Abstract
Altered glycosylation is a common feature of cancer cells. It takes a variety of forms, which includes loss of expression or excessive expression of some structures, the accumulation of precursors, the appearance of novel structures, etc. Notably, these changes in glycan structure do not occur as a random consequence of disorder biology. Only a limited subset of oligosaccharides is found frequently enriched on the tumor cell surface and implicated in different tumor phenotypes. Among these, altered sialylation has long been associated with metastatic cell behaviors such as invasion and enhanced cell survival and accumulating evidence points to the alteration occurring in the sialic acid linkage to other sugars, which normally exists in three main configurations: α2,3, α2,6, and α2,8, catalyzed by a group of sialyltransferases. The aberrant expression of all three configurations has been described in cancer progression. However, the increased α2,6 sialylation catalyzed by β-galactoside α2,6 sialyltranferase 1 (ST6Gal I) is frequently observed in many types of the cancers. In this review, we describe the findings on the role of ST6Gal I in cancer progression, and highlight in particular the knowledge of how ST6Gal I-mediated α2,6 sialylated glycans or sialylated carrier proteins regulate cell signaling to promote the malignant phenotype of human carcinoma.
Keywords: cancer metastasis, sialylation, ST6Gal I
1. Introduction
It has long been known that cell surface glycans undergo dramatic changes upon carcinogenesis. During the past decades, a large number of studies have demonstrated the importance of these carbohydrates in tumor progression and deepened our understanding of the molecular mechanisms linking altered glycosylation to tumor behavior. Many reviews have discussed the roles of different tumor-associated glycans in different stages of human cancer progression [1,2,3,4]. Among these, altered sialylation on cell surface is always a part that could not be ignored. Sialic acids are a diverse group of negatively charged monosaccharides typically found as terminal components attached to cell surface glycoconjugates including N-glycans, O-glycans and glycosphingolipids [5,6]. Sialic acids have been primarily described in mammals, however they are also found in lower vertebrates and in invertebrates [7,8]. Sialic acids comprise more than 50 naturally occurring derivatives of the nine-carbon sugar neuraminic acid, which represent their first level of diversity [9]. In mammalian cells, the most common sialic acids are i-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc), although only the former is present in human cells [10]. The second level of diversity arises from the sialic acid linkage to sugar chains. To date, sialic acids are known to be linked via an α2,3 or α2,6 bond to Gal/GalNAc, or α2,8 bond to sialic acid in proteins through a group of sialyltransferases. Given the relatively strong electronegative charge of sialic acids and their location at the outmost reaches of the cell surface, it is not surprising that sialic acids modulate the conformation and stabilization of molecules and membranes, interactions with the environment, as well as normal processes including transmembrane signaling, fertilization, growth, differentiation and apoptosis [11,12]. On the other hand, altered sialylation has long been associated with the malignancy of carcinoma. High expression of sialic acids has been proposed to protect cancer cells from recognition and eradication by the immune system [13]. However, there is limited information regarding the molecular details of how distinct sialylated structures or sialylated carrier proteins regulate cell signaling to control metastatic cell behaviors including invasion and enhanced cell survival. Many cancer associated sialylated structures, which includes sialyl Thomsen-nouvelle antigen (sialyl Tn), sialyl Lewis antigen (sLe), α2,6 sialylated lactosamine, polysialic acid and gangliosides have been identified and their potential functions are well documented by some reviews and books [11,14,15,16,17]. Altered expression of these structures in cancer cells could result from multiple mechanisms. Loss of expression or excessive expression of certain sialyltransferases is frequently observed. Table 1 shows the sialyltransferases identified in human and most of them have been reported to express abnormally in cancers and are proposed to contribute to cancer progression [16]. The roles of different sialyltransferases in tumor progression have been comprehensively described in several reviews [18,19]. Here, we are focusing on the β-galactoside α2,6 sialyltranferase 1 (ST6Gal I), an enzyme catalyzing the α2,6 sialylation on N-glycans, because the altered expression of ST6Gal I are observed in many types of cancers (Table 1) and increasing evidence indicates its fundamental roles in tumor malignancy. In detail, we describe the recent findings on ST6Gal I in cancer progression, where the mechanistic roles of ST6Gal I in tumor malignant progression are highlighted and the mechanisms governing the cell surface α2,6 sialylation are discussed.
