Abstract
Two new classes of dendrimers bearing 8 and 32 fluorene donor groups have been synthesized. The first and second generations of these porphyrin-PAMAM-fluorene dendrimers were characterized by 1H-NMR, 13C-NMR, FTIR, UV-vis spectroscopy, elemental analyses and MALDI-TOF mass spectrometry. The UV-vis spectra showed that the individual properties of donor and acceptor moieties were preserved, indicating that the new dendrimers could be used as photosynthetic antennae. Furthermore, for fluorescent spectroscopy, these dendrimers showed good energy transfer.
Keywords: dendrimers, fluorene, PAMAM, porphyrin
1. Introduction
In the last few years, porphyrin-core dendrimer systems have been attracting much attention because the terminal substituent groups on the photoactive core can modulate their physico-chemical properties [1,2,3,4]. This has been extensively studied for a number of different applications, such as: shape-selective catalysts [3], organic solar cells [4], photoelectrochemical devices [5], light-emitting diodes [6], photodynamic therapy [7], chemical sensors [8], optical limiters [9], and so forth. In the past few years, there has been a considerable increase in the number of reports dealing with porphyrins decorated with dendrimers. A large number of different porphyrins bearing pendant linear oligofluorenyl arms have been reported [10,11,12] and it is possible to join them with PAMAM dendrimers. Poly(amidoamine) (PAMAM) species are a novel class of commercial dendrimers which possess an established molecular composition with different terminal functional groups. These macromolecules were first synthesized and investigated by Tomalia [1]. The design and modification of PAMAM dendrimers with fluorescent units could give them new interesting properties and expand their areas of application. In this paper we report on the synthesis and characterization of some new modified first and second generation PAMAM derivatives using diethylenetriamine-bearing fluorene fluorescent units at their peripheries and their photophysical characteristics.
2. Results and Discussion
Diethylenetriamine was used to obtain dendritic molecules with high molecular weight. Here, it is used as the building block in the synthesis of dendron-coated porphyrins. The porphyrin used was 5,10,15,20-tetrakis(4-hydroxyphenyl)-21H,23H-porphine, as a free base. The addition between the hydroxy groups of the porphyrin was conducted with methyl bromoacetate in acetone and K2CO3 (Scheme 1). Following with an amidation reaction with diethylenetriamine to obtain the first-generation dendrimer 3 (Scheme 1).
Scheme 1.
Synthesis of first-generation porphyrin dendrimer 3.
A mixture of the amide 3 and methyl acrylate in methanol was stirred in a nitrogen atmosphere at 90 °C. The MeOH and excess of methyl acrylate, as volatiles, were evaporated on a rotary evaporator, thus obtaining compound 4. Finally the second-generation dendrimer 5 was obtained from compound 4 with an excess of diethylenetriamine in a mixture of methanol and toluene 1:1 at reflux for 24 h. (Scheme 2). It should be pointed out that compounds 2, 3, 4 and 5 have good solubility in most organic solvents.
Scheme 2.
Synthesis of second-generation porphyrin-dendrimers: (a) methyl acrylate, methanol, reflux; (b) diethylenetriamine, methanol, toluene, reflux.
Finally the fluorene-2-carboxaldehyde moiety was attached to the porphyrin-PAMAM dendrimers in methanol/benzene 1:1 at reflux for 3 days. The compounds 6 and 7 were washed with methanol to remove the excess of fluorene (Scheme 3).
Scheme 3.
Synthesis of dendrimers 6 and 7 with peripheral fluorene moieties.
Complete functionalization of the porphyrin was confirmed using NMR (1H, 13C) and matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). In Figure 1, the MALDI-TOF mass spectra for dendrimers 6 and 7 show a peak at m/z = 2659 and 9398, respectively.
Figure 1.
MALDI-TOF mass spectra of dendrimers 6 (a) and 7 (b).
2.1. Optical Properties in Solution
The absorption spectra of 6 or 7 contain the characteristic fluorene group peaks 272 and 309 nm, as well as the porphyrin Soret band at 419 and 436 nm and the Q bands between 557 and 655 nm for the first and second generation dendrimers, respectively (Figure 2a). The spectra are quite similar, but the maximum absorption spectrum of the second-generation dendrimer is higher and broader than the spectrum of the first generation dendrimer. This band is also slightly red shifted compared to 419 nm for the first generation, but not as much as for the second generation (436 nm). This could be due to presence of the 32 fluorene moieties and the length of the PAMAM spacer groups.
