Abstract
The essential oils obtained by steam distillation from needles of six China endemic Pinus taxa (P. tabulaeformis, P. tabulaeformis f. shekanensis, P. tabulaeformis var. mukdensis, P. tabulaeformis var. umbraculifera, P. henryi and P. massoniana) were analysed by GC/MS. A total of 72 components were separated and identified by GC/MS from the six taxa. The major constituents of the essential oils were: α-pinene (6.78%–20.55%), bornyl acetale (3.32%–12.71%), β-caryophellene (18.26%–26.31%), α-guaiene (1.23%–8.19%), and germacrene D (1.26%–9.93%). Moreover, the essential oils were evaluated for antioxidant potential by three assays (DPPH, FRAP and ABTS) and tested for their total phenolic content. The results showed that all essential oils exhibited acceptable antioxidant activities and these strongly suggest that these pine needles may serve as a potential source of natural antioxidants for food and medical purposes.
Keywords: pine needle, essential oil, GC/MS, antioxidant activity
1. Introduction
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and their elimination by protective mechanisms, referred to as antioxidants [1]. Because of their oxidant properties, these species can damage all components of the cell, including proteins, lipids, and DNA [2] and consequently leading to cell injury and the development of various physiological and pathological abnormalities such as aging, neurodegenerative diseases (Alzheimer’s, Parkinson’s and Huntington’s) [3], cardiovascular diseases (atherosclerosis, heart failure) and many cancers [4]. Interest in extracts and biologically active compounds isolated from popular plant species has recently increased. Large number of plants worldwide has been investigated for their antioxidant activity [5,6,7,8]. This antioxidant capacity can be explored in food industry to maintain food quality by using plants as a source of antioxidants to prevent the rancidity and oxidation of lipids. Recent years, researches were mainly focused on medicinal plants to extract natural antioxidants that can replace synthetic additives such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) that might be toxic and even carcinogenic [9].
The genus Pinus belongs to the family Pinaceae and comprises about 115 species. It is the largest genus of conifers occurring naturally in the Northern hemisphere, especially in the Mediterranean region, Caribbean area, Asia, Europe, North and Central American [10]. A total of 22 species and 10 varieties of the genus Pinus are distributed in China, and 11 of them are endemic [11]. The needles of the genus Pinus are widely used in folk medicine and as food additives due to their numerous pharmacological properties, such as anti-aging and anti-inflammatory effects [12]. The leaves of the genus Pinus have been used to prepare drinks in Asia and pine needles have been used in traditional medicine for liver diseases, skin diseases, and hypertension [13,14]. Essential oils constitute of the most important group of pharmacologically active components of the genus Pinus [15,16,17]. Essential oils, volatile products of a plant’s secondary metabolism, possess well-known antioxidant properties [5,6,7,8,9]. The needles of the genus Pinus contain essential oils and the components of their essential oils have been established through chromatographic techniques [17,18,19]. However, most of these efforts used different experimental conditions and there is little comprehensive information on the volatile compounds and biological activities of the essential oils of pine needles.
In the present study the essential oils of needles from six of the Pinus taxa endemic to China (including P. tabulaeformis, P. tabulaeformis f. shekanensis, P. tabulaeformis var. mukdensis, P. tabulaeformis var. umbraculifera, P. henryi and P. massoniana) were obtained by steam distillation. Their chemical components were separated and identified by gas chromatography/mass spectrometry (GC/MS). In addition, total phenolic content, DPPH free radical scavenging activity, ferric reducing antioxidant power (FRAP) and ABTS radical cation scavenging activity assays were applied to accurately evaluate the antioxidant properties of the oils. Hopefully, this study will provide sufficient experimental evidence of antioxidant activity and potential for further development and utilization of these Pinus taxa.
2. Results and Discussion
2.1. Essential Oil Composition
Clear yellow volatile oils were obtained by hydrodistillation of needles from P. tabulaeformis, P. tabulaeformis f. shekanensis, P. tabulaeformis var. mukdensis, P. tabulaeformis var. umbraculifera, P. henryi and P. massoniana at 0.51%, 0.50%, 0.47%, 0.42%, 0.48% and 0.53% yield, respectively (Table 1). Bo et al. observed that the yield of essential oil from P. massoniana and P. sylvestris was 0.50% and 0.43% [20], while Zafar et al. reported that the yield of essential oil from P. roxburghaii needles was 0.11% [21]. The content of essential oil is influenced by pretreatment of the leaves, ratio of water and leaves, extraction time and collection season [22].
Table 1.
