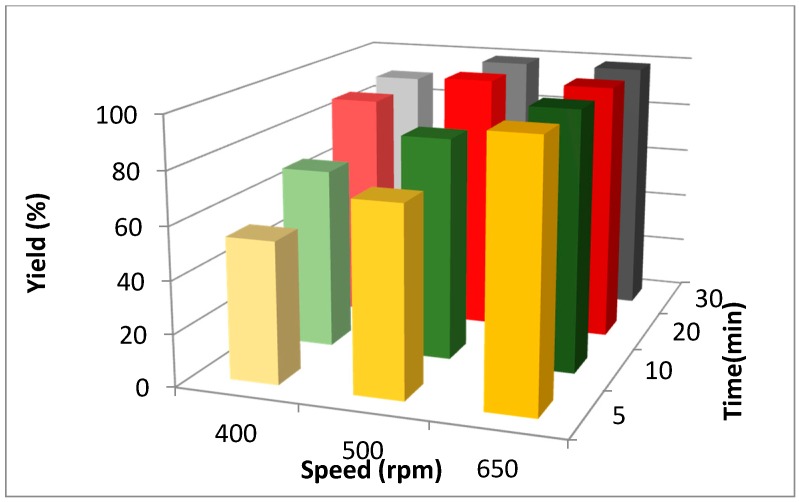
Two other relevant parameters were investigated, namely reaction time and PM rotation frequency (rpm, min−1). An increase in kinetic energy promoted the cycloaddition and a higher rotational speed enhanced conversion (Figure 3). Complete conversion was achieved in only 5 min at 650 rpm.
Figure 3.
Influence of rotation frequency and milling time on product yield (3a). Reaction conditions: Octyl azide (1a) (1 mmol), phenylacetylene (2a) (1 mmol), Cu powder (1 mmol); stainless steel jar (50 mL), 1500 small balls (Ø = 2 mm) and 48 medium balls (Ø = 5 mm).
Using the optimized procedure (1 eq. Cu powder, 650 rpm, 5 min) as a basis, the octyl azide and phenylacetylene reaction was scaled-up to 10 mmol in a 50 mL jar. As depicted in Table 2, a 95% reaction yield was observed and mechanical energy transfer efficacy was confirmed in the planetary ball mill, even in the presence of small amounts of Cu powder (630 mg vs. 2.88 mL of reagents). In addition, the reaction was repeated on a larger scale (50 mmol) using a 250 mL jar charged with 10 balls (30 mm Ø) and 48 medium balls (5 mm Ø), in order to broaden the scope of the study and evaluate the versatility of the protocol. The isolated yield was around 95% and gave ca. 10 g of 1-octyl-4-phenyl-1H-1,2,3-triazole.
Table 2.
Scale-up of mechanochemical CuAAC.
| Entry | Octyl Azide (1a) | Yield a | ||
|---|---|---|---|---|
| mmol | g | % | g | |
| 1 b | 1 | 0.1552 | 99 | 0.254 |
| 2 b | 10 | 1.5524 | 98 | 2.519 |
| 3 c | 50 | 7.7620 | 95 | 12.208 |
a: Isolated yield; b: 1 eq Cu powder, 5 min, 650 rpm; 50 mL jar, 1500 small balls (Ø = 2 mm) and 48 medium balls (Ø = 5 mm); c: 1 eq Cu powder, 5 min, 650 rpm; 250 mL jar, 48 medium balls (Ø = 5 mm) and 10 very large balls (Ø = 30 mm).
In an attempt to further confirm the versatility of the method, a selection of different benzyl azides and several alkynes were tested and the stability of iodo- and chloro- substituted phenyl moieties were thus confirmed (Entries 2 and 3, Table 3). As an efficient mechanochemical Knoevenagel-condensation has already been reported [52], alkynylalcohol was tested in order to evaluate the hydroxyl group’s susceptibility to dehydratation towards conjugated π double bonds (please check meaning) (Entry 9, Table 3). A diyne was tested so as to explore the efficiency of the protocol in the preparation of dimers.