Figure 2.
(a) Absorption (b) and (c) Fluorescence in chloroform at room temperature (λex 250, 420 and 620 nm) of 6 and 7 in CH2Cl2 at room temperature.
The dendrimers 6 and 7, after excitation at 250 nm, showed one broad band at 300 nm, the intensity of which was higher for the second-generation dendrimer 7 than for the first generation dendrimer 6 (Figure 2b). When the dendrimers are excited at 420 nm in the Soret band, the emission spectra reveal a strong red fluorescence with a peak maximum at 653 nm and a weak shoulder at 716 nm (Figure 2c) and almost no residual emission of the fluorene for both compounds. Thus, there is apparently a good energy transfer between fluorene and porphyrin, since the essentially red emission of the porphyrin is observed in comparison to blue emission of fluorene at around 300 nm.
In fact, the porphyrin core emits almost all the UV energy absorbed by the fluorene units. The excitation spectra obtained after exciting in the strongest emission band at around 620 nm reveal an emission from the Soret state, the three first Q states, as well as from the fluorene. This indicates that excitation over all the 250–620 nm region leads to all the fluorescent excited states of the porphyrin. Table 1 shows the absorption spectral data of compounds 6 and 7 in CH2Cl2 at 24 °C, where the molar extinction coefficient (ε) of the absorbance of the dendrimers 6 and 7 showed an increase in the second-generation dendrimer 7, while the absorption maximum (λmax) for the dendrimers 6 and 7 was slightly red shifted from 419 to 436 nm (solvatochromism) with an increase in ε.
Table 1.
Summarized absorbance data (CH2Cl2).
| Sample | Fluorenemax (nm) | Soret Bandmax (nm) | Qmax (nm) | ɛ (×10−5 M−1 cm−1) |
|---|---|---|---|---|
| 6 | 272, 309 | 419 | 557 | 0.235 |
| 7 | 266, 307 | 436 | 564 | 0.247 |
2.2. EPR Studies
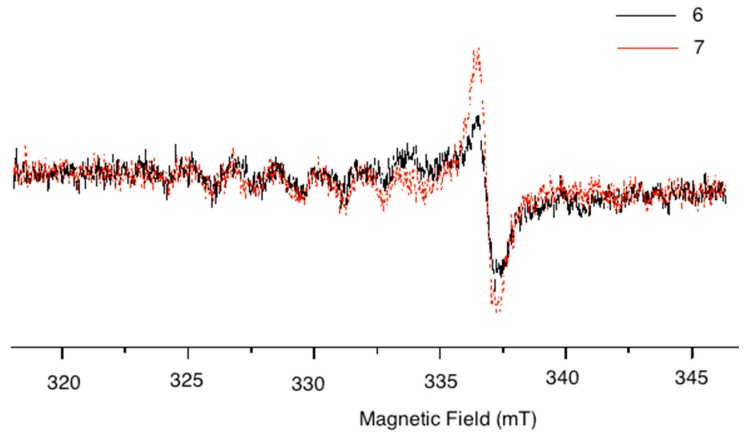
Porphyrins have an extensive system of delocalized π electrons. The EPR signals of porphyrin-compounds are generated by the interaction of the delocalized π electrons of the porphyrin with the magnetic field. The EPR spectra of fluorene-porphyrins 6 and 7 are shown in Figure 3.
Figure 3.
EPR spectra of compounds 6 and 7.
In the EPR spectrum of the solid sample, the overlap of two signals in the central field was observed. One of them corresponds to a singlet with g = 2.0036; this signal has been assigned to the formation of oxygen radicals by the interaction of O2 with the porphyrin ring [13], but the signal intensity is sensitive to the saturation effect of the microwave power related to the isoelectric point (pH value) [14].
The other signal presents hyperfine structure due the interaction of the unpaired electron with the magnetic cores (mainly nitrogen atoms) of the porphyrin macrocycle, (nitrogen atoms IN = 1 and hydrogen atoms IH = 1/2) [14], this signal exhibited nine lines due to the coupling between the porphyrins’ nitrogen atoms due to coupling of unpaired electrons with the conjugated porphyrin system [15]. The low intensity of this signal agrees with the presence of an electron-withdrawing group linked to the porphyrin macrocycle ring [16].