Chemical composition of the essential oils of the six Pinus taxa.
| No. | Name of Components | RI | Relative Peak Area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Ptf | Pvm | Pvu | Ph | Pm | |||||||
| 1 | Tricyclene | 921 | 1.81 | 1.11 | 0.39 | - | 0.21 | - | ||||
| 2 | α-Thujene | 925 | 0.13 | 1.48 | 0.24 | - | 1.72 | 2.02 | ||||
| 3 | α-Pinene | 932 | 11.08 | 6.78 | 16.55 | 20.55 | 9.68 | 8.16 | ||||
| 4 | Camphene | 945 | 0.38 | 0.45 | 0.58 | - | 0.34 | 0.63 | ||||
| 5 | Sabinene | 970 | 0.15 | - | - | - | 0.18 | - | ||||
| 6 | β-Pinene | 978 | 2.29 | 0.95 | 1.45 | - | 0.35 | 2.99 | ||||
| 7 | Myrcene | 982 | - | - | 0.72 | - | 1.04 | - | ||||
| 8 | α-Phellandrene | 1003 | 0.26 | - | - | - | - | - | ||||
| 9 | δ-3-Carene | 1008 | 0.31 | 0.19 | 0.38 | - | 0.13 | 0.13 | ||||
| 10 | α-Terpinene | 1014 | - | - | 0.11 | - | 0.12 | 0.29 | ||||
| 11 | Limonene | 1024 | 0.41 | - | - | - | 0.09 | - | ||||
| 12 | β-Phellandrene | 1027 | 0.12 | 0.30 | 0.57 | - | 0.37 | 0.66 | ||||
| 13 | β-Ocimene | 1041 | - | - | 0.22 | - | 0.15 | - | ||||
| 14 | γ-Terpinene | 4056 | 0.48 | 0.22 | 0.33 | - | 0.13 | 0.52 | ||||
| 15 | α-Terpinolene | 4086 | 0.24 | 0.37 | 0.95 | - | 0.59 | 0.17 | ||||
| 16 | Terpinen-4-ol | 1161 | - | - | - | - | 0.15 | - | ||||
| 17 | α-Terpineol | 1175 | 3.43 | 2.62 | 3.32 | - | 1.32 | 3.34 | ||||
| 18 | Bornyl acetale | 1267 | 4.13 | 3.32 | 3.93 | 12.71 | 2.96 | 3.83 | ||||
| 19 | α-Cubebene | 1346 | 0.21 | 0.32 | 0.28 | - | 0.26 | 0.24 | ||||
| 20 | α-Rlangene | 1371 | 0.24 | 0.26 | 0.30 | - | 0.31 | 0.24 | ||||
| 21 | α-Copaene | 1379 | - | - | 0.20 | - | - | 0.14 | ||||
| 22 | β-Bourbonene | 1388 | - | - | - | 1.13 | 0.12 | - | ||||
| 23 | β-Elemene | 1391 | 1.26 | 2.13 | 1.82 | - | 1.41 | 2.93 | ||||
| 24 | Longifolene | 1411 | 0.54 | 0.29 | 0.12 | 1.52 | 0.24 | - | ||||
| 25 | β-Caryophellene | 1421 | 22.36 | 20.83 | 24.08 | 26.31 | 18.26 | 18.48 | ||||
| 26 | α-Caryophellene | 1423 | 3.72 | - | - | - | 2.64 | 3.36 | ||||
| 27 | α-Guaiene | 1437 | 2.80 | 2.07 | 1.23 | 8.19 | 2.48 | 3.67 | ||||
| 28 | Aromadendrene | 1440 | 2.15 | 3.18 | 3.54 | - | 2.22 | 2.31 | ||||
| 29 | (E)-β-Farnesene | 1456 | 1.45 | 0.77 | - | 1.25 | 2.59 | - | ||||
| 30 | β-Santalene | 1458 | 0.47 | - | 0.83 | 1.27 | - | - | ||||
| 31 | α-Humulene | 1461 | - | - | 2.65 | - | 0.85 | - | ||||
| 32 | γ-Muurolene | 1472 | 1.06 | - | - | 2.30 | - | - | ||||
| 33 | Germacrene D | 1482 | 7.43 | 8.97 | 9.93 | 1.26 | 2.71 | 9.78 | ||||
| 34 | α-Amorphene | 1484 | 0.42 | 0.86 | 0.60 | 0.27 | 0.72 | 0.80 | ||||
| 35 | Aristolochene | 1486 | 2.10 | 3.56 | 2.95 | - | 3.03 | 3.71 | ||||
| 36 | β-Selinene | 1488 | 1.60 | - | 0.22 | 1.01 | 1.23 | - | ||||
| 37 | Phenylethyl-3 methyl butanoate | 1490 | 0.41 | 0.47 | - | 1.18 | 0.27 | - | ||||
| 38 | Phenylethyl isovalerate | 1491 | 0.74 | 1.69 | 1.49 | - | 0.14 | 1.23 | ||||
