Abstract
In this paper, we report the synthesis and biological evaluation of picolylamide-based diselenides with the aim of developing a new series of diselenides with O···Se non-bonded interactions. The synthesis of diselenides was performed by a simple and efficient synthetic route. All the products were obtained in good yields and their structures were determined by 1H-NMR, 13C-NMR and HRMS. All these new compounds showed promising activities when tested in different antioxidant assays. These amides exhibited strong thiol peroxidase-like (TPx) activity. In fact one of the compounds showed 4.66 times higher potential than the classical standard i.e., diphenyl diselenide. The same compound significantly inhibited iron (Fe)-induced thiobarbituric acid reactive species (TBARS) production in rat’s brain homogenate. In addition, the X-ray structure of the most active compound showed non-bonded interaction between the selenium and the oxygen atom that are in close proximity and may be responsible for the increased antioxidant activity. The present study provides evidence about the possible biochemical influence of nonbonding interactions on organochalcogens potency.
Keywords: diselenides, 2-picolylamide, TPx, TBARS
1. Introduction
Organochalcogen compounds have been explored extensively in many research articles [1,2], reviews [3,4] and books [5,6,7] for their synthetic versatility. Among them, selenium-containing compounds have been successfully employed in certain reactions [8] as catalysts [9], ionic liquids [10], and intermediates in total synthesis [3,4,5,6,7,11]. In this context, the formation of C-Se bonds has contributed to the access to a wide range of biologically active molecules [12,13,14] and functional materials [15].
Synthetic organoselenium compounds have gained special interest since the 1970s, after the identification of various selenoproteins [4,5,13]. These proteins are involved in different physiological processes in mammals, such as antioxidant defense, thyroid hormone production and immune responses [16]. The presence of the selenium atom in such proteins plays a key role in the mode of action, which cannot be performed by sulfur (its closest relative in the chalcogen family) [17]. Due to these findings, a number of new selenium-based pharmaceutical agents are under development for therapeutic use as anticancer, antioxidant, anti-inflammatory and antidepressant drugs, among other applications [18]. Therefore, in pharmaceutical companies and academic laboratories the design and synthesis of biologically relevant small molecules containing selenium constitutes an important research topic.
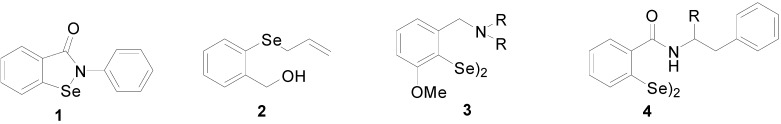
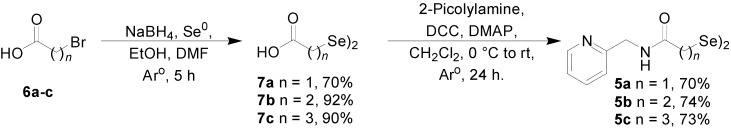
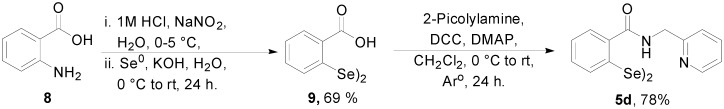
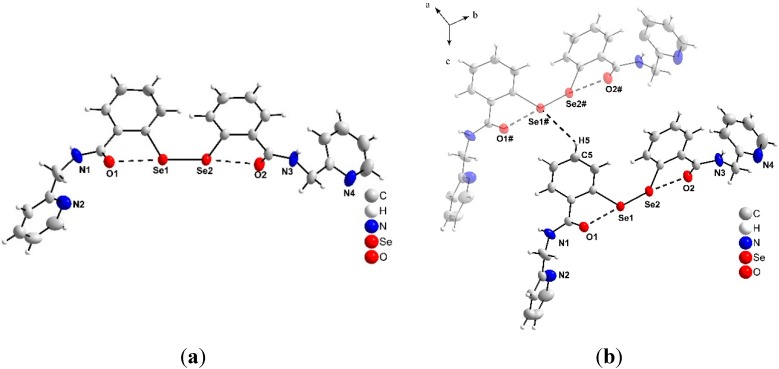
The literature has shown that in many compounds the selenium atom shows interesting interactions with various heteroatoms like N, O and even S, which lead to possible hypervalent selenium species. This may lead to either intermolecular [19,20] or intramolecular interactions [21,22]. X-ray crystallography [22,23,24], 77Se NMR [23,24,25] and modern computational methods [26,27] have been used to explore and provide insights into these possible nonbonding interactions. There are many examples available where organoselenium compounds, e.g., 2–3 (Figure 1) [28,29] with proximal interaction showed significant biological activities in comparison to ebselen (1; a mimic of GPx that also reacts with peroxynitrite) [5,12].
Figure 1.
Ebselen (1) and organoselenium compounds 2–4 with a heteroatom in close proximity.
In this context, our group has recently synthesized diselenides with heteroatoms in close proximity to the selenium atom with interesting GPx-like activity [30,31]. In fact, we have provided conclusive and baseline data that the presence of proximal heteroatom drastically affect GPx-like activity which furthermore enhances both in vitro and in vivo antioxidant activity with negligible toxicity [32,33,34].
Similarly, 2-picolylamine has been used in the synthesis of many biologically active compounds. For example, there are many reports where picolylamine derivatives were used to form metal complexes as models that mimic both the structure and reactivity of metal ion sites in complex biological systems, showing that picolylamine possess a broad spectrum of biological activities [35,36,37,38].
As part of our wider research program aimed at designing and developing biologically active new organoselenium compounds [15,32,33,34], herein we report a series of 2-picolylamide-based diselenides with proximal non-bonded interactions. The focus of the present paper is to address and explore the synthetic avenues and biosignificance of diselenides with amide groups in close proximity i.e., 2.711 Å. Indeed, pure in vitro enzyme mimetic activity of glutathione peroxidase (GPx) and inhibition of iron (Fe II)-induced lipid peroxidation will be assessed in rat’s brain tissue homogenate.