Table 3.
Synthetic results of mechanochemical CuAAC a.
| Entry | Alkyne | Azide | Product | Yield b % (Conv. c) |
|---|---|---|---|---|
| 1 d | 2a |
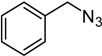 1b |
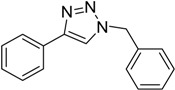 3b |
98 (>99) |
| 2 d | 2a |
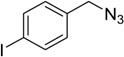 1c |
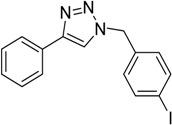 3c |
97 (>99) |
| 3 | 2a |
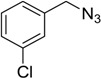 1d |
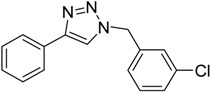 3d |
95 (>99) |
| 4 d | 2a |
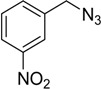 1e |
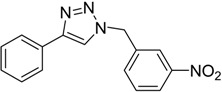 3e |
91 (95) |
| 4 d | 2a |
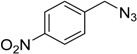 1f |
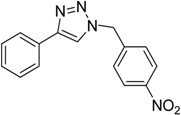 3f |
94 (97) |
| 5 d | 2a |
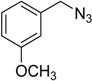 1g |
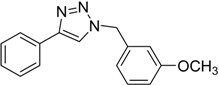 3g |
97 (>99) |
| 6 | 2a |
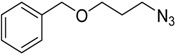 1h |
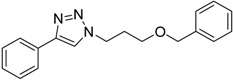 3h |
90 (92) |
| 7 d |
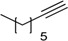 2b |
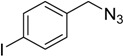 1i |
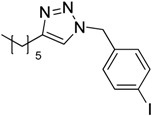 3i |
98 (>99) |
| 8 |
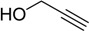 2c |
1a |
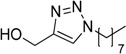 3j |
92 (96) |
| 9 |
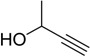 2d |
1a |
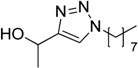 3k |
88 (91) |
| 10 e |
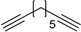 2e |
1a |
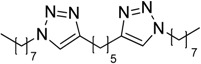 3l |
98 (>99) |
| 11 f | 2a |
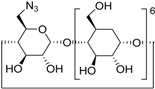 1j |
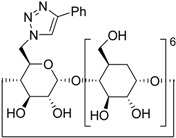 3m |
81 |
a: Reaction conditions: azide 1 (1 mmol), alkyne 2 (1 mmol), Cu powder (1 mmol), 5 min, 650 rpm; stainless steel jar (50 mL), 1500 small balls and 48 medium balls; b: Isolated yield, compound purity proved by 1H-NMR and 13C-NMR (see Supporting Info); c: Determined by GC-MS; d: Reaction time 10 min; e: Excess 1a (2 mmol); f: Reaction condition: 0.1 mmol 1j (6-monoazido-β-CD) (0.1 mmol), 2a (0.5 mmol), Cu powder (0.1 mmol), 30 min.
As depicted in Table 3, all the reactions afforded pure triazole derivatives in high yields via simple Cu powder filtration. The high efficiency of the method was proven by the fast double 1,3-dipolar cycloadditions (5 min) of entry 10 (Table 3).
CuAAC has been widely used in cyclodextrin (CD) functionalization, however Cu(II) salts tend to generate green-greyish coloured CD adducts because of the CD cavity’s excellent cation complexation properties [53]. This means that time-consuming competitive chelant purification methods become necessary. This drawback can be avoided by means of a solid supported Cu catalyst or using metallic copper. We have seen [35,54], that the click reaction afforded the desired 6-monotriazolyl-β-CD derivative when reacted in DMF in the presence of copper power under US or a combined MW/US irradiation. Gratifyingly, even better results can be achieved in PM (30 min) in solvent-free conditions, giving the CD derivatives as white powders and in high yields, as shown in Table 3 (entry 11). Considering the typical complications that occur in CD functionalization (low yield and low regioselectivity), the outstanding efficiency of CuAAC under mechanochemical activation may open the door to a totally new synthetic approach.