| 39 | epi-Cubebol | 1495 | 0.44 | 0.24 | - | 1.37 | 1.17 | - | ||||
| 40 | α-Selinene | 1496 | 1.72 | 2.42 | 2.34 | - | 3.53 | 2.91 | ||||
| 41 | Bicyclogermacrene | 1498 | - | 0.35 | - | 0.83 | 2.97 | - | ||||
| 42 | α-Muurolene | 1500 | 0.62 | - | - | 1.79 | - | - | ||||
| 43 | β-Cadinene | 1508 | 2.00 | - | 1.07 | - | - | 0.92 | ||||
| 44 | γ-Cadinene | 1517 | 3.72 | 9.74 | 2.19 | 3.45 | 2.23 | 1.62 | ||||
| 45 | δ-Cadinene | 1521 | 2.33 | 1.31 | - | - | 2.57 | - | ||||
| 46 | Cadina-1,4-diene | 1525 | - | - | - | - | 0.12 | 1.55 | ||||
| 47 | α-Cadinene | 1533 | - | - | - | - | 0.19 | 1.36 | ||||
| 48 | α-Calacorene | 1542 | 0.61 | 0.16 | 0.35 | - | 0.11 | 0.51 | ||||
| 49 | (E)-Nerolidol | 1546 | 0.24 | 1.31 | 0.35 | - | 1.17 | 2.50 | ||||
| 50 | Occidentalol | 1553 | - | - | - | - | 2.33 | - | ||||
| 51 | Germacrene B | 1557 | 0.20 | 1.50 | 0.81 | - | 0.22 | 0.12 | ||||
| 52 | (Z)-3-Hexenyl benzoate | 1567 | 0.16 | 0.18 | 0.58 | - | 0.71 | 1.22 | ||||
| 53 | Longipinaol | 1573 | - | 0.15 | - | - | 1.77 | 0.64 | ||||
| 54 | Spathulenol | 1579 | 0.23 | - | 0.60 | 1.25 | 0.65 | - | ||||
| 55 | Caryophyllene oxide | 1585 | 0.30 | 3.59 | 1.16 | - | - | 1.74 | ||||
| 56 | Globulol | 1593 | 0.15 | 0.45 | 1.40 | - | 2.62 | 1.18 | ||||
| 57 | β-Calacorene | 1596 | 2.25 | 1.40 | 2.44 | 3.64 | 1.28 | - | ||||
| 58 | β-Oplopenone | 1608 | 0.49 | 2.87 | 0.81 | - | 0.18 | 1.91 | ||||
| 59 | δ-Cadinol | 1646 | - | 0.44 | 0.61 | - | 2.98 | 0.91 | ||||
| 60 | α-Cadinol | 1652 | - | - | - | 1.55 | - | - | ||||
| 61 | α-Eudesmol | 1654 | 0.17 | 0.74 | - | - | 1.74 | 1.76 | ||||
| 62 | β-Bisabolal | 1672 | 0.15 | - | - | - | 0.76 | 1.98 | ||||
| 63 | Pentadecanal | 1718 | 0.17 | 0.18 | - | - | 0.48 | 1.21 | ||||
| 64 | Benzyl benzoate | 1733 | 1.10 | 0.90 | 0.69 | - | - | - | ||||
| 65 | Octadecane | 1798 | 0.23 | 2.07 | - | - | 2.54 | - | ||||
| 66 | Cubitene | 1877 | 0.56 | - | 0.29 | 0.48 | - | - | ||||
| 67 | Laurenene | 1881 | 0.52 | - | - | - | 0.36 | - | ||||
| 68 | Pimaradiene | 1943 | 0.23 | 0.62 | - | - | 0.47 | - | ||||
| 69 | Neocembrene | 1960 | 0.14 | 0.11 | - | 1.69 | 1.87 | 0.18 | ||||
| 70 | Sclareol | 1973 | 0.28 | 0.89 | 0.26 | - | 1.38 | - | ||||
| 71 | Manoyl oxide | 1993 | 0.57 | - | - | - | - | - | ||||
| 72 | Palustradiene | 2005 | - | 0.22 | - | - | 0.36 | 2.18 | ||||
| Total percentage a | 0.51 | 0.50 | 0.47 | 0.42 | 0.53 | 0.48 | ||||||
| Essential oil (%) content | 93.76 | 95.03 | 95.93 | 95.00 | 95.77 | 97.03 | ||||||
RI: retention index according to n-hydrocarbons (C6–C22) on the HP-5MS column; Pt: P. tabulaeformis; Ptf: P. tabulaeformis f. shekanensis; Pvm: P. tabulaeformis var. mukdensis; Pvu: P. tabulaeformis var. umbraculifera; Ph: P. henryi; Pm: P. massoniana; a Percentage of the total peak area. Components with percentage ≥ 0.1% are presented; -: not detected.
