Abstract
Here the aromatic formylation mediated by TiCl4 and dichloromethyl methyl ether previously described by our group has been explored for a wide range of aromatic rings, including phenols, methoxy- and methylbenzenes, as an excellent way to produce aromatic aldehydes. Here we determine that the regioselectivity of this process is highly promoted by the coordination between the atoms present in the aromatic moiety and those in the metal core.
Keywords: formylation, TiCl4, aromatic rings, aldehydes, dichloromethyl methyl ether
1. Introduction
The high reactivity of aldehydes makes them a key functional group in organic chemistry. This group is widespread in Nature, and its use in the synthesis of natural products is noteworthy. Furthermore, as efficient electrophiles, aldehydes can undergo further transformations to be converted into an extensive range of functional groups, such as hydroxyls, carboxylic acids, double bonds, and alkanes, among others [1]. As a result of this feature, aldehydes are widely used as active pharmaceutical ingredients and also commonly found in food and cosmetics [2]. Thus the synthesis and manipulation of this kind of compound is a continuous focus of research. In this regard, there is increased interest in the development of mild and efficient methods for the introduction of the aldehyde moiety into organic structures [3,4,5,6,7,8,9,10,11]. Traditionally, these methods involve the oxidation of alcohols, the selective reduction of esters, the reductive ozonolysis of alkenes, and so on [12,13,14].
During recent years, our group has channeled much research effort into developing new strategies for solid-phase peptide synthesis (SPPS) [15,16,17,18,19]. Special attention has been devoted to the development of protecting groups and linkers, which are the cornerstones of SPPS. Most of these are based on the benzyl (Bzl) moiety. To make this moiety more acid-labile and therefore more user friendly, we and other groups have developed linkers [20] and protecting groups [19,21] based on electron-rich aromatic compounds. The relatively higher acid lability of these groups when compared with the naked benzyl group is due to the stability of the carbocation formed in the removal process [22]. In this regard, electron-rich aromatic aldehydes, which can be easily transformed into hydroxymethyl- or aminomethyl benzyl-type moieties, are key intermediates for the development of new protecting groups and/or linkers. There is currently a wide range of choice of approaches regarding the introduction of a formyl group into aromatic rings [3,6]; however, the organic chemist continues to face the challenge of formylation through C-C bond formation [23], which is the most convenient approach for the case of aromatic aldehydes. One of the most widely used procedures is the well-known Vilsmeier-Haack reaction [24], whereby, in the presence of N,N-dimethylformamide (DMF) and phosphorous oxychloride, the activated aromatic compound furnishes the corresponding aromatic aldehyde. Additionally, the procedures described by Duff [25] or Casiraghi-Skattebøl [26] are typically used for phenolic formylation.
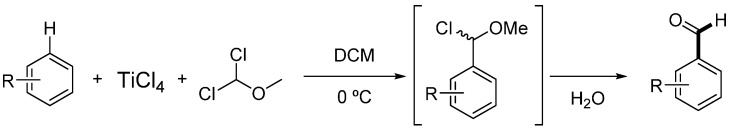
We have reported the successful ortho-formylation of electron-rich phenols mediated by dichloromethyl methyl ether and titanium (IV) tetrachloride [27], as well as a description of the reaction mechanisms in phenolic compounds [28]. This methodology was based on the outstanding procedure pioneered by Gross [29] and Cresp [30] that affords aromatic aldehydes (Scheme 1).
Scheme 1.
Titanium-mediated formylation reaction.
Herein we report an extensive study of this formylation procedure, where the dichloromethyl methyl ether (Cl2CHOMe) acts as a formylating agent for pre-activated aromatic rings in the presence of titanium tetrachloride.
2. Results and Discussion
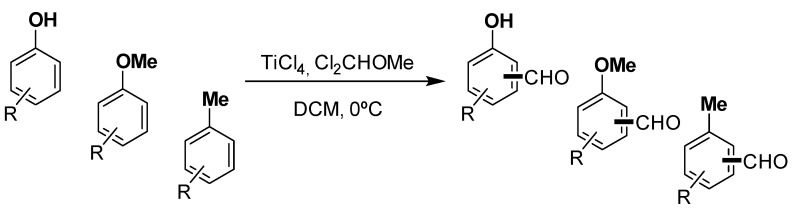
Aiming to explore the range of applications of formylation by CHCl2OMe and TiCl4 in aromatic rings, we tested the reaction with three benzene-like activated rings. These substrates are structurally based on phenols, methoxy- and methylbenzenes (Scheme 2).
Scheme 2.
Formylated scaffolds examined in the present study.
The general formylation procedure was carried out using the corresponding aromatic ring (1 eq.) mixed with TiCl4 (2.2 eq.) in dry dichloromethane (DCM) for 1 h under Ar at 0 °C. CHCl2OMe (1.1 eq.) was immediately added to the solution and stirred for 45 min, after which the reaction was quenched with a saturated aqueous solution of NH4Cl to furnish a mixture of regioisomers, giving satisfactory results in terms of regioselectivity, purity of the reaction crude products, and yields. This result contrasts with other common procedures that render less regioselectivity and more complex reaction crude products [24,25].
