Abstract
Plants of the Calycanthaceae family, which possesses four genera and about 15 species, are mainly distributed in China, North America and Australia. Chemical studies on the Calycanthaceae have led to the discovery of about 14 alkaloids of different skeletons, including dimeric piperidinoquinoline, dimeric pyrrolidinoindoline and/or trimeric pyrrolidinoindolines, which exhibit significant anti-convulsant, anti-fungal, anti-viral analgesic, anti-tumor, and anti-melanogenesis activities. As some of complex tryptamine-derived alkaloids exhibit promising biological activities, the syntheses of these alkaloids have also been a topic of interest in synthetic chemistry during the last decades. This review will focus on the structures and total syntheses of these alkaloids.
Keywords: biological activity, biosynthesis, Calycanthaceae alkaloids, structure, synthesis
1. Introduction
The small family of the Calycanthaceae comprises four genera, namely Chimonanthus Lindley, Sinocalycanthus Cheng & S. Y. Chang, Calycanthus L., and Idiospermum, which globally include ca. 15 species [1,2,3]. The plants of the Chimonanthus and Sinocalycanthus genera are ornamental shrubs endemically distributed in China, and those of the Calycanthus and Idiospermum originate from North America and Australia, respectively. The classification of the species of Chimonanthus genus is still a tough task and has been a subject of debate for a long time [4]. The early literature categorized this genus into three species, i.e., Ch. nitens Oliv., Ch. praecox (Linn.) Link, and Ch. salicifolius S.Y. Hu [5]. Recently it was proposed that this genus be classified in 10 species based on morphological evidence, including Ch. nitens Oliv., Ch. praecox (Linn.) Link, Ch. salicifolius S.Y. Hu, Ch. nitens, Ch. zhejiangensis M.C. Liu, Ch. campanulatus, Ch. baokangensis, Ch. anhuiensis, Ch. caespitosa, Ch. campanulatus var. guizhouensis [6]. In addition, only one species, C. chinensis Cheng et S.Y. Chang, is attributed to the Sinocalycanthus genus. Three plants named C. floridus var. floridus, C. floridus var. laevigatus, and C. occidentalis Hook. et Arn. pertain to the genus Calycanthus. The plant Idiospermum australiense (Diels) S. T. Blake a rare tree that occurs only in the North Queensland region of Australia is the sole member of the Idiospermum genus. These Calycanthaceae plants are primitive angiosperms and popular ornamental flowers with a pleasant aroma. The Calycanthaceae plants have long been used in Traditional Chinese Medicines (TCMs), to treat rheumatic arthritis, coughs, throat wounds, dizziness, nausea, fever, detoxification, and enteral disease [7,8,9].
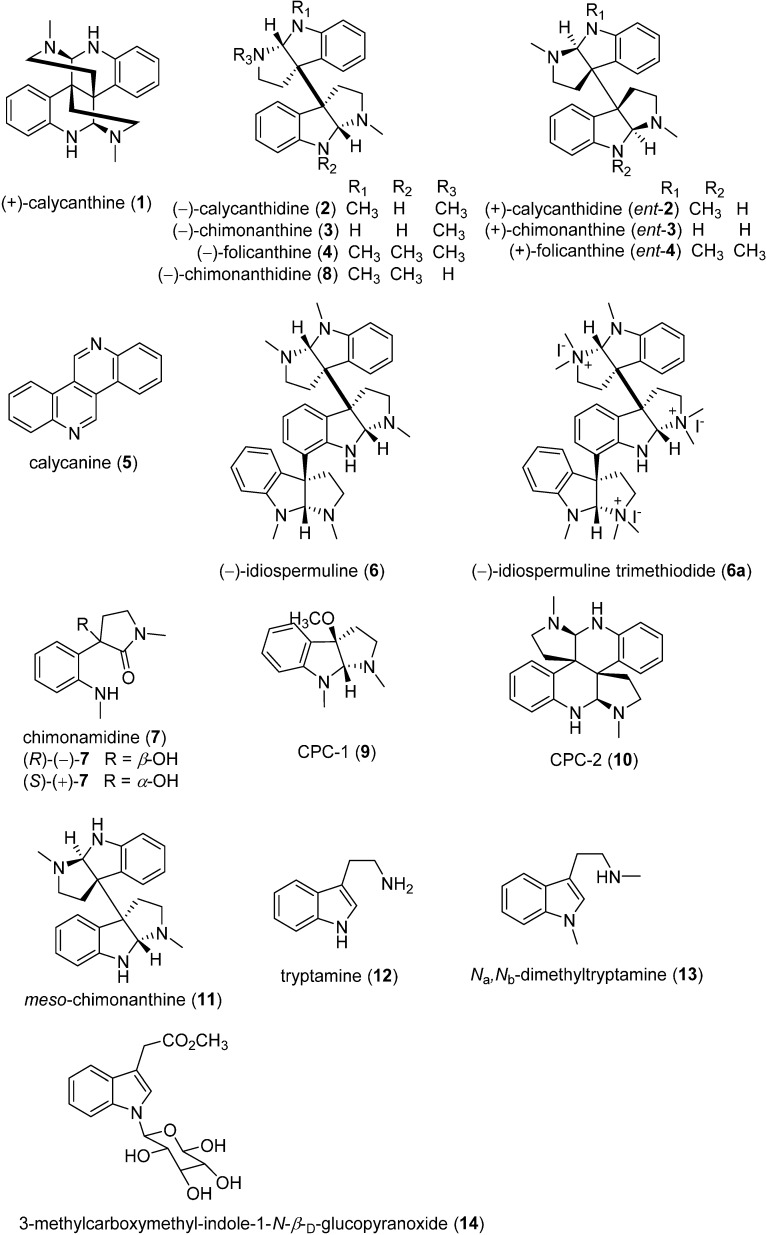
The chemical investigation of the Calycanthaceae plants started more than one hundred years ago in 1888, which led to the isolation of a large amount of alkaloids, flavonoids [10], lignans [6,11], coumarins [12,13,14], terpenoids [15,16,17], and essential oils [7,18,19,20]. It is important to note that the discovery of the Calycanthaceae alkaloids was reminiscent of the history of development of science. Looking back to this history, only 14 alkaloids (Figure 1) whose discoveries were full of hardship and arduousness were characterized from this family. During past decades, there has been a trend towards e synthetic approaches of these structurally interesting and bioactive alkaloids. This review attempts to provide timely and comprehensive coverage of the chemical and biological studies related to the Calycanthaceae alkaloids, with a specific focus on summarizing the great amount of synthetic work performed in this area.
Figure 1.
Structures of the Calycanthaceae alkaloids.
2. Structures, Biological Activities, and Biosynthetic Origins of Calycanthaceae Alkaloids
2.1. Structures of Calycanthaceae Alkaloids and Their Discovery
The phytochemical investigation of the plants Calycanthaceae was first described in 1888, which led to isolation of the first Calycanthaceae alkaloid, (+)-calycanthine (1, Figure 1) with a dimeric piperidinoquinoline skeleton, from the seeds of C. glaucus Willd. by Eccles. One year later, Wiley proved the high content of this alkaloid in the seeds of the same plant [21]. In 1905, further progress was made by Gordin whereby this principal alkaloid was crystalized in different forms at ordinary temperature and deduced to possess a molecular formula C11H14N2 containing no oxygen atom [21,22]. A few years later, Späth and Stroh expressed their disagreement on Gordin’s work and stated that the empirical formula of (+)-calycanthine should be doubled C11H14N2 [23]. Soon Manske put forward the possibility that this molecular formula might be C22H26N4 [24], which was finally proved by Barger’s group in 1939 [25]. The structure of this alkaloid (+)-calycanthine, C22H26N4, was finally established uniequivocally by means of X-ray crystal structural analysis of its dihydrobromide dehydrate by Hamor’s group in 1960 [26].
In 1905, Gordin also reported a second alkaloid, isocalycanthine, from the seeds of Chimonanthus genus, which possessed a different melting point and showed different behavior with respect to the removal of water of crystallization from the hydrated base with those of calycanthine [27,28]. However, Manske expressed doubts about the existence of isocalycanthine. In his study on the species of C. floridus L., a great quantity (1.2%) of calycanthine was. Gordin’s seed extract consisted in reality of C. fertilis. In the same report, Gordin also discribed the isolation process of (+)-calycanthine (2.6%) from the seeds of Meratia praecox (C. praecox) [24]. In 1992, the occurrence of isocalycanthine in a closely related species Psychotria forsteriana (Rubiaceae) was reported by Kuballa’s group [29].
In 1938, Barger’s group isolated a minor alkaloid, (−)-calycanthidine (2), from the seeds of C. floridus, which molecular formula was deduced to be C13H16N2 [30]. This conclusion was corrected by Saxton in 1962, who established both the formula C23H28N4 and the structure of (−)-calycanthidine [31]. The alkaloid (−)-chimonanthine (3) was firstly obtained from Ch. fragrans. Lindle (Ch. praecox) by Hodson’s group [32], and its structure was determined on the basis of detailed X-ray analysis of chimonanthine dihydrobromide by Grant’s group [33,34]. As for (−)-folicanthine (4), it was first isolated by Hodson’s group in 1957 and was identified to be a dimeric pyrrolidinoindoline alkaloid, close to (−)-calycanthidine (2), and (−)-chimonanthine (3) [32]. From then on, this seemed to be an end for the structural elucidation of (−)-calycanthidine, (−)-chimonanthine, (−)-folicanthine, until in 2000, the total syntheses of these alkaloids were completed by Overman’s group, proving that (−)-calycanthidine, (−)-chimonanthine, (−)-folicanthine should be drawn as compounds 2, 3, and 4 (Figure 1), respectively; therefore the structures of compounds ent-2, ent-3, and ent-4 reported in the previous papers should be (+)-calycanthidine, (+)-chimonanthine, (+)-folicanthine, respectively [35]. Calycanine (5) with a molecular formula C16H10N2 was a product of calycanthine and chimonanthine obtained by Zn dehydrogenation. Its structure was first incorrectly proposed by Barger, and then revised by Woodward’s group via synthesis [32,34,36].