3. Experimental Section
3.1. General Information
All chemicals were purchased from Sigma-Aldrich (Milan, Italy) and used without further purification. Cu reduced powder RPE, purity >98%, was purchased from Carlo Erba (Milan, Italy). β-CD was kindly provided by Wacker Chemie (München, Germany). Reactions were monitored by TLC on Merck 60 F254 (0.25 mm) plates (Milan, Italy), which were visualized by UV inspection and/or by heating after a spraying with 5% H2SO4 in ethanol or phosphomolybdic acid. Mechanochemical reactions were carried out in a Planetary Ball Mill (PM100 Retsch GmbH, Haan, Germany) using either 50 or 250 mL grinding jars and milling balls (both made in stainless steel). NMR spectra (300 MHz and 75 MHz for 1H and 13C, respectively) were recorded on a Bruker 300 Avance instrument (Milan, Italy) at 25 °C. Chemical shifts were calibrated to the residual proton and carbon resonances of the solvent; DMSO-d6 (δH = 2.54, δC = 39.5), CDCl3 (δH = 7.26, δC = 77.16). Chemical shifts (δ) are given in ppm, and coupling constants (J) in Hz. GC-MS analyses were performed in a GC Agilent 6890 (Agilent Technologies, Santa Clara, CA, USA) that was fitted with a mass detector Agilent Network 5973, using a 30 m capillary column, i.d. of 0.25 mm and film thickness 0.25 μm. GC conditions were: injection split 1:20, injector temperature 250 °C, detector temperature 280 °C. Gas carrier: helium (1.2 mL/min), temperature program: from 70 °C (2 min) to 300 °C at 5 °C/min. HRMS was determined using a MALDI-TOF mass spectra (Bruker Ultraflex TOF mass spectrometer, Milan, Italy).
3.2. General Reaction Protocols
3.2.1. General Procedure A for Alkyne-Azide Click Reaction (Preparation of 3a, 3d, 3h)
The milling jar (50 mL; stainless steel) were equipped with 1500 milling balls (d = 2 mm, stainless steel) and 48 medium balls (d = 5 mm, stainless steel). Afterwards the alkyne (1 mmol), the azide (1 mmol) and Cu powder (1 mmol, 63 mg) were added in the given order. Milling was accomplished at 650 rpm for 5 min. After cooling of the milling jar to room temperature, the crude products were filtered on Büchner funnel with a sintered glass disc using diethyl acetate (3 × 10 mL). The solvent was evaporated in vacuum, the crude products were dried and analyzed by GC-MS, 1H-, 13C-NMR spectroscopy and MALDI-TOF mass spectrometry after dissolution in an appropriate solvent.
3.2.2. General Procedure B for Alkyne-Azide Click Reaction (Preparation of 3b, 3c, 3e, 3f, 3g, 3i)
The milling jar (50 mL; stainless steel) were equipped with 1,500 milling balls (d = 2 mm, stainless steel) and 48 medium balls (d = 5 mm, stainless steel). Afterwards the alkyne (1 mmol), the azide (1 mmol) and Cu powder (1 mmol, 63 mg) were added in the given order. Milling was accomplished at 650 rpm for 10 min. After cooling of the milling jar to room temperature, the crude products were filtered on Büchner funnel with a sintered glass disc using diethyl acetate (3 × 10 mL). The solvent was evaporated in vacuum, the crude products were dried and analyzed by GC-MS, 1H-, 13C-NMR spectroscopy and MALDI-TOF mass spectrometry after dissolution in an appropriate solvent.
3.2.3. General Procedure C for Alkyne-Azide Click Reaction (Preparation of 3l)
The milling jar (50 mL; stainless steel) were equipped with 1,500 milling balls (d = 2 mm, stainless steel) and 48 medium balls (d = 5 mm, stainless steel). Afterwards the alkyne (1 mmol), the azide (2 mmol) and Cu powder (1 mmol, 63 mg) were added in the given order. Milling was accomplished at 650 rpm for 5 min. After cooling of the milling jar to room temperature, the crude product was filtered on Büchner funnel with a sintered glass disc using diethyl acetate (3 × 10 mL). The solvent was evaporated in vacuum, the crude products were dried and analyzed by GC-MS, 1H-, 13C-NMR spectroscopy and MALDI-TOF mass spectrometry after dissolution in an appropriate solvent.
