Abstract
Two new compounds, fumitremorgin 12-methoxy-13-[5'-hydroxy-2'-(1''-hydroxy-3''-methoxy-5''-methylbenzoyl)-3'-methoxy]benzoic acid methyl ester (fumitremorgin D, 1) and 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (2) were isolated from the cultured endophytic isolated fungus Aspergillus fumigatus, together with fourteen known compounds. Their structures were elucidated by 1-D and 2-D NMR analyses. The cytotoxicity profile of the compound against the human hepatocellular carcinoma cell line HepG2 was evaluated by MTT antiproliferative assays.
Keywords: Diphylleia sinensis L, Aspergillus fumigates, indolediketopiperazine alkaloids, steroid, cytotoxicity
1. Introduction
Many microorganism-originated secondary metabolites have been utilized as drugs and/or lead compounds in the pharmaceutical industry [1,2]. The specific metabolic pathways, habitats and bioactivities of endophytic fungi make them a good source of structurally novel and/or biologically active secondary metabolites [3,4]. Fungi of the genus Aspergillus (Moniliaceae) have been reported as prolific producers of bioactive compounds [5,6,7,8]. In the course of our investigation of endophytic fungi harbored in plant tissues, the fungus Wrq12 was isolated from Diphylleia sinensis. L and identified as Aspergillus fumigatus. Diphylleia sinensis. L (also called “WO-ER-CHI” in Traditional Chinese Medicine), is mainly distributed in the midwest of China, and is generally used for the treatment of rheumatic arthritis, lumbocrural pain, traumatic injury, irregular menstruation, etc. [9]. Further fermentation and fractionation of the chloroform extract of A. fumigatus mediums led to the isolation of two new compounds 1–2 along with the fourteen known compounds fumitremorgin C (3) [10], 12,13-dihydroxyfumitremorgin C (4) [11], verruculogen (5) [12], 13-oxoverruculogen (6) [7], ergosteryl peroxide (7) [13], helvolic acid (8) [14], emodin 1,6-dimethyl ether (9) [15], isorhodoptilometrin (10) [16], monomethylsulochrin (11) [17], trypacidin (12) [18], fumigaclavine C (13) [19], fumigaclavine A (14) [19], fumiquinazoline C (15) [20] and pseurotin A (16) [21]. The structures of the compounds were established on the basis of spectroscopic analyses and by comparison of their data with literature values.
2. Results and Discussion
Compound 1 was obtained as a yellow amorphous powder. The IR spectroscopic data indicated the presence of ether groups (1107 cm−1 and 1241 cm−1), amide groups (1631 cm−1), an amine group (3431 cm−1) and an ester group (1723 cm−1). Its molecular formula was determined as C40H41N3O11 by HR-ESIMS (m/z 740.2806 [M+H]+, C40H42N3O11+ calc. 740.2814), requiring 22 sites of unsaturation for the whole molecule. All 40 carbons and 38 of 41 protons can be identified in the 13C- and 1H-NMR spectra of compound 1, and HSQC correlations suggest the presence of three exchangeable protons. Salient features of the molecule including seven methyl singlets, three sp3-hybridized methylenes, three sp3-hybridized and eight sp2-hybridized methines, one sp3-hybridized and fourteen sp2-hybridized quaternaries, one ketone (δ 199.9) and three ester or amide carbonyls were indicated by the NMR data.