(−)-Idiospermuline (6), a trimeric pyrrolidinoindoline, together with two known dimeric alkaloids, (+)-calycanthine and (−)-chimonanthine were isolated by the bioassay-guided method from the seeds of Idiospermum australiense (Diels) S.T. Blake, a native species from North Queensland, Australia. The structure of (−)-idiospermuline was determined by NMR and MS data and the absolute stereochemistry was established by X-ray crystallographic study of idiospermuline trimethiodide (6a) [37].
In 2004, Takayama’s group investigated the alkaloidal constituents of the seeds of Ch. praecox L., leading to the isolation of two new tryptamine-related alkaloids, chimonamidine (7) and chimonanthidine (8), together with the known (+)-calycanthine, (−)-chimonanthine, (−)-folicanthine, and (−)-calycanthidine. They conducted the total synthesis of (±)-chimonamidine to establish its absolute structure. As a result, natural chimonamidine showed optical rotation = −12.6, which was significantly different with those of (R)-(−)-chimonamidine ( = −178) and (S)-(+)-chimonamidine ( = +171), suggesting that natural chimonamidine is a mixture slightly enriched with the (R)-(−)-enantiomer. Chimonanthidine (8) was determined to be Nb-monodemethylfolicanthine by total synthesis, with the absolute configuration eventually confirmed by a combined strategy of comparing the CD spectrum with that of known (−)-folicanthine [38]. In his paper of 2006, a further phytochemical study on the seeds and rinds of Ch. praecox (L.) f. concolor resulted in the isolation of a new pyrrolidinoindoline-type alkaloid, CPC-1 (9), and one new tetrahydroquinoline dimeric alkaloid, CPC-2 (10), together with eight known alkaloids, (+)-calycanthine, (−)-chimonanthine, (−)-folicanthine, (−)-calycanthidine, (−)-chimonanthidine, meso-chimonanthine (11), tryptamine (12), and Na,Nb-dimethyltryptamine (13) [39].
In 2009, an antifungal activity-guided phytochemical investigation on the defatted seeds of Ch. praecox, a species grown in Shaanxi Province of China, afforded two dimeric alkaloids, (+)-calycanthine and (−)-folicanthine [40]. Additionally, an additional indole-derived glycoside, 3-methylcarboxymethyl-indole-1-N-β-d-glucopyranoside (14), and the four known compounds (+)-calycanthine, (−)-chimonanthine, (−)-folicanthine, (−)-calycanthidine were obtained from the fruits and leaves of C. praecox in 2011 [15]. A recent phytochemical study on the flower buds of Ch. praecox also led to the isolation of five known dimeric alkaloids and several other compounds [41].
2.2. Biological Activities
The Calycanthaceae plants have been used as Traditional Chinese Medicines (TCMs) for the treatment of colds, and as sedative, antitussive, anti-hypertension, antioxidation, anti-inflammatory, and antitumor medicines [14,15]. As the important components of these plants, the Calycanthaceae alkaloids showed biological activities such as anti-convulsant, anti-fungal, anti-viral, analgesic, anti-tumor, and melanogenesis inhibitory properties.
The main representative alkaloid, calycanthine (1), has been recognized as a powerful centrally acting anti-convulsant for a long time [42,43]. It was reported that calycanthine may mediate its convulsant action predominantly by inhibiting of the inhibitory neurotransmitter GABA as a result of interactions with l-type Ca2+ channels and by inhibiting GABA-mediated chloride currents at GABAA receptors [44].
(+)-Calycanthine (1) and (−)-folicanthine (4) were evaluated for their antifungal activities against five plant pathogenic fungi, Exserohilum turcicum, Bipolaris maydis, Alternaria solani, Sclerotinia sderotiorum, and Fusarium oxysportium. It turned out to be that B. maydis was the most susceptible to 1 with an EC50 value of 29.3 μg/mL, and then S. sderotiorum to 4 with an EC50 of 61.2 μg/mL [40]. (−)-Chimonanthine (3) and (−)-folicanthine (4) also showed weak antiviral activities against porcine respiratory and reproductive syndrome virus (PRRSV) with IC50 values of 68.9 ± 3.1 μM and 58.9 ± 10.2 μM, respectively [45].
Chimonanthines were tested on μ- and κ-opioid binding assay, and on the tail-flick and the capsaicin-induced pain models. As a result, (−)-chimonanthine (3), (+)-chimonanthine (ent-3), and meso-chimonanthine (11) showed strong binding affinities towards μ-opioid receptors with Ki values of 271 ± 85 nM, 652 ± 159 nM, and 341 ± 29 nM, respectively, indicating their significant analgesic activities [46,47].
It was also recently reported that the methanol extract of the flower buds of Ch. praecox showed an inhibitory effect against melanogenesis in ophylline-stimulated B16 melanoma 4A5 cells. A further investigation revealed that the principal alkaloids of (+)-calycanthine (1), (−)-chimonanthine (3), and (−)-folicanthine (4) showed the most potent melanogenesis inhibitory activity, with IC50 values of 0.93, 1.4, and 1.8 μM, respectively. Abutin (174 μM), a commercially tyrosinase inhibitor, was used as a positive control. In this paper, (+)-chimonanthine (ent-3), and meso-chimonanthine (11) showed cytotoxicity at 10 μM [41]. In another assay, compounds 2–4 were screened for the cytotoxicity against a small panel of human cancer lines, showing cytotoxic effects against gastric carcinoma NUGC3 and hepatocarcinoma SNU739 cancer cells with IC50 values ranging from 10.3 to 19.7 μM [15].
2.3. Biosynthetic Origins
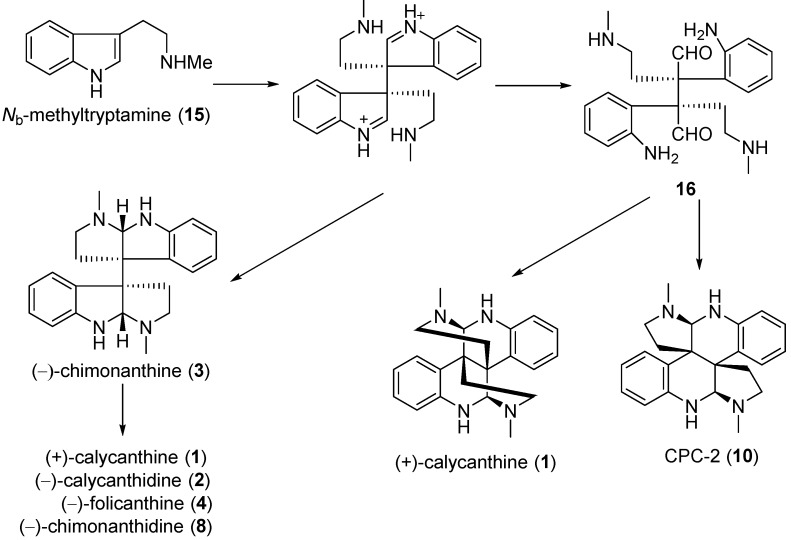
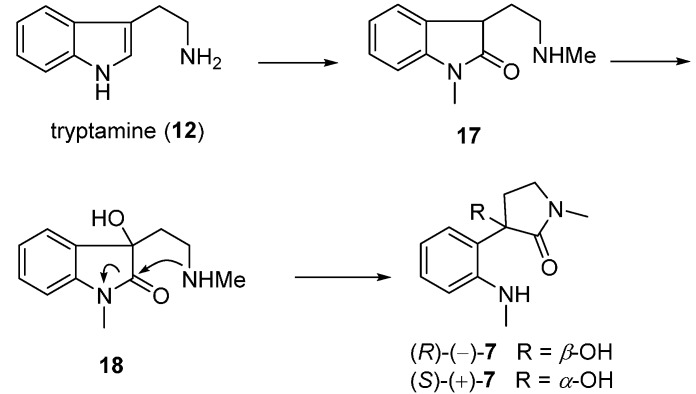
Calycanthine, calycanthidine, chimonanthine, folicanthine, chimonanthidine, and CPC-2 are a series of tryptamine-derived dimeric alkaloids, which were proposed to be originated from Nb-methyltryptamine (15). The oxidative dimerization of two molecules of Nb-methyltryptamine forms the key tetraaminodialdehyde intermediate 16, which undergoes several enzyme-catalyzed reactions and modifications yielding calycanthine and CPC-2 (Scheme 1) [39]. A possible biosynthesis of chimonanmidine (7) is shown in Scheme 2. The intermediate 17 was derived from tryptamine (12) by oxidation, and subsequently converted into 18 by introduction a hydroxy function at the benzylic position. Chimonanmidine (7) was finally produced by the transannulation of the lactam ring of 18 [38,48].
Scheme 1.
Potential biogenetic pathway of tryptamine-derived dimeric Calycanthaceae alkaloids.
Scheme 2.
Potential biogenetic pathway of chimonamidine (7).