3.2.4. General Procedure D for Alkyne-Azide Click Reaction (Preparation of 3m)
The milling jar (50 mL; stainless steel) were equipped with 1500 milling balls (d = 2 mm, stainless steel) and 48 medium balls (d = 5 mm, stainless steel). Afterwards the alkyne (0.5 mmol), the azide (0.1 mmol) and Cu powder (0.1 mmol, 6.3 mg) were added in the given order. Milling was accomplished at 650 rpm for 30 min. After cooling of the milling jar to room temperature, the crude product was was purified by flash chromatography on reverse phase (RP18, water-methanol). The solvent was evaporated in vacuum, the crude products were dried and analyzed by GC-MS, 1H, 13C-NMR spectroscopy and MALDI-TOF mass spectrometry after dissolution in an appropriate solvent.
4. Conclusions
In conclusion, we have developed an efficient and versatile mechanochemical CuAAC procedure using copper powder as a catalyst. The reaction was performed in a planetary ball mill without solvent and we have proven that the method is fast and efficient, affording the products in high yield after a simple work-up. The scaling-up of the octyl azide and phenylacetylene reaction followed a simple numbering-up procedure from milligrams to multigram scale production, and afforded the corresponding triazole derivative in an almost quantitative yield and a short reaction time (5 min).
Acknowledgments
The University of Turin is acknowledged for its financial support (Fondi Ricerca Locale 2013).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/02/2837/s1.
Author Contributions
L. Rinaldi, K. Martina and G. Cravotto designed the investigation and wrote the paper; L. Rinaldi, F. Baricco and L. Rotolo performed all the experiments and the analyses. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 3a–3m are available from the authors.
References
- 1.Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A., editor. Volume 1. Wiley; New York, NY, USA: 1984. pp. 1–176. [Google Scholar]
- 2.Padwa A. Intermolecular 1,3-dipolar cycloaddition. In: Trost B.M., editor. Comprehensive Organic Synthesis. Volume 4. Pergamon; Oxford, UK: 1991. pp. 1069–1109. [Google Scholar]
- 3.Tornøe C.W., Christensen C., Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002;67:3057–3062. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 4.Kolb H.C., Finn M.G., Sharpless K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Meldal M., Tornøe C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 7.Whiting M., Tripp J.C., Lin Y.C., Lindstrom W., Olson A.J., Elder J.H., Sharpless K.B., Fokin V.V. Rapid discovery and structure-activity profiling of novel inhibitors of human immunodeficiency virus type 1 protease enabled by the copper(I)-catalyzed synthesis of 1,2,3-triazoles and their further functionalization. J. Med. Chem. 2006;49:7697–7710. doi: 10.1021/jm060754+. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q., Chan T.R., Hilgraf R., Fokin V.V., Sharpless K.B., Finn M.G. Bioconjugation by copper(I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 9.Hawker C.J., Fokin V.V., Finn M.G., Sharpless K.B. Bringing Efficiency to Materials Synthesis: The Philosophy of Click Chemistry. Aust. J. Chem. 2007;60:381–383. doi: 10.1071/CH07107. [DOI] [Google Scholar]
- 10.Lévêque J.-M., Cravotto G. Microwaves, power ultrasound, and ionic liquids. A new synergy in green organic synthesis. Chimia. 2006;60:313–320. doi: 10.2533/000942906777836255. [DOI] [Google Scholar]