Table 1.
Cloned sialyltransferases and their involvement in human cancers.
| Sialyltransferases | Acceptor Sequence(s)/(Carrier Type) | Types of Cancers in Which the Altered Expression Observed | References ** |
|---|---|---|---|
| ST3Gal I | Galβ1-3GalNAc *, Galβ1-3GlcNAc/(O-glycans, glycolipids) | Breast, bladder, colon | [20,21,22] |
| ST3Gal II | Galβ1-3GalNAc/(O-glycans, glycolipids) | Prostate, colon | [22,23] |
| ST3Gal III | Galβ1-3*/4GlcNAc(N-glycans, O-glycans, glycolipids) | Stomach, pancreas, extrahepatic bile duct, cervix | [24,25,26,27] |
| ST3Gal IV | Galβ1-3GalNAc, Galβ1-3/4*GlcNAc/(N-glycans, O-glycans, glycolipids) | Renal cell, stomach | [28,29] |
| ST3Gal V | Galβ1-4Glc/(glycolipids) | Pediatric leukemia | [30] |
| ST3Gal VI | Galβ1-3/4*GlcNAc/(N-glycans, O-glycans, glycolipids) | ||
| ST6Gal I | Galβ1-4GlcNAc/(N-glycans) | Colon, breast, cervix, choriocarcinomas, acute myeloid leukemias, liver, brain | [18,31,32,33,34,35,36,37,38,39,40] |
| ST6Gal II | Galβ1-4GlcNAc/(N-glycans) | ||
| ST6GalNAc I | Galβ1-3GalNAc/(O-glycans) | Stomach, pancreas, colon, ovary, breast | [15,41,42,43,44] |
| ST6GalNAc II | Galβ1-3GalNAc/(O-glycans) | Colon | [42,45] |
| ST6GalNAc III | Galβ1-3GalNAc, GM1b/(O-glycans, glycolipids) | ||
| ST6GalNAc IV | Galβ1-3GalNAc, GM1b/(O-glycans, glycolipids) | ||
| ST6GalNAc V | GM1b/(glycolipids) | Colon, breast | [46,47] |
| ST6GalNAc VI | GM1b, GT1b/(glycolipids) | Colon | [48] |
| ST8Sia I | GM3/(glycolipids) | Breast cancer, pediatric acute leukemia | [30,49] |
| ST8Sia II | Sia2-3/6/8Galβ1-4GlcNAc/(N-glycans) | Liver | [37] |
| ST8Sia III | GT3, Siaα2-3Galβ1-4GlcNAc/(N-glycans, glycolipids) | Glioblastoma | [50] |
| ST8Sia IV | Sia2-3/6/8Galβ1-4GlcNAc/(N-glycans) | ||
| ST8Sia V | GD3, GM1b GD1a, GT1b, GQ1c/(glycolipids) |
* The preferred acceptor sequence; ** The authors apologize to many researchers, whose outstanding papers are not cited here due to a space limitation.
