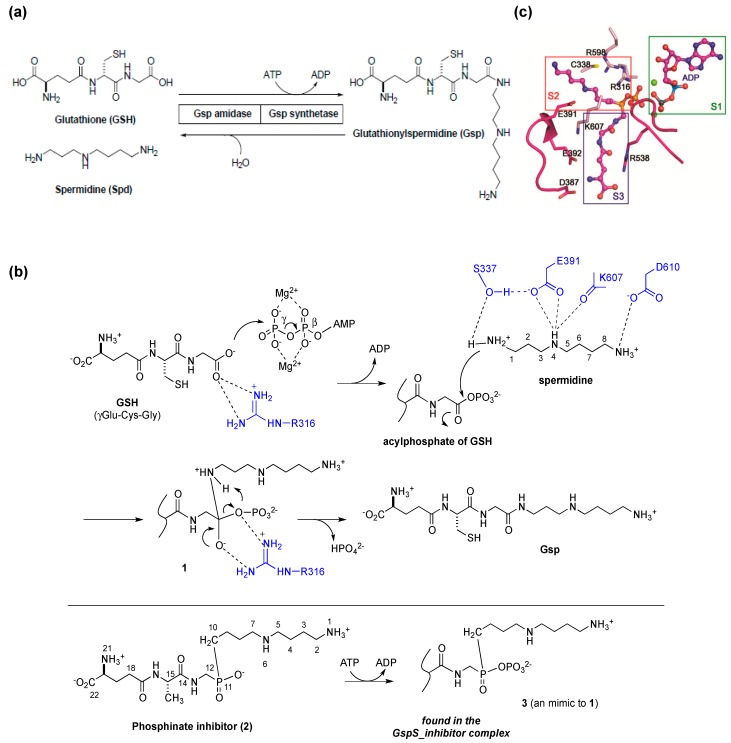
Figure 2.
(a) The reactions catalyzed by E. coli glutathionylspermidine synthetase/amidase (GspSA). GspSA from E. coli is a bifunctional protein that contains an N-terminal amidase domain and a C-terminal synthetase domain. The two catalyzed reactions are shown at the molecular level; (b) Proposed reaction mechanism of GspS. The conjugation reaction of GSH with spermidine proceeds in two steps—the C terminus of GSH is initially phosphorylated by γ-phosphate of ATP to form an acylphosphate, followed by the nucleophilic attack of N1-spermidine to the acylphosphate; (c) Structure of inhibitor-bound GspS (GspS_inhibitor) at the S2 and S3 substrate-binding pockets near four negatively charged residues Asp387, Asp389, Glu391 and Glu392. Figure 2a was reproduced from [26] © The American Society for Biochemistry and Molecular Biology. Figure 2b,c were reproduced from [30] © European Molecular Biology Organization.