2. Results and Discussion
2.1. Chemistry
We started our studies from the preparation of carboxylic acid diselenide derivatives that were to be used as intermediates to prepare the target compounds. For the synthesis of aliphatic acid diselenides 7a–c, a previously reported method was modified [39,40] starting from the reaction of appropriate bromocarboxylic acids 6a–c with Na2Se2 (generated in situ) as outlined in Scheme 1. The reaction mixture was purified just by solvent extraction, which after vacuum evaporation afforded the pure desired products 7 in good to excellent yields. In the following step, a number of coupling agents were screened to couple carboxylic acid diselenides 7 with 2-picolylamine to form the amide linkage. It was found that DCC and catalytic amount of DMAP constituted the best conditions, resulting in the desired amide 5 in good yields as illustrated in Scheme 1. It is important to mention that when other coupling reagents, such as DIC/HOBt, EDC or BOP were used, the reactions were either not successful or resulted in a complex mixture of byproducts and the starting materials.
Scheme 1.
Synthesis of aliphatic diselenide derivatives of 2-picolylamide 5a–c.
The aromatic derivative of amide 5d was prepared from anthranilic acid (8, Scheme 2). Compound 8 was first converted to the bis(2-carboxyphenyl) diselenide 9 through the diazonium salt by treating it with disodium diselenide (generated in situ) which on purification by solvent extraction afforded 9 in 69% yield [41]. Subsequently, the diselenide 9 was coupled with 2-picolylamine by the method described previously in Scheme 1.
Scheme 2.
Synthesis of the aromatic 2-picolylamide diselenide derivative 5d.
By applying the strategy shown in Scheme 1 and Scheme 2, 2-picolylamine was easily joined with organoselenium compounds and the biological activities of these diselenides 5a–d were then explored. Furthermore, modification of the distance between amide and selenium through variation of the chain length and the conformation as open or restricted for the interaction between these groups provides some evidence of the influence of the Se-heteroatom interaction between selenium and the amide group on the antioxidant activity of the synthesized molecules.
2.2. X-ray Crystallography
Aromatic diselenide 5d was obtained as solid product, which was crystalized and analysed by the XRD technique. The XRD analysis of 5d (Figure 2a) shows that the structural parameters of the diselenide are similar to those found in others ligands [42,43]. The Se1-Se2 bond distance [2.3300(3) Å] and the Se-C(Ph) bond distance [1.931(2) and 1.934(2) Å, respective to the Se1-C1 and Se2-C14] are in good agreement with the distances found in other diselenides [44,45].
Figure 2.
(a) Molecular structure of 5d with 40% thermal ellipsoids (using DIAMOND software); (b) Molecular and hydrogen interaction assembly of 5d. Symmetry operations used to generate equivalent atoms: (#) x, y, −1 + z.
On the other hand, the Se-O bond distance [2.711(2) and 2.735(2) Å, respective to Se1···O1 and Se2···O2] is longer than the sum of the covalent radii. However, compared to reports in the literature (O···Se 2.793 and 2.852 Å) the distances are in compliance [46], suggesting an intramolecular nonbonding interaction between them. In addition to the intramolecular Se···O interactions, some intermolecular interactions between selenium and hydrogen atoms were also found in the crystal lattice. The stark deviation from the interaction of Se2-O2 can be attributed to a weak intermolecular hydrogen interaction between the Se and H (Figure 2b) from the C5 carbon (allowing the visualization of supramolecular assemblies along the two-dimensional plane bc.), C5-H5···Se1# [H5···Se1# = 3.082(1) Å]. The variation that occurs between the distances of Se1-O1 and Se2-O2 atoms provided by hydrogen interactions suggests that on the solid state, there is a difference in reactivity between the selenium atoms. Symmetry transformations was used to generate equivalent atoms: (#) x, y, −1 + z.
2.3. Biological Evaluation
Reactive species (RS) play a significant role in normal physiological progressions like, immune function, various signaling cascades and cellular processes (e.g., proliferation, growth, differentiation, apoptosis, migration, cytoskeletal regulation and contraction) etc. [47,48,49]. At the same time, RS causes severe damage and injuries to biomolecules like proteins, lipids, lipoproteins and DNA [47,48,49]. As previously discussed, the nonbonding selenium interaction with heteroatoms plays a pivotal role in biological activities. The aim of the present study was to investigate the antioxidant properties of novel chalcogenides. Two different in vitro antioxidant assays were performed.
2.3.1. Thiol Peroxidase-Like (TPx) Activity
The catalytic activities of synthesized diselenides 5a–d were examined and the results are summarized in Table 1. Diphenyl diselenide (DPDS), a well-known GPx-mimic, was used as a positive control. The concentrations of new compounds used were 15, 25, 75 and 100 µM. The control or basal GPx-like activity was carried out in the absence of selenium catalysts and the rate of thiophenol oxidation was negligible. Interestingly all compounds exhibited significantly higher catalytic activity.
Table 1.
Thiol peroxidase like-activity (Δ/Minutes × 1000) of 2-picolylamide-based diselenides 5.
| Compound | Concentration (µM) | |||
|---|---|---|---|---|
| 15 | 25 | 75 | 100 | |
| 5a | 1.16 | 3.88 | 27.60 | 34.00 |
| 5b | 0.01 | 0.01 | 1.72 | 3.40 |
| 5c | 9.71 | 14.97 | 24.03 | 31.91 |
| 5d | 20.74 | 31.34 | 41.66 | 46.48 |
| DPDS | 9.96 | |||
It is apparent from Table 1 that aromatic diselenide 5d has considerably higher TPx activity (entry 4) than compounds 5a–c (entries 1–3) and DPDS (entry 5). By a closer inspection of the diselenides structures, and specifically 5d, it is apparent that an adjacent heteroatom, i.e., oxygen, in close proximity of the selenium may possibly generate small nonbonding interactions (between selenium and oxygen). In fact, the experimental data obtained from X-Ray (XRD) analysis of 5d (Figure 1) confirmed that Se···O distance is 2.711 and 2.735 Å, for Se1···O1 and Se2···O2, respectively. Based on interatomic distances (between Se and O1 and O2), we can speculate that Se-O1 may be the initial prime target of the first thiol (PhSH) attacking the Se-Se bond to yield selenyl sulfide.