2. The Structure of ST6Gal I Gene and Protein as well as the Lectins Specifically Recognizing α2,6 Sialylated Sugar Chains
Among the sialyltransferases identified, ST6Gal I is the first to be cloned and biochemical, genetic studies have continued to use this enzyme as a paradigm for understanding Golgi glycosylation [51]. The initial report on the structure of ST6Gal I gene and protein is derived from rat liver [51]. It has been shown that this sialyltransferase comprise 403 residues coded by approximately 4.7 kb mRNA and the topology of the enzyme in the Golgi apparatus consists of a short NH2-terminal cytoplasmic domain, a 17-residue hydrophobic sequence which serves as the membrane anchor and signal sequence, and a large luminal, catalytic domain. Further study showed that the rat ST6Gal I gene produces three different sized mRNA through alternative splicing and promoter utilization in tissue-specific fashion [52]. Similarly, this tissue-specific fashion is also observed in human ST6Gal I transcripts [53,54]. Three major mRNA species have been identified, which share a common protein coding region but diverge in the 5'-untranslated regions. The first cloned from a placenta cDNA library contains the 5'-untranslated exons Y and Z (Y+Z form) [55]. The second lacks exons Y and Z but contains 5'-untranslated exon X [56]. The third species initially characterized from HepG2 hepatoma cells lacks Y, Z and X. Instead, it contains a short specific sequence in front of exon I [57]. The different transcripts of ST6Gal I have been shown to result from the regulation of its multiple and distinct promoter regions [58]. Although the regulatory mechanism remains unclear, this strategy provides a reasonable explanation for the observation that most tissues express ST6Gal1 in humans, but the level of expression varies dramatically. Under pathological conditions, cells seems to have different preference for ST6Gal I transcripts. Both the Y+Z form and hepatic transcripts were detectable in normal and cancer tissues of colon but that latter form had a marked tendency to accumulate in cancer [58]. The regulation of the different promoter of ST6Gal I will be discussed later in this review.
The study of biological functions of ST6Gal I mediated α2,6-sialylation under physiological and pathological conditions are facilitated with the discovery of lectins which specifically recognize the Sia(α2-6)Gal/GalNAc sequence. The earliest identified is the lectin named Sambucus nigra agglutinin (SNA) [51,59]. The level of α2,6 sialylation of cell glycoproteins determined by SNA lectin has been demonstrated to closely correlate with the level of ST6Gal I enzyme activity in colon cancer cell lines, and the colon cancer tissues with the high expression ST6Gal I present a high reactivity to SNA [38,54]. In addition to SNA, another lectin Trichosanthes japonica agglutinin I (TJA-I) has also been reported to specifically recognize the Sia(α2-6)Gal/GalNAc residues [60]. Histochemical study with TJA-I lectin showed that normal mucosa and benign adenoma tissues from the patients with colonic adenocarcinomas were not stained and 83% of well and moderately differentiated colon adenocarcinomas reacted with this lectin, indicating a potential application of TJA-I staining for early diagnosis of colon cancers [61]. Both of these lectins have been widely utilized and become useful and convenient tools for the indication of the α2,6-sialylation level.
3. Functions of ST6Gal I in Cancer Progression
The up-regulated expression of ST6Gal I was first described in colon cancer, but successively confirmed in other carcinomas of breast, liver, cervix, choriocarcinomas, acute myeloid leukemias and some malignancies of the brain as well [18,31,32,33,34,35,36,37,38,39,40]. The α2,6 sialylated blood group type 2H catalyzed by ST6Gal I in colon cancer has been reported to be predictive markers of poor prognosis [62]. It is worth mentioning that altered epression of ST6Gal I is observed in hepatocarcimoma, but not the cirrhosis [40]. All this information suggests that ST6Gal I plays important roles in tumor progression. So, how does this enzyme benefit the tumor cells? The hallmark of early carcinogenesis is the acquisition of a highly proliferative activity by the transforming cells. However, ST6Gal I seems to have no effect on it, because quantitative lectin-histochemical and immune-histochemical studies on the occurrence of α2,3 and α2,6 sialic acid residues in colorectal carcinomas showed that 2,6 sialylated glycoconjugates did not display any association with local tumor growth, while α2,3 sialylation positively correlated with tumor growth and significantly increased at tumor Stage I and Stage II, but decreased in advanced carcinomas [63]. Consistent with the observation in colon cancer, increased ST6Gal I expression in breast cancer was observed only by a group of patients mainly of Stage III [31]. A study from Varki’s group showed that mammary tumors developed by PyMT mice in a ST6Gal I null background displayed increased differentiation but the same growth rate as those grown in the PyMT mice expressing ST6Gal I [64]. However, paradoxically, overexpression of ST6Gal I in colon cancer cells has also been reported to reduce tumorigenicity [65] and conversely, inhibition of ST6Gal I expression increased cell proliferation and tumor growth in vitro and in vivo [66]. Given that ST6Gal I and α2,3 sialyltransferases share common substrates and overexpression of ST6Gal I could lead to the decrease in α2,3 sialylation through substrate competition mechanisms [67], it is reasonable to hypothesize that the inhibitory effects of ST6Gal I overexpression on tumor growth is the direct result of decreased α2,3 sialylation.