3. Total Synthesis of Calycanthaceae Alkaloids
3.1. Calycanthines and Chimonanthines
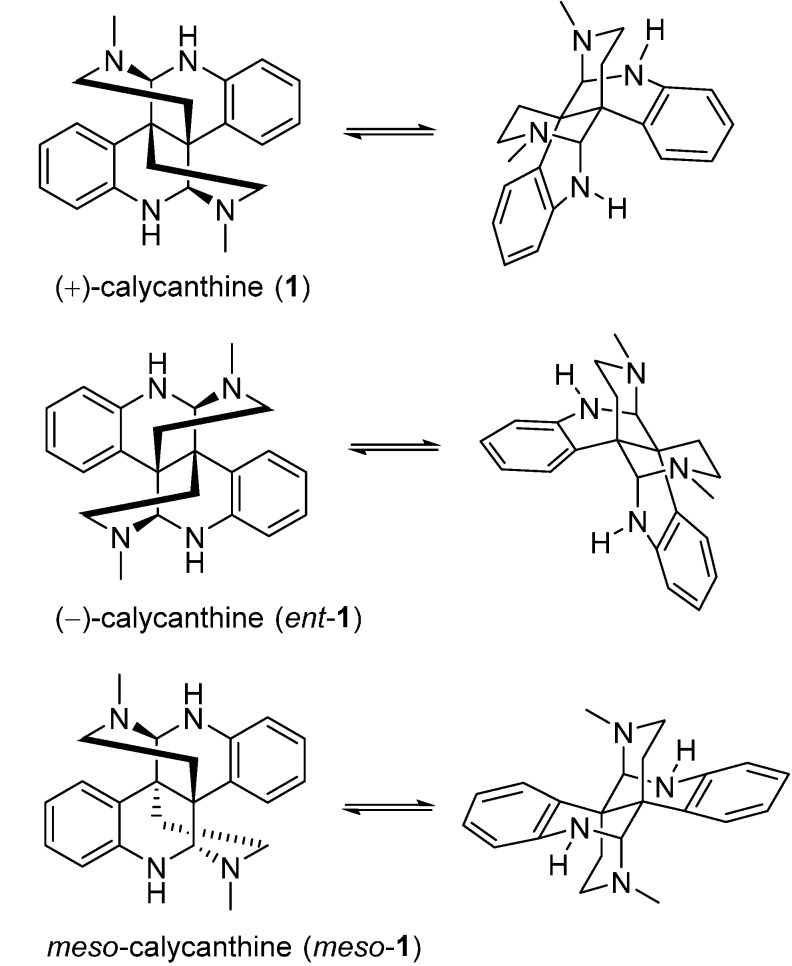
The dimeric piperidinoquinoline and pyrrolidinoindoline alkaloids have long been synthetic topics, and efforts have been made on the total synthesis of these two skeletons. Owing to their characteristic C2-symmetrical bridged bicycles and four chiral centers, three possible calycanthine diastereomers including (+)-calycanthine from Calycanthaceae plants, (−)- and meso-calycanthine from Psychotria forsteriana [29], were identified (Figure 2). Likewise, the C2-symmetrical chimonanthines also comprised (±)- and meso-chimonanthine. The total syntheses of these alkaloids have been intensively studied for decades. Hino speculated that the structure of 1,1'-dimethyl-3,3'-bis(2-aminoethyl)-3,3'-bioxindole was the key intermediate for the syntheses of calycanthine and (±)-folicanthine [49,50]. Some other groups made synthetic efforts to these bis(pyrroloindoline) scaffold by oxidation dimerization of indole [51] and/or oxindoles derivatives [52,53]. The synthetic challenges were attributed to the two structural features of the dimeric pyrroloindole core, including C3a-C3'a σ bond and its vicinal quaternary stereogenic carbons.
Figure 2.
Structures of calycanthines.
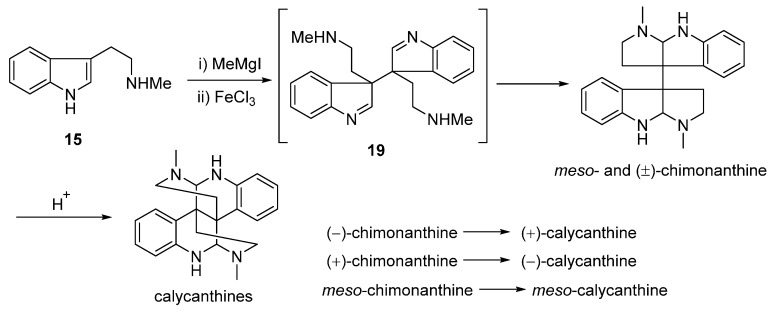
In 1964, Scott’s group established an approach using Nb-methyltryptamine (15) as starting material to form the magnesium salt of methyl tryptamine in presence of methyl magnesium iodide (CH3MgI). The intermediate was treated with iron(III) chloride to produce the indolenine dimer 19, which was subsequently transformed to meso- and (±)-chimonanthine in one step. Moreover, (±)-calycanthine was accessible by treating (±)-chimonanthine with aqueous acid, demonstrating that structure of 1 was the thermodynamically preferred scaffold (Scheme 3) [54].
Scheme 3.
Biomimetic syntheses of chimonanthines and calycanthines proposed by Soctt.
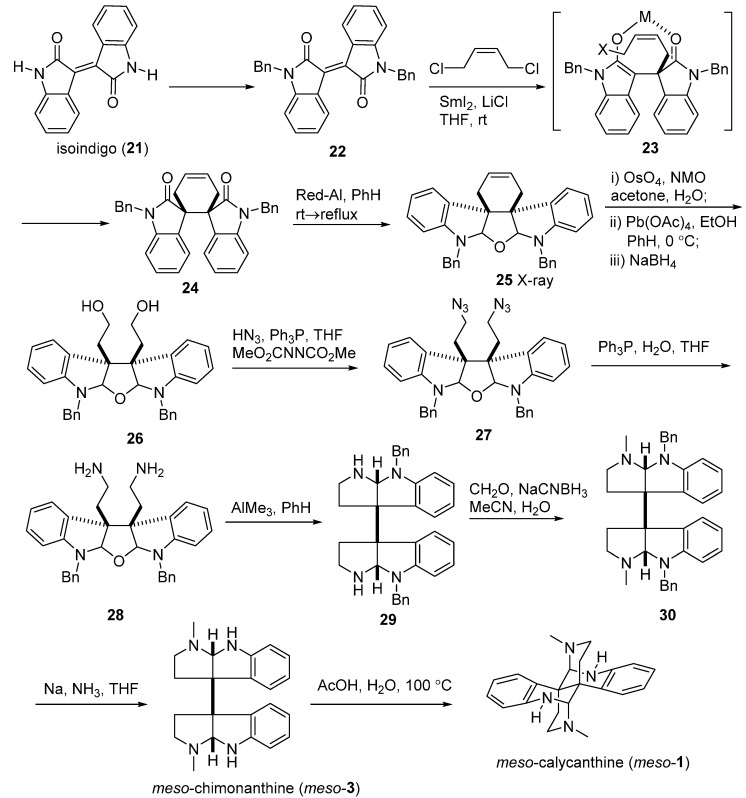
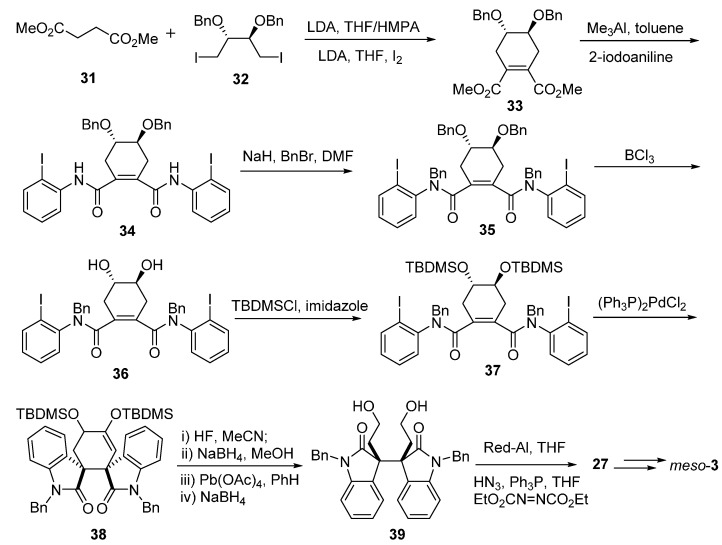
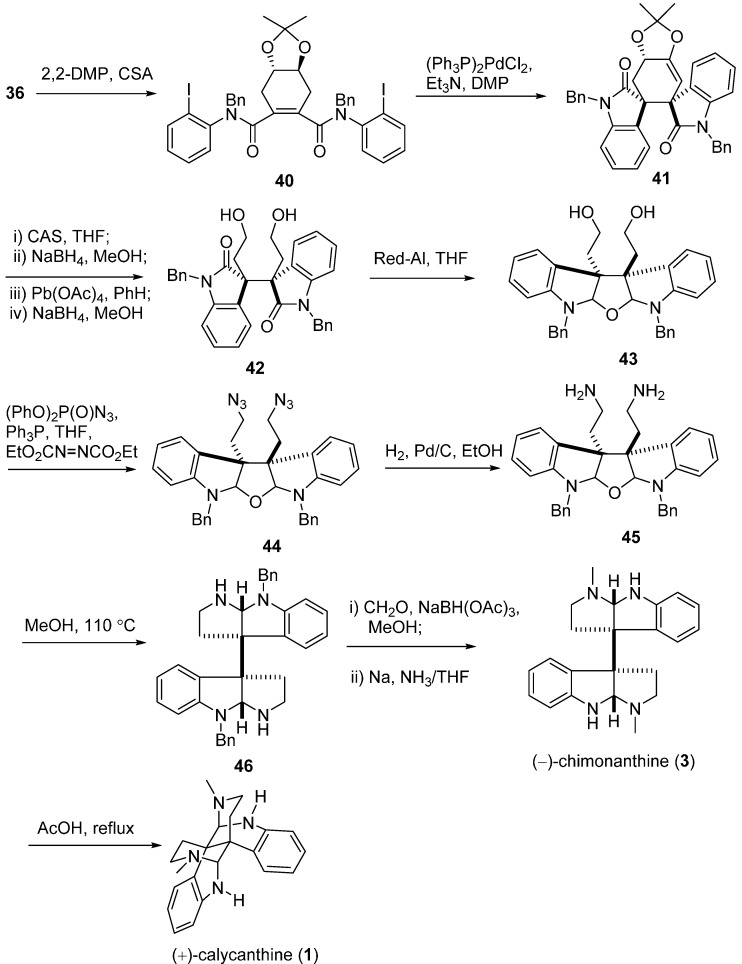
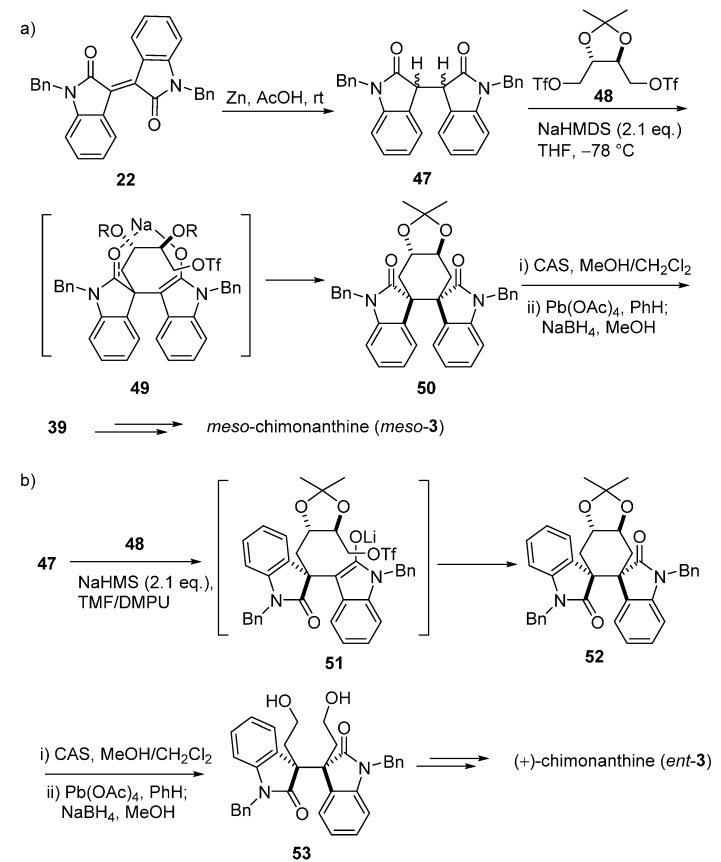
Overman’s group also proposed systemic syntheses for these Calycanthaceae alkaloids. In 1996, he attempted to stereocontrolly synthesize meso-chimonanthine (11) and meso-calycanthine (meso-1) via a samarium-mediated reductive dialkylation (Scheme 4). Isoindigo (21) was converted to N-benzyl derivative 22, which then generated 24 by the treatment of 2 equiv. of SmI2 in the presence of 10 equiv. of LiCl with cis-1,4-dicholoro-2-butene. Compound 24 was subsequently transformed into hexacycle 25 with sodium bis(2-methoxyethoxy)aluminum hydride (Red-Al). The cyclohexene of 25 was cleaved to yield 26, which was immediately reduced to diamine 28 via diazide product 27. Intermediate 28 was then treated with excess Me3Al at room temperature to provide bis(pyrroloindoline) 29. The desired meso-chimonanthine (meso-3) was produced from 29 by a cascade of methylation and deprotection reactions. The final product meso-calycanthine (meso-1) was obtained by exposure of meso-3 to hot dilute acetic acid [55]. In 1999, a flexible approach to meso-chimonanthine (Scheme 5), (−)-chimonanthine, and (+)-calycanthine (Scheme 6) using an intramolecular Heck reaction cascade was present by Overman et al. [56]. One year later, they put forward to a highly efficient synthesis of 3a,3a'-bispyrrolidino[2,3-b]indolines, a precursor for the meso-chimonanthine (Scheme 7a) and (+)-chimonanthine (Scheme 7b). This dialkylation route utilized the reactivities of dienolate (chelated or nonchelated) and the chirality of a tartrate-derived dielectrophile to control the relative configuration and absolute stereochemistry, respectively [35].