Using the TPx-like mechanism of the classical standard i.e., DPDS, we can speculate that the Se···O interaction in 5d may promote the conversion of selenyl sulfide to selenol/selenolate. The ease of formation of selenolate ion is a fundamental and significant step. Furthermore, The H2O2 can oxidize selenolate ion to produce selenenic acid and can regenerate the parent molecule i.e., 5d, upon reaction with PhSH. It is worthy to note that the presence of adjacent nitrogen (N) in 5d, may facilitate the attack of incoming thiol (PhSH) on diselenide. The catalytic role of the nitrogen in deprotonation of thiol (PhSH) and consequently a high concentration of thiolate cannot be neglected in this regard [32,50].
In the same context, the literature has demonstrated that the presence of amines in close proximity may possibly interact with selenium and may contribute towards selenolate stabilization and ultimately higher potential [51]. We have recently reported the concise influence of nonbonding nitrogen interaction on the biochemical efficacies of chalcogenides [32,50]. However, it is interesting to note that employing butoxycarbonyl (BOC group), which usually blocks the interactions in synthetic routes, significantly reduced the TPx-like potential of chalcogenides [32]. In fact, the addition of BOC to amino-acid derived diselenides increased the toxic potential apparent from considerably increased ALT activity, serum creatinine and triglyceride levels after in vivo treatment in mice [34]. Although not very relevant to selenium molecules, but only to depict the diverse scenarios, we have obtained persuasive evidence that nonbonding interactions can also significantly interact with sulfur (disulfides) [32].
Our results are in strong agreement to above, which may be employed to explain the enhanced TPx potential of 5d as compared with other analogues 5a–c and the prototypal diselenide DPDS. The TPx-like activity of 5a–c also provides concrete evidence that the length of the carbon chain is important with respect to the interaction between the selenium and the heteroatom (oxygen) in close proximity. Aromatization of this side chain restricts the possible conformations and thus favors the interaction. Based on experimental TPx activity, XRD analysis and relevant literature [32,34,50,51] we can assume the important catalytic influence of nonbonding interactions.
2.3.2. Inhibition of Thiobarbituric Acid Reactive Substances (TBARS) Production in Brain Homogenates
Lipid peroxidation is an important mechanism involved in cellular toxicity. The occurrence of lipid peroxidation specifies the potential disruption of biomembrane integrity and therefore is an important biomarker of oxidative stress. Consequently, the protective effect against lipid peroxidation has an importance for designing new clinically effective antioxidants. TBARS are formed by the reaction of byproducts of lipid peroxidation, mainly malondialdehyde with thiobarbituric acid (TBA). Thus, the stable colored product of TBA with malondialdehyde can be used to quantify lipid peroxidation in biological samples [32,33]. Brain homogenates (S1) can be used as a source of biomembranes and our group has been using this model for some time as an index of lipid peroxidation [32]. During this study Fe(II) was used as an inducer of brain lipid peroxidation as it caused a considerable increase in the TBARS production.
It is worthy to note that Tomoda and Iwaoka method was followed for the determination of TPx-like potential under irrelevant physiological conditions such as using organic solvent (methanol or ethanol) and nonbiological thiols (benzenethiol or thiophenol). At the same time, H2O2 is used which is a non-reactive oxygen metabolite in the absence of metal ions compared to the hydroxyl radical. Whereas TBARS analysis is performed in complex biological homogenates containing different thiols, metal ions (including iron in different oxidation state), enzymes that can reduce selenium containing molecules (for instance, thioredoxin reductase) lipids, proteins and nucleic acids [52]. Thus, it is rather difficult to do direct comparisons between the GPx- or TPx-like activity and the anti-lipid peroxidation properties of diselenides.
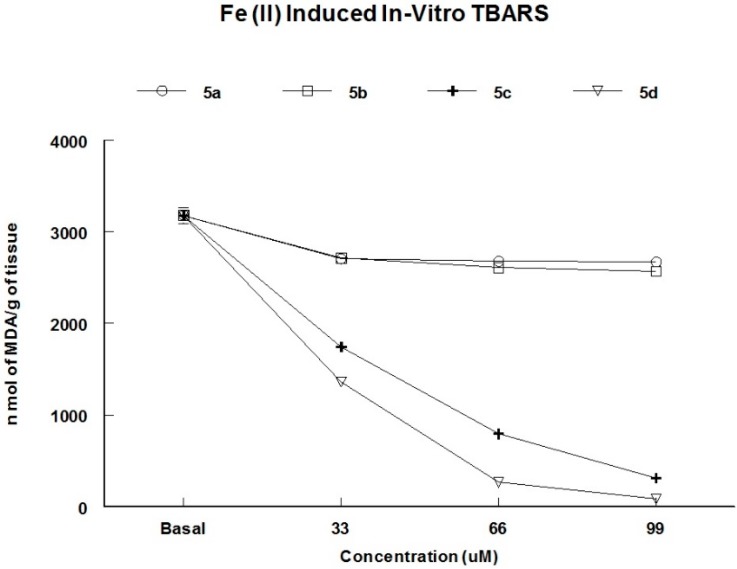
TBARS assay was performed for the synthesized compounds and the results are outlined in Figure 3. Compounds 5c and 5d significantly inhibited TBARS production as compared with 5a–b. The TBARS inhibition potential of 5d can be attributed to its high TPx-like potential (Table 1). However, compound 5a, which showed TPx-like activity did not protect lipid peroxidation, and rather 5c showed interesting protective efficacy in TBARS assay.
Figure 3.
Effect of 2-picolylamide based diselenides 5a–d on Fe (II) induced TBARS formation in low-speed supernatant (S1) from brain homogenate. Fe (II) sulfate at 20 µM was used as pro-oxidant. TBARS are expressed as nmol of MDA/g of tissue. Data are presented as mean ± S.E.M. (n = 5).