In contrast to the elusive role in the tumor growth, increasing evidence indicates that ST6Gal I is critical for the tumor malignancy including metastasis and invasion. It has been shown in colon cancer that metastatic tumor growth is accompanied by a significant increase of α2,6 sialylated carbohydrate sequences produced by ST6Gal I [63]. Knockdown of ST6Gal I significantly inhibits the cell metastasis in diverse carcinomas [68,69,70]. Conversely, forced expression of ST6Gal I in MDA-MB-435 human mammary tumor cells and OV4 ovarian carcinoma cells leads to reduced cell–cell adhesion and enhanced capacity for invasion [69,71]. Animal models also implicate ST6Gal I in tumor metastasis. Neuraminidase treatment of metastatic murine cell lines dramatically decreases the amount of liver metastasis after splenic injection [72]. Despite the well-established link between ST6Gal I and cell migration, the underlying mechanisms remain unclear. Several groups including us recently demonstrate that ST6Gal I promotes the cell motility by activating the PI3K/Akt signaling pathway [37,73]. Also, in vitro studies show that the effects of ST6Gal I on cell migratory response are mediated, at least in part, by the α2,6 sialylation of the β1 integrin because cells deficient in β1 integrin do not exhibit differential invasion upon forced expression of ST6Gal I expression [71,74,75].
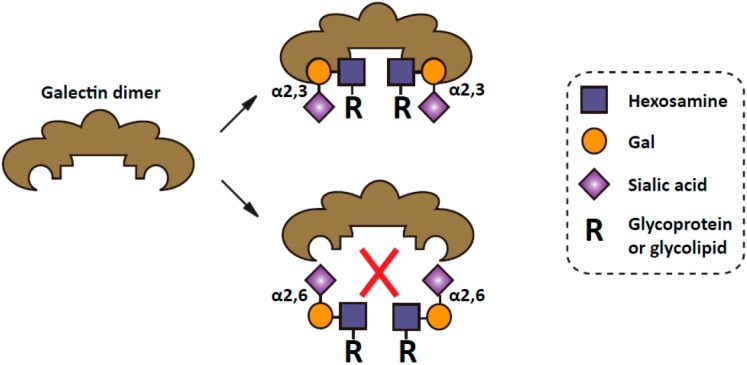
On the other hand, the selective enrichment of α2,6 sialic acids produced by ST6Gal I on tumor cells has also been shown to render the cells resistant to apoptosis. One striking example is the effect of α2,6 sialylation on the apoptosis signaling mediated by cell galectins. Galectins are a family of animal lectins with affinity for β-galactosides [76,77,78]. A number of galectins have been shown to interact with cell-surface and extracellular matrix glycoconjugates through lectin–carbohydrate interactions. Through this action, some galectins are capable of inducing apoptosis [79,80,81]. However, recent study shows that α2,6 sialylation of galactose serves as a generic inhibitor of galectin binding and upregulation of cell surface α2,6 sialylation is able to block the binding of pro-apoptotic galectins, thereby promoting tumor cell survival [82,83,84]. In this regard, it is worth mentioning that the anti-apoptotic effect of α2,6 sialylation is specific as compared with α2,3 sialylation in that, unlike α2,6 sialic acids, α2,3 sialic acids have little effects on galectin binding (Figure 1). In addition to the galectin-mediated pathway, it has also been shown that α2,6 sialylation could elicit its anti-apoptotic effects through inhibiting the cell death pathways initiated by Fas and TNFR1 [85,86]. Reduced Fas-mediated apoptosis is a well-established factor in tumor survival and Fas expression is down-regulated in many different tumor types [87,88,89]. However, some cells express high levels of Fas, but are yet resistant to Fas-induced apoptosis [90,91,92]. In fact, it has been shown that Fas pro-apoptotic activity is masked by sialylation [93,94,95]. Recent work clearly defines that α2,6 sialic acid linkage is functionally important, as α2,6 sialylation of Fas (but not α2,3 sialylation) could inhibit Fas apoptotic activity by interfering the formation of death inducing signaling complex and restraining Fas receptor internalization [85]. Similar to Fas, ST6Gal I mediated α2,6 sialylation of TNFR death receptor blocks apoptosis directed by the TNFR1 ligand, TNFα [86]. Consistent with the inhibitory effect of α2,6 sialylation on apoptosis through galectins, Fas and TNFR1, upregulation of ST6Gal I was reported to confer radiation resistance in colon cancer cell lines, as well as multidrug resistance in human acute myeloid leukemia [96,97].