Scheme 4.
Stereocontrolled syntheses of meso-chimonanthine and meso-calycanthine by Overman.
Scheme 5.
Double Heck cyclizations for meso-chimonanthine by Overman.
Scheme 6.
Double Heck cyclizations for (−)-chimonanthine and (+)-calycanthine by Overman.
Scheme 7.
Enantioselective dialkylation approach for meso-chimonanthine (a) and (+)-chimonanthine (b) proposed by Overman.
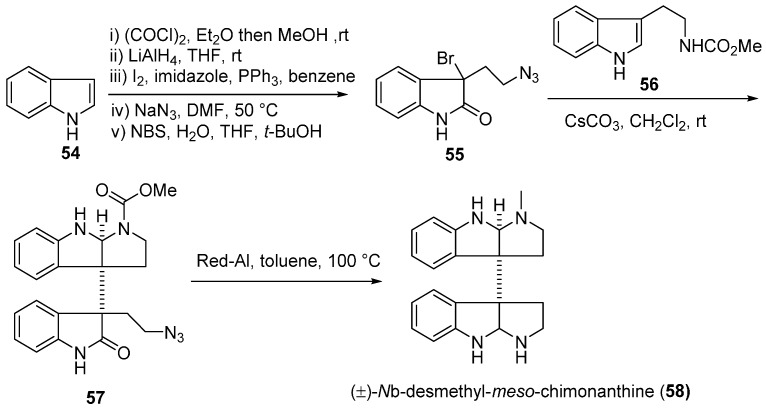
In 2006, Dalko’s group provided an elegant synthesis of a homologous compound of meso-chimonanthine, Nb-desmethyl-meso-chimonanthine. They investigated a tandem [4 + 2]-cycloaddition-cyclisation of a conveniently functionalized bromoxidole (55) and tryptamine derivative 56 to construct the meso-chimonanthine core in a highly diastereoselective manner (Scheme 8) [57].
Scheme 8.
Synthesis of (±)-Nb-desmethyl-meso-chimonanthine (58) via a tandem [4 + 2]-cycloaddition-cyclisation by Dalko.
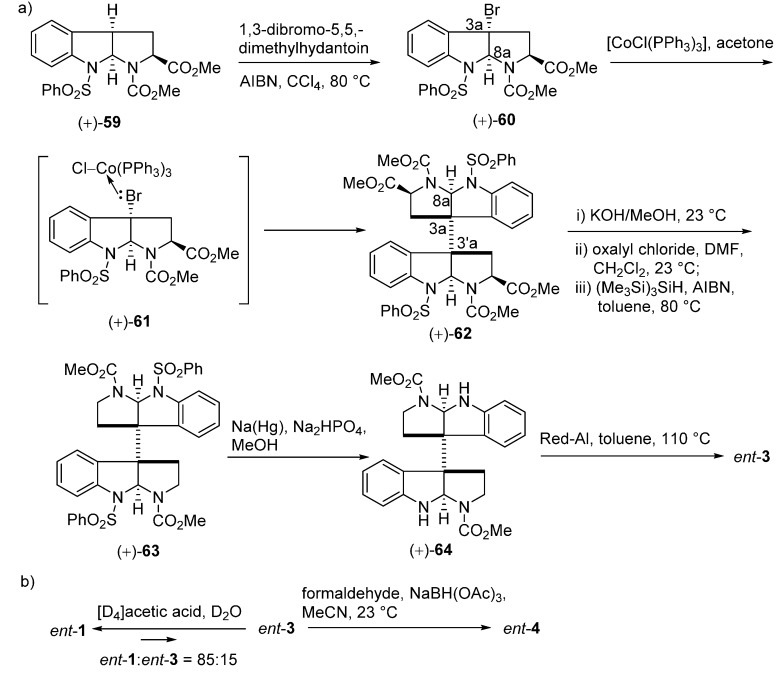
In 2007, an alternative strategy for the total synthesis of (−)-calycanthine (ent-1) and (+)-chimonanthine (ent-3) using a reductive CoI-promoted dimerization of endo bromide (+)-60 was proposed by Movassaghi. The vital homodimerization requiring a stoichiometric amount of metal catalyst was the key step to secure the vicinal quaternary stereocenters, which was directed by the stereochemistry at the C8a-position of (+)-60 (Scheme 9a). The further treatment of ent-3 with [D4]acetic acid and deuterium oxide provided (−)-calycanthine (ent-1) (Scheme 9b) [58]. In 2014, Movassaghi’s group also described an enhanced diazene-based method for heterodimerization to enantioselectively synthesize the (−)-calycanthidine (2), meso-chimonanthine (11) and (±)-desmethyl-meso-chimonanthine (58) [59,60].
Scheme 9.
Synthesis of (+)-chimonanthine (ent-3) (a), (+)-folicanthine (ent-4) and (−)-calycanthine (ent-1) (b) via a reductive CoI-promoted homodimerization by Movassaghi.
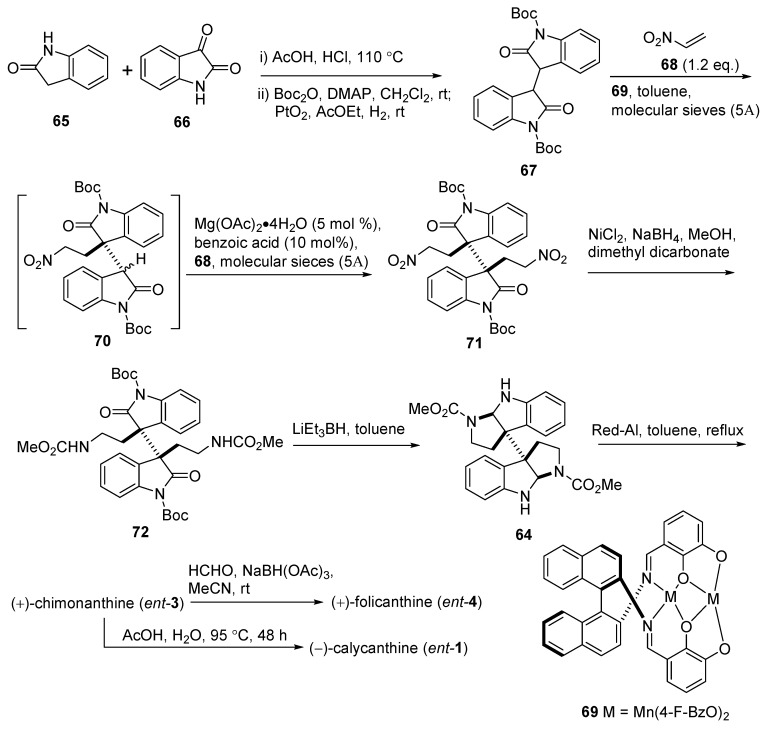
In 2012, Kanai and Matsunaga’s group illustrated a straightforward catalytic asymmetric total synthesis to achieve enantioselective (−)-calycanthine (ent-1) and (+)-chimonanthine (ent-3) in seven steps (Scheme 10). A one-pot double Michael reaction from 3,3'-bioxindole 67 [61] with the base catalyst Mn(4-F-BzO)2/Schiff (69) produced the key dialkylated adduct 71 in 69% yield and 95% ee. Then the intramolecular dicyclization of 72 was available by the treatment with LiEt3BH in toluene [62]. Recently, a new strategy using a double intramolecular carbamoylketene-alkene [2 + 2] cycloaddition for the synthesis of the racemic chimonanthine was accomplished by Shishido’s group (see Chapter 3.2) [63].