The chemical composition of the essential oils from the six Pinus taxa was analysed by GC-MS. Qualitative and quantitative analytical results are shown in Table 1. In total, 72 constituents were identified in the essential oils, while 57 (representing 93.76% of the total amount) were found in P. tabulaeformis; 47 (representing 95.03% of the total amount) in P. tabulaeformis f. shekanensis; 46 (representing 95.93% of the total amount) in P. tabulaeformis var. mukdensis; 23 (representing 95.00% of the total amount) in P. tabulaeformis var. umbraculifera; 61 (representing 95.77% of the total amount) in P. henryi and 43 (representing 97.03% of the total amount) in P. massoniana. β-Caryophellene and α-pinene were the main constituents of all oils; this has also been reported elsewhere [23,24]. The contents of β-caryophellene for P. tabulaeformis, P. tabulaeformis f. shekanensis, P. tabulaeformis var. mukdensis, P. tabulaeformis var. umbraculifera, P. henryi and P. massoniana were 22.36%, 20.83%, 24.08%, 26.31%, 18.26% and 18.48%, respectively, and the contents of α-pinene were 11.08%, 6.78%, 16.55%, 20.55%, 9.68% and 7.81%, respectively. For germacrene D the values were 7.43% (P. tabulaeformis), 8.97% (P. tabulaeformis f. shekanensis), 9.93 (P. tabulaeformis var. mukdensis) and 9.78% (P. massoniana). Other major compounds were bornyl acetale (12.71%), α-guaiene (8.19%) in P. tabulaeformis var. umbraculifera and γ-cadinene (9.74%) for P. tabulaeformis f. shekanensis. Based on the results, the essential oils of the six Pinus taxa have different chemical compositions in terms of major constituents. Aside from the genetic variation, some reports have emphasized the influence of the age of the plant, the harvest period, the climate and the geographic circumstances on the components of the essential oil [17,25], which explains the chemical composition differences among the six Pinus taxa.
2.2. Total Phenolic Content
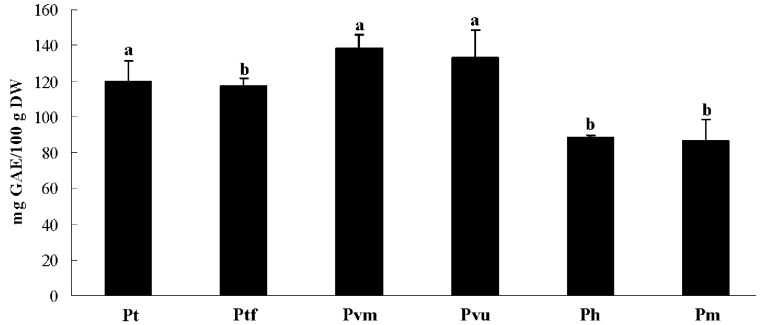
Phenolic compounds are often correlated to the antioxidant activity due to their capability to act as electron donors in free radical reactions [26]. In this study, total phenolic content (TPC) was estimated by the Folin-Ciocalteu colorimetric method using gallic acid as standard. The TPCs of the extracts ranged from 86.60 to 138.34 mg GAE/100 g DW, with P. tabulaeformis var. mukdensis showing the highest value of 138.34 mg GAE/100 g DW, followed by P. tabulaeformis var. umbraculifera, P. tabulaeformis, P. tabulaeformis f. shekanensis, P. henryi and P. massoniana with TPC values of 133.35, 119.68, 117.08, 88.61 and 86.60 mg GAE/100 g DW, respectively (Figure 1). Generally, aside from genetic differences, it has been shown that temperature and altitude are one of the major environmental factors that affect plant composition and properties [27,28,29]. Wildi and Lutz found that the plant samples collected from lower temperature sites showed higher phenolic content. In this work, P. tabulaeformis var. mukdensis and P. tabulaeformis var. umbraculifera were collected from a lower temperature site (Liaoning Province) compared to the other four taxa (Shaanxi Province). In addition to genetic differences, this might be one of the reasons why P. tabulaeformis var. mukdensis and P. tabulaeformis var. umbraculifera had higher phenolic content than the other taxa.
Figure 1.
Total phenolics content of six Pinus tax extracts. Means with different letters indicate significant differences at the 0.05 level. Taxon codes are identified in Table 1.
2.3. Antioxidant Activity
Antioxidant activity is a complex process usually occurring through several mechanisms. The evaluation of the antioxidant activity should be carried out by more than one test method [30]. In this study, three antioxidant assays, DPPH free radical scavenging activity, ferric reducing antioxidant power (FRAP) and ABTS radical cation scavenging activity were applied to accurately evaluate the antioxidant properties of the six Pinus taxa. The three assays gave very different values in absolute terms (i.e., μmol trolox equivalent (TE)/g DW), but showed the same relative pattern. Among taxa, the highest antioxidant activity was observed in P. tabulaeformis var. Mukdensis, regardless of the assay method used.
The antioxidant activity of all extracts determined by DPPH radical scavenging ability ranged from 892.45 to 1851.65 μmol TE/g DW. The highest antioxidant activity was found in the needle extract of P. tabulaeformis var. mukdensis, followed by P. tabulaeformis f. shekanensis, P. tabulaeformis var. umbraculifera, P. tabulaeformis, P. massoniana and P. henryi (Table 2). The greatest ferric reducing antioxidant power of 1134.45 μmol TE/g DW was detected in P. tabulaeformis var. mukdensis, while the P. massoniana had the lowest power of 477.78 μmol TE/g DW. Based on the mean value of each taxon, the ferric ion reducing power rank was P. tabulaeformis var. mukdensis > P. tabulaeformis > P. tabulaeformis f. shekanensis > P. tabulaeformis var. umbraculifera > P. henryi > P. massoniana. The ferric reducing antioxidant power of all taxa listed in Table 2 shows a different order from the DPPH assay results. These differences could be attributed to the different stoichiometry of the DPPH and FRAP assay reactions. In addition, the compositional differences in extracts and their different solubility in the test systems may also affect their antioxidant activity [31,32]. In the ABTS radical cation scavenging activity assay (Table 2), the rank order based on the average TE values was as follows: P. tabulaeformis var. mukdensis (3584.41 μmol TE/g DW) > P. tabulaeformis var. umbraculifera > P. tabulaeformis > P. tabulaeformis f. shekanensis > P. henryi > P. Massoniana (1461.01 μmol TE/g DW).