Figure 1.
α2,6 sialylation inhibits the galectin binding to the carbohydrate [11]. The free hydroxyl group on the six carbon of galactose is required for the binding to galectins [83]. The addition of α2,6 linked sialic acids at this site by sialyltransferases, therefore, could block their interaction. In contrast, α2,3 sialic acids have little effects on galectin binding.
Cancer stem cells (CSCs) refer to a minority population of cancer cells that are capable of self-renewal and generation of differentiated progeny. Eradication of this rare population is a new insight in cancer treatment. Intriguingly, high expression of ST6Gal I has been correlated with human induced pluripotent stem cells and CSCs, indicating that ST6Gal I activity may be involved in maintaining some aspect of stem like cell behavior [98]. Considering the fact that tumors induced in ST6Gal I knockout mice were more differentiated compared with those in the wild type background [64], it is postulated that ST6Gal I could be important for an immature or undifferentiated cell phenotype. On the other hand, epithelial-mesenchymal transition (EMT) has been regarded as one mechanism for the generation of CSCs. EMT describes a trans-differentiation process that allows fully polarized epithelial cells to undergo multiple biochemical changes, enabling them to acquire a mesenchymal identity with properties of stem-like cells. Recently, our group showed that ST6Gal I expression was required for the TGF-β induced EMT [99]. Knockdown of ST6Gal I prevented TGF-β-induced increase in cell migration. Therefore, ST6Gal I in CSCs is highly likely to contribute to maintaining the metastatic property as well. In addition, as mentioned above, a number of reports show that ST6Gal I confers resistance to apoptosis. Thus, we could not exclude the possibility that expression of ST6Gal I reduces the apoptosis sensitivity in response to various stimuli, thereby extending the cell lifespan of the CSCs.
The functions of ST6Gal I in cancer progression could be more complicated than discussed above. The pro-migratory and anti-apoptotic roles have been challenged by several reports that: (1) forced expression of ST6Gal I suppressed the cell migration and enhanced the cell death induced by chemotherapeutic agents in glioma cells [67,100]; (2) ST6Gal I loss was associated with increasing invasiveness in bladder carcinogenesis [101]. In glioma cells, it was shown that instead of ST6Gal I, ST3Gal IV contributed to the cell migration and survival and overexpression of ST6Gal I could suppress the ST3Gal IV mediated α2,3 sialylation by competing for their common substrates. Whether a similar mechanism occurs in bladder carcinomas has to be confirmed. On the other hand, a recent study directly demonstrated that ST6Gal I is also involved in tumor angiogenesis. Increased ST6Gal I mediated sialylation suppressed VEGF-independent angiogenesis in tumor growth by preventing Gal1 binding and elimination of α2,6 linked sialic acids conferred resistance to anti-vascular endothelial growth factors-targeted treatment [102].