Scheme 10.
Synthesis of (+)-chimonanthine (ent-3), (+)-folicanthine (ent-4) and (−)-calycanthine (ent-1) via double Michael reaction of bisoxindole by Matsunaga.
3.2. Folicanthines
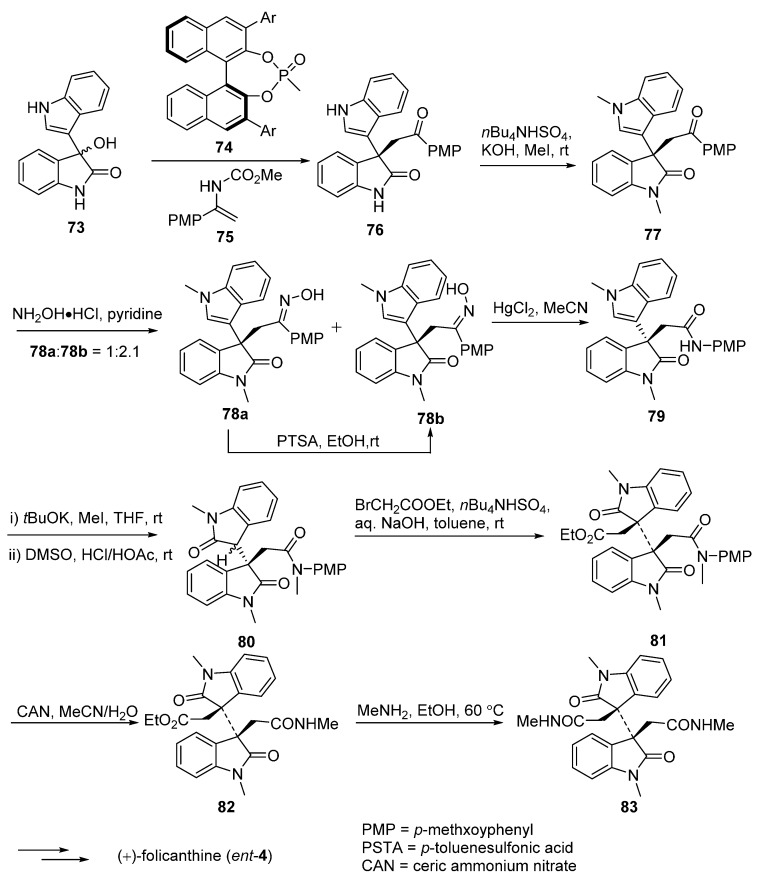
The structures of the folicanthines are also representatives of the dimeric hexahydropyrroloindole alkaloid family. (+)-Folicanthine (ent-4) could be easily obtained in a quantitative yield from (+)-chimonanthine (ent-3) by treatment with formaldehyde and sodium triacetoxyborohydride [NaBH(OAc)3] (Schemes 9b and scheme10) [58,62]. In striking contrast to the elegant synthetic procedures towards calycanthines and chimonanthines, Gong’s group have developed a highly enantioselective nucleophilic substitution reaction of 3-hydroxyoxindoles with an enecarbamate catalyzed by chiral phosphoric acids.
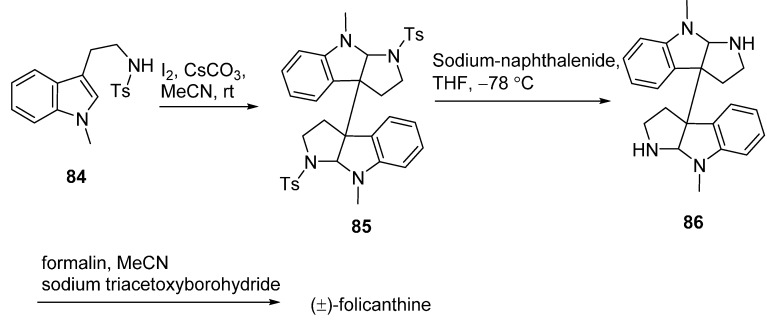
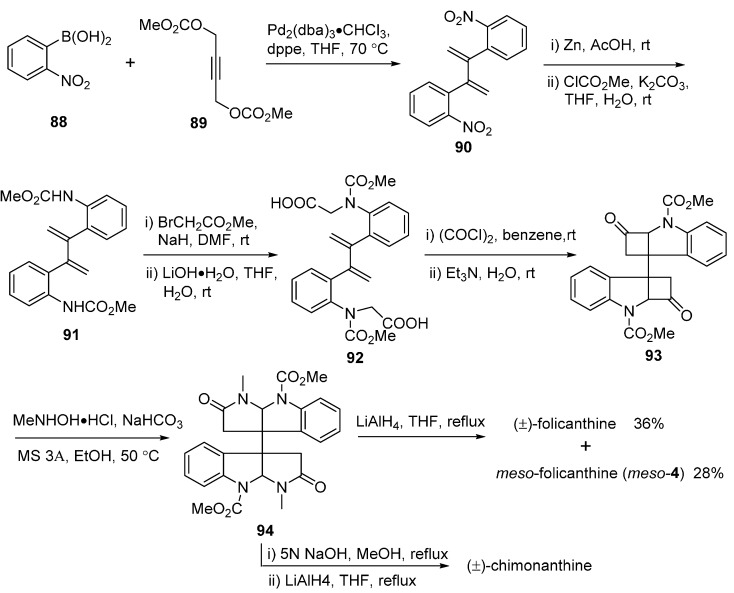
In Scheme 11, the vital enantioselective substitution reaction of 3-hydroxy-3,3'-bisindolin-2-one (73) with 75 was catalyzed by a special chiral phosphoric acids 74 to give 76. The dimethylation of 76 provided 77, which was followed with a Beckmann rearrangement to convert the ketones into amide derivatives 78a and 78b. A second Beckmann rearrangement of 78b by the treatment with mercury(II) chloride (Hg2Cl) could furnish amide 79. After introducing a methyl group at the amide nitrogen atom, the product 80 underwent an alkylation to produce key intermediate 81, which was finally converted to the desired product (+)-folicanthine (ent-4) after several step sequences [64]. Contemporaneously, Liang’s group described a concise three-step synthesis of (±)-folicanthine using two molecules of 2-(1-methyl-1H-indole-3-yl)-N-tosylethaneamine (84, Scheme 12). The core structure 85 of (±)-folicanthine could be easily obtained by a one-step cyclization-dimerization of substituted tryptophan in high yield. In general, this synthetic route had the advantages of being highly efficient, atom-economic, and metal-free, but it has no enantioselectivity [65]. Recently, Shishido’s group also developed a total synthesis route to access the folicanthines and chimonanthines in racemic form, employing a double intramolecular carboamoylketene-alkene [2 + 2] cycloaddition reaction as the key step (Scheme 13). 2,2'-(Buta-1,3-diene-2,3-diyl)bis(nitrobenzene) (90), obtained by a Pd-catalyzed preparation, underwent the key double [2 + 2] cycloaddition to yield bis-carboxylic acid 92. After a three-step sequence, the mixture of (±)-folicanthine and meso-folicanthine (meso-4) was obtained, which was separated by preparative TLC in 36% and 20% yields, respectively [63].
Scheme 11.
Synthesis of (+)-folicanthine (ent-4) via asymmetric organocatalytic substitution reaction by Gong.
Scheme 12.
Concise synthesis of (±)-folicanthine by Liang.
Scheme 13.
Synthesis of folicanthines and (±)-chimonanthine via a double intramolecular carbamoylketene-alkene [2 + 2] cycloaddition by Shishido.
3.3. (−)-Idiospermuline (6)
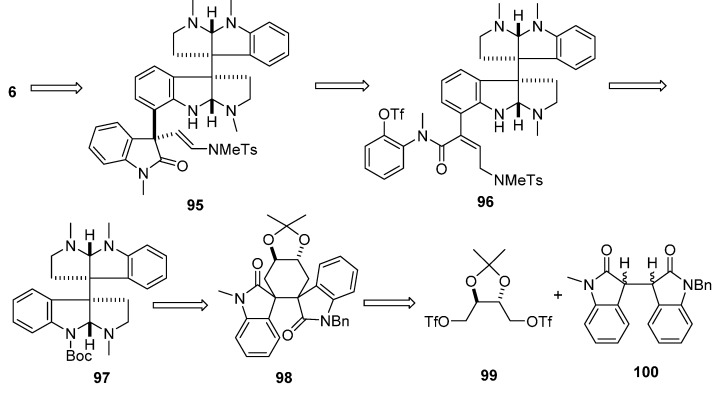
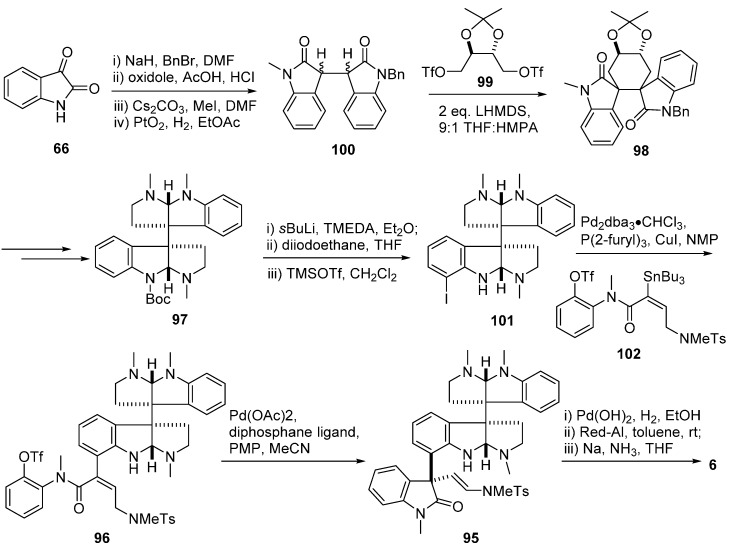
(−)-Idiospermuline (6), a typical trispyrrolidinoindoline alkaloid, contains three all-carbon quaternary centers, consisting of one (−)-chimonanthine unit and one pyrrolidinoindoline unit with a C3''a–C7' σ bond. Its retrosynthetic analysis was similar to that of hodgkinsines [66] and is presented in Scheme 14, which guided the total enantioselective synthesis of (−)-idiospermuline (Scheme 15). The first step in the synthesis focused on the dimeric pyrrolidinoindoline derivative 97 similar to that of (+)-chimonanthine in Scheme 7. The introduction of the readily available stannyl butenanilide 102 furnished the intermediate 96. Heck cyclization of 96 treating with chelating diphosphane ligands like bis(1,4-diphenylphosphanyl)butane (dppb) diastereoselectively furnished 3a''R precursor 95. Catalytic hydrogenation of 95 with Pd(OH)2 and H2, followed by reduction of the carbonyl group with Red-Al in toluene, then Na in NH3, provided the desired (−)-idiospermuline (6) [67,68,69,70].