3. Experimental Section
3.1. General Methods and Materials
1H- and 13C-NMR spectra were obtained at 200/50 MHz on a Bruker AC-200 NMR spectrometer or at 400/100 MHz on a Varian AS-400 NMR spectrometer. Spectra were recorded in CDCl3 solutions. Chemical shifts are reported in ppm, referenced to the solvent peak of CDCl3 or tetramethylsilane (TMS) as the external reference. Data are reported as follows: Chemical shift (δ), multiplicity, coupling constant (J) in Hertz and integrated intensity. Abbreviations to denote the multiplicity of a particular signal are: s (singlet), d (doublet), t (triplet), q (quartet), quint (quintet), sext (sextet) and m (multiplet). The NMR spectra of compounds 5a–d are shown in the Supplementary Material. High-resolution mass spectra were recorded on a Bruker micrOTOF-Q II ESI mass spectrometer equipped with an automatic syringe pump for sample injection. Infrared spectra were recorded on a Bruker Optics Alpha benchtop FT-IR spectrometer and are reported in frequency of absorption (cm−1). The melting points were determined in a Microquimica MQRPF-301 digital model equipment with a heating plate. Column chromatography was performed using Silica Gel (230–400 mesh). Thin layer chromatography (TLC) was performed using Merck Silica Gel GF254, 0.25 mm thickness. For visualization, TLC plates were either placed under ultraviolet light, or stained with iodine vapor and acidic vanillin. Most reactions were monitored by TLC for disappearance of starting material. All commercially available starting materials were used without further purification. The solvents were distilled and dried according to standard procedures.
Single crystal X-ray diffraction patterns of 5d were collected with a Bruker APEX II CCD area-detector diffractometer and graphite-monochromatized Mo–Kα radiation. The structure was solved by direct methods using SHELXS [53]. Subsequent Fourier-difference map analyses yielded the positions of the non-hydrogen atoms. Refinements were carried out with the SHELXL package [53]. All refinements were made by full-matrix least-squares on F2 with anisotropic displacement parameters for all non-hydrogen atoms. Hydrogen atoms were included in the refinement in calculated positions but the atoms (of hydrogens) that are commenting performing special bond were located in the Fourier map. Drawings were done using DIAMOND for Windows [54].
3.1.1. General Procedure for the Synthesis of Diselenide 7
The synthesis was carried out at ambient temperature under inert atmosphere and vigorous stirring. Elemental selenium (200 mesh, 2 g, 0.025 mol) was placed in a two necked round-bottomed flask followed by addition of commercial 80% EtOH (70 mL). NaBH4 (1.9 g, 0.05 mol) was then slowly added to the solution, until complete dissolution of selenium and formation of colorless suspension with white-gray solid. Subsequently, DMF (50 mL) was added to the solution and the reaction mixture stirred until the color turned to red-brown followed by addition of commercial 80% EtOH (25 mL). The solution was stirred vigorously until termination of gas evolution and then selenium powder (200 mesh, 2 g, 0.025 mol) was added to the solution and stirred until complete dissolution and formation of clear dark-red solution. The appropriate bromocarboxylic acids 6 (0.05 mol) was slowly added in small portions to the solution, changing the red color of the solution to yellow. After 4 h the reaction was quenched by addition of water (150 mL) and the reaction mixture was extracted with diethyl ether (100 mL) three times. The combined organic phases were washed with water (250 mL) for three times, with brine (100 mL), and was dried over MgSO4. The solvent was removed under reduced pressure using rotary evaporator and the crude diselenides 7a–c were used without further purification. Diselenide 7a: Light yellow solid, 70% yield, mp 101–104 °C (lit. [55] mp 104.5–105.5 °C); 7b: Yellow solid, 92% yield, mp 132–135 °C (lit. [56] mp 134.5–135.5 °C); 7c: Yellow solid, 90% yield, mp 86–87 °C (lit. [57] mp 88 °C).
3.1.2. Procedure for the Synthesis of Diselenide 9
Sodium nitrite (6.9 g, 0.1 mmol,) in water (28 mL) was added dropwise to a stirred solution of anthranilic acid 9 (0.856 g, 6.24 mmol) in 37% hydrochloric acid (20 mL) and water (100 mL) cooled in an ice bath (0–5 °C). The solution of the resulting diazonium salt was stirred for 20 min.
In another flask a mixture of Se powder (16 g, 0.2 mmol) and KOH pellets (24 g, 0.43 mmol) was heated in a round-bottomed flask to 300–360 °C for 2–3 h. The mixture was cooled and distilled water (250 mL) was added. This solution was added dropwise to the about solution of diazonium salt while maintaining the temperature at 0–5 °C which was subsequently, allowed to gain room temperature and left for stirring overnight. The reactions mixture was filtered over Celite. The red solution was acidified with 10% hydrochloric acid forming the precipitate which was collected, resuspended in methanol and refluxed. The suspension was filtered off, and the filtrate was evaporated in vacuo yielding the target acid 9 in 69% yield. Diselenide 9: Yellow solid, mp 297–300 °C (lit. [41] mp 296–297 °C).
3.1.3. General Procedure for the Synthesis of 2-Picolyamide Derivatives of Diselenides 5a–d
The synthesis was carried out at 0 °C under an inert atmosphere under vigorous stirring. The appropriate diselenide (0.005 mol) in dry CH2Cl2 (5 mL) was placed in a two neck flask followed by addition of a catalytic amount of DMAP (0.366 g, 0.3 mol %). The reaction mixture was stirred for 5–7 min at 0 °C. Next DCC (2.1 g, 0.01 mol, slight excess) was then added to the reaction mixture and further stirred for 15 min at 0 °C. 2-Picolylamine (1.30 mL, 0.010 mol) was added to the reaction mixture and the reaction is left for overnight at ambient temperature. The formation of white precipitate in the reaction was due to dicyclohexyl urea. The reaction mixture was filtered off and then after drying over MgSO4, the solvent was evaporated in vacuo and the crude product was purified by flash chromatography (ethyl acetate:hexane).