4. Mechanistic Roles of ST6Gal I in Cancer Progression
While not completely understood, the functions of α2,6 sialylation described above may be exerted by affecting the structures of attached glycans or carrier proteins. In the first case, as mentioned earlier, the addition of α2,6 sialic acid to the terminal galactose of N-glycans masks the galectin recognition sites for binding of β-galactoses, which in turn switches off the galectin functions including adhesion, migration and apoptosis. In contrast to the inhibitory effect on the glycan–galectin binding, α2,6 sialic acids have also been reported to bind specifically to the siglec-2 family of lectins [103]. Since siglec-2 are mainly expressed by immune cells, the potential functions of siglecs in tumor biology has been envisioned that changes in tumor cell sialylation could affect the activity of siglec-expressing immune cells, and consequently modulate the anti-tumor immune response. Clearly, further evidence is needed for this hypothesis. On the other hand, α2,6 sialic acids have direct effects on the structure/function of specific sialylated glycoproteins. α2,6 sialylation has been shown to alter conformation of the β1 integrin [104], clustering of the CD45 [105], EGFR [106] and PECAM [107], cell surface retention of PECAM [107] and Fas death receptor [85]. There is also evidence that α2,6 sialylation of galectin receptors causes release from the galectin lattice, leading to receptor internalization [108]. Given the relatively large size and negative charge of sialic acids, these findings should not be surprising. Taken together, abundant literature indicates that α2,6 sialylation holds potential to influence tumor cell behaviors through many different mechanisms.
5. Regulatory Mechanisms of ST6Gal I Expression in Cancer Progression
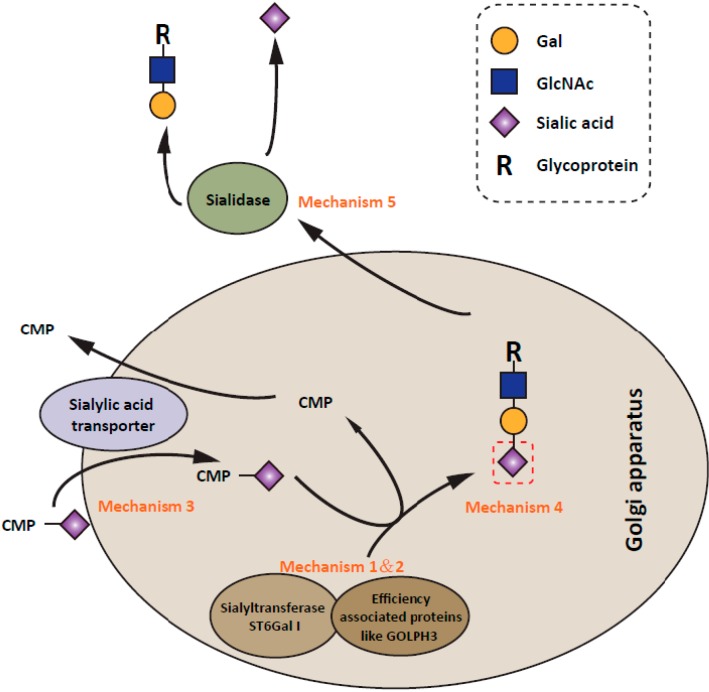
Given the accumulating evidence for the importance of ST6Gal I mediated α2,6 sialylation in cancer progression, much attention has been paid to elucidating the regulatory mechanisms of its expression. It has been shown that the expression of α2,6 sialylation on tumor cell surface can be modulated at different levels (Figure 2). The most frequently observed is the modulation of the ST6Gal I transcription. ST6Gal I expression is positively regulated by oncogenic N-ras and H-ras and negatively regulated by the tumor suppressor transcription factor RUN3 [109,110,111,112,113]. On the other hand, caveolin-1, a gene with a prevalent tumor suppressor activity, has been reported to stimulate ST6Gal transcription [114]. In spite of the fact that many proteins have been reportedly involved in the modulation of ST6Gal I transcription, the information regarding how ST6Gal I is transcriptionally regulated still remains obscure. Expression of ST6Gal I has been shown to be regulated by different promoters (designated as P1, P2 and P3) in different cancers [115]. The activity of P1 promoter is specifically enhanced in cervical cancer tissue [27,116]. Further analysis of P1 promoter by luciferase assays in cervical and hepatic cell lines showed that mutation of Sp1 or HNF1 binding sites affected the promoter activity only in HepG2 cell line, but not in C33A cells, indicating that the regulation of ST6Gal I promoter activity is cell type specific [117]. Moreover, in contrast to the enhