Table 3.
Synthetic results of mechanochemical CuAAC a.
| Entry | Alkyne | Azide | Product | Yield b % (Conv. c) |
|---|---|---|---|---|
| 1 d | 2a |
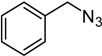 1b |
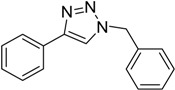 3b |
98 (>99) |
| 2 d | 2a |
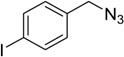 1c |
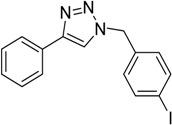 3c |
97 (>99) |
| 3 | 2a |
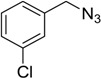 1d |
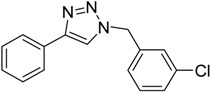 3d |
95 (>99) |
| 4 d | 2a |
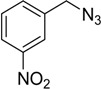 1e |
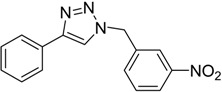 3e |
91 (95) |
| 4 d | 2a |
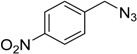 1f |
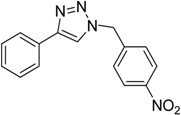 3f |
94 (97) |
| 5 d | 2a |
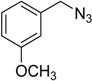 1g |
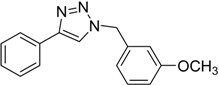 3g |
97 (>99) |
| 6 | 2a |
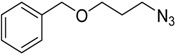 1h |
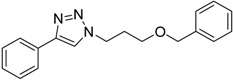 3h |
90 (92) |
| 7 d |
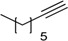 2b |
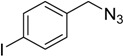 1i |
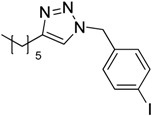 3i |
98 (>99) |
| 8 |
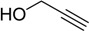 2c |
1a |
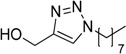 3j |
92 (96) |
| 9 |
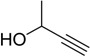 2d |
1a |
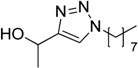 3k |
88 (91) |
| 10 e |
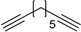 2e |
1a |
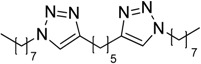 3l |
98 (>99) |
| 11 f | 2a |
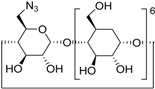 1j |
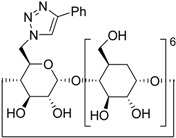 3m |
81 |
a: Reaction conditions: azide 1 (1 mmol), alkyne 2 (1 mmol), Cu powder (1 mmol), 5 min, 650 rpm; stainless steel jar (50 mL), 1500 small balls and 48 medium balls; b: Isolated yield, compound purity proved by 1H-NMR and 13C-NMR (see Supporting Info); c: Determined by GC-MS; d: Reaction time 10 min; e: Excess 1a (2 mmol); f: Reaction condition: 0.1 mmol 1j (6-monoazido-β-CD) (0.1 mmol), 2a (0.5 mmol), Cu powder (0.1 mmol), 30 min.