2,2′-Diselanediylbis(N-(pyridin-2-ylmethyl)acetamide) (5a). Obtained as a yellow viscous liquid in 70% yield; 1H-NMR (400 MHz, CDCl3) δ: 3.73 (s, 2 H), 4.53 (d, J = 5.4 Hz, 2 H), 7.17 (dd, J = 7.5, 4.9 Hz, 1 H), 7.30 (d, J = 7.9 Hz, 1 H), 7.66–7.61 (m, 1 H), 8.28 (broad s, 1 H), 8.50–8.47 (m, 1 H); 13C-NMR (100 MHz, CDCl3): 33.0, 45.7, 123.1, 123.2, 137.7, 149.5, 157.6, 171.0; IR (KBr); 3253, 3057, 2926, 2823, 1645, 1560, 1474, 1303, 1215, 758, 528 cm−1; ESI-HRMS m/z: Calcd. for C16H19N4O2Se2 [M + H]+ 458.9836, found 458.9835.
3,3′-Diselanediylbis(N-(pyridin-2-ylmethyl)propanamide)(5b). Obtained as a yellow viscous liquid in 74% yield; 1H-NMR (200 MHz, CDCl3) δ: 2.69 (t, J = 7.0 Hz, 2 H), 3.08 (t, J = 7.0 Hz, 2 H), 4.49 (d, J = 5.1 Hz, 2 H), 7.16 (dd, J = 6.9, 5.2 Hz, 1 H), 7.27 (d, J = 7.7 Hz, 1 H), 7.63 (td, J = 7.7, 1.7 Hz, 2 H), 8.44 (d, J = 5.1 Hz, 1 H); 13C-NMR (50 MHz, CDCl3): 24.3, 37.2, 44.5, 122.4, 122.5, 137.1, 148.7, 156.7, 171.6; IR (KBr); 3291, 3065, 2926, 2823, 1650, 1550, 1435, 1350, 1246, 756, 501 cm−1; ESI-HRMS m/z: Calcd. for C18H23N4O2Se2 [M + H]+ 487.0149, found 487.0146.
4,4′-Diselanediylbis(N-(pyridin-2-ylmethyl)butanamide)(5c). Obtained as a yellow viscous liquid in 73% yield; 1H-NMR (400 MHz, CDCl3) δ: 2.14–2.05 (m, 2 H), 2.39 (t, J = 7.3 Hz, 2 H) 2.92 (t, J = 7.2 Hz, 2 H), 4.51 (d, J = 5.0 Hz, 2 H), 7.17 (dd, J = 7.3, 5.2 Hz, 2 H), 7.24 (d, J = 7.8 Hz, 1 H), 7.64 (td, J = 15.3, 1.7 Hz, 1 H), 8.50 (d, J = 5.0 Hz, 1 H); 13C-NMR (100 MHz, CDCl3): 26.5, 28.7, 35.6, 44.4, 122.1, 122.3, 136.8, 148.9, 156.5, 172.2.; IR (KBr); 3283, 3056, 2926, 1650, 1548, 1435, 1352, 1229, 1176, 756, 511 cm−1; ESI-HRMS m/z: Calcd. for C20H27N4O2Se2 [M + H]+ 515.0463, found 515.0460.
2,2′-Diselanediylbis(N-(pyridin-2-ylmethyl)benzamide)(5d). Obtained as a yellow solid in 78% yield; m.p. 138–140 °C; 1H-NMR (400 MHz, DMSO-d6) δ: 4.63 (d, J = 5.8 Hz, 2H), 7.30 (dd, J = 7.0, 5.8 Hz, 1 H), 7.44–7.35 (m, 3 H), 7.72 (d, J = 7.7 Hz, 1 H), 7.80 (td, J = 7.7, 1.7 Hz, 1 H), 7.95 (d, J = 7.5 Hz, 1 H), 8.55 (d, J = 4.7 Hz ,1 H), 9.41 (broad s, 1 H); 13C-NMR (100 MHz, DMSO-d6): 44.5, 120.8, 121.9, 125.9, 127.8, 129.6, 131.6, 131.9, 132.2, 136.7, 148.5, 158.0, 167.2.; IR (KBr); 3324, 3055, 2926, 2851, 1625, 1560, 1474, 1433, 1242, 734, 546 cm−1; ESI-HRMS m/z: Calcd. for C26H23N4O2Se2 [M + H]+ 583.0151, found 583.0154.
3.2. General Methods and Materials
3.2.1. Glutathione-Peroxidase-Like Activity Assay
The catalytic activity of the nitrogen-containing diselenides as a GPx model enzyme was evaluated according to the Tomoda and Iwaoka method [58]. The selenium agent (final concentration: 0.1 mM), thiophenol, PhSH (final concentration 2 mM) at 25(±3) °C were mixed. After 120 s, the catalytic GPx model reaction (H2O2 + 2PhSH → 2H2O + PhSSPh) was initiated by the addition of H2O2 (final concentration: 5 mM). The reduction of H2O2 was monitored through the UV absorption increase at 305 nm, due to diphenyl disulfide formation.
3.2.2. Animals
Adult male Wistar rats (250–350 g) from our own breeding colony (Animal House-holding, UFSM, Santa Maria-RS, Brazil) were maintained in room with controlled temperature (22–25 °C), on a 12 h light/dark cycle, with water and food ad libitum. Animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources from Federal University of Santa Maria (23081.002435/2007-16).
3.2.3. Thiobarbituric Acid Reactive Substances (TBARS) Assay
Lipid peroxidation was determined by measuring TBARS as described by Ohkawa and coworkers [59] in S1 of brain. Aliquots of S1 (200 μL) were mixed to incubating medium containing Tris-HCl (0.01 mM) pH 7.4, the compounds at different concentrations. Iron (II) as pro-oxidant was added as positive control for lipid peroxidation. The mixture was incubated at 37 °C for 60 min. The basal or pro-oxidant-induced TBARS production was stopped by adding 0.5 mL of acetic acid buffer (pH 3.5), and lipid peroxidation products were measured by the addition of 0.5 mL of TBA 0.6%. Tubes were then incubated in boiling water for 60 min and their contents were subjected to spectrophotometric analysis. The amount of TBARS produced was measured at 532 nm, using MDA as an external standard. TBARS levels were expressed as nmol MDA (malondialdehyde)/g of tissue.