2.3. Crystal Structure Determination
The structure of dendrimer generation 0.5 molecule 2 was confirmed by X-ray diffraction analysis of a single crystal prepared by recrystallization from chloroform at room temperature. The perspective view and crystal-packing diagram of molecule 2 are shown in Figure 4.
Figure 4.
Crystal structure and crystal-packing of dendrimer 2.
The suitable crystal of compound 2 was rolled in epoxy resin and mounted on glass fiber. A Bruker Apex AXS CCD area detector X-ray diffractometer was the instrument used for the determination. The data were first reduced and corrected for absorption using psi-scans and then solved using the program SHELL-XS. All non-hydrogen atoms were refined with anisotropic thermal parameters and the hydrogen atoms were refined at calculated positions with thermal parameters constrained to the carbon atom on which they were attached. A summary of the key crystallographic information is given in Table 2.
Table 2.
Crystal data and structure refinement.
| Empirical formula | C56 H46 N4O12 |
| Formula weight | 966.97 |
| Temperature | 298(2) K |
| Wavelength | 1.54178 Å |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 12.3084(3) Å α = 90° |
| b = 16.4548(4) Å β = 98.2840(10)° | |
| c = 11.8932(2) Å γ = 90° | |
| Volume | 2383.62(9) Å3 |
| Z | 2 |
| Density (calculated) | 1.347 Mg/m3 |
| Absorption coefficient | 0.789 mm−1 |
| F(000) | 1012 |
| Crystal size | 0.364 × 0.105 × 0.017 mm3 |
| Theta range for data collection | 3.629 to 68.264° |
| Index ranges | −14 ≤ h ≤ 14, −19 ≤ k ≤ 19, −14 ≤ l ≤ 12 |
| Reflections collected | 27,160 |
| Independent reflections | 4359 [R(int) = 0.1102] |
| Completeness to theta = 67.679° | 99.8% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4359/1/330 |
| Goodness-of-fit on F2 | 1.048 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0668, wR2 = 0.1691 |
| R indices (all data) | R1 = 0.0988, wR2 = 0.1936 |
| Largest diff. peak and hole | 0.747 and −0.269 e·Å−3 |
CCDC 1063702 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-122-333-6033; E-Mail: deposit@ccdc.cam.ac.uk.
3. Experimental Section
3.1. General Information
Solvents and reagents were purchased as reagent grade and used without further purification. Ether and toluene were distilled from sodium and benzophenone, acetone was distilled over calcium chloride, and methanol was distilled over calcium oxide powder. Column chromatography was performed on Merck silica gel 60 Å (70–230 mesh). 1H- and 13C-NMR spectra were recorded on a Varian Unity-300 MHz instrument with tetramethylsilane (TMS) as an internal reference. Infrared (IR) spectra were measured on a Nicolet FT-SSX spectrophotometer. Elemental analysis was determined by Galbraith Laboratories, Inc. (Knoxville, TN, USA). Matrix-assisted laser desorption ionization time-of-flight mass spectroscopy (MALDI-TOF-MS) was performed on a JEOL JMS AX505 HA instrument using 9-nitroanthracene (9NA) as a matrix. The UV-Vis absorption spectra were obtained at room temperature with a Shimadzu 2401 PC spectrophotometer, while a Perkin-Elmer LS-50 spectro-fluorimeter was used for the fluorescence spectra. The excitation wavelengths used for the emission properties are reported in the text. The EPR measurements were made in a quartz tube at room temperature in the solid sample, with a Jeol JES-TE300 spectrometer operating in X-Band fashion at 100 KHz modulation frequency and a cylindrical cavity in the mode TE011. The external calibration of the magnetic field was made with a Jeol ES-FC5 precision gauss meter and microwave frequency with a HP 5350B frequency counter. Spectral acquisition, manipulations and simulation were performed using the program ES-IPRITS-TE. The EPR spectrum was recorded as a first derivation.
3.2. Synthesis of Generation 0.5 Dendrimer 2
A mixture of the methyl bromoacetate (1.60 mmol), potassium carbonate (3.20 mmol mmol) in dry acetone (50 mL) was heated to reflux and stirred vigorously in a nitrogen atmosphere for 20 min. Compound 1 (1.60 mmol) dissolved in dry acetone (40 mL) was added dropwise and the reaction was continued for 6 hours. The mixture was filtered. The filtrate was evaporated to dryness under pressure. The residue dissolved in diethyl ether was washed with an aqueous solution of 5% Na2CO3 (three times). The organic layer was dried and evaporated to give the ester 2 (0.27 mmol) as a purple solid in 90% yield. m.p: ˃300 °C. FTIR (pellet, KBr, cm−1): 2954, 2920, 1750, 1602, 1503, 1430, 1298, 1207, 1173, 1140, 1076, 963, 806, 708, 604, 593. UV-vis (CH2Cl2): λmax 418, 447, 515, 548, 591, 650. 1H-NMR (CDCl3) δH: −2.77 (br, 2H, NH), 3.95 (s, 6H, O-CH3), 4.96 (s, 8H CH2-O), 7.30 (d, 8H, Ar, J = 8.7 Hz), 8.16 (d, 8H, Ar, J = 8.7 Hz), 8.87 (br, 8H, pyrrole). 13C-NMR (CDCl3) δC: 29.6 (C-CO), 32.4 (O-CH3), 68.0 (CH2-O), 112.9 (Ar), 119.4 (Aripso), 130.9 (pyrrole), 135.6 (Ar), 135.7 (pyrroleipso), 157.63(Aripso-O), 169.4 (C=O). MS (FAB+) m/z: 966 (M+). Anal. Calcd. for C56H46N4O12. C 69.56%, H 4.79%, N 5.79% O 19.85%. Found: C, 69.56; H, 4.77%.
3.3. Synthesis of Generation 1.0 Dendrimer 3
A mixture of ester-containing derivative 2 (0.22 mmol) and diethylenetriamine (0.92 mmol) in methanol (15 mL) and toluene (15 mL) were refluxed for 24 h in an atmosphere of nitrogen. The organic solvent and the excess diethylenetriamine were removed in vacuo. The residue was washed several times with alcohol to obtain the amide compound 3 (0.16 mmol) as a purple solid in 85% yield. m.p: ˃300 °C. FTIR (pellet, KBr, cm−1): 3297, 2938, 1651, 1545, 1470, 1348, 1290, 1235, 1176, 1120, 797, 731, 587. UV-vis (CH2Cl2): λmax 206, 426, 518, 559, 603, 647. 1H-NMR (CDCl3) δH: −2.79 (br, 2H, NH), 1.90–1.96 (m, 16H, CH2-NH2), 3.30–3.54 (m, 16H, CH2-NH2), 4.90 (s, 8H, CH2-O), 7.41 (br, 8H, Ar), 8.08–8.11 (br, 8H, Ar), 8.85 (br, 8H, pyrrole). 13C-NMR (CDCl3) δC: 38.8 (CH2-NH2), 40.4 (CH2-NH2), 41.6 (CH2-NH2), 43.4 (CH2-NH2), 53.7 (CH2-N), 57.8 (CH2-N), 58.4 (CH2-O), 69.1 (CH2-O) 108.6 (Ar), 115.0 (Aripso), 137.3 (pyrrole), 164.6 (C=O), 171.9 (C=O), 175.2 (C=O), 175.6 (C=O). MS (FAB+) m/z: 1,250 (M+). Anal. Calcd. for C68H82N16O8. C 65.26%, H 6.60%, N 17.91%. Found: C, 65.6; H, 6.58%.
3.4. Synthesis of Generation 1.5 Dendrimer 4
A mixture of the amide 3 (0.14 mmol) and methyl acrylate (2.32 mmol) in methanol (20 mL) was stirred in a nitrogen atmosphere at 90 °C for 48 h. Methanol and excess methyl acrylate were removed in vacuo. The residue was washed with hexane and methyl acetate to obtain the ester compound 4 (0.12 mmol) as a purple oil in 80% yield. FTIR (film, cm−1): 3391, 3320, 2952, 2847, 1732, 1672, 1606, 1507, 1353, 1243, 1177, 1053, 992, 966, 844, 805, 735, 605. UV-vis (CH2Cl2): λmax 421, 454, 518, 554, 594, 649, 685. 1H-NMR (CDCl3) δH: −2.74 (br, 2H, NH), 2.53 (m, 32H, CH2-CO), 2.83 (m, 32H, CH2-N), 2.60 (m, 16H, CH2-N), 3.69 (m, 16H, CH2-NCO), 3.72 (m, 48H, O-CH3), 4.86 (s, 8H CH2-O), 7.41 (d, 8H, Ar, J = 8.7 Hz), 8.20 (d, 8H, Ar, J = 8.1 Hz), 8.90 (br, 8H, pyrrole). 13C-NMR (CDCl3) δC: 29.67 (CH2-CO), 32.47 (CH2-NCO), 36.81 (CH2-CO), 49.69 (CH2-N), 51.59 (CH3-O), 53.2 (CH2-N), 67.62 (CH2-O), 113.1 (Ar), 119.4 (Aripso), 131.1 (pyrrole), 135.7 (Ar), 157.25 (Aripso), 168.1 (C=O), 172.9 (C=O), 173.4 (C=O). MS MALDI TOF m/z: 2627 (M+). Anal. Calcd. for C132H178N16O40. C 60.31%, H 6.82%, N 8.52%. Found: C, 60.31; H, 6.82, N, 8.54%.
3.5. Synthesis of Generation 2.0 Dendrimer 5
A mixture of ester-containing derivative 4 (0.11 mmol) and diethylenetriamine (2.31 mmol) in methanol (15 mL) and toluene (15 mL) were refluxed for 24 h in an atmosphere of nitrogen. The organic solvent and the excess diethylenetriamine were removed in vacuo. The residue was washed several times with alcohol to obtain the amide compound 5 (0.09 mmol) as purple oil in 82% yield. (film, cm−1): 3257, 3074, 1644, 1547, 1433, 1368, 1293, 1239, 1178, 1043, 998, 642, 597. UV-vis (CH2Cl2): λmax 209, 418, 517, 555, 593, 648. 1H-NMR (CDCl3) δH: −2.74 (br, 2H, NH), 1.92–2.00 (m, 64H, CH2-NH2), 2.73–2.78 (m, 16H, CH2-N), 2.86–2.90 (m, 16H, CH2-N), 2.98 (m, 64H, CH2-N), 3.05–3.18 (m, 16H, CH2-N), 3.61 (s, 32H, CH2-N), 3.95 (s, 8H, CH2-NCO), 3.99 (s, 8H CH2-NCO), 4.86 (s, 8H CH2-O), 7.15–7.47 (m, 8H, Ar) 8.05–8.16 (m, 8H, Ar), 8.52 (br, 2H, pyrrole), 8.65 (br, 2H, pyrrole), 8.85 (br, 4H, pyrrole). 13C-NMR (CDCl3) δC: 21.2 (CH2-NH2), 38.9 (CH2-N), 46.2 (CH2-N), 36.0 (CH2-CO), 40.4 (CH2-N), 42.5 (CH2-N), 32.9 (CH2-N), 55.6 (CH2-N), 67.1 (CH2-O), 113.0 (Ar), 119.54 (Aripso), 127.4 (pyrrole), 135.7 (Ar), 157.7 (Aripso), 124.8 (C=C), 154.8 (C=C), 162.8 (C=O), 164.9 (C=O), 170.0 (C=O), 172.3 (C=O), 173.7 (C=O). MS MALDI TOF m/z: 3,764 (M+). Anal. Calcd. for C180H322N64O24. C 57.39%, H 8.62%, N 23.80%. Found: C 57.36; H 8.59; N 23.82%.
3.6. Synthesis of Dendrimers 6 and 7 with Fluorene in the Periphery
To a solution of dendrimers 3 and 5 (0.5 mmol) in methanol (15 mL), fluorene-2-carboxaldehyde (0.5 mmol) in methanol (15 mL) was added, the mixture was stirred in a nitrogen atmosphere at 45–50 °C for 3 day. The methanol was removed in vacuo. The residue was washed several times with methanol first and then with CH2Cl2 to obtain the desired products 6 and 7.