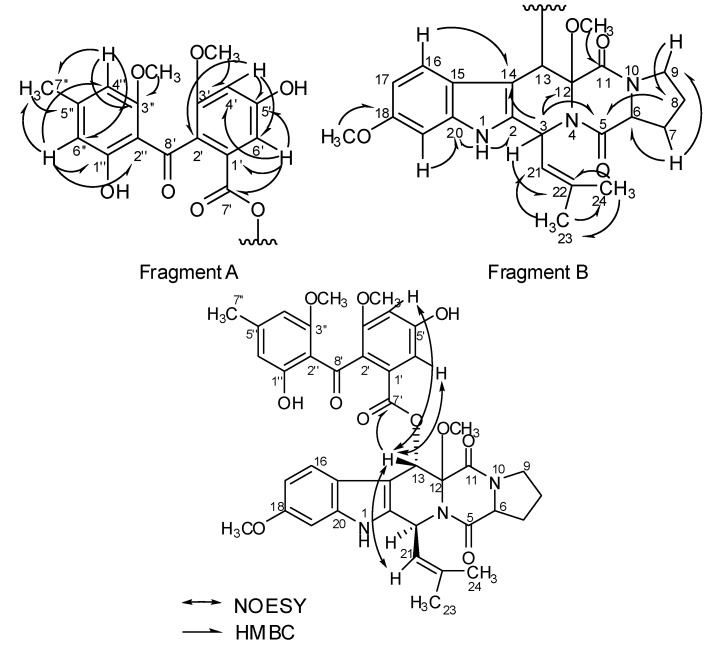
By comparison with the literature data [11], fragment B in compound 1 was most likely an indole-diketopiperazine skeleton (Figure 1), which is indicated by the carbon combination, along with the presence of an amide group and amine group. This deduction was further confirmed by a correlative interpretation of its NOESY and HMBC spectrum (Table 1). Although no direct HMBC correlation between the methoxyl group (δ 3.62 (3H, s), δ 52.1) and C-12 (δ 83.1) was observed, the chemical shift of C-12 at δ 83.1 indicated that the methoxyl group was linked at C-12.
Figure 1.
Key HMBC and NOESY correlations for compound 1.
Table 1.
NMR Spectroscopic Data (400 MHz, CDCl3) for compound 1.
| fumitremorgin D (1) | ||||||
|---|---|---|---|---|---|---|
| Position | δC, type | δH ( J in Hz) | HMBC | NOESY | ||
| 1-NH | 8.02, s | 2, 14, 15, 20 | ||||
| 2 | 130.1, C | |||||
| 3 | 50.2, CH | 5.82, d (9.5) | 2, 5, 12, 14, 21, 22, | H-16, H-21, H-24 | ||
| 4 | ||||||
| 5 | 171.1, C | |||||
| 6 | 58.8, CH | 4.45, dd (8.0, 12.0) | 5, 7 | H-7, H-8 | ||
| 7 | 29.1, CH2 | 2.06, 2.45, m | 6, 8, 9 | H-6, H-8 | ||
| 8 | 22.5, CH2 | 1.94, 2.08, m | 5 | H-6, H-9 | ||
| 9 | 45.3, CH2 | 3.64, d (8.0) | 7, 8 | H-7 | ||
| 10 | ||||||
| 11 | 166.4, C | |||||
| 12 | 83.1, C | |||||
| 13 | 68.7, CH | 5.75, s | 2, 12, 14, 7' | H-16, H-21, H-4', H-6' | ||
| 14 | 105.2, C | |||||
| 15 | 120.7, C | |||||
| 16 | 121.1, CH | 7.78, d (8.0) | 14, 18, 20 | H-13, H-19 | ||
| 17 | 109.8, CH | 6.78, d (8.0) | 15, 19 | 18-OCH3, H-16 | ||
| 18 | 156.6, C | |||||
| 19 | 95.1, CH | 6.80, s | 15, 17, 18, 20 | 18-OCH3, H-16 | ||
| 20 | 137.6, C | |||||
| 17 | 109.8, CH | 6.78, d (8.0) | 15, 19 | 18-OCH3, H-16 | ||
| 21 | 123.8, CH | 4.78, d (9.3) | 23, 24 | H-3, H-23 | ||
| 22 | 134.7, C | |||||
| 23 | 25.6, CH3 | 1.64, s | 21, 22, 24 | H-12, H-24 | ||
| 24 | 18.2, CH3 | 1.96, s | 21, 22, 23 | H-23 | ||
| 1' | 128.4, C | |||||
| 2' | 127.5, C | |||||
| 3' | 157.0, C | |||||
| 4' | 103.4, CH | 6.59, s | 108.1; 127.5; 156.9 | H-13, 3'-OCH3 | ||
| 5' | 156.9, C | |||||
| 6' | 108.1, CH | 7.00, s | 103.4; 127.5; 156.9; 166.2 | H-13 | ||
| 7' | 166.2, C | |||||
| 8' | 199.9, C | |||||
| 1'' | 164.0, C | |||||
| 2'' | 110.5, C | |||||
| 3'' | 161.0, C | |||||
| 4'' | 103.0, CH | 6.06, s | 8', 3'', 6'', 7'' | H-7'', 3''-OCH3 | ||
| 5'' | 148.0, C | |||||
| 6'' | 110.9, CH | 6.45, s | 1'', 2'',4'', 7'' | H-7'' | ||
| 7'' | 22.4, CH3 | 2.28, s | 4'', 5'' | H-4'', H-6'' | ||