4. Conclusions
In conclusion, we have described the synthesis in good yields of new 2-picolylamide-based diselenide derivatives using flexible methodologies. All diselenides were evaluated for their antioxidant capacity to shed light on the SAR on this series of compounds. All these new compounds showed good antioxidant activities when tested in different antioxidant capacity assays. Significantly higher GPx-mimic activity and lipid peroxidation inhibition potential was observed for the synthesized compounds as compared with diphenyl diselenide. In addition, in the present study, crystal structure of the most active compound i.e., 5d, showed strong nonbonding interaction between the selenium atom and the amide oxygen.
Acknowledgments
We gratefully acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), The World Academy of Sciences (TWAS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Institutos Nacionais de Ciência e Tecnologia-Catálise (INCT-Catálise), Fundação de Amparo à Pesquisa e Inovação do Estado de São Paulo-GlaxoSmithKline (FAPESP-GSK) and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina-Programa de Apoio a Núcleos de Excelência (FAPESC-Pronex) for financial support. J.R. and S.S. would like to thank CAPES, CNPq and TWAS for doctoral and postdoctoral fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are also grateful to Laboratório Central de Biologia Molecular Estrutural (CEBIME) for the HRMS analysis.
Supplementary Materials
Additional material available from Cambridge Crystallographic Data Center as Deposition No. CCDC 1061963 comprises H-atom coordinates, thermal parameters and remaining bond lengths and angles. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; E-Mail: deposit@ccdc.cam.ac.uk. 1H-NMR, 13C-NMR, IR and HRMS spectra of 5a–d can be accessed at http://www.mdpi.com/1420-3049/20/06/10095/s1.
Author Contributions
J.R. and S.S. designed and performed the synthesis of the diselenides; J.R., S.S., T.E.A.F. and R.F.S.C. performed spectral analysis and characterization; W.H., J.R., S.S., E.P.W., M.J. and J.B.T.D.R. evaluated the biological activity; D.F.B. performed XRD-analysis of compound 5d; A.L.B., J.R., S.S. and R.F.S.C. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 5a–d are available from the authors.
References
- 1.Back T.G. Design and synthesis of some biologically interesting natural and unnatural products based on organosulfur and selenium chemistry. Can. J. Chem. 2009;87:1657–1674. doi: 10.1139/V09-133. [DOI] [Google Scholar]
- 2.Saba S., Rafique J., Braga A.L. Synthesis of unsymmetrical diorganyl chalcogenides under greener conditions: Use of an iodine/DMSO system, solvent- and metal-free approach. Adv. Synth. Catal. 2015;357:1446–1452. doi: 10.1002/adsc.201500024. [DOI] [Google Scholar]
- 3.Mugesh G., du Mont W.-W., Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001;101:2125–2180. doi: 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]
- 4.Bhabak K.P., Mugesh G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc. Chem. Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- 5.Rappoport Z. The Chemistry of Organic Selenium and Tellurium Compounds. Volume 4 Wiley & Sons, Ltd.; Chichester, UK: 2014. [Google Scholar]
- 6.Devillanova F.A., du Mont W.-W. Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium. 2nd ed. RSC; Cambridge, UK: 2013. [Google Scholar]
- 7.Back T.G. Organoselenium Chemistry—A Practical Approach. 1st ed. Oxford University Press; Oxford, UK: 1999. [Google Scholar]
- 8.Godoi M., Paixão M.W., Braga A.L. Chiral organoselenium-transition-metal catalysts in asymmetric transformations. Dalton Trans. 2011;40:11347–11355. doi: 10.1039/c1dt11022e. [DOI] [PubMed] [Google Scholar]
- 9.Singh F.V., Wirth T. Facile oxidative rearrangements using hypervalent iodine reagents. Org. Lett. 2011;13:6504–6507. doi: 10.1021/ol202800k. [DOI] [PubMed] [Google Scholar]
- 10.Kim S.H., Kim Y.J., Lee H., Park K.Y., Lee C., Chin C.S. Ionic liquids containing anionic selenium species: Applications for the oxidative carbonylation of aniline. Angew. Chem. Int. Ed. 2002;41:4300–4303. doi: 10.1002/1521-3773(20021115)41:22<4300::AID-ANIE4300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaour K.C., Edmonds D.J., Bulger P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 12.Braga A.L., Rafique J. Synthesis of biologically relevant small molecules containing selenium. Part A. Antioxidant compounds. In: Rappoport Z., editor. The Chemistry of Organic Selenium and Tellurium Compounds. Volume 4. Wiley; Chichester, UK: 2013. pp. 989–1052. [Google Scholar]
- 13.Braga A.L., Rafique J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer Compounds. In: Rappoport Z., editor. The Chemistry of Organic Selenium and Tellurium Compounds. Volume 4. Wiley; Chichester, UK: 2013. pp. 1053–1117. [Google Scholar]
- 14.Braga A.L., Rafique J. Synthesis of biologically relevant small molecules containing selenium. Part C. Miscellaneous biological activities. In: Rappoport Z., editor. The Chemistry of Organic Selenium and Tellurium Compounds. Volume 4. Wiley; Chichester, UK: 2013. pp. 1119–1174. [Google Scholar]