Scheme 14.
Retrosynthesis of (−)-idiospermuline (6).
Scheme 15.
Total synthesis of (−)-idiospermuline (6).
3.4. Chimonamidines (7)
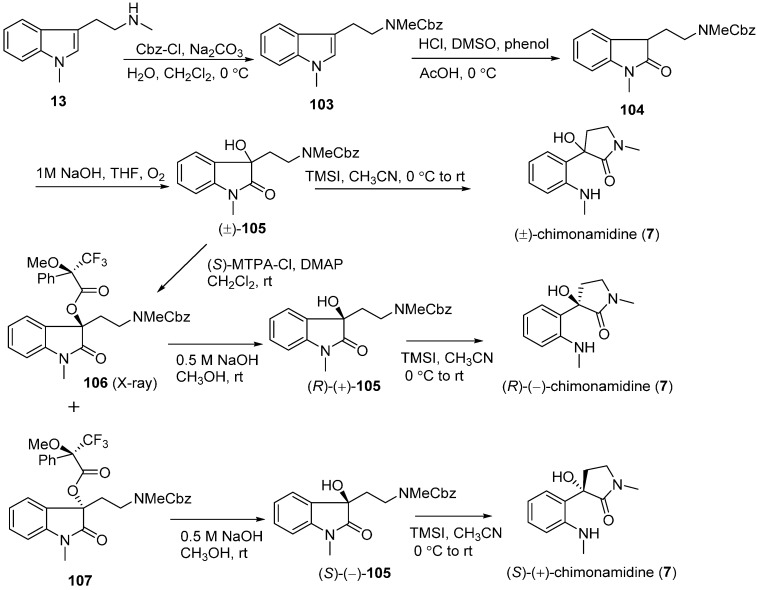
Takayama’s group conducted a biomimetic synthesis (Scheme 16) in order to confirm the absolute structure of chimonamidine (7). Based on its plausible biogenetic pathway shown in Scheme 2, the precursor Na,Nb-dimethyltryptamine (13) was treated with benzyl chloroformate (Cbz-Cl) and Na2CO3 to give Na,Nb-dimethyl-Nb-carbobenzyloxytryptamine (103). Oxidation of 103 with dimethyl sulfoxide and hydrochloric acid yielded 104, which was then converted to the racemic mixture of (±)-hydroxyketones 105 by introduction of a hydroxy group at the benzylic position. The target racemic mixture of (±)-chimonamidine (7) was obtained by removing the Nb-Cbz protecting group and forming a new lactam ring under the condition of trimethylsilyl iodide (TMSI). Meanwhile, the chiral synthesis of chimonamidine was also performed by Takayama. After several attempts, a strategy that involved the separation of racemic mixtures by (+)-MTPA chloride and SiO2 column chromatography succeeded to yield two diastereomeric esters 106 and 107. Two more steps that involved the hydrolysis with aqueous alkaline solution and cyclization with TMSI of 106 and 107 were conducted to afford two enantiomerically pure compounds (R)-(−)-chimonamidine and (S)-(+)-chimonamidine, respectively. A comparison of the optical rotation between synthesized ( = −178 for (R)-(−)-7; = +171 for (S)-(+)-7) and natural chimonamidine ( = −12.6) came to the conclusion that natural chimonamidine was a mixture slightly enriched with (R)-(−)-chimonamidine [38].
Scheme 16.
Total synthesis of (±)-chimonamidine (7).
3.5. Chimonanthidine (8)
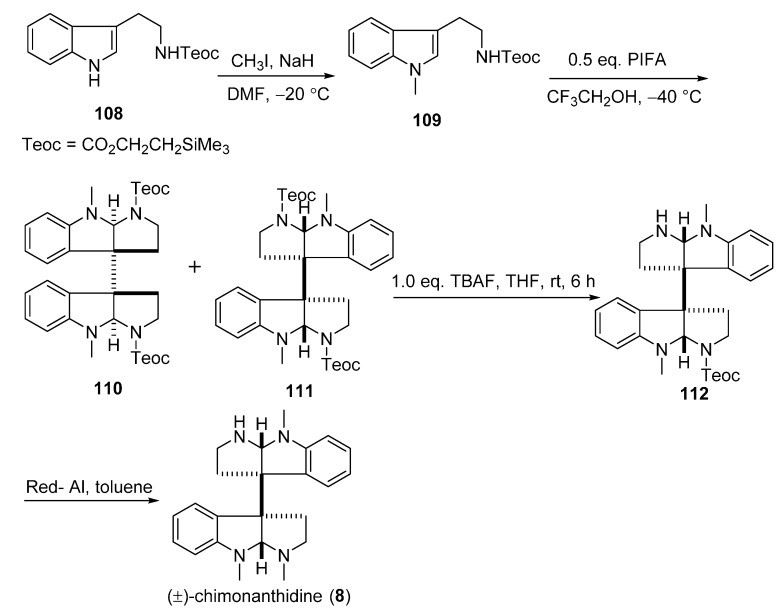
A synthetic approach that uses hypervalent iodine(III) reagents for the dimerization of indole derivatives was developed for the total synthesis of (±)-chimonanthidine (8) by Takayama’s group (Scheme 17). The methylation of the known compound 108 with methyl iodide (CH3I) and sodium hydride (NaH) in DMF produced Na-Methyl-Nb-trimethylsilyethoxy-carbonyl (Teoc) tryptamine (109), which was then treated with 0.5 equiv. phenyliodine(III) bis(trifluoroacetate) (PIFA) in CF3CH2OH to yield two dimeric diastereoisomers 110 and 111. (±)-Chimonanthidine (8) was obtained from the monodeprotected amine 112, which was transformed from 111 by treatment with tetrabutylammonium fluoride (TBAF) in THF [38].
Scheme 17.
Total synthesis of (±)-chimonanthidine (8).
3.6. Rac-CPC-1 (Rac-9)
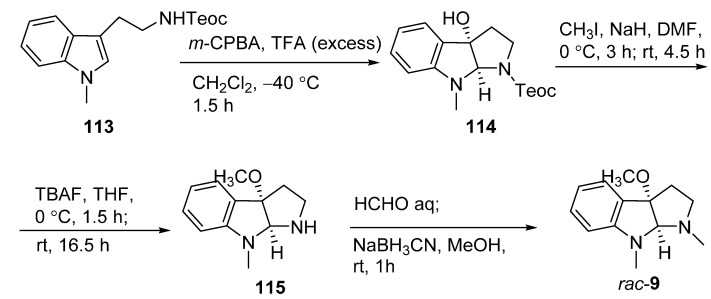
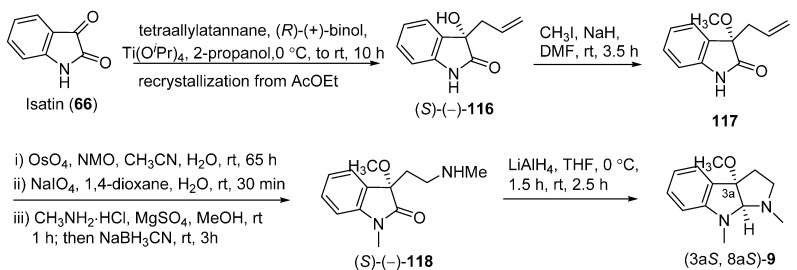
The total synthesis of racemic CPC-1 was initially performed to confirm the structure of CPC-1 (Scheme 18). Compound 113 was treated with m-CPBA in the presence of excess trifluoroacetic acid (TFA) in CH2Cl2 to afford 3a-hydroxypyrrolidinoindoline (114). Methylation of the hydroxy group of 114 and then removal of the Nb-Teoc group yielded the intermediate 115, which was finally treated with formalin and then NaBH3CN to give rac-9. To further establish the absolute configuration of 9, its chiral total synthesis was conducted (Scheme 19). This synthetic approach started from isatin (66), which was treated with (R)-(+)-binol, Ti(OiPr)4, and tetraallylstannane to yield allylated compound 116. Two recrystallizations of 116 from EtOAc afford enantiomerically pure (S)-(−)-116. Methylations of Na and hydroxy group of (S)-(−)-116 gave the dimethyl compound 117. The intermediate 117 was treated with OsO4 and N-methylmorpholine N-oxide (NMO) followed by NaIO4 to provide the aldehyde, which was directly subjected to reductive amination by condensing with CH3NH2 and then reduced with NaBH3CN to give (S)-(−)-118. The desired product (3aS, 8aS)-9 was obtained by the reductive cyclization of 118. The optical rotation value of (3aS, 8aS)-9 ( = +101) was determined to be opposite of that of 9 ( = −88), indicating the absolute configuration of natural CPC-1 to be 3aR,8aR [39].
Scheme 18.
Total synthesis of rac-9.
Scheme 19.
Total synthesis of (3aS, 8aS)-9.