Dendrimer 6. Purple solid, 85% yield. m.p: ˃300 °C. FTIR (pellet, KBr, cm−1): 3297, 2938, 1651, 1545, 1470, 1348, 1290, 1235, 1176, 1120, 797, 731, 587. UV-vis (CH2Cl2): λmax 206, 426, 518, 559, 603, 647. 1H-NMR (CDCl3) δH: −2.79 (br, 2H, NH), 1.90–1.96 (m, 16H, CH2-N), 3.30–3.54 (m, 16H, CH2-N), 4.06 (br, 16H, CH2-fluorene), 4.90 (s, 8H, CH2-O), 7.41 (br, 8H, Ar), 7.57–8.40 (br, 56H, fluorene, Ar-O), 8.08–8.11 (br, 8H, Ar), 8.85 (br, 8H, pyrrole). 13C-NMR (CDCl3) δC: 38.8 (CH2-NH2), 40.4 (CH2-N), 41.6 (CH2-N), 42.2 (CH2-fluorene), 43.4 (CH2-N), 53.7 (CH2-N), 57.8 (CH2-N), 58.4 (CH2-O), 69.1 (CH2-O) 108.6 (Ar), 115.0 (Aripso), 137.3 (pyrrole), 164.6 (C=O), 171.9 (C=O), 175.2 (C=O), 175.6 (C=O). MS MALDI TOF m/z: 2,659 (M+). Anal. Calcd. for C180H146N16O8. C 81.24%, H 5.53%, N 8.42%. Found: C, 81.26; H, 5.55; N 8.40%.
Dendrimer 7. Purple oil, 82% yield. (film, cm−1): 3257, 3074, 1644, 1547, 1433, 1368, 1293, 1239, 1178, 1043, 998, 642, 597. UV-vis (CH2Cl2): λmax 209, 418, 517, 555, 593, 648. 1H-NMR (CDCl3) δH: −2.86 (br, 2H, NH), 2.71 (br, 64H, CH2-N), 3.36 (br, 96H, CH2-N), 3.77 (br, 64H, CH2-N), 4.06 (br, 64H, CH2-fluorene), 4.80 (br, 8H CH2-O), 7.36 (br, 8H, Ar-O), 7.57–8.40 (br, 232H, fluorene, Ar-O), 8.79 (m, 8H, pyrrole), 9.39 (br, 32H, CH=N). 13C-NMR (CDCl3) δC: 29.4 (CH2-NH2), 35.7 (CH2-N), 37.4 (CH2-N), 42.2 (CH2-fluorene), 45.1 (CH2-N), 48.7 (CH2-N), 50.3 (CH2-N), 56.4 (CH2-N), 60.4 (CH2-N), 67.3 (CH2-O), 112.8 (Ar), 119.1 (Aripso), 121.7 (Ar), 122.57 (Ar), 123,7 (Ar), 124.3 (Ar), 125.5 (Ar), 125.8 (Ar), 126.3 (Ar), 126.9 (Ar), 128.5 (pyrrole), 130.1 (Ar), 130.5 (Ar), 130.6 (Ar), 131.0 (Ar), 135.4 (Ar), 156.6 (CH=N), 168.6 (C=O), MS MALDI TOF m/z: 9405 (M+). Anal. Calcd. for C628H578N64O24. C 80.19%, H 6.19%, N 9.53%. Found: C 80.16; H 6.19; N 9.52%.
4. Conclusions
Two new classes of dendrimers bearing 8 and 32 fluorene donor groups have been synthesized. The first and second generation porphyrin-PAMAM-fluorene dendrimers were obtained in good yields. The UV-vis spectra showed that the individual properties of donor and acceptor moieties are preserved, indicating that the new dendrimers could be used as photosynthetic antennae. Also, for fluorescence spectroscopy, these dendrimers showed an efficient energy transfer.
Acknowledgments
This work was supported by DGAPA-UNAM (IN-107814) grant. We would also like to thank Rios O. H., Velasco L., Huerta S. E., Patiño M. M. R., Peña Gonzalez M. A., Rios Ruiz L. and Garcia Rios E. for technical assistance.