- 15.Frizon T.E., Rafique J., Saba S., Bechtold I.H., Gallardo H., Braga A.L. Synthesis of functionalized organoselenium materials: Selenides and diselenides containing cholesterol. Eur. J. Org. Chem. 2015:3470–3476. doi: 10.1002/ejoc.201500124. [DOI] [Google Scholar]
- 16.Shamberger R.J. Biochemistry of Selenium. Plenum Press; New York, NY, USA: 1983. [Google Scholar]
- 17.Wessjohann L.A., Schneider A., Abbas M., Brandt W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007;388:997–1006. doi: 10.1515/BC.2007.138. [DOI] [PubMed] [Google Scholar]
- 18.Tiekink E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealised. Dalton Trans. 2012;41:6390–6395. doi: 10.1039/c2dt12225a. [DOI] [PubMed] [Google Scholar]
- 19.Tiecco M., Testaferri L., Santi C., Tomassini C., Santoro S., Marini F., Bagnoli L., Temperini A. Intramolecular non-bonding interaction between selenium and sulfur. Spectroscopic evidences and importance in asymmetric synthesis. Eur. J. Org. Chem. 2006:4867–4873. doi: 10.1002/ejoc.200600517. [DOI] [Google Scholar]
- 20.Bleiholder C., Werz D.B., Koppel H., Gleiter R. Theoretical investigations on chalcogen-chalcogen interactions: What makes these nonbonded interactions bonding? J. Am. Chem. Soc. 2006;128:2666–2674. doi: 10.1021/ja056827g. [DOI] [PubMed] [Google Scholar]
- 21.Vargas-Baca I., Chivers T. Weakly bonding interactions in organochalcogen chemistry. Phorphorus Sulfur Silicon Relat. Elem. 2000;164:207–227. doi: 10.1080/10426500008045247. [DOI] [Google Scholar]
- 22.Nakanishi W., Hayashi S., Itoh N. First linear alignment of five C–Se…O…Se–C atoms in anthraquinone and 9-(methoxy)anthracene bearing phenylselanyl groups at 1,8-positions. Chem. Commun. 2003:124–125. doi: 10.1039/b209261a. [DOI] [PubMed] [Google Scholar]
- 23.Mugesh G., Panda A., Singh H.B., Butcher R.J. Intramolecular Se…N nonbonding interactions in low-valent organoselenium derivatives: A detailed study by 1H and 77Se NMR spectroscopy and X-Ray crystallography. Chem. Eur. J. 1999;5:1411–1421. [Google Scholar]
- 24.Iwaoka M., Tomoda S. Nature of the Intramolecular Se…N nonbonded interaction of 2-selenobenzylamine derivatives. An experim,ental evaluation by 1H, 77Se, and 15N-NMR spectroscopy. J. Am. Chem. Soc. 1996;118:8077–8084. doi: 10.1021/ja953358h. [DOI] [Google Scholar]
- 25.Iwaoka M., Komatsu H., Katsuda T., Tomoda S. Experimental and theoretical studies on the nature of weak nonbonded interactions between divalent selenium and halogen atoms. J. Org. Chem. 2005;70:321–327. doi: 10.1021/jo048436a. [DOI] [PubMed] [Google Scholar]
- 26.Minkin V.I., Minyaev R.M. Cyclic aromatic systems with hypervalent centers. Chem. Rev. 2001;101:1247–1265. doi: 10.1021/cr990358h. [DOI] [PubMed] [Google Scholar]
- 27.Roy D., Patel C., Liebman J.F., Sunoj R.B. Probing intramolecular interactions in arylselenides using a property descriptor based approach. J. Phys. Chem. A. 2008;112:8797–8803. doi: 10.1021/jp8041395. [DOI] [PubMed] [Google Scholar]
- 28.Press D.J., Mercier E.A., Kuzma D., Back T.G. Substituent effects upon the catalytic activity of aromatic cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2008;73:4252–4255. doi: 10.1021/jo800381s. [DOI] [PubMed] [Google Scholar]
- 29.Bhabak K.P., Mugesh G. A simple and efficient strategy to enhance the antioxidant activities of amino-substituted glutathione peroxidase mimics. Chem. Eur. J. 2008;14:8640–8651. doi: 10.1002/chem.200800963. [DOI] [PubMed] [Google Scholar]
- 30.Nasimento V., Ferreira N.L., Canto R.F.S., Schott K.L., Waczuk E.P., Sancineto L., Santi C., Rocha J.B.T., Braga A.L. Synthesis and biological evaluation of new nitrogen-containing diselenides. Eur. J. Med. Chem. 2014;87:131–139. doi: 10.1016/j.ejmech.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Nascimento V., Alberto E.E., Tondo D.W., Dambrowski D., Detty M.R., Nome F., Braga A.L. GPx-like activity of selenides and selenoxides: Experimental evidence for the involvement of hydroxy perhydroxy selenane as the active species. J. Am. Chem. Soc. 2012;134:138–141. doi: 10.1021/ja209570y. [DOI] [PubMed] [Google Scholar]
- 32.Hassan W., Narayanaperumal S., Rahman A.U., Braga A.L., Rodrigues O.E.D., Rocah J.B.T., Gul K. Modulation of diorganoyl dichalcogenides reactivity by non-bonded nitrogen interactions. Chem. Biol. Interact. 2012;199:96–105. doi: 10.1016/j.cbi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim M., Hassan W., Anwar J., Deobald A.M., Kamdem J.P., SOUZA D.O., Rocha J.B.T. 1-(2-(2-(2-(1-aminoethyl) phenyl)diselanyl)phenyl)ethanamine: An amino Organoselenium compound with interesting antioxidant profile. Toxicol. In Vitro. 2014;28:524–530. doi: 10.1016/j.tiv.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Hassan W., Schair V.P.P., Santos D.B., Duarte M.M.M.F., Varga F., Nogueira C.W., Zeni G., Braga A.L., Rocha J.B.T. Addition of butoxycarbonyl group to phenylalanine derived chalcogenide increases the toxic potential: Importance of non-bonding nitrogen interaction. Chem. Biol. Interact. 2014;207:24–25. doi: 10.1016/j.cbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Pingawer R., Tongraung P., Woracharteewan A., Nantasenamet C., Prahayasittikul S., Ruchiarwat S., Prachayasittikul V. Cytotoxicity and QSAR study of (thio)ureas derived from phenylalkylamines and pyridylalkylamines. Med. Chem. Res. 2013;22:4016–4029. [Google Scholar]