4. Conclusions
In conclusion, the Calycanthiaceae plants are rich in promising bioactive dimeric and/or oligomeric piperidinoquinoline and hexahydropyrroloindole alkaloids, which are characterized by unique vicinal quaternary stereocenters. These alkaloids have been a longstanding challenge as total synthetic targets. During the last decades, stereocontrolled total synthetic approaches, such as metal-catalyzed dialkylation, intramolecular double Heck reaction, tandem [4 + 2]-cycloaddition-cyclisation, CoI-promoted reductive homodimerization, double Michael reaction, double Beckmann rearrangement, and intramolecular double carbamoylketene-alkene [2 + 2] cycloaddition, have extensively explored. Taken together, these results will keep research on the metabolites of the Calycanthaceae plants as a hot topic for the scientific community in the future.
Acknowledgments
This work was supported by the funds from the National Natural Science Foundation of China (No. 81303305), Major Science & Technology Project of Zhejiang Province (No. 2012C12014-1), Lishui Science & Technology Bureau Research Fund (No. 20140212037) and Lishui Agricultural Bureau Research Fund (No. LS20140010). We thank Wei Li of Fudan University for the help with the wording and grammar in the paper.
List of Abbreviations
- Ac
acetyl
- Bn
benzyl
- Boc
tert-butoxycarbonyl
- CAN
ceric ammonium nitrate
- CAS
camphorsulfonic acid monohydrate
- Cbz
carbobenzoxy
- m-CPBA
m-cholorperoxybenzoic acid
- dba
trans,trans-dibenzylideneacetone
- dppe
1,2-bis(diphenylphosphino)ethane
- dppb
bis(1,4-diphenylphosphanyl)butane
- DMA
N,N-dimethylacetamide
- DMF
2,5-dimethylfuran
- DMSO
dimethyl sulfoxide
- 2,2-DMP
2,2-dimethioxypropane
- DMPU
1,3-dimethylhexahydro-2-pyrimidinone
- HMDS
1,1,1,3,3,3-hexamethyldisilazane
- HMPA
hexamethylphoramide
- NaHMDS
sodium hexamethyldisilazide
- NMO
4-methylmorpholine-N-oxide
- PIFA
phenyliodine(III) bis(trifluoroacetate)
- PMP
1,2,2,6,6-pentamethylpiperidine
- PSTA
p-toluenesulfonic acid
- Red-Al
sodium bis(2-methoxyethoxy)aluminum hydride
- TBAF
tertrabutylammonium fluoride
- Tf
trifluoromethanesulfonyl
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- TMEDA
N,N,N,N-tetramethylethylenediamine
- TMS
trimethylsilyl
Author Contributions
The basic ideas were thought by K.-J. C; the whole paper was written by J.-B. X and polished by K.-J. C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li Y., Li B.T. Cladistic analysis of calycanthaceae. J. Trop. Subtrop. 2000;8:275–281. [Google Scholar]
- 2.Zhou S.L., Renner S.S., Wen J. Molecular phylogeny and intra- and intercontinental biogeography of Calycanthaceae. Mol. Phylogenetics Evol. 2006;39:1–15. doi: 10.1016/j.ympev.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Chen L.Q. Research advances on Calycanthaceae. Chin. Landsc. Archit. 2012;8:49–53. [Google Scholar]
- 4.Dai P.F., Yang J., Zhou T.H., Huang Z.H., Feng L., Su H.L., Liu Z.L., Zhao G.F. Genetic diversity and differentiation in Chimonanthus praecox and Ch. salicifolius (Calycanthaceae) as revealed by inter-simple sequence repeat (ISSR) markers. Biochem. Syst. Ecol. 2012;44:149–156. [Google Scholar]
- 5.Jiang Y., Li B.T., Li Y.H. Chinese Flora (Zhongguo Zhiwu Zhi) Volume 30. Science Press; Beijing, China: 1979. p. 5. [Google Scholar]
- 6.Wang L.Y., Zhang Z.B., Zou Z.R., Zhu D. Advances of studies on chemical composition and pharmacological activity of Chimonanthus Lindl. LiShiZhen Medicaine Mater. Med. Res. 2012;23:3103–3106. [Google Scholar]
- 7.Lv J.S., Zhang L.L., Chu X.Z., Zhou J.F. Chemical composition, antioxidant and antimicrobial activity of the extracts of the flowers of the Chinese plant Chimonanthus praecox. Nat. Prod. Res. 2012;26:1363–1367. doi: 10.1080/14786419.2011.602828. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z.Z., Xi J., Schröder S., Wang W.G., Xie T.P., Wang Z.G., Bao S.S., Fei J. Chimonanthus nitens var. salicifolius aqueous extract protects against 5-fluorouracil induced gastrointerstinal mucostis in a mouse model. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/789263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao P.G. Modern Chinese Materia Medica. Volume 3. Chemical Industry Press; Beijing, China: 2001. p. 388. [Google Scholar]
- 10.Sun L.R., He M.Z., Feng Y.L., Chen K.P., Jian H., Wang Y.S., Yang S.L. Studies on the chemical constituents of Chimonanthus nitens. Chin. Tradit. Hreb. Drugs. 2009;40:1214–1216. [Google Scholar]
- 11.Shu R.G., Li S.S., Hu H.W., Zhang P.Z. Studies on chemical constituents of Chimonanthus nitens Oliv. Chin. Pharm. J. 2010;45:1134–1135. [Google Scholar]
- 12.Li Q.J., Wang M.L., Yang X.S., Ma L., Hao X.J. Two new coumarins glycosides from Chimonanthus nitens. J. Asian Nat. Prod. Res. 2013;15:270–275. doi: 10.1080/10286020.2012.762766. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Hua J.W., Wang X.Y., Cheng W.L., Lei H.X., Cheng K.J., Yu P.Z. Chemical constituents of chloroform fraction from leaves of Chimonanthus salicifolius. Zhongguo Zhongyao zazhi (China J. Chin. Matera Med.) 2013;38:2661–2664. [PubMed] [Google Scholar]
- 14.Xiao B.K., Liu Y.M., Feng S.X., Huang R.Q., Luo C.H., Dong J.X. Studies on the chemical constituents of Chimonanthus nitens Oliv (I) Chin. Tradit. Herb. Drugs. 2005;36:187–189. [Google Scholar]
- 15.Wang W.X., Cao L., Xiong J., Xia G., Hu J.F. Constituents from Chimonanthus praecox (wintersweet) Phytochem. Lett. 2011;4:271–274. [Google Scholar]
- 16.Kashiwada Y., Nishimura K., Kurimoto S., Takaishi Y. New 29-nor-cycloartanes with a 3,4-seco- and a novel 2,3-seco-structure from the leaves of Sinocalycanthus chinensis. Bioorg. Med. Chem. 2011;19:2790–2796. doi: 10.1016/j.bmc.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Collins R.P., Halim A.F. Essential leaf oils in Calycanthus floridus. Planta Med. 1971;20:241–243. doi: 10.1055/s-0028-1099699. [DOI] [PubMed] [Google Scholar]
- 18.Yu C.L., Kuang Y., Yang S.X., Liu L., Liu C.G. Chemical composition, antifungal activity and toxicity of essential oils from leaves of Chimonanthus praecox and Chimonanthus zhejianggensis. Asian J. Chem. 2014;26:254–256. [Google Scholar]
- 19.Zhao Y., Zhang Y., Wang Z.Z. Chemical composition and biological activities of essential oil from flower of Chimonanthus praecox (L.) Link. LiShiZhen Med. Mater. Med. Res. 2010;21:622–625. [Google Scholar]
- 20.Deng C., Song G., Hu Y. Rapid determination of volatile compounds emitted from Chimonanthus praecox flowers by HS-SPME-GC-MS. Z. Naturforschung C. 2004;59:636–640. doi: 10.1515/znc-2004-9-1005. [DOI] [PubMed] [Google Scholar]
- 21.Gordin H.M. On the crystalline alkaloid of Calycanthus glaucus. J. Am. Chem. Soc. 1905;27:144–155. [Google Scholar]
- 22.Gordin H.M. On the crystalline alkaloid of Calycanthus glaucus. Second paper. J. Am. Chem. Soc. 1905;27:1418–1429. [Google Scholar]
- 23.Späth E., Stroh W. Zur Kenntnis des Caly-canthins. Ber. Dtsch. Chem. Ges. 1925;58:2131–2132. [Google Scholar]
- 24.Manske R.H.F. Calycanthine, I. The isolation of calycanthine from Meratia praecox. J. Am. Chem. Soc. 1929;51:1836–1839. [Google Scholar]
- 25.Barger G., Madinaveitia J., Streuli P. Calycanthine. J. Chem. Soc. 1939:510–517. [Google Scholar]
- 26.Hamor T.A., Robertson J.M., Shrivastava H.N., Silverton J.V. The structure of calycanthine. Proc. Chem. Soc. 1960:78–80. [Google Scholar]
- 27.Gordin H.M. On the crystalline alkaloid of Calycanthus glaucus. Third paper. On isocalycanthine, isomeric with calycanthine. J. Am. Chem. Soc. 1905;31:1305–1312. [Google Scholar]
- 28.Gordin H.M. The crystalline alkaloid of Calycanthus glaucus. Fourth paper. Some salts of a new quaternary base obtained by methylating isocalycanthine. J. Am. Chem. Soc. 1911;33:1626–1632. [Google Scholar]
- 29.Adjibade Y., Weniger B., Quirion J.C., Kuballa B., Cabaline P., Anton R. Dimeric alkaloids from Psychotria forsteriana. Phytochemistry. 1992;31:317–319. [Google Scholar]
- 30.Barger G., Jacob A., Madinaveitia J. Calycanthidine, a new simple indole alkaloid. Recl. Trav. Chim. Pays-Bas. 1938;57:548–554. [Google Scholar]
- 31.Saxton J.E., Bardsley W.G., Smith G.F. The structure of calycanthidine. Proc. Chem. Soc. 1962:148. [Google Scholar]
- 32.Hodson H.F., Robinson B., Smith G.F. Chimonanthine, a new calycanthaceous alkaloid. Proc. Chem. Soc. 1961:465–466. [Google Scholar]