| C1''-OH | 12.99, s | 1'', 5'', 6'' | H-3''-OCH3 | |||
| 18-OCH3 | 55.7, CH3 | 3.81, s | 18 | H-19 | ||
| 3''-OCH3 | 55.7, CH3 | 3.36, s | 13'' | H-4'' | ||
| 3'-OCH3 | 56.1, CH3 | 3.63, s | 3' | H-4' | ||
| 12-OCH3 | 52.1, CH3 | 3.62, s | 11 | H-8 | ||
The remaining signals of compound 1 were identified by the HMBC data (Figure 1) and comparisons with the literature data [17]. The HMBC correlations between δ 6.06 (s, H-4'') and carbon resonance at δ 22.4 (7''-CH3), δ 110.9 (C-6'') and δ 161.0 (C-3'') together with the correlations between δ 6.45 (s, H-6'') and carbon resonance at δ 22.4 (7''-CH3), δ 103.0 (C-4''), δ 110.5 (C-2'') and δ 164.0 (C-1'') suggested a 1'', 2'', 3'' and 5''-tetrasubstituted aromatic ring. Likewise, another 1', 2', 3' and 5'-tetrasubstituted aromatic ring was identified by the HMBC correlations. The structure of fragment A was confirmed as rhizoctonic acid. It can directly be located at C-13 by key HMBC correlation from δ 5.75 (H-13) to δ 166.2 (C-7'). This linkage was also supported by the key NOESY correlation (Figure S10–S11 Supplementary Files) between H-13 (δ 5.75) and both H-4' (δ 6.59) and H-6' (δ 7.00). The observation of a NOESY correlation between H-13 (δ 5.75) and H-21 (δ 4.78) showed H-13 was cis to the 2-methylprop-1-ene moiety and trans to H-3 as shown (Figure 1). Thus, the gross structure of compound 1 was determined as 12-methoxy-13-[5'-hydroxy-2'-(1''-hydroxy-3''-methoxy-5''-methyl-benzoyl)-3'-methoxy]benzoic acid methyl ester-fumitremorgin, which was named fumitremorgin D.
Compound 2 was isolated as an amorphous solid. Its molecular formula was determined as C32H46O6 by HR-ESI(+)MS [M+Na]+ m/z 509.2878 (calcd for C29H42NaO6+, 509.2874), requiring nine sites of unsaturation. The 1H- and 13C-NMR data (Table 2) revealed 27 carbon resonance lines and all 42 protons. Scrutiny of its 1H- and 13C-NMR data, in correlation with DEPT and HSQC experiments, indicated the 13C resonances of 14-CH3 and 23-CH3 overlap (δ 17.5). The 13C resonances appearing in δ 40.5 (C-15), was covered by the DMSO peaks. Thus, the 1H, 13C, DEPT and HSQC NMR data for compound 2 revealed the presence of eight methyl singlets, five sp3-hybridized methylenes, five sp3-hybridized and three sp2-hybridized methines, three sp3-hybridized and one sp2-hybridized quaternaries, two ketones (δ 200.8 and 209.3) and two ester carbons. These carbon combinations indicated that compound 2 was most likely a helvolic acid derivative [14].
Table 2.
NMR data for compound 2 in DMSO-d6.