Table 2.
Antioxidant activity determined by the DPPH, FRAP and ABTS assays of the needles extracts from the six Pinus taxa.
| Taxa | DPPH | FRAP | ABTS |
|---|---|---|---|
| Pt | 1775.22 ± 138.17 NF | 1036.68 ± 51.14 a | 3078.52 ± 278.59 a |
| Ptf | 1844.19 ± 180.55 | 904.72 ± 90.73 b | 2467.85 ± 141.63 b |
| Pvm | 1851.65 ± 151.19 | 1134.45 ± 36.14 ab | 3584.41 ± 315.63 a |
| Pvu | 1817.25 ± 131.19 | 814.72 ± 112.41 b | 3486.33 ± 140.75 c |
| Ph | 918.28 ± 25.37 | 584.78 ± 68.67 c | 2151.43 ± 215.03 b |
| Pm | 892.45 ± 78.31 | 477.78 ± 48.67 c | 1461.01 ± 131.03 c |
All values are mean ± SD; a–c Values with different letters in the same column were significantly (p < 0.05) different; NF: not significant. Taxon codes are identified in Table 1.
The results of this study showed that the aqueous extracts of the six Pinus taxa had strong antioxidant activity, including DPPH radical, ferric reducing antioxidant power (FRAP) and ABTS radical cation scavenging capacity. Among the tested Pinus taxa, P. tabulaeformis var. mukdensis had the greatest antioxidant activities, while P. massoniana displayed the lowest antioxidant capacities. Compared with some popular plant species, the six tested Pinus taxa had higher antioxidant capacities than Phyllostachys pubescens [33], Ascophyllum nodosum [34], Sorghum bicolor [35], Vaccinium corymbosum [36] and Phaseolus vulgarisbut [37], but lower than some other popular plant species, such as Camellia sinensis [6] and Oenocarpus bacaba [38], as measured by their radical cation scavenging activity. Pine needles have been used to prepare drinks in Eastern Asia and as a traditional medicine for several centuries in China [13,14], thus the results in this study indicate that pine needles could be a potential source of natural antioxidant foods and also provide data to health professionals and food policy makers for encouraging the population to consume these plants.
2.4. Correlation Analysis
Correlation analysis was used to explore the relationships amongst the different antioxidant variables measured for extracts of the six Pinus taxa (Table 3). Regarding the different methods, a significant correlation between methods was confirmed with the three methods (DPPH, FRAP and ABTS). The correlation coefficient was 0.90 between DPPH and FRAP, and 0.84 between DPPH and ABTS at 0.05 level. Moreover, the results of TPC in the studied taxa correlated significantly and positively with their antioxidant capacity determined by DPPH (r = 0.94), FRAP (r = 0.93) and ABTS (r = 0.86) methods at 0.01 level. This was in agreement with earlier reports which stated that plants with higher content of polyphenolic compounds possess stronger antioxidant activity [39,40,41]. Therefore, the total phenolics assay could be a suitable candidate for measuring the antioxidant capacity of the six Pinus taxa.
Table 3.
Linear correlation coefficients between phenolic content and antioxidant capacity (panel A), and among the different methods for quantifying antioxidant capacity (panel B).
| DPPH | FRAP | ABTS | |
|---|---|---|---|
| Panel A | |||
| TPC | 0.94 ** | 0.93 ** | 0.86 * |
| Panel B | |||
| DPPH | 1.00 | ||
| FRAP | 0.90 * | 1.00 | |
| ABTS | 0.84 * | 0.63 | 1.00 |
* Correlation is significant at the 0.05 level. ** Correlation is significant at the 0.01 level.
3. Experimental Section
3.1. Chemicals and Plant Materials
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Tokyo Chemical Co. (Tokyo, Japan). 2,4,6-Tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetram-ethylchroman-2-carboxylic acid (Trolox), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), gallic acid, rutin, Folin-Ciocalteu reagent and a mixture of aliphatic hydrocarbons (C6–C22) were from Sigma Chemical Co. (St. Louis, MO, USA). All the other chemicals used were analytical grade.