- 11.Cravotto G., Cintas P. The Combined Use of Microwaves and Ultrasound. In: de La Hoz A., Loupy A., editors. Methods and Practice, Microwaves in Organic Synthesis. 3rd ed. Volume 1. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2012. pp. 541–562. [Google Scholar]
- 12.Bruckmann A., Krebs A., Bolm C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008;10:1131–1141. doi: 10.1039/b812536h. [DOI] [Google Scholar]
- 13.Wang G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013;42:7668–7700. doi: 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- 14.Barge A., Tagliapietra S., Binello A., Cravotto G. Click chemistry under microwave or ultrasound irradiation. Curr. Org. Chem. 2011;15:189–203. doi: 10.2174/138527211793979826. [DOI] [Google Scholar]
- 15.Kappe C.O., van der Eycken E. Click chemistry under non-classical reaction conditions. Chem. Soc. Rev. 2010;39:1280–1290. doi: 10.1039/b901973c. [DOI] [PubMed] [Google Scholar]
- 16.Strack M., Langklotz S., Bandow J.E., Metzler-Nolte N., Albada H.B. Silyl-Based Alkyne-Modifying Linker for the Preparation of C-Terminal Acetylene-Derivatized Protected Peptides. J. Org. Chem. 2012;77:9954–9958. doi: 10.1021/jo302305d. [DOI] [PubMed] [Google Scholar]
- 17.Capicciotti C.J., Trant J.F., Leclere M., Ben R.N. Synthesis of C-Linked Triazole-Containing AFGP Analogues and their Ability to Inhibit Ice Recrystallization. Bioconjugate Chem. 2011;22:605–616. doi: 10.1021/bc100394k. [DOI] [PubMed] [Google Scholar]
- 18.Glowacka I.E., Balzarini J., Wroblewski A.J.E. Synthesis and biological evaluation of novel 1,2,3-triazolonucleotides. Arch. Pharm. 2013;346:278–291. doi: 10.1002/ardp.201200421. [DOI] [PubMed] [Google Scholar]
- 19.Isobe H., Fujino T., Yamazaki N., Guillot-Nieckowski M., Nakamura E. Triazole-linked analogue of deoxyribonucleic acid (TLDNA): Design, synthesis, and double-strand formation with natural DNA. Org. Lett. 2008;10:3729–3732. doi: 10.1021/ol801230k. [DOI] [PubMed] [Google Scholar]
- 20.Barge A., Caporaso M., Cravotto G., Martina K., Tosco P., Aime S., Carrera C., Gianolio E., Pariani G., Corpillo D. Design and Synthesis of a γ(1)β(8)-Cyclodextrin Oligomer: A New Platform with Potential Application as a Dendrimeric Multicarrier. Chemistry. 2013;19:12086–12092. doi: 10.1002/chem.201301215. [DOI] [PubMed] [Google Scholar]
- 21.Carmona T., Marcelo G., Rinaldi L., Martina K., Cravotto G., Mendicuti F. Soluble cyanine dye/β-cyclodextrin derivatives: Potential carriers for drug delivery and optical imaging. Dyes Pigments. 2015;114:204–214. doi: 10.1016/j.dyepig.2014.11.014. [DOI] [Google Scholar]
- 22.Li Y., Wang J., Cai C.-Z. Rapid Grafting of Azido-Labeled Oligo(ethylene glycol)s onto an Alkynyl-Terminated Monolayer on Nonoxidized Silicon via Microwave-Assisted “Click” Reaction. Langmuir. 2011;27:2437–2445. doi: 10.1021/la104060j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daugaard A.E., Hansen T.S., Larsen N.B., Hvilsted S. Microwave assisted click chemistry on a conductive polymer film. Synth. Met. 2011;161:812–816. doi: 10.1016/j.synthmet.2011.02.005. [DOI] [Google Scholar]
- 24.Martina K., Baricco F., Berlier G., Caporaso M., Cravotto G. Efficient green protocols for the preparation of highly functionalized β-cyclodextrin grafted silica. ACS Sustain. Chem. Eng. 2014;2:2595–2603. doi: 10.1021/sc500546e. [DOI] [Google Scholar]