| 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (2) | |||||
|---|---|---|---|---|---|
| Position | δC, type | δH ( J in Hz) | HMBC | COSY | NOESY |
| 1 | 158.4, CH | 7.42, d (10.0) | 3, 5, 10 | ||
| 2 | 126.8, CH | 5.76, d (10.0) | 10 | H-1 | |
| 3 | 200.8, C | ||||
| 4 | 39.5, CH | 2.74, m | 4-CH3 | ||
| 4-CH3 | 12.3, CH3 | 1.12, d (7.2) | 3, 4, 5 | H-4 | H-6 |
| 5 | 45.6, CH | 2.41, m | 4 | H-4 | H-6, H-8-CH3 |
| 6 | 73.0, CH | 5.04, s | 7, 8, 10, 6-OAc | H-5, H-4-CH3 | |
| 6-OAc | 169.0, C | ||||
| CH3 | 20.4, CH3 | 2.09, s | 6-OAc | ||
| 7 | 209.3, C | ||||
| 8 | 52.3, CH | ||||
| 8-CH3 | 17.5, CH3 | 1.10, s | 7, 8, 9, 14 | H-5, H-15 | |
| 9 | 41.2, CH | 2.54, m | |||
| 10 | 37.7, C | ||||
| 10-CH3 | 27.1, CH3 | 1.39, s | 1, 5, 9, 10 | H-4 | |
| 11 | 23.3, CH2 | 1.57, 1.87, m | 12 | ||
| 12 | 25.5, CH2 | 1.70, 2.29, m | H-13 | ||
| 13 | 29.0, CH2 | 2.31, 1.22, m | H-12 | ||
| 14 | 46.1, C | ||||
| 14-CH3 | 17.5, CH3 | 0.81, s | 8, 14, 15 | ||
| 15 | 40.5, CH2 | 1.64, 2.05, d (8.4) | 13, 16, 14-CH3 | ||
| 16 | 73.3, CH | 5.70, br | 14 | H-17 | |
| 16-OAc | 169.9, C | ||||
| CH3 | 20.5, CH3 | 1.87, s | 16-OAc | ||
| 17 | 28.1, CH2 | 1.99; 2.09, m | H-16 | ||
| 18 | 124.1, CH | 5.11, t (8.0) | H-17 | ||
| 19 | 130.8, C | ||||
| 20 | 17.5, CH3 | 1.58, s | 21, 22, 24 | ||
| 21 | 20.5, CH3 | 1.65, s | 21, 22, 23 | ||
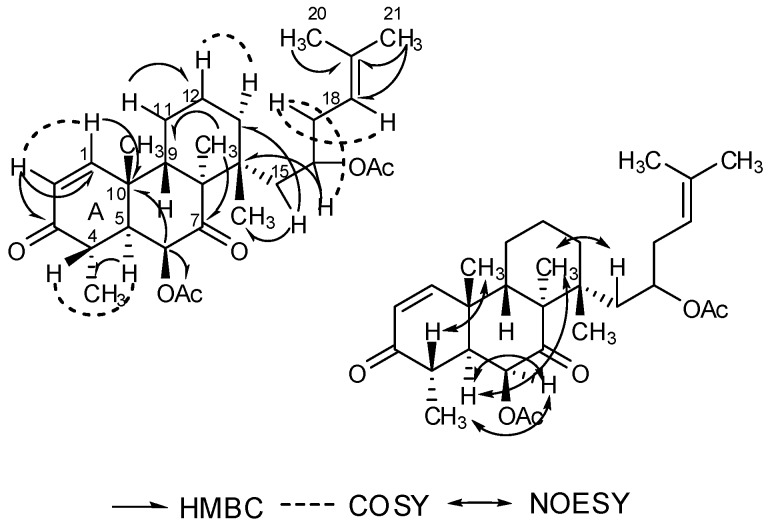
We confirmed the assignment of a tricyclic skeleton (A, B and C ring) by correlative interpretation of its COSY, NOESY, HSQC and HMBC spectroscopic data and comparison with literature [14]. The HMBC data from H-15 (δ 1.64) to 14-CH3 (δ 17.5) and from H-16 (δ 5.70) to C-14 (δ 46.1) suggest a side chain connected at the site of C-14 of the skeleton. HMBC correlations from δ 1.58 and δ 1.65 to δ 124.1 and δ 130.8 confirmed a 2-methy-2-yl-butene. The COSY data between H-18 (δ 5.11) and H-17 (δ 2.09), and H-16 (δ 5.70) and H-17 (δ 2.09) indicated the connection between C-16 and C-17 (Figure 2).
Figure 2.
Key NMR correlations for compound 2.
The relative configuration of compound 2 was determined by the NOESY correlations and comparison with literature data [14]. The NOESY correlations between H-15 (δ 2.05) and 8-CH3 (δ 1.10) showed that the substituent group and 8-CH3 were oriented cis to each other. Comprehensive analyses of MS and NMR data led to the structural elucidation of compound 2 as 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione. Structurally, this compound is the first example of a helvolic acid derivative possessing a phenanthrene skeleton.