- 33.Grant I.J., Hamor T.A., Robertson J.M., Sim G.A. The structure of chimonanthine. Proc. Chem. Soc. 1962:148–149. [Google Scholar]
- 34.Grant I.J., Hamor T.A., Robertson J.M., Sim G.A. The structure of Chimonanthine: X-ray ananlysis of Chimonanthine dihydrobromide. J. Chem. Soc. 1965:5678–5696. [Google Scholar]
- 35.Overman L.E., Larrow J.F., Steaens B.A., Vance J.M. Enantioselective construction of vicinal stereogenic quaternary centers by dialkylation: practical total syntheses of (+)- and meso-chimonanthine. Angew. Chem. Int. Ed. 2000;39:213–215. doi: 10.1002/(sici)1521-3773(20000103)39:1<213::aid-anie213>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Woodward R.B., Yang N.C., Katz T.J., Clark V.M., Harley-Mason J., Ingleby R.F.J., Sheppard N. Calycanthine: The structure of the alkaloid and its degradation product, calycanine. Proc. Chem. Soc. 1960:76–78. [Google Scholar]
- 37.Duke R.K., Allan R.D., Johnston G.A.R., Mewett K.N., Mitrovic A.D. Idiospermuline, a trimeric pyrrolidinoindoline alkaloid from the seed of Idiospermum australiense. J. Nat. Prod. 1995;58:1200–1208. doi: 10.1021/np50122a007. [DOI] [PubMed] [Google Scholar]
- 38.Takayama H., Matsuda Y., Masubuchi K., Ishida A., Kitajima M., Aimi N. Isolation, structure elucidation, and total synthesis of two new Chimonanthus alkaloids, chimonamidine and chimonanthidine. Tetrahedron. 2004;60:893–900. [Google Scholar]
- 39.Kitajima M., Mori I., Arai K., Kogure N., Takayama H. Two new tryptamine-derived alkaloids from Chimonanthus praecox f. concolor. Tetrahedron Lett. 2006;47:3199–3202. [Google Scholar]
- 40.Zhang J.W., Gao J.M., Xu T., Zhang X.C., Ma Y.T., Jarussophon S., Konishi Y. Antifungal activity of alkaloids from the seeds of Chimonanthus praecox. Chem. Biodivers. 2009;6:838–845. doi: 10.1002/cbdv.200800089. [DOI] [PubMed] [Google Scholar]
- 41.Morikawa T., Nakanishi Y., Ninomiya K., Matsuda H., Nakashima S., Miki H., Miyashita Y., Yoshikawa M., Hayakawa T., Muraoka O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014;68:539–549. doi: 10.1007/s11418-014-0832-1. [DOI] [PubMed] [Google Scholar]
- 42.Chen A.L., Powell C.E., Chen K.K. The action of calycanthine. J. Am. Pharm. Assoc. 1942;31:513–516. [Google Scholar]
- 43.Adhibadé Y., Hue B., Pelhate M., Anton R. Action of calycanthine on nervous transmission in cockroach central nervous system. Planta Med. 1991;57:99–101. doi: 10.1055/s-2006-960040. [DOI] [PubMed] [Google Scholar]
- 44.Chebib M., Duke R.K., Duke C.C., Connor M., Mewett K.N., Johnston G.A.R. Convulsant actions of calycanthine. Toxicol. Appl. Pharmacol. 2003;190:58–64. doi: 10.1016/s0041-008x(03)00149-2. [DOI] [PubMed] [Google Scholar]
- 45.Shi L.J., Yang S.X., Bi J.L., Yin G.F., Wang Y.H. Chemical constituents from the branches and leaves of Chimonanthus praecox with antiviral activity investigations. Nat. Prod. Res. Dev. 2012;24:1335–1338. [Google Scholar]
- 46.Amador T.A., Verotta L., Nunes D.S., Elisabetsky E. Antinociceptive profile of hodgkinsine. Planta Med. 2000;66:770–772. doi: 10.1055/s-2000-9604. [DOI] [PubMed] [Google Scholar]
- 47.Verotta L., Orsini F., Sbacchi M., Scheildler M.A., Amador T.A., Elisabetsky E. Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids. Bioorg. Med. Chem. 2002;10:2133–2142. doi: 10.1016/s0968-0896(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 48.May J.A., Stoltz B. The structural and synthetic implications of the biosynthesis of the calycanthaceous alkaloids, the communesins, and nomofungin. Tetrahedron. 2006;62:5262–5271. [Google Scholar]
- 49.Hino T. Synthetic approaches to Calycanthuaceae alkaloids. II. A synthesis of 1,1'-dimethyl-3,3'-bis(2-aminoethyl)-3,3'-bioxindole. Chem. Pharm. Bull. 1961;9:979–988. [Google Scholar]
- 50.Hino T., Yamada S. Total synthesis of (±)-folicanthine. Tetradedron. Lett. 1963;4:1757–1760. [Google Scholar]
- 51.Hall E.S., McCapra F., Scott A.I. Biogenetic-type synthesis of the calycanthaceous alkaloids. Tetrahedron. 1967;23:4131–4141. doi: 10.1016/s0040-4020(01)97925-6. [DOI] [PubMed] [Google Scholar]
- 52.Hendrickson J.B., Göschke R., Rees R. Total synthesis of calycanthaceous alkaloids. Tetrahedron. 1964;20:565–579. [Google Scholar]
- 53.Fang C.L., Horne S., Taylor N., Rodrigo R. Dimerization of a 3-substituted oxindole at C-3 and its application to the synthesis of (±)-folicanthine. J. Am. Chem. Soc. 1994;116:9480–9486. [Google Scholar]
- 54.Scott A.I., McCapra F., Hall E.S. Chimonanthine, a one-step synthesis and biosynthetic model. J. Am. Chem. Soc. 1964;86:302–303. [Google Scholar]
- 55.Link J.T., Overman L.E. Stereocontrolled total syntheses of meso-chimonanthine and meso-calycanthine via a novel samarium mediated reductive dialkylation. J. Am. Chem. Soc. 1996;118:8166–8167. [Google Scholar]
- 56.Overman L.E., Paone D.V., Stearns B.A. Direct stereo- and enantiocontrolled synthesis of vicinal stereogenic quaternary carbon centers. Total syntheses of meso- and (−)-chimonanthine and (+)-calycanthine. J. Am. Chem. Soc. 1999;121:7702–7703. [Google Scholar]
- 57.Menozzi C., Dalko P.I., Cossy J. Concise synthesis of the Nb-desmethyl-meso-chimonanthine. Chem. Commun. 2006:4638–4640. doi: 10.1039/b610497e. [DOI] [PubMed] [Google Scholar]
- 58.Movassaghi M., Schmidt M.A. Concise total synthesis of (−)-calycanthine, (+)-chimonanthine, and (+)-folicanthine. Angew. Chem. Int. Ed. 2007;46:3725–3728. doi: 10.1002/anie.200700705. [DOI] [PubMed] [Google Scholar]
- 59.Lathrop S.P., Movassaghi M. Application of diazene-directed fragment assembly to the total synthesis and stereochemical assignment of (+)-desmethyl-meso-chimonanthine and related heterodimeric alkaloids. Chem. Sci. 2014;5:333–340. doi: 10.1039/C3SC52451E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Movassaghi M., Ahmad O.K., Lathrop S.P. Directed heterodimerization: Stereocontrolled assembly via solvent-caged unsymmetrical diazene fragmentation. J. Am. Chem. Soc. 2011;133:13002–13005. doi: 10.1021/ja2057852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis J.M., Overman L.E., Tanner H.R., Wang J. A versatile synthesis of unsymmetical 3,3'-bioxindoles: Stereoselective Mukaiyama Aldol reactions of 2-siloxyindoles with isatins. J. Org. Chem. 2008;73:9151–9154. doi: 10.1021/jo801867w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitsunuma H., Shibasaki M., Kanai M., Matsunaga S. Catalytic asymmetric total synthesis of chimonanthine, folicanthine, and calycanthine through double Michael reaction of bisoxindole. Angew. Chem. Int. Ed. 2012;51:5217–5221. doi: 10.1002/anie.201201132. [DOI] [PubMed] [Google Scholar]
- 63.Araki T., Manabe Y., Fujioka K., Yokoe H., Kanematsu M., Yoshida M., Shishido K. Total syntheses of (±)-folicanthine and (±)-chimonanthine via a double intramolecular carbamoylketene-alkene [2 + 2] cycloaddition. Tetrahedron Lett. 2013;54:1012–1014. [Google Scholar]
- 64.Guo C., Song J., Huang J.Z., Chen P.H., Luo S.W., Gong L.Z. Core-structure-oriented asymmetric organocatalytic substitution of 3-hydroxyoxindoles: Application in the enantioselective total synthesis of (+)-folicanthine. Angew. Chem. Int. Ed. 2012;51:1046–1050. doi: 10.1002/anie.201107079. [DOI] [PubMed] [Google Scholar]
- 65.Li Y.X., Wang H.X., Ali S., Xia X.F., Liang Y.M. Iodine-mediated regioselective C2-amination of indoles and a concise total synthesis of (±)-folicanthine. Chem. Commun. 2012;48:2343–2345. doi: 10.1039/c2cc16637b. [DOI] [PubMed] [Google Scholar]
- 66.Kodanko J.J., Overman L.E. Enantioselective total syntheses of the cyclotryptamine alkaloids hodgkinsine and hodgkinsine B. Angew. Chem. Int. Ed. 2003;42:2528–2531. doi: 10.1002/anie.200351261. [DOI] [PubMed] [Google Scholar]
- 67.Overman L.E., Peterson E.A. Enantioselective total synthesis of the cyclotryptamine alkaloid idiospemuline. Angew. Chem. Int. Ed. 2003;42:2525–2528. doi: 10.1002/anie.200351260. [DOI] [PubMed] [Google Scholar]
- 68.Overman L.E., Peterson E.A. Enantioselective synthesis of (−)-idiospemuline. Tetrahedron. 2003;59:6905–6919. [Google Scholar]
- 69.Dounay A.B., Overman L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003;103:2945–2963. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- 70.Somei M., Yamada F. Simple indole alkaloids and those with a non-rearranged monoterpenoid unit. Nat. Prod. Rep. 2005;22:73–103. doi: 10.1039/b316241a. [DOI] [PubMed] [Google Scholar]