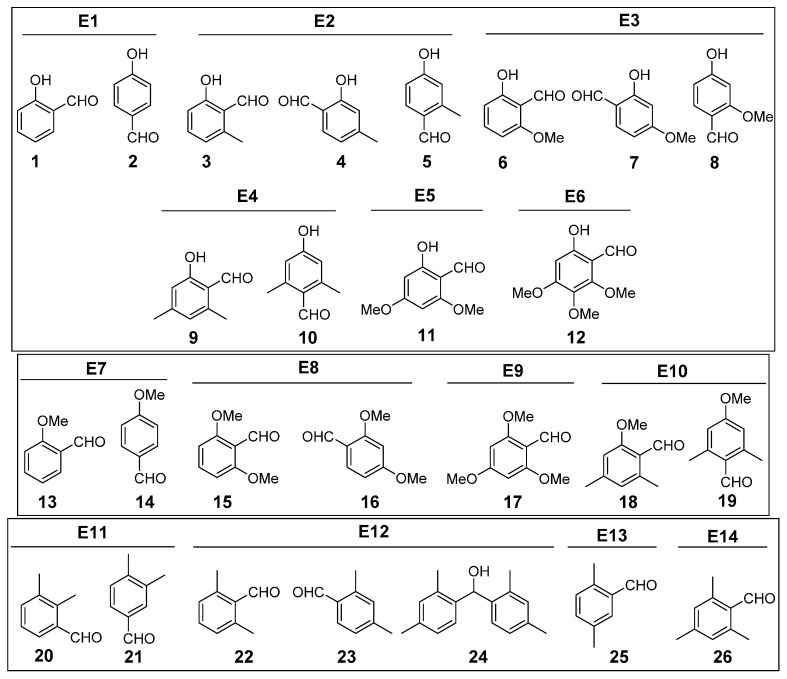
Table 1 provides a summary of the reactions. Some reactions furnish only one main product, as a result of the symmetry of the starting materials (entries 6, 9, 13 and 14). Meanwhile, non-symmetric starting reagents yielded distinct regioisomers.
Table 1.
Formylation of phenols, methoxy- and methylbenzenes.
| Entry | Reactant | Formylation Conversion [a] %, [%] | Main Product | Regioisomeric Ratio [b] [mp: :▲] :▲] |
||
|---|---|---|---|---|---|---|
| Aldehyde | Yield (%) | |||||
| Phenols | ||||||
| 1 | 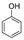 |
68 [22] | 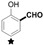 |
▬ [c] | [2.8:1] | |
| 2 | 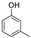 |
94 [6] | 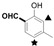 |
40 [d] | [3:1.6:1] | |
| 3 | 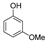 |
97 | 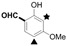 |
44 [d] | [3.7:1.3:1] | |
| 4 | 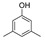 |
98 [2] | 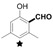 |
65 | [5:1] | |
| 5 | 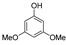 |
80 [11] | 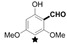 |
63 | [30:1] | |
| 6 | 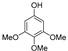 |
64 [25] | 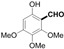 |
56 | ▬ | |
| Methoxybenzenes | ||||||
| 7 | 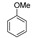 |
>99 | 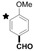 |
97 [d] | [1.1:1] | |
| 8 | 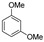 |
>99 | 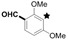 |
61 | [3:1] | |
| 9 | 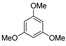 |
62 [38] | 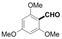 |
44 | ▬ | |
| 10 | 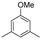 |
>99 | 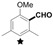 |
15 | [3.5:1] | |
| Methylbenzenes | ||||||
| 11 | 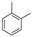 |
89 {4} | 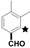 |
70 [d] | [3.2:1] | |
| 12 | 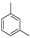 |
>99 | 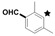 |
62 [d] | [32:1] | |
| 13 | 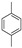 |
95% {3%} | 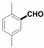 |
97 | ▬ | |
| 14 | 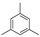 |
97 | 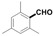 |
96 | ▬ | |
Notes: [a] The % corresponds to the chromatographic peak area in the reaction crude determined by HPLC: total % of formylated products, [%] remaining starting material and {%} dimerization by-product. [b]mp: :▲ indicates the ratio for the formylation of the main product and the other regioisomers. [c] Degradation during the purification. [d] The final products were isolated as mixture of regioisomers.
:▲ indicates the ratio for the formylation of the main product and the other regioisomers. [c] Degradation during the purification. [d] The final products were isolated as mixture of regioisomers.
2.1. Phenols
Regarding the formation of phenolic aldehydes (entries 1–6), the formylation takes place preferably in the ortho position with respect to the hydroxyl group (entries 1–5). This common behaviour is due to coordination between the metal atom and the oxygen of the OH, as previously studied by our group [27] and others [30]. After taking into account the role of the metal coordination, the steric hindrance caused by substituents also plays a significant role in formylation. Thus the formyl group was introduced in the less hindered position in each studied compound (entries 2, 3, 8, 10, 11 and 12).
2.2. Methoxybenzenes
In the case of the methoxy group (entries 7–10), Hamilton and co-workers demonstrated that TiCl4 are able to coordinate with several ethers [31], but it showed a weaker coordination effect in comparison with the hydroxyl one. Hence, the steric hindrance had a considerable effect on the formylated position. Thus for entry 7, the para substitution was favored, taking into account the presence of two ortho positions. The result in entry 7 reinforces the regioselectivity shown in entry 1. A special case of discussion is entry 10 vs. 7. In the first case, where the ortho and para positions were flanked by two substituents, the ortho one was favored. This observation indicates that both coordination and steric effects play a key role in the regioselectivity of formylation.