The cytotoxic activities of all compounds were evaluated by an MTT assay using the HepG2 cell line. Compounds 1 and 2 both showed weak cytotoxicity in this assay, with IC50 values of 47.5 μM and 139.9 μM, respectively. It was noteworthy that 12,13-dihydroxyfumitremorgin C (4), and verruculogen (5) showed cytotoxic activity against the HepG2 cell line with IC50 values of 4.5 μM and 9.8 μM, respectively. Despite the lack of a macrocyclic structure, 12,13-dihydroxyfumitremorgin C (4) showed improved activity over verruculogen (5), suggesting that the macrocyclic linking at 1-N and 3-C does not play a crucial role in the observed cytotoxicity. Meanwhile, compounds 1, 3 and 6 lacking C-12 and/or C-13 hydroxyls, showed IC50 values of 47.5, 156.5 and 44.9 μM, respectively. Based on above results, the simultaneous presence of hydroxyls at C-12 and C-13 showed an important structure-activity relationship (SAR) to the cytotoxic activity of indolediketopiperazine alkaloids against the HepG2 cell line.
3. Experimental Section
3.1. General Procedures
Chemical shifts are given in δ (ppm) with the residual solvent peak referenced to δH 7.27 and δC 77.0 for CDCl3 and δH 3.41, 2.51 and δC 39.5 for DMSO. Column chromatography: (10–40 μm; Marine Chemical Factory, Qingdao, China); Sephadex LH-20 (Amersham Pharmacia Biotech, Uppsala, Sweden); RP-C18 gel (ODS LiChrosorb RP-18, Merck, Darmstadt, Germany) were used for column chromatography. NMR spectroscopic data: Bruker Avance III 400MHz; δ in ppm with SiMe4 as internal standard. MS: Bruker micrOTOF-QII mass spectrometer for HR-ESI; IR spectroscopic data: Nexus 670 (Nicolet, Waltham, MA, USA).
3.2. Isolation and Cultivation of the Fungus
All plant tissues (mainly roots or rhizomes) of D. sinensis L. which were collected from Honghegu (Shanxi Province, China), were cleaned in running tap water and any visibly diseased or damaged material was eliminated. All tissues were surface disinfected with 75% ethanol for 1 min and thrice in sterile distilled water, then disinfected for 5 min in 0.1% mercuric chloride solution and thrice in sterile distilled water and step 1 was then repeated again. After surface sterilization and removal of epidermis the phloem was cut into 0.5–1 cm fragments and inoculated on potato dextrose agar medium (five pieces each) for 3–10 days at 28 °C. Individual colonies were transferred onto new potato dextrose agar medium for further analysis and maintenance. Based on 16s rDNA sequence analysis, strain Wrq12 was classified as A. fumigatus. The strain A. fumigatus Wrq12 is deposited in the China General Microbiological Culture Collection Center (CGMCC No. 3785).
3.3. Preparative Cultivation and Isolation
Forty 500-mL round-bottomed flasks of rice mediums were inoculated with A. fumigatus. The flasks were incubated at 27 °C in a constant temperature incubator for 30 days, and then extracted with CDCl3 five times to yield 26.4 g of extract after solvent removal. The extract was then partitioned using CHCl3/MeOH in a gradient 10:0 to 0:10 elution silica gel column to yield 15 fractions (Fr. 1-Fr. 17). Fraction 12 was chromatographed using CHCl3/MeOH in a gradient (10:0 to 3:7) to yield compounds 13–14. Fraction 10 was fractionated by silica gel column chromatography using CHCl3/MeOH in a gradient (10:0 to 0:10) to yield 12 fractions (Fr.10-1-Fr.10-12). Fraction 10-6 was chromatographed over a Sephadex LH-20 column, as eluting solvent CHCl3/MeOH (1:1) to afford nine fractions (Fr.10-6-9-1-Fr.10-6-9-9). Fractions were further fractionated by repeated column chromatography on Sephadex LH-20 using CHCl3/MeOH (1:1) together with ODS column chromatography using MeOH/H2O as eluting solvent to afford compounds 1, 2, 4, 8 and 10. Fraction 10-5 was fractionated by an ODS column using MeOH/H2O in a gradient (1:1 to 8:2) to yield compound 11. Fraction 10-4 was further fractionated by Sephadex LH-20 chromatography using CHCl3/MeOH (1:1) as eluting solvent to afford compounds 5–7 and 9. Fraction 10-2 was fractionated by silica gel column chromatography using CHCl3/MeOH in a gradient (9:1 to 7:3) to yield compound 12. Fraction 10-3 was further fractionated by ODS column chromatography using MeOH/H2O in a gradient (1:1 to 9:1) to yield compounds 3 and 15. Fraction 10-7 was fractionated by silica gel column chromatography using CHCl3/MeOH in a gradient (10:0 to 9:1) to yield compound 16. In TLC tests, all compounds isolated showed spots with the same Rf value and TLC color display as the CDCl3 extract, indicating that all compounds were isolated from the raw extract.
3.4. Cytotoxic Activity Assays
Cytotoxicity was evaluated by the MTT method using HepG2 cell lines. The cell line was grown in DMEM supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Then 100 μL of these cell suspensions at a density of 5 × 103 cell per well was plated in 96-well plates and incubated for 16–18 h under the above condition. Then the test compound solutions (in DMSO) were mixed with culture medium and cells were treated with them at gradient concentrations (12.75 μM, 25 μM, 50 μM, 100 μM, 200 μM). After further incubation under the same condition for 24 h, 10 μL of the MTT solution (5 mg/mL in DMEM medium) was added to each well and incubated for 4 h. The old medium containing MTT was then gently replaced by DMSO and standing 20 min to dissolve formazan crystals. Absorbance was then determined on an IMark (Bio-Rad, Hercules, CA, USA) plate reader at 490 nm.
3.5. Analytical Data
Fumitremorgin 12-methoxy-13-[5'-hydroxy-2'-(1''-hydroxy-3''-methoxy-5''-methylbenzoyl)-3'-methoxy]-benzoic acid methyl ester (fumitremorgin D, 1): Amorphous yellow solid; IR νmax 1107, 1241, 1631, 3431, 1723 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 12.99 (1H, s, C1''-OH), 8.02 (1H, s, H-1) , 7.78 (1H, d, J = 8.0, H-16), 7.00 (1H, s, H-6'), 6.80 (1H, s, H-19), 6.78 (1H, d, J = 8.0, H-17), 6.59 (1H, s, H-4') , 6.45 (1H, s, H-6''), 6.06 (1H, s, H-4''), 5.82 (1H, d, J = 9.5 Hz, H-3), 5.75 (1H, s, H-13), 4.78 (1H, d, J = 9.3, H-21), 4.45 (1H, dd, J = 8.0, 12.0 Hz, H-6), 3.81 (3H, s, 18-OCH3), 3.64 (2H, d, J = 8.0, H-9), 3.63 (3H, s, 3'-OCH3), 3.62 (3H, s, 12-OCH3), 3.36 (3H, s, 3''-OCH3), 2.45–2.06 (2H, m, H-7), 2.28 (3H, s, H-7''), 2.08–1.94 (2H, m, H-8), 1.96 (3H, s, H-24), 1.64 (3H, s, H-23); 13C-NMR (CDCl3, 100 MHz) δ 130.1 (C, C-2), 50.2 (CH, C-3), 171.1 (C, C-5), 58.8 (CH2, C-6), 29.1 (CH2, C-7), 22.5 (CH2, C-8), 45.3 (CH2, C-9), 166.4 (C, C-11), 83.1 (C, C-12), 68.7 (CH, C-13), 105.2 (C, C-14), 120.7 (C, C-15), 121.1 (CH, C-16), 109.8 (CH, C-17), 156.6 (C, C-18), 95.1 (CH, C-19), 137.6 (C, C-20), 123.8 (CH, C-21), 134.7 (C, C-22), 25.6 (CH3, C-23), 18.2 (CH3, C-24), 128.4 (C, C-1'), 127.5 (C, C-2'), 157.0 (C, C-3'), 103.4 (CH, C-4'), 156.9 (C, C-5'), 108.1 (CH, C-6'), 166.2 (C, C-7'), 199.9 (C, C-8'), 164.0 (C, C-1''), 110.5 (C, C-2''), 161.0 (C, C-3''), 103.0 (CH, C-4''), 148.0 (C, C-5''), 110.9 (CH, C-6''), 22.4 (CH3, C-7''), 55.7 (CH3, 18-OCH3), 55.7 (CH3, 3''-OCH3), 56.1 (CH3, 3'-OCH3), 52.1 (CH3, 12-OCH3); HR-ESI(+)MS [M+H]+ m/z 740.2806 (calcd for C40H42N3O11+, 740.2814).
4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (2): Amorphous white solid; 1H-NMR (DMSO-d6, 400 MHz) δ 7.42 (1H, d, J = 10.0 Hz, H-1), 5.76 (1H, d, J = 10.0, H-2), 2.74 (1H, m, H-4), 1.12 (3H, d, J = 7.2, 4-CH3), 2.41 (1H, m, H-5), 5.04 (1H, s, H-6), 2.09 (3H, s, 6-OAc-CH3), 1.10 (3H, s, 8-CH3), 2.54 (1H, m, H-9), 1.39 (3H, s, 10-CH3), 1.57, 1.87 (each 1H, m, H-11), 1.70, 2.29 (2H, m, H-12), 2.31, 1.22 (2H, m, H-13), 0.81 (3H, s, 14-CH3), 1.64, 2.05 (each 1H, d, J = 8.4, H-15), 5.70 (CH, d, J = 8.4, H-16), 1.87 (3H, s, 16-OAc-CH3), 1.99, 2.09 (2H, m, H-17), 5.11 (1H, t, J = 8.0, H-18), 1.58 (3H, s, H-20), 1.65 (3H, s, H-21); 13C-NMR (DMSO-d6, 100 MHz) δ 158.4 (CH, C-1), 126.8 (CH, C-2), 200.8 (C, C-3), 39.5 (CH, C-4), 12.3 (CH3, 4-CH3), 45.6 (CH, C-5), 73.0 (CH, C-6), 169.0 (C, 6-OAc), 20.4 (CH3, 6-OAc-CH3), 209.3 (C, C-7), 52.3 (CH, C-8), 17.5 (CH3, 8-CH3), 41.2 (CH, C-9), 37.7 (C, C-10), 27.1 (CH3, 10-CH3), 23.3 (CH2, C-11), 25.5 (CH2, C-12), 29.0 (CH, C-13), 46.1 (C, C-14), 17.5 (CH3, 14-CH3), 40.5 (CH2, C-15), 73.3 (CH, C-16), 169.9 (C, 16-OAc), 20.5 (CH3, 16-OAc-CH3), 28.1 (CH2, C-17), 124.1 (CH, C-18), 130.8 (C, C-19), 17.5 (CH3, C-20), 25.5 (CH3, C-21); HR-ESI(+)MS [M+Na]+ m/z 509.2878 (calcd for C29H42NaO6+, 509.2874).
4. Conclusions
In our investigation, sixteen compounds were isolated from the chloroform extract of rice mediums of the endophytic isolated fungus A. fumigatus, including two new compounds 12-methoxyl-13-[5'-hydroxy-2'-(1''-hydroxy-3''-methoxy-5''-methylbenzoyl)-3'-methoxy]benzoic acid methyl ester- fumitremorgin (fumitremorgin D, 1) and 4,8,10,14-tetramethyl-6-acetoxy-14-[16-acetoxy-19-(20,21-dimethyl)-18-ene]-phenanthrene-1-ene-3,7-dione (2). A SAR study of the cytotoxicity of indolediketopiperazine alkaloids against the HepG2 cell line was also discussed.
Acknowledgments
This project was supported by National Science Foundation of China (grants 81173505) and Ministry of Science and Technology of China (grant 2012BAC01B05-7).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/01/1424/s1.
Author Contributions
Changqi Zhao has supervised the study. Zizhen Liang and Tiantian Zhang have written and revised the paper. Zizhen Liang performed the isolation of new compounds and has analyzed the data. Zizhen Liang and Tiantian Zhang performed the biological assays and analyzed the results of MTT antiproliferative assay. Xiaoqian Zhang and Jia Zhang prepared the plant. Zizhen Liang and Jia Zhang prepared the fungus.
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Sample Availability: Samples of the all compounds are available from the authors.
References
- 1.Tan R., Zou W. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- 2.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 3.Ge H.M., Yu Z.G., Zhang J., Wu J.H., Tan R.X. Bioactive alkaloids from endophytic aspergillus fumigatus. J. Nat. Prod. 2009;72:753–755. doi: 10.1021/np800700e. [DOI] [PubMed] [Google Scholar]
- 4.Scherlach K., Boettger D., Remme N., Hertweck C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010;27:869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 5.Kusari S., Lamshöft M., Spiteller M. Aspergillus fumigatus fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009;107:1019–1030. doi: 10.1111/j.1365-2672.2009.04285.x. [DOI] [PubMed] [Google Scholar]
- 6.Li X.-J., Zhang Q., Zhang A.-L., Gao J.-M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 7.Wang F., Fang Y., Zhu T., Zhang M., Lin A., Gu Q., Zhu W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron. 2008;64:7986–7991. [Google Scholar]
- 8.Ge H.M., Zhang W.Y., Ding G., Saparpakorn P., Song Y.C., Hannongbua S., Tan R.X. Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture. Chem. Commun. 2008:5978–5980. doi: 10.1039/B812144C. [DOI] [PubMed] [Google Scholar]
- 9.Ying J.-S., Chen D.-Z. Flora of China. Volume 19 Beijing Science Press; Beijing, China: 2001. [Google Scholar]
- 10.Cui C.-B., Kakeya H., Okada G., Onose R., Osada H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996;49:527–533. doi: 10.7164/antibiotics.49.527. [DOI] [PubMed] [Google Scholar]
- 11.Abraham W.-R., Arfmann H.-A. 12, 13-Dihydroxy-fumitremorgin C from Aspergillus fumigatus. Phytochemistry. 1990;29:1025–1026. [Google Scholar]
- 12.Kato N., Suzuki H., Takagi H., Uramoto M., Takahashi S., Osada H. Gene disruption and biochemical characterization of verruculogen synthase of Aspergillus fumigatus. ChemBioChem. 2011;12:711–714. doi: 10.1002/cbic.201000562. [DOI] [PubMed] [Google Scholar]
- 13.Rösecke J., König W.A. Constituents of the fungi Daedalea quercina and Daedaleopsis confragosa var. tricolor. Phytochemistry. 2000;54:757–762. doi: 10.1016/s0031-9422(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.-Y., Kinoshita H., Ihara F., Igarashi Y., Nihira T. Identification of novel derivative of helvolic acid from Metarhizium anisopliae grown in medium with insect component. J. Biosci. Bioeng. 2008;105:476–480. doi: 10.1263/jbb.105.476. [DOI] [PubMed] [Google Scholar]
- 15.Danielsen K., Aksnes D.W., Francis G.W. NMR study of some anthraquinones from rhubarb. Magn. Reson. Chem. 1992;30:359–360. [Google Scholar]
- 16.Ren H., Tian L., Gu Q., Zhu W. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharm. Res. 2006;29:59–63. doi: 10.1007/BF02977469. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Li Y., Liu J., Song Y., Tan R. Anti-Helicobacter pylori metabolites from Rhizoctonia sp. Cy064, an endophytic fungus in Cynodon dactylon. Fitoterapia. 2004;75:451–456. doi: 10.1016/j.fitote.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro E.A.A., Carvalho J.M., dos Santos D.C.P., Feitosa A.D.O., Marinho P.S.B., Guilhon G.M.S.P., de Souza A.D.L., da Silva F.M.A., Marinho A.M.D.R. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2013;27:1633–1638. doi: 10.1080/14786419.2012.750316. [DOI] [PubMed] [Google Scholar]
- 19.Cole R.J., Kirksey J.W., Dorner J.W., Wilson D.M., Johnson J.C., Jr, Johnson A.N., Bedell D.M., Springer J.P., Chexal K.K. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. J. Agric. Food. Chem. 1977;25:826–830. doi: 10.1021/jf60212a015. [DOI] [PubMed] [Google Scholar]
- 20.Numata A., Takahashi C., Matsushita T., Miyamoto T., Kawai K., Usami Y., Matsumura E., Inoue M., Ohishi H., Shingu T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992;33:1621–1624. [Google Scholar]
- 21.Boot C.M., Gassner N.C., Compton J.E., Tenney K., Tamble C.M., Lokey R.S., Holman T.R., Crews P. Pinpointing pseurotins from a marine-derived Aspergillus as tools for chemical genetics using a synthetic lethality yeast screen. J. Nat. Prod. 2007;70:1672–1675. doi: 10.1021/np070307c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.