- 25.Tagliapietra S., Cravotto G., Gaudino E.C., Visentin S., Mussi V. Functionalization of Single-Walled Carbon Nanotubes through 1,3-Cycloaddition of Carbonyl Ylides under Microwave Irradiation. Synlett. 2012;23:1459–1462. doi: 10.1055/s-0031-1290681. [DOI] [Google Scholar]
- 26.Nagao Y., Takasu A. “Click polyester”: Synthesis of polyesters containing triazole units in the main chain via safe and rapid “click” chemistry and their properties. J. Polym. Sci. Pol. Chem. 2010;48:4207–4218. doi: 10.1002/pola.24206. [DOI] [Google Scholar]
- 27.Marullo S., D’Anna F., Rizzo C., Noto R. The ultrasound-ionic liquids synergy on the copper catalyzed azide–alkyne cycloaddition between phenylacetylene and 4-azidoquinoline. Ultrason. Sonochem. 2015;23:317–323. doi: 10.1016/j.ultsonch.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Cintas P., Palmisano G., Cravotto G. Power ultrasound in metal-assisted synthesis: From classical Barbier-like reactions to click chemistry. Ultrason. Sonochem. 2011;18:836–841. doi: 10.1016/j.ultsonch.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Cintas P., Carnaroglio D., Rinaldi L., Cravotto G. Complementary and synergic effects of microwaves and ultrasound in metal-assisted synthesis. Chem. Today. 2012;30:33–35. [Google Scholar]
- 30.Cravotto G., Calcio Gaudino E., Cintas P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 2013;42:7521–7534. doi: 10.1039/c2cs35456j. [DOI] [PubMed] [Google Scholar]
- 31.Antilla J.C., Klapars A., Buchwald S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002;124:11684–11688. doi: 10.1021/ja027433h. [DOI] [PubMed] [Google Scholar]
- 32.Himo F., Lovell T., Hilgraf R., Rostovtsev V.V., Noodleman L., Sharpless K.B., Fokin V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 33.Gommermann N., Gehrig A., Knochel P. Enantioselective Synthesis of Chiral α-Aminoalkyl-1,2,3-triazoles Using a Three-Component Reaction. Synlett. 2005:2796–2798. doi: 10.1055/s-2005-918931. [DOI] [Google Scholar]
- 34.Cintas P., Barge A., Tagliapietra S., Boffa L., Cravotto G. Alkyne-azide click reaction catalyzed by metallic copper under ultrasound. Nat. Prot. 2010;5:607–616. doi: 10.1038/nprot.2010.1. [DOI] [PubMed] [Google Scholar]
- 35.Cravotto G., Fokin V.V., Garella D., Binello A., Boffa L., Barge A. Ultrasound-Promoted Copper-Catalyzed Azide-Alkyne Cycloaddition. J. Comb. Chem. 2010;12:13–15. doi: 10.1021/cc900150d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cravotto G., Cintas P. Harnessing mechanochemical effects with ultrasound-induced reactions. Chem. Sci. 2012;3:295–307. doi: 10.1039/c1sc00740h. [DOI] [Google Scholar]
- 37.Cintas P., Martina K., Robaldo B., Garella D., Boffa L., Cravotto G. Improved protocols for microwave-assisted Cu(I)-catalyzed Huisgen 1,3-dipolar cycloadditions. Collect. Czech. Chem. C. 2007;72:1014–1024. doi: 10.1135/cccc20071014. [DOI] [Google Scholar]
- 38.Balaz P., Achimovicova M., Balaz M., Billik P., Cherkezova-Zheleva Z., Criado J.M., Delogu F., Dutkova E., Gaffet E., Gotor F.J., et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013;42:7571–7637. doi: 10.1039/c3cs35468g. [DOI] [PubMed] [Google Scholar]
- 39.Stolle A., Schmidt R., Jacob K. Scale-up of organic reactions in ball mills: Process intensification with regard to energy efficiency and economy of scale. Faraday Discuss. 2014;170:267–286. doi: 10.1039/C3FD00144J. [DOI] [PubMed] [Google Scholar]