2.3. Methylbenzenes
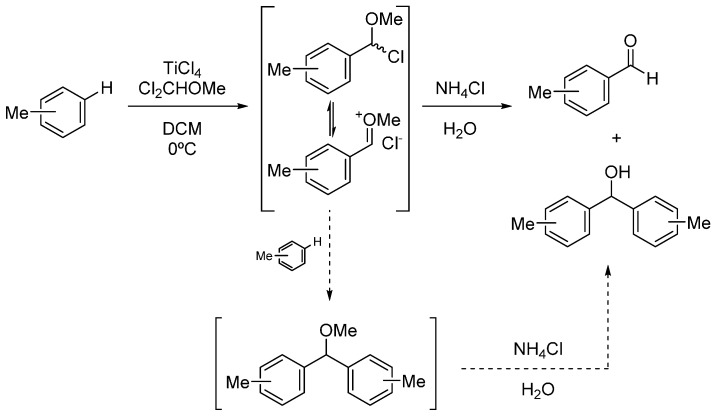
Concerning methylbenzene derivatives (entries 11–14), when formylation took place in the absence of oxygen atoms, coordination with the metal atom was not possible and therefore the less hindered isomer was favored. In this case, it is believed that the reaction mechanism occurs through the formation of an activated complex involving a π-interaction between the transition metal and the aromatic ring, as Calderazzo and co-workers proposed [32]. Indeed, another interesting point was the observed dimerization of two aromatic rings, affording diphenylmethanol compounds, as the proposed mechanism indicates (Scheme 3).
Scheme 3.
Proposed dimerization side-reaction mechanisms.
Accordingly, the chloride intermediate or the plausible oxocarbenium species [23] may undergo nucleophilic attack by an unreactive aromatic ring. This process provided the condensed diphenyl methoxy ether compound, which, with the acidolytic cleavage mediated by aqueous NH4Cl and the HCl obtained from the hydrolysis of TiCl4 (TiCl4 + 2 H2O → TiO2 + 4 HCl) [33,34], furnished the diphenylmethanol derivative in small quantities.
In general, most of the reactions tested showed high formylation conversions ranging from 64% to more than 99%. Accordingly, the titanium tetrachloride and Cl2CHOMe combination can be considered an effective formylating reagent.
3. Experimental Section
3.1. General Information
The commercially available products were used as received without further purification. Glass equipment was oven-dried, and DCM was dried using 4-Å molecular sieves under argon and protected from the light. All the reactions were carried out under argon. IR spectra were recorded on a Nicolet 510 FT-IR spectrometer equipped with an ATR Smart Orbit adaptor and are reported as frequency of absorption (cm−1). NMR spectra were recorded on a Varian Mercury-400 (1H/400 MHz and 13C/100 MHz). 1H data is reported as follows: chemical shift (δ ppm), [integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and coupling constant (J in Hz)]. Data for 13C-NMR are reported in terms of chemical shift. NMR spectra are referenced by tetramethylsilane (TMS). Melting points were measured with a Nikon Eclipse polarized microscope (MOP), which contains a Linkam THMS E600 thermal tray and a CI 93 temperature programmer. The HPLC reversed-phase column Xbridge C18 (75 × 4.6 mm, 3.5 μm) 4.6 × 3 × 150 mm, 5 µm was from Waters (Dublin, Ireland). Analytical HPLC (HPLC A and B) was carried out on using HPLC A: a Waters instrument comprising two solvent-delivery pumps (Waters 1525), an automatic injector (Waters 717 auto sampler), diode array wavelength detector (Waters 2487), and linear gradients of MeCN (+0.036% TFA) into H2O (+0.045% TFA) at 1 mL/min; or using HPLC B: a Shimadzu system comprising two solvent-delivery pumps (LC-20AD), an automatic injector (SIL-10ADvp), a variable wavelength detector (SPD-20A; 220 nm) and linear gradients of MeCN (+0.036% TFA) into H2O (+0.045% TFA) at 1 mL/min, which are specified in each case. The average in the chromatograms was determined by the area integration of the chromatographic peaks at λ = 220 nm. The thin-layer chromatography plates (TLC) used was purchased from Merck (TLC Silica gel 60 F254, silica-plated aluminium sheets). Column chromatography was performed on wet packed silica (Merck Silica gel 60, 0.2 mm). The automatic purification was performed by CombiFlash® Rf Teledyne ISCO with a Waters detector 2487 Dual λ Absorbance using pre-packed Redisep Rf Gold C18 (20–40 μm, 100 Å) from Teledyne Technology Company.
3.2. General Formylation Procedure
The appropriate benzene derivative (3.2–10.6 mmol) was dissolved in dry DCM (10–20 mL), purged with Ar, and cooled with an ice bath to 0 °C. Next, TiCl4 (2.2 eq.) was added dropwise. The reaction mixture was stirred for 1 h. Afterwards, dichloromethyl methyl ether (1.1 eq.) was added, and the mixture was left to react for a further 45 min. As a reaction quencher, a saturated solution of NH4Cl (25 mL) was added. The mixture was then left for 2 h. The organic layer was separated and washed with 0.1 N HCl solution (3 × 50 mL) and brine (3 × 50 mL). The organic layer was dried over MgSO4 and filtered, and the solvent was evaporated under vacuum to furnish the desired aldehydes (Figure 1). The purified products were homogeneous by HPLC and were characterized and purified by using various physical techniques.
Figure 1.
Obtained compounds.
3.3. Phenols
3.3.1. Entry 1
Reaction of phenol (1.0 g, 10.63 mmol), TiCl4 (2.6 mL, 26.71 mmol), dichloromethyl methyl ether (1.0 mL, 11.02 mmol) and DCM (20 mL) gave a purple oil. The products decomposed during SiO2 purification and only aldehyde 2 could be isolated (10 mg, 0.75%). HPLC detected 18%, 22% and 50% of 4-hydroxyaldehyde (2), the starting material, and 2-hydroxybenzaldehyde (1) respectively. 2-Hydroxybenzaldehyde (1): HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 4.9 min. 4-Hydroxybenzaldehyde (2): Appearance: White solid. HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 3.6 min. 1H-NMR (C3D6O) δ 9.86 (1H, s), 9.38 (1H, s), 7.83–7.77 (2H, m), 7.03–6.99 (2H, m) ppm. 13C-NMR (C3D6O) δ 191.0, 163.9, 132.8, 130.5, 116.7 ppm.