- 36.Xue L., Wang H.-H., Wang X.-J., Jiang H. Modulating affinities of di-2-picolylamine (dpa)-substituted quinoline sensors for zinc ions by varying pendant ligands. Inorg. Chem. 2008;47:4310–4318. doi: 10.1021/ic702393z. [DOI] [PubMed] [Google Scholar]
- 37.Pullimamidi R.R., Nomula R., Pallepogu R., Shaik H. Picolinic acid based Cu(II) complexes with heterocyclic bases—Crystal structure, DNA binding and cleavage studies. Eur. J. Med. Chem. 2014;79:117–127. doi: 10.1016/j.ejmech.2014.03.084. [DOI] [PubMed] [Google Scholar]
- 38.Lee P.-K., Law W.H.T., Liu H.-W., Lo K.K.-W. Luminescent cyclometalated iridium(iii) polypyridine di-2-picolylamine complexes: Synthesis, photophysics, electrochemistry, cation binding, cellular internalization, and cytotoxic activity. Inorg. Chem. 2011;50:8570–8579. doi: 10.1021/ic201153d. [DOI] [PubMed] [Google Scholar]
- 39.Krief A., Derock M. Condition-driven selective syntheses of dialkyl diselenides involving elemental selenium and sodium borohydride. Synlett. 2005;6:1012–10140. doi: 10.1055/s-2005-864814. [DOI] [Google Scholar]
- 40.Back T.G., Moussa Z. Diselenides and allyl selenides as glutathione peroxidase mimetics. Remarkable activity of cyclic seleninates produced in situ by the oxidation of allyl ω-hydroxyalkyl selenides. J. Am. Chem. Soc. 2003;125:13455–13460. doi: 10.1021/ja0357588. [DOI] [PubMed] [Google Scholar]
- 41.Luo Z., Sheng J., Sun Y., Yang S., Lu C., Yan J., Liu A., Luo H.-B., Huang L., Li X. Synthesis and evaluation of multi-target-directed ligands against alzheimer’s disease based on the fusion of donepezil and ebselen. J. Med. Chem. 2013;56:9089–9099. doi: 10.1021/jm401047q. [DOI] [PubMed] [Google Scholar]
- 42.Selvakumar K., Shah P., Singh H.B., Butcher R.J. Synthesis, structure, and glutathione peroxidase-like activity of amino acid containing ebselen analogues and diaryl diselenides. Chem. Eur. J. 2011;17:12741–12755. doi: 10.1002/chem.201100930. [DOI] [PubMed] [Google Scholar]
- 43.Sarma B.K., Mugesh G. Antioxidant activity of the anti-inflammatory compound ebselen: A reversible cyclization pathway via selenenic and seleninic acid intermediates. Chem. Eur. J. 2008;14:10603–10614. doi: 10.1002/chem.200801258. [DOI] [PubMed] [Google Scholar]
- 44.Miyake Y., Nishibayashi Y., Uemura S. Asymmetric baeyer-villiger oxidation of cyclic ketones using chiral organoselenium catalysts. Bull. Chem. Soc. Jpn. 2002;75:2233–2237. doi: 10.1246/bcsj.75.2233. [DOI] [Google Scholar]
- 45.Mugesh G., Panda A., Singh H.B., Punekar N.S., Butcher R.J. Glutathione peroxidase-like antioxidant activity of diaryl diselenides- a mechanistic study. J. Am. Chem. Soc. 2001;123:839–850. doi: 10.1021/ja994467p. [DOI] [PubMed] [Google Scholar]
- 46.Bhabak K.P., Mugesh G. Amide-based glutathione peroxidase mimics: Effect of secondary and tertiary amide substituents on antioxidant activity. Chem. Asian J. 2009;4:974–983. doi: 10.1002/asia.200800483. [DOI] [PubMed] [Google Scholar]
- 47.Stadtman E.R., Levine R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 48.Yla-Herttuala S. Oxidized LDL and atherogenesis. Ann. N. Y. Acad. Sci. 1999;874:134–137. doi: 10.1111/j.1749-6632.1999.tb09231.x. [DOI] [PubMed] [Google Scholar]
- 49.Marnett L.J. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 50.Nogueira C.W., Zeni G., Rocha J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004;104:6255–6286. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- 51.Epp O., Ladenstein R., Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2 nm resolution. Eur. J. Biochem. 1983;133:51–59. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- 52.Freitas A.S., Prestes A.S., Wagner C., Sudati J.H., Alves D., Porciúncula L.O., Kade I.J., Rocha J.B.T. Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: A possible novel pathway for their antioxidant activity. Molecules. 2010;15:7699–7714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheldrick G.M. A short history of SHELX. Acta Cryst. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 54.Brandenburg K. Diamond 3.1a. 1997–2005. Crystal Impact GbR; Bonn, Germany: 2005. Version 1.1a. [Google Scholar]
- 55.Kurz J.L., Harris J.C. Substituent effect transmission from heavy atoms. Microscopic dissociation constants of selenoglycolic acid. J. Org. Chem. 1970;35:3086–3090. doi: 10.1021/jo00834a048. [DOI] [Google Scholar]
- 56.Painter E.P., Franke K.W., Gortner R.A. Organic selenium compounds. Their decomposition in alkaline solutions, and other properties related to the behavior of selenium compounds in cereals. J. Org. Chem. 1940;5:579–589. doi: 10.1021/jo01212a003. [DOI] [Google Scholar]
- 57.Günther W.H.H. Hypophosphorous acid, a novel reagent for the reduction of diselenides and the selenol-catalyzed reduction of disulfides. J. Org. Chem. 1966;31:1202–1205. doi: 10.1021/jo01342a052. [DOI] [Google Scholar]
- 58.Iwaoka M., Tomoda S. A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J. Am. Chem. Soc. 1994;116:2557–2561. doi: 10.1021/ja00085a040. [DOI] [Google Scholar]
- 59.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.