- 40.Braga D., Addario D.D., Polito M., Grepioni F. Mechanically induced expeditious and selective preparation of disubstituted pyridine/pyrimidine ferrocenyl complexes. Organometallics. 2004;23:2810–2812. doi: 10.1021/om049958j. [DOI] [Google Scholar]
- 41.Ranu B.C., Stolle A. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges. Royal Society of Chemistry; London, UK: 2014. [Google Scholar]
- 42.Cravotto G., Garella D., Carnaroglio D., Calcio Gaudino E., Rosati O. Solvent-free chemoselective oxidation of thioethers and thiophenes by mechanical milling. Chem. Commun. 2012;48:11632–11634. doi: 10.1039/c2cc36365h. [DOI] [PubMed] [Google Scholar]
- 43.Trotzki R., Hoffmann M.M., Ondruschka B. Studies on the solvent-free and waste-free Knoevenagel condensation. Green Chem. 2008;10:767–772. doi: 10.1039/b801661e. [DOI] [Google Scholar]
- 44.Tan Y.-J., Zhang Z., Wang F.-J., Wu H.-H., Li Q.-H. Mechanochemical milling promoted solvent-free imino Diels-Alder reaction catalyzed by FeCl3: Diastereoselective synthesis of cis-2,4-diphenyl-1,2,3,4-tetrahydroquinolines. RSC Adv. 2014;4:35635–35638. doi: 10.1039/C4RA05252H. [DOI] [Google Scholar]
- 45.Groote R., Szyja B.M., Leibfarth F.A., Hawker C.J., Doltsinis N.L., Sijbesma R.P. Strain-Induced Strengthening of the Weakest Link: The Importance of Intermediate Geometry for the Outcome of Mechanochemical Reactions. Macromolecules. 2014;47:1187–1192. doi: 10.1021/ma4022339. [DOI] [Google Scholar]
- 46.Zhu S.-E., Li F., Wang G.-W. Mechanochemistry of fullerene and related materials. Chem. Soc. Rev. 2013;42:7535–7570. doi: 10.1039/c3cs35494f. [DOI] [PubMed] [Google Scholar]
- 47.Schneider F., Szuppa T., Stolle A., Ondruschka B., Hopf H. Energetic assessment of the Suzuki-Miyaura reaction: A curtate life cycle assessment as an easily understandable and applicable tool for reaction optimization. Green Chem. 2009;11:1894–1899. doi: 10.1039/b915744c. [DOI] [Google Scholar]
- 48.Thorwirth R., Stolle A., Ondruschka B., Wild A., Schubert U.S. Fast, ligand- and solvent-free copper-catalyzed click reactions in a ball mill. Chem. Commun. 2011;47:4370–4372. doi: 10.1039/c0cc05657j. [DOI] [PubMed] [Google Scholar]
- 49.Tan D., Mottillo C., Katsenis A.D., Štrukil V., Friščić T. Development of C-N coupling using mechanochemistry: Catalytic coupling of arylsulfonamides and carbodiimides. Angew. Chem. Int. Ed. 2014;53:9321–9324. doi: 10.1002/anie.201404120. [DOI] [PubMed] [Google Scholar]
- 50.Boldyrev V.V. ; Mechanochemistry and sonochemistry. Ultrason. Sonochem. 1995;2:S143–S145. doi: 10.1016/1350-4177(95)00019-3. [DOI] [Google Scholar]
- 51.Cave G.W.V., Raston C.L., Scotta J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001:2159–2169. doi: 10.1039/B106677N. [DOI] [PubMed] [Google Scholar]
- 52.Polshettiwar V., Varma R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008;41:629–639. doi: 10.1021/ar700238s. [DOI] [PubMed] [Google Scholar]
- 53.Stolle A., Szuppa T., Leonhardt S.E.S., Ondruschka B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011;40:2317–2329. doi: 10.1039/c0cs00195c. [DOI] [PubMed] [Google Scholar]
- 54.Klufers P., Piotrowski H., Uhlendorf J. Homoleptic Cuprates(II) with Multiply Deprotonated α-Cyclodextrin Ligands. Chem. Eur. J. 1997;3:601–608. doi: 10.1002/chem.19970030416. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.