3.3.2. Entry 2
Reaction of 3-methylphenol (336 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL) afforded a pink solid. The mixture of the regioisomers was chromatographed on SiO2 (hexane/AcOEt, 9:1) to yield 4-hydroxy-2-methylbenzaldehyde (5) (69.8 mg, 16%) and a mixture of inseparable products, 2-hydroxy-6-methylbenzaldehyde (3) and 2-hydroxy-4-methylbenzaldehyde (4) in a 1:3.6 ratio, as determined by 1H-NMR (175.8 mg of the mixture was obtained, 40% yield of the formylation reaction). HPLC and 1H-NMR detected a small amount of the starting material (5.6%). 4-Hydroxy-2-methylbenzaldehyde (5): Appearance: White solid. Mp = 106–108 °C. TLC: Rf = 0.1 (hexane/AcOEt, 9:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 4.1 min. 1H-NMR (C3D6O) δ 10.1 (1H, s), 9.21 (1H, s), 7.71 (1H, d, J = 8.4 Hz), 6.86–6.81 (1H, m), 6.78–6.74 (1H, m), 2.58 (4H, s) ppm. 13C-NMR (C3D6O) δ 191.3, 162.9, 144.0, 135.7, 128.3, 119.1, 114.1, 19.7 ppm. IR (ATR, Smart orbit): = 3118.7, 1657.5, 1556.0, 1307.4 (max) cm−1.
3.3.3. Entry 3
Reaction of 3-methoxyphenol (352 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL) gave an orange solid. The mixture of regioisomers was chromatographed on SiO2 (hexane/AcOEt, 8:2) to yield 4-hydroxy-2-methoxy- benzaldehyde (8) (81.1 mg, 17%) and a mixture of inseparable products: 6-hydroxy-2-methoxy- benzaldehyde (6) and 2-hydroxy-4-methoxybenzaldehyde (7) in a 1:3.2 ratio determined by 1H-NMR (212.7 mg of the mixture was obtained, 44% yield of the formylation reaction). 4-Hydroxy-2-methoxybenzaldehyde (8): Appearance: White solid. Mp = 155–157 °C. TLC: Rf = 0.1 (hexane/AcOEt, 8:2). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 4.4 min. 1H-NMR (C3D6O) δ 10.2 (1H, s), 9.35 (1H, s), 7.64 (1H, d, J = 8.5 Hz), 6.57 (1H, d, J = 2.1 Hz), 6.53 (1H, ddd, J = 8.5, 2.1, 0.6 Hz), 3.91 (3H, s) ppm. 13C-NMR (C3D6O) δ 186.4, 164.7, 164.1, 129.7, 118.1, 108.2, 98.8, 55.2 ppm. IR (ATR, Smart orbit): = 3137.8, 1654.3, 1634.6, 1569.3 (max) cm−1.
3.3.4. Entry 4
Reaction of 3,5-dimethylphenol (395.8 mg, 3.24 mmol), TiCl4 (790 μL, 7.21 mmol), dichloromethyl methyl ether (330 μL, 3.65 mmol) and DCM (10 mL) gave an orange solid which was chromatographed on SiO2 (hexane/AcOEt, 9:1) to yield 2-hydroxy-4,6-dimethylbenzaldehyde (9) (318.2 mg, 65%) and 4-hydroxy-2,6-dimethylbenzaldehyde (10) (61.7 mg, 13%). The ratio of the products 9:10 was 5:1 and the global yield 78%. 2-Hydroxy-4,6-dimethylbenzaldehyde (9): Appearance: Pale yellow solid. Mp = 48–50 °C. TLC: Rf = 0.77 (hexane/AcOEt, 8:2). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 5.1 min. 1H-NMR (CDCl3) δ 11.9 (1H, m), 10.2 (1H, m), 6.62 (1H, s), 6.53 (1H, m), 2.55 (3H, s), 2.30 (3H, s) ppm. 13C-NMR (100 MHz, CDCl3) δ 194.7, 163.6, 149.4, 142.0, 123.3, 116.7, 116.3, 77.5, 77.2, 76.8, 22.3, 18.1 ppm. IR (ATR, Smart orbit): = 2879.8, 1640.8, 1625.6 (max), 1567.2 cm−1. 4-Hydroxy-2,6-dimethylbenzaldehyde (10): Appearance: White solid. Mp = 195–197 °C. TLC: Rf = 0.23 (hexane/AcOEt, 8:2). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 6.9 min. 1H-NMR (C3D6O) δ 10.4 (1H, s), 6.60 (2H, s), 2.54–2.53 (6H, m). 13C-NMR (C3D6O) δ 206.1, 191.7, 145.3, 126.2, 117.2, 21.0 ppm. IR (ATR, Smart orbit): = 3138.5, 1649.9, 1602.3, 1555.7, 1313.9, 1151.3 ( max) cm−1.
3.3.5. Entry 5
Reaction of 3,5-dimethoxyphenol (502.8 mg, 3.26 mmol), TiCl4 (790 μL, 7.21 mmol), dichloromethyl methyl ether (330 μL, 3.65 mmol) and DCM (10 mL) afforded an orange solid, which was chromatographed on SiO2 (hexane/AcOEt, 9:1) to yield 6-hydroxy-2,4-dimethoxybenzaldehyde (11) (339.0 mg, 63%). 6-Hydroxy-2,4-dimethoxybenzaldehyde (11): Appearance: Pale orange solid. Mp = 70–72 °C. TLC: Rf = 0.28 (hexane/AcOEt, 8:2). HPLC B (H2O/MeCN from 95:5 to 0:100 over 11 min): tR = 8.3 min. 1H-NMR (CDCl3) δ 12.5 (1H, s), 10.1 (1H, s), 6.01 (1H, d, J = 2.2 Hz), 5.91 (1H, d, J = 2.2 Hz), 3.85 (3H, s), 3.83 (3H, s) ppm. 13C-NMR (CDCl3) δ 191.8, 168.1, 166.4, 163.5, 106.0, 92.9, 90.6, 55.7, 55.7 ppm. IR (ATR, Smart orbit): = 1642.3, 1619.4, 600.7 (max) cm−1.
3.3.6. Entry 6
Reaction of 3,4,5-trimethoxyphenol (579.6 mg, 3.15 mmol), TiCl4 (760 μL, 6.93 mmol), dichloro-methyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL) provided a pale orange solid, which was chromatographed on SiO2 (DCM/hexane, 9:1) to yield 6-hydroxy-2,3,4-trimethoxybenzaldehyde (12) (372.0 mg, 56%). The HPLC analysis of the isolated mixture reveals 25% of starting material. 6-Hydroxy-2,3,4-trimethoxybenzaldehyde (12): Appearance: Pale yellow solid. Mp = 63–65 °C. TLC: Rf = 0.52 (DCM/hexane, 9:1). HPLC B (H2O/MeCN from 95:5 to 0:100 over 11 min): tR = 8.1 min. 1H-NMR (CDCl3) δ 12.1 (1H, s), 10.1 (1H, s), 6.19 (1H, s), 4.04 (3H, s), 3.90 (3H, s), 3.79 (3H, s) ppm. 13C-NMR (CDCl3) δ 192.8, 162.2, 161.3, 155.6, 134.0, 108.5, 95.4, 62.2, 61.4, 56.4 ppm. IR (ATR, Smart orbit): = 2945.4, 2850.4, 1638.5, 1623.2 (max) cm−1.
3.4. Methoxybenzenes
3.4.1. Entry 7
Reaction of anisole (352 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL) were used to obtain a pale rosy oil that contained the two products. The mixture were not possible to isolate by SiO2 and ratio of 1:1.1 was determined by 1H-NMR for 2-methoxybenzaldehyde (13) and 4-methoxybenzaldehyde (14) respectively (424.4 mg, 97% global yield). 2-Methoxybenzaldehyde and 4-methoxybenzaldehyde (13 and 14): TLC: Rf = 0.43–0.37 (DCM/hexane, 1:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR (13) = 6.0 min, tR (14) = 6.3 min. 1H-NMR and 13C-NMR see SI.
3.4.2. Entry 8
Reaction of 1,3-dimethoxybenzene (420 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloro-methyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL) gave a grey solid, which was purified on SiO2 (DCM) to yield 2,6-dimethoxybenzaldehyde (15) (97.4 mg, 18%) and 2,4-dimethoxybenzaldehyde (16) (323.9 mg, 61%). The ratio of regioisomers 16:15 was 3:1 and the global yield 79%. 2,6-Dimethoxybenzaldehyde (15): Appearance: Pale yellow solid. Mp = 94–97 °C. TLC: Rf = 0.21 (DCM). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 4.9 min. 1H-NMR (CDCl3) δ 10.5 (1H, s), 7.45 (1H, t, J = 8.5 Hz), 6.58 (2H, d, J = 8.5 Hz), 3.90 (6H, s) ppm. 13C-NMR (CDCl3) δ 189.6, 162.4, 136.0, 114.5, 104.0, 56.2 ppm. IR (ATR, Smart orbit): = 2844.9, 2796.7, 1671.7, 1589.9, 1576.0, 1109.6 () cm−1. 2,4-Dimethoxybenzaldehyde (16): Appearance: White solid. Mp = 68–70 °C. TLC: Rf = 0.51 (DCM). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 5.7 min. 1H-NMR (CDCl3) δ 10.3 (1H, d, J = 0.7 Hz), 7.80 (1H, d, J = 8.7 Hz), 6.54 (1H, ddd, J = 8.7, 2.2, 0.7 Hz), 6.44 (1H, d, J = 2.2 Hz), 3.90 (3H, s), 3.87 (3H, s) ppm. 13C-NMR (CDCl3) δ 188.5, 166.3, 163.8, 130.9, 119.2, 105.9, 98.1, 55.8, 55.7 ppm. IR (ATR, Smart orbit): = 2855.6, 2779.3, 1663.6, 1596.3, 1578.4, 826.5 (max) cm−1.
3.4.3. Entry 9
Reaction of 1,3,5-trimethoxybenzene (533.5 mg, 3.17 mmol), TiCl4 (760 μL, 6.93 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM(10 mL), afforded a purple solid, which was purified on SiO2 (DCM/hexane, 9:1) to yield 2,4,6-trimethoxybenzaldehyde (17) (275.0 mg, 44%). 2,4,6-Trimethoxybenzaldehyde (17): Appearance: Orange solid. Mp = 118–121 °C. TLC: Rf = 0.37 (DCM/hexane, 9:1). HPLC B (H2O/MeCN from 95:5 to 0:100 over 11 min): tR = 7.0 min. 1H-NMR (CDCl3) δ 10.4 (1H, s), 6.07 (2H, s), 3.88 (6H, s), 3.87 (3H, s) ppm. 13C-NMR (CDCl3) δ 187.9, 166.3, 164.2, 109.0, 90.4, 56.1, 55.6 ppm. IR (ATR, Smart orbit): = 2843.2, 2796.0, 1661.3, 1596.1, 1574.3, 806.6 (max) cm−1.
3.4.4. Entry 10
Reaction of 3,5-dimethylanisole (453 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL), gave a violet oily solid, which was purified by reverse-phase liquid chromatography (Semipreparative HPLC: Xbridge Prep BEH 130 C18, 5 μm OBDTM 19 × 100 mm column, gradient H2O/MeCN from 55:45 to 50:50 over 30 min) to yield a 3.5:1 ratio of 2-methoxy-4,6-dimethylbenzaldehyde (18) (77.4 mg, 14.7%) and 4-methoxy-2,6-dimethylbenzaldehyde (19) (22.1 mg, 4.2%) respectively. 2-Methoxy-4,6-dimethylbenzaldehyde (18): Appearance: Yellow solid. Mp = 48–50 °C. TLC: Rf = 0.50 (DCM). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 5.6 min. 1H-NMR (CDCl3) δ 10.6 (1H, s), 6.63 (1H, s), 6.62 (1H, s), 3.88 (3H, s), 2.54 (3H, s), 2.35 (3H, s) ppm. 13C-NMR (CDCl3) δ 191.9, 163.5, 145.8, 142.2, 125.2, 121.1, 109.9, 55.9, 22.3, 21.6 ppm. IR (ATR, Smart orbit): = 2965.3, 2926.5, 1671.3, 1598.5, 1319.1, 1146.5 cm−1. 4-Methoxy-2,6-dimethylbenzaldehyde (19): Appearance: White solid. Mp = 49–50 °C. TLC: Rf = 0.24 (DCM). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 4.7 min. 1H-NMR (CDCl3) 10.5 (1H, s), 6.59 (2H, s), 3.84 (3H, s), 2.61 (6H, s) ppm. 13C-NMR (CDCl3) 191.8, 162.9, 144.6, 126.2, 115.0, 55.4, 21.3 ppm. IR (ATR, Smart orbit): = 2960.2, 2921.5, 1678.8, 1610.3, 1465.1, 1299.4, 1205.1, 1097.2, 836.8 (max) cm−1.
3.5. Methylbenzenes
3.5.1. Entry 11
Reaction of o-xylene (386 μL, 3.20 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL), afforded a green oil, which was purified on SiO2 (hexane/AcOEt, 8:2). During the purification, partial decomposition was observed. Both regioisomers were isolated as a 3.2:1 mixture of 3,4-dimethylbenzaldehyde (21) and 2,3-dimethylbenzaldehyde (20) respectively, determined by the 1H-NMR of the isolated mixture (see SI). TLC: Rf = 0.48–0.53 (hexane/AcOEt, 8:2). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 6.7 min.
3.5.2. Entry 12
Reaction of m-xylene (394 μL, 3.21 mmol), TiCl4 (780 μL (7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL), afforded a yellow oil, which was purified on SiO2 (treated with 1% of NEt3) (hexane/AcOEt, 19:1) to yield a mixture of 2,6-dimethylbenzaldehyde (22) and 2,4-dimethylbenzaldehyde (23) (267.0 mg, 62%) in a 1:32 ratio, as determined by 1H-NMR of the isolated mixture. Moreover, bis(2,4-dimethylphenyl)methanol (24) was isolated as a dimerization by-product (38.0 mg, 9.9%). Mixture of 22 and 23: TLC: Rf = 0.25–0.27 (hexane/AcOEt, 19:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 6.8 min. bis(2,4-Dimethylphenyl)methanol (24): Appearance: White solid. TLC: Rf = 0.12 (hexane/EtOAc, 19:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 8.3 min. 1H-NMR (CDCl3) δ 7.17 (2H, m), 6.99 (4H, m), 6.07 (1H, s), 2.32 (6H, s), 2.25 (6H, s) ppm. 13C-NMR (CDCl3) δ 138.2, 137.2, 135.7, 131.4, 126.8, 126.6, 70.1, 21.1, 19.1 ppm.
3.5.3. Entry 13
Reaction of p-xylene (394 μL, 3.21 mmol), TiCl4 (780 μL, 7.11 mmol), dichloromethyl methyl ether (320 μL, 3.54 mmol) and DCM (10 mL), gave a rosy oil which was not purified (95% purity). The dimerization byproduct (3.4% of the peak area) was observed in the chromatographic traces. 2,5-Dimethylbenzaldehyde (25): Appearance: Rosy oil. TLC: Rf = 0.25–0.27 (hexane/EtOAc, 19:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 6.9 min. 1H-NMR (400 MHz, CDCl3) δ 10.23 (1H, s), 7.59 (1H, d, J = 1.2 Hz), 7.28 (1H, dd, J = 7.8, 1.5 Hz), 7.14 (1H, d, J = 7.8 Hz), 2.61 (3H, s), 2.37 (3H, s) ppm. 13C-NMR (100 MHz, CDCl3) δ 192.4, 144.6, 140.7, 132.6, 132.5, 132.0, 127.1, 21.7, 19.6 ppm. IR (ATR, Smart orbit): = 2923.8, 2722.1, 1690.5 (max), 1611.3, 1504.9, 1240.7, 1155.2 cm−1.
3.5.4. Entry 14
Reaction of mesytilene (1000 μL, 7.19 mmol), TiCl4 (1.5 mL, 13.68 mmol), dichloromethyl methyl ether (720 μL, 7.96 mmol) and DCM (25 mL), gave a colorless oil (1.02 g, 96%), which was not purified (97% purity). 2,4,6-Trimethylbenzaldehyde (26): Appearance: Colorless oil. TLC: Rf = 0.54 (DCM/hexane, 1:1). HPLC (H2O/MeCN from 95:5 to 0:100 over 8 min): tR = 6.0 min. 1H-NMR (CDCl3) δ 10.5 (1H, s), 6.87 (2H, s), 2.55 (6H, s), 2.29 (3H, s) ppm. 13C-NMR (CDCl3) δ 192.87, 143.78, 141.43, 130.51, 129.95, 21.43, 20.46 ppm. IR (NaCl): =2921.4, 2865.3, 2783.1, 1687.1 (max), 1608.4 cm−1.
4. Conclusions
In short, the formylation studies presented here demonstrate the potential of aromatic formylation using TiCl4 and dichloromethyl methyl ether as a straightforward and versatile reaction that affords a wide range of functionalized aldehydes. Of note, only for phenol derivatives did the oxygen-metal interaction contribute significantly to determining o-formylation. We consider that this reaction will allow the development of a new set of protecting groups and linkers for further application in SPPS.
Acknowledgments
I.R.T. thanks the Generalitat de Catalunya for a pre-doctoral fellowship. This work was funded in part by the following: the CICYT (CTQ2012-30930), the Generalitat de Catalunya (2014 SGR 137), and the Institute for Research in Biomedicine Barcelona (IRB Barcelona) (Spain); the National Research Foundation and the University of KwaZulu Natal (South Africa); and SENESCYT (Ecuador).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/04/5409/s1.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. I.R.-T., J.M.B., I.P.R.M., E.N. and F.A. designed the experiments. I.R.-T. and M.P.-B. performed the experiments and characterized all the compounds. I.R.-T., E.N. and F.A. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Lawrence N.J. Aldehydes and ketones. J. Chem. Soc. Perkin Trans. 1. 1998;10:1739–1750. doi: 10.1039/a800646f. [DOI] [Google Scholar]
- 2.Tenson T., Mankin A. Antibiotics and the ribosome. Mol. Microbiol. 2006;59:1664–1677. doi: 10.1111/j.1365-2958.2006.05063.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson L.N. The synthesis of aromatic aldehydes. Chem. Rev. 1946;38:227–254. doi: 10.1021/cr60120a002. [DOI] [PubMed] [Google Scholar]
- 4.Casnati G., Crisafulli M., Ricca A. A new method for the selective ortho-formylation of phenols. Tetrahedron Lett. 1965;6:243–245. doi: 10.1016/S0040-4039(00)89947-5. [DOI] [Google Scholar]
- 5.Hofsløkken N.U., Skattebøl L. Convenient method for the ortho-formylation of phenols. Acta Chem. Scand. 1999;53:258–262. doi: 10.3891/acta.chem.scand.53-0258. [DOI] [Google Scholar]
- 6.Kantlehner W. New Methods for the preparation of aromatic aldehydes. Eur. J. Org. Chem. 2003;14:2530–2546. doi: 10.1002/ejoc.200200653. [DOI] [Google Scholar]
- 7.Hoover J.M., Stahl S.S. Highly practical copper(I)/TEMPO catalyst system for chemoselective aerobic oxidation of primary alcohols. J. Am. Chem. Soc. 2011;133:16901–16910. doi: 10.1021/ja206230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neves Â.C.B., Calvete M.J.F., Pinho e Melo T.M.V.D., Pereira M.M. Immobilized catalysts for hydroformylation reactions: A versatile tool for aldehyde synthesis. Eur. J. Org. Chem. 2012;2012:6309–6320. doi: 10.1002/ejoc.201200709. [DOI] [Google Scholar]
- 9.Cheng C., Brookhart M. Efficient reduction of esters to aldehydes through iridium-catalyzed hydrosilylation. Angew. Chem. Int. Ed. Engl. 2012;51:9422–9424. doi: 10.1002/anie.201205154. [DOI] [PubMed] [Google Scholar]
- 10.Li H., Misal Castro L.C., Zheng J., Roisnel T., Dorcet V., Sortais J.-B., Darcel C. Selective reduction of esters to aldehydes under the catalysis of well-defined NHC-iron complexes. Angew. Chem. Int. Ed. Engl. 2013;52:8045–8049. doi: 10.1002/anie.201303003. [DOI] [PubMed] [Google Scholar]
- 11.Feng Q., Song Q. Aldehydes and ketones formation: Copper-catalyzed aerobic oxidative decarboxylation of phenylacetic acids and α-hydroxyphenylacetic acids. J. Org. Chem. 2014;79:1867–1871. doi: 10.1021/jo402778p. [DOI] [PubMed] [Google Scholar]
- 12.Tamura N., Aoyama T., Takido T., Kodomari M. Novel [4-Hydroxy-TEMPO + NaCl]/SiO2 as a reusable catalyst for aerobic oxidation of alcohols to carbonyls. Synlett. 2012;23:1397–1401. doi: 10.1055/s-0031-1290980. [DOI] [Google Scholar]
- 13.De M. Muñoz J., Alcázar J., de la Hoz A., Díaz-Ortiz A. Application of flow chemistry to the selective reduction of esters to aldehydes. Eur. J. Org. Chem. 2012;2:260–263. doi: 10.1002/ejoc.201101458. [DOI] [Google Scholar]
- 14.Nicolaou K.C., Adsool V.A., Hale C.R.H. An expedient procedure for the oxidative cleavage of olefinic bonds with PhI(OAc)2, NMO, and catalytic OsO4. Org. Lett. 2010;12:1552–1555. doi: 10.1021/ol100290a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isidro-Llobet A., Alvarez M., Albericio F. Amino acid-protecting groups. Chem. Rev. 2009;109:2455–2504. doi: 10.1021/cr800323s. [DOI] [PubMed] [Google Scholar]
- 16.Góngora-Benítez M., Tulla-Puche J., Albericio F. Multifaceted roles of disulfide bonds. peptides as therapeutics. Chem. Rev. 2013;114:901–926. doi: 10.1021/cr400031z. [DOI] [PubMed] [Google Scholar]
- 17.Postma T., Albericio F. Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in fmoc-based solid-phase peptide synthesis. Org. Lett. 2014;16:1772–1775. doi: 10.1021/ol5004725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postma T.M., Giraud M., Albericio F. Trimethoxyphenylthio as a highly labile replacement for tert-butylthio cysteine protection in Fmoc solid phase synthesis. Org. Lett. 2012;14:5468–5471. doi: 10.1021/ol3025499. [DOI] [PubMed] [Google Scholar]
- 19.Góngora-Benítez M., Mendive-Tapia L., Ramos-Tomillero I., Breman A.C., Tulla-Puche J., Albericio F. Acid-labile cys-protecting groups for the Fmoc/tBu strategy: Filling the gap. Org. Lett. 2012;14:5472–5475. doi: 10.1021/ol302550p. [DOI] [PubMed] [Google Scholar]
- 20.García O., Nicolás E., Albericio F. Solid-phase synthesis: A linker for side-chain anchoring of arginine. Tetrahedron Lett. 2003;44:5319–5321. [Google Scholar]
- 21.Garcia O., Bofill J.M., Nicolas E., Albericio F. 2,2,4,6,7-Pentamethyl-2,3-dihydrobenzofuran-5-methyl (Pbfm) as an alternative to the trityl group for the side-chain protection of cysteine and asparagine/glutamine. Eur. J. Org. Chem. 2010;19:3631–3640. doi: 10.1002/ejoc.201000201. [DOI] [Google Scholar]
- 22.Ramos-Tomillero I., Mendive-Tapia L., Góngora-Benítez M., Nicolás E., Tulla-Puche J., Albericio F. Understanding acid lability of cysteine protecting groups. Molecules. 2013;18:5155–5162. doi: 10.3390/molecules18055155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohsawa K., Yoshida M., Doi T. A direct and mild formylation method for substituted benzenes utilizing dichloromethyl methyl ether-silver trifluoromethanesulfonate. J. Org. Chem. 2013;78:3438–3444. doi: 10.1021/jo400056k. [DOI] [PubMed] [Google Scholar]
- 24.Vilsmeier A., Haack A. Action of phosphorus halides on alkylformanilides. A new method for the preparation of secondary and tertiary p-alkylaminonobenzaldehydes. Ber. Dtsch. Chem. Ges. A/B. 1927;60:119–122. doi: 10.1002/cber.19270600118. [DOI] [Google Scholar]
- 25.Duff J.C., Bills E.J. Reactions between hexamethylenetetramine and phenolic compounds. Part I. A new method for the preparation of 3- and 5-aldehydosalicylic acids. J. Chem. Soc. 1932:2861–2862. [Google Scholar]
- 26.Hansen T.V., Skattebøl L. Ortho-Formylation of phenols; Preparation of 3-bromosalicylaldehyde. Org. Synth. 2005;82:64–68. doi: 10.15227/orgsyn.082.0064. [DOI] [Google Scholar]
- 27.Garcıa O., Nicolás E., Albericio F. o-Formylation of electron-rich phenols with dichloromethyl methyl ether and TiCl4. Tetrahedron Lett. 2003;44:4961–4963. doi: 10.1016/S0040-4039(03)01168-7. [DOI] [Google Scholar]
- 28.Heras C., Ramos-Tomillero I., Caballero M., Paradís-Bas M., Nicolás E., Albericio F., de P. R. Moreira I., Bofill J.M. On the mechanism of phenolic formylation mediated by ticl4 complexes: Existence of diradical intermediates induced by valence tautomerism. Eur. J. Org. Chem. 2015 doi: 10.1002/ejoc.201403548. [DOI] [Google Scholar]
- 29.Gross H., Rieche A., Matthey G. Über α-Halogenäther, XIII. Neue verfahren zur darstellung von phenolaldehyden. Chem. Ber. 1963;96:308–313. [Google Scholar]
- 30.Cresp T.M., Sargent M.V., Elix J.A., Murphy D.P.H. Formylation and bromination ortho to the hydroxy-group of 2-carbonylsubstituted phenols in the presence of titanium(VI)chloride. J. Chem. Soc. Perkin Trans. 1. 1973:340–345. doi: 10.1039/p19730000340. [DOI] [Google Scholar]
- 31.Hamilton P.M., McBeth R., Bekebrede W., Sisler H.H. Molecular addition compounds of titanium tetrachloride with several ethers. J. Am. Chem. Soc. 1953;75:2881–2883. doi: 10.1021/ja01108a027. [DOI] [Google Scholar]
- 32.Calderazzo F., Ferri I., Pampaloni G., Troyanov S. η6-Arene derivatives of titanium(IV), zirconium(IV) and hafnium(IV) J. Organomet. Chem. 1996;518:189–196. doi: 10.1016/0022-328X(96)06194-3. [DOI] [Google Scholar]
- 33.Martín R., Murruzzu C., Pericàs M.A., Riera A. General approach to glycosidase inhibitors. Enantioselective synthesis of deoxymannojirimycin and swainsonine. J. Org. Chem. 2005;70:2325–2328. doi: 10.1021/jo048172s. [DOI] [PubMed] [Google Scholar]
- 34.Andrus M.B., Liu J., Ye Z., Cannon J.F. Asymmetric glycolate aldol reactions using cinchonium phase-transfer catalysts. Org. Lett. 2005;7:3861–3864. doi: 10.1021/ol051096a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.