Author Contributions
MMG and SCM designed research; KGG, UOM, ABM, VGV and SHO performed research and analyzed the data; SCM, VGV and MMG wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Tomalia D.A., Fréchet J.M.J. Dendrimers and other Dendritic Polymers. Wiley; New York, NY, USA: 2001. [Google Scholar]
- 2.Hecht S., Fréchet J.M.J. Dendritic Encapsulation of Function: Applying Nature’s Site Isolation Principle from Biomimetics to Materials Science. Angew. Chem. Int. Ed. 2001;40:74–91. doi: 10.1002/1521-3773(20010105)40:1<74::AID-ANIE74>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Piotti M.E., Rivera F., Jr., Bond R., Hawker C.J., Frechet J.M.J. Synthesis and Catalytic Activity of Unimolecular Dendritic Reverse Micelles with ‘Internal’ Functional Groups. J. Am. Chem. Soc. 1999;121:9471–9472. doi: 10.1021/ja991879p. [DOI] [Google Scholar]
- 4.Gunes S., Neugebauer H., Sariciftci N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007;107:1324–1338. doi: 10.1021/cr050149z. [DOI] [PubMed] [Google Scholar]
- 5.Kira A., Umeyama T., Matano Y., Yoshida K., Isoda S., Park J.K., Kim D., Imahori H. Supramolecular Donor−Acceptor Heterojunctions by Vectorial Stepwise Assembly of Porphyrins and Coordination-Bonded Fullerene Arrays for Photocurrent Generation. J. Am. Chem. Soc. 2009;131:3198–3200. doi: 10.1021/ja8096465. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama N., Stapert H.R., Zhang G.-D., Takasu D., Jiang D.-L., Nagano T., Aida T., Kataoka K. Light-Harvesting Ionic Dendrimer Porphyrins as New Photosensitizers for Photodynamic Therapy. Bioconjugate Chem. 2003;14:58–66. doi: 10.1021/bc025597h. [DOI] [PubMed] [Google Scholar]
- 7.Finikova O., Galkin A., Rozhkov V., Cordero M., Hägerhäll C., Vinogradov S. Porphyrin and Tetrabenzoporphyrin Dendrimers: Tunable Membrane-Impermeable Fluorescent pH Nanosensors. J. Am. Chem. Soc. 2003;125:4882–4893. doi: 10.1021/ja0341687. [DOI] [PubMed] [Google Scholar]
- 8.Kiran P.P., Reddy D.R., Maiya B.G., Dharmadhikari A.K., Kumar G.R., Desai N.R. Enhanced optical limiting and nonlinear absorption properties of azoarene-appended phosphorus (V) tetratolylporphyrins. Appl. Opt. 2002;41:7631–7636. doi: 10.1364/AO.41.007631. [DOI] [PubMed] [Google Scholar]
- 9.Minghao S., Zhishan B. Tuning the optical properties of hyperbranched polymers through the modification of the end groups (pages 111–124) J. Polym. Sci. Part A: Polym. Chem. 2007;45:111–124. doi: 10.1002/pola.21810. [DOI] [Google Scholar]
- 10.Poriel C., Ferrand Y., Le-Maux P., Paul-Roth C., Simonneaux G., Rault-Berthelot J. Anodic oxidation and physicochemical properties of various porphyrin-fluorenes or -spirobifluorenes: Synthesis of new polymers for heterogeneous catalytic reactions. J. Electroanal. Chem. 2005;583:92–103. doi: 10.1016/j.jelechem.2005.05.005. [DOI] [Google Scholar]
- 11.Paul-Roth C., Rault-Berthelot J., Simonneaux G. New polymers for catalytic carbene transfer: Electropolymerization of tetrafluorenylporphyrinruthenium carbon monoxide. Tetrahedron. 2004;60:12169–12175. doi: 10.1016/j.tet.2004.10.013. [DOI] [Google Scholar]
- 12.Kazuya O., Naoyuki M., Yoshiaki K. Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties. Int. J. Mol. Sci. 2013;14:322–331. doi: 10.3390/ijms14010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannistraro S., van Vorst A., Jorit G. EPR studied on singlet oxygen production by porphyrins. Photochem. Photobiol. 1978;28:257–279. doi: 10.1111/j.1751-1097.1978.tb07704.x. [DOI] [Google Scholar]
- 14.Lin Q.S., Zhang T.L., Yuan L.B. Study of ESR spectra and photochemical reaction of porphyrin derivatives. Chin. J. Appl. Chem. 1988;5:57–61. [Google Scholar]
- 15.Minbo L., Hongli Z., Huihui Y., Chengrui J., Shaohua Z., Ying J. Absorption and EPR spectra of some porphyrins and metalloporphyrins. Dyes Pigm. 2007;74:357–362. doi: 10.1016/j.dyepig.2006.02.018. [DOI] [Google Scholar]
- 16.Wenqi Z., Ning S., Lianxiang Y., Xingqiao W. UV-visible, fluorescence and EPR properties of porphyrins and metalloporphyrins. Dyes Pigm. 2008;77:153–157. doi: 10.1016/j.dyepig.2007.04.007. [DOI] [Google Scholar]