The needles of P. tabulaeformis, P. tabulaeformis f. shekanensis, P. tabulaeformis var. mukdensis, P. tabulaeformis var. umbraculifera, P. henryi and P. massoniana were collected from different regions in China (Table 4). The samples were dried in a shady ventilated place, then cut into 1 cm pieces and separately hydrodistilled for 4 h (200 g sample in 500 mL of distilled water) in a Clevenger-type apparatus with a water cooled receiver, in order to reduce hydrodistillation overheating artifacts. The essential oil was taken up in diethyl ether, dried over sodium sulphate and reduced in volume at room temperature under vacuum on a rotatory evaporator. The oil obtained was stored at 4 °C prior to studies. The yields of the essential oils were calculated by the formula:
Table 4.
The origins of the six Pinus taxa with their geographical characteristics.
| Taxa | Origin | Latitude(°N)/Longitude(°E) | Elevation (m) |
|---|---|---|---|
| P. tabulaeformis | Huanglong, Shaanxi | 35.632/109.772 | 1127 |
| P. tabulaeformis f. shekanensis | Fuxian, Shaanxi | 35.998/108.690 | 1316 |
| P. tabulaeformis var. mukdensis | Anshan, Liaoning | 40.960/123.147 | 294 |
| P. tabulaeformis var. umbraculifera | Anshan, Liaoning | 41.009/123.124 | 250 |
| P. massoniana | Yangxian, Shaanxi | 33.326/107.624 | 722 |
| P. henryi | Nanzheng, Shaanxi | 32.857/106.586 | 1254 |
3.2. Identification of the Chemical Components of the Essential Oils
The GC/MS analysis was carried out using splitless injection mode on a ULTR-Polaris Q GC-MS instrument (Thermo Electron Corporation, Waltham, MA, USA), equipped with a HP-5MS capillary column (30 m × 0.32 mm, 0.25 μm film thicknesses). Helium was used as carrier gas at a flow rate of 1 mL/min in the split mode (split ratio 1:50), with an injection vol. 0.2 µL. Oven temperature was programmed from 50 °C (3 min) to 260 °C (5 min) at 2 °C/min and injector heater 250 °C. The mass-spectrometer was operating (full scan-mode) in the EI-mode at 70 eV. The components of essential oils were identified by matching their recorded mass spectra with the data bank mass spectra (NIST 98, NIST 02, NIST 05, and NIST 08) and by comparing their retention indices relative to a series of n-hydrocarbons (C6–C22) with literature values [42]. For each compound on the gas chromatogram, the percentage of peak area relative to the total peak area of all compounds was determined and reported as relative amount of that compound, without using correction factors.
3.3. Determination of Total Phenolic
The total phenolic content (TPC) was determined using Folin-Ciocalteu method [43]. In this method, extract (0.1 mL, 1 mg/mL) was reacted with Folin-Ciocalteu reagent (0.1 mL) and then neutralized with sodium carbonate (10 mL, 7%, v/v; in distilled water). After 60 min of incubation, the absorbance of the solution was measured at 765 nm. The results were expressed as the equivalent to milligrams of gallic acid per 100 gram of dry weight (mg GAE/100 g).
3.4. Antioxidant Capacity Determined by DPPH
The ability to scavenge DPPH free radicals was determined based on the method of Brand-Williams et al. with minor modifications [44]. Briefly, 0.1 mM DPPH solution (190 μL) was mixed with each test sample solution (10 μL) in 96-well plates. After the reaction was allowed to take place in the dark for 30 min, the absorbance at 517 nm was recorded to determine the concentration of remaining DPPH. Results were expressed as trolox equivalent antioxidant capacity.
3.5. Antioxidant Capacity Determined by Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was determined according to Benzie and Strain with some modifications [45]. FRAP reagent consist of 10 mM TPTZ in 40 mM HCl, 20 mM ferric chloride and 300 mM acetate buffer (pH 3.6) in the ratio of 1:1:10 (v/v/v). For this assay, FRAP reagent (300 μL) was mixed at 37 °C with each test sample solution (10 μL) in 96-well plates. After 10 min, the coloured products were then taken at 593 nm. Results were expressed as trolox equivalent antioxidant capacity.
3.6. Antioxidant Capacity Determined by Radical Cation (ABTS)
The ABTS assay was based on the method of Re et al. with slight modifications [46]. ABTS radical cation (ABTS+) was produced by reacting 7 mM ABTS solution with 2.45 mM potassium persulphate and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. For this assay, ABTS radical solution (190 μL) was mixed with each test sample solution (10 μL) in 96-well plates. After 6 min, the decrease of absorbance was measured at 734 nm using a micro plate reader. Results were expressed as trolox equivalent antioxidant capacity.
3.7. Statistical Analysis
All analyses were performed in triplicate and the results expressed as the mean ± standard deviation (SD). Data analysis was carried out by one-way ANOVA followed by Tukey’s post hoc test at p < 0.05 using the SPSS version 18.0 for Windows. A two-tailed Pearson’s correlation test was processed to determine the correlations among means.
4. Conclusions
This paper describes a comparative study of the chemical composition and antioxidant activity of essential oils from needles of the six Pinus taxa endemic to China. In light of the results obtained, we can conclude that there are obvious differences in the relative contents of chemical constituents among the six Pinus taxa’ essential oils, although their main constituents were similar and differed slightly in amount. Antioxidant evaluation (DPPH free radical scavenging activity, ferric reducing antioxidant power (FRAP) and ABTS radical cation scavenging activity assays) of the extracts showed that the antioxidant potency correlated well with the total phenolic content (TPC) and revealed that all essential oils exhibit acceptable antioxidant activities, which strongly suggests that these pine needles may serve as a potential source of natural antioxidants for food and medical purposes.
Acknowledgments
The authors would like to thank the National Natural Science Foundation of China (Grant # 30972382) for financial support.
Author Contributions
Zhouqi Li designed the research; Qing Xie and Zhihong Liu performed the experiments; Zhouqi Li and Qing Xie wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the essential oils are available from the authors.
References
- 1.Reuter S., Gupta S.C., Chaturvedi M.M., Aqqarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pincemail J., Bonjean K., Cayeux K., Defraigne J.O. Mecanismes physiologiques de la defense antioxidant. Clin. Nutr. Metab. 2002;16:233–239. doi: 10.1016/S0985-0562(02)00166-8. [DOI] [Google Scholar]
- 3.Glizczynska-Swiglo A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006;96:131–136. doi: 10.1016/j.foodchem.2005.02.018. [DOI] [Google Scholar]
- 4.Weisburger J.H. Lycopene and tomato products in health promotion. Exp. Biol. Med. 2002;227:924–927. doi: 10.1177/153537020222701014. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H.Y., Li R.X., Chuang L.Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules. 2012;17:7294–7304. doi: 10.3390/molecules17067294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L.S., Kim S.H., Kim Y.B., Kim Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules. 2014;19:9173–9186. doi: 10.3390/molecules19079173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaurinovic B., Popovic M., Vlaisavljevic S., Raseta M. Antioxidant activities of Melittis melissophyllum L. (Lamiaceae) Molecules. 2011;16:3152–3167. doi: 10.3390/molecules16043152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi W.P., Fang B., Zhao Q.Y., Jiao B.N., Zhou Z.Q. Falvonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014;161:230–238. doi: 10.1016/j.foodchem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Lagha-Benamrouche S., Madani K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crop. Prod. 2013;50:723–730. doi: 10.1016/j.indcrop.2013.07.048. [DOI] [Google Scholar]
- 10.Graikou K., Gortzi O., Mantanis G., Chinou I. Chemical composition and biological activity of the essential oil from the wood of Pinus heldreichii Christ. var. leucodermis. Eur. J. Wood Prod. 2012;70:615–620. doi: 10.1007/s00107-012-0596-9. [DOI] [Google Scholar]
- 11.Song Z.Q. Oleoresin Characteristics and Chemical Classification of Pinus. 2rd ed. University of Science and Technology of China Press; Hefei, China: 2009. p. 7. [Google Scholar]
- 12.Watanabe K., Momose F., Handa H. Interaction between influenza virus pine cone antitumor substances that inhibit the virus multiplication. Biochem. Biophys. Res. Commun. 1995;214:318–323. doi: 10.1006/bbrc.1995.2290. [DOI] [PubMed] [Google Scholar]
- 13.Kim K.Y., Chung H.J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000;48:1269–1272. doi: 10.1021/jf9900229. [DOI] [PubMed] [Google Scholar]
- 14.Lee E. Effects of powdered pine needle (Pinus densiflora seib et Zucc.) on serum and liber lipid composition and antioxidative capacity in rats fed high oxidized fat. J. Korean Soc. Food Sci. Nutr. 2003;32:926–930. [Google Scholar]
- 15.Kim H., Lee B., Yun K.W. Comparison of chemical composition and antimicrobial activity of essential oils from three Pinus species. Ind. Crop. Prod. 2013;44:323–329. doi: 10.1016/j.indcrop.2012.10.026. [DOI] [Google Scholar]
- 16.Su X.Y., Wang Z.Y., Liu J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009;117:681–686. doi: 10.1016/j.foodchem.2009.04.076. [DOI] [Google Scholar]
- 17.Bo C.Y., Zheng G.Y., Song Q. Comparative study on chemical components of essential oils from Pinus massoniana, P. sylvestris var. mongolica and Abies nephrolepis needles. Chem. Ind. For. Prod. 2010;30:45–50. [Google Scholar]
- 18.Zafar I., Mohammd Z.U.R., Shaista J.K., Aneela F., Shahid M. GC-MS studies of needles essential oils of Pinus roxburghaii and their antimicrobial activity. Pak. J. Biochem. Mol. Biol. 2011;44:36–38. [Google Scholar]
- 19.Yang J.K., Kang B.K., Kim T.H., Hong S.C., Seo W.T., Choi M.S. Efficient extraction methods and analysis of essential oil from softwood leaves. Korean J. Biotechnol. Bioeng. 2002;17:357–364. [Google Scholar]
- 20.Amri I., Hamrouni L., Hanana M., Gargouri S., Fezzani T., Jamoussi B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013;29:91–106. doi: 10.1080/01448765.2013.764486. [DOI] [Google Scholar]
- 21.Dob T., Berramdane T., Chelgoum C. Chemical composition of essential oil of Pinus halepensis Miller growing in Algeria. C. R. Chim. 2005;8:1939–1945. [Google Scholar]
- 22.Chang C.W., Chang W.L., Chang S.T., Cheng S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008;42:278–286. doi: 10.1016/j.watres.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S.S., Chua M.T., Chang E.H., Huang C.G., Chen W.J., Chang S.T. Variations in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresour. Technol. 2008;100:465–470. doi: 10.1016/j.biortech.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 24.Kılıç Ö., Koçak A. Essential oil composition of six Pinus L. taxa (Pinaceae) from Canada and their chemotaxonomy. J. Agric. Sci. Technol. 2014;4:67–73. [Google Scholar]
- 25.Jin Y.J., Wu J.K., Sun F., Deng W.H., Wang H.J., Sun W.C. Terpene composition of needle oil from Pinus tabulaeformis and the comparison with other two-needle pines. J. Beijing For. Coll. 1994;16:38–47. [Google Scholar]
- 26.Velioglu Y.S., Mazza G., Gao L., Oomah B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 27.Giorgi A., Madeo M., Speranza G., Cocucci M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010;24:1546–1559. doi: 10.1080/14786419.2010.490656. [DOI] [PubMed] [Google Scholar]
- 28.Rieger G., Muller M., Guttenberger H., Bucar F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008;56:9080–9086. doi: 10.1021/jf801104e. [DOI] [PubMed] [Google Scholar]
- 29.Wildi B., Lutz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–146. doi: 10.1111/j.1365-3040.1996.tb00235.x. [DOI] [Google Scholar]
- 30.Aruoma O. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003;523–524:9–20. doi: 10.1016/S0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 31.Lissi E.A., Modak B., Torres R., Escobar J., Urzua A. Total antioxidant potential of resinous exudates from Heliotropium sp. A comparison of ABTS and DPPH methods. Free Radic. Res. 1999;30:471–477. doi: 10.1080/10715769900300511. [DOI] [PubMed] [Google Scholar]
- 32.Mathew S., Abraham T.E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Sun S.N., Cao X.F., Xu F., Sun R.C., Jones G.L. Structural features and antioxidant activities of lignins from steam-exploded bamboo (Phyllostachys pubescens) J. Agric. Food Chem. 2014;62:5939–5947. doi: 10.1021/jf5023093. [DOI] [PubMed] [Google Scholar]
- 34.Peinado I., Girón J., Koutsidis G., Ames J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014;66:36–44. doi: 10.1016/j.foodres.2014.08.035. [DOI] [Google Scholar]
- 35.Woo K.S., Ko J.Y., Jeong H.S. Effect of milling time on antioxidant compounds and activities of methanol extracts of sorghum [Sorghum bicolor (L.) Moench] Food Sci. Biotechnol. 2014;23:1741–1746. doi: 10.1007/s10068-014-0238-6. [DOI] [Google Scholar]
- 36.Pertuzatti P.B., Barcia M.T., Rodrigues D., Cruz P.N., Hermosín-Gutiérrez I., Smith R., Godoy H.T. Antioxidant activity of hydrophilic and lipophilic extracts of Brazilian blueberries. Food Chem. 2014;164:81–88. doi: 10.1016/j.foodchem.2014.04.114. [DOI] [PubMed] [Google Scholar]
- 37.Pereira M.P., Tavano O.L. Use of different spices as potential natural antioxidant additives on cooked beans (Phaseolus vulgaris). Increase of DPPH radical scavenging activity and total phenolic content. Plant Foods Hum. Nutr. 2014;69:337–343. doi: 10.1007/s11130-014-0439-4. [DOI] [PubMed] [Google Scholar]
- 38.Fernanda D.B., Abadio F., Dietmar R.K., Reinhold C., Tseng W.H., Böser S., Graeve L. Antioxidant activity and characterization of phenolic compounds from bacaba (Oenocarpus bacaba Mart.) fruit by HPLC-DAD-MS. J. Agric. Food Chem. 2012;22:7665–7673. doi: 10.1021/jf3007689. [DOI] [PubMed] [Google Scholar]
- 39.Du G.M., Li M.J., Ma F.W., Liang D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. doi: 10.1016/j.foodchem.2008.08.025. [DOI] [Google Scholar]
- 40.Wang L.J., Su S., Wu J., Du H., Li S.S., Huo J.W., Zhang Y., Wang L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chem. 2014;160:357–364. doi: 10.1016/j.foodchem.2014.03.081. [DOI] [PubMed] [Google Scholar]
- 41.Gharibi S., Tabatabaei B.E.S., Saeidi G., Goli S.A.H., Talebi M. Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind. Crop. Prod. 2013;50:154–158. doi: 10.1016/j.indcrop.2013.07.038. [DOI] [Google Scholar]
- 42.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing Co.; Carol Stream, IL, USA: 1995. [Google Scholar]
- 43.Singleton V.L., Orthofer R., Lamuela-Raventos R.M., Lester P. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 44.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 45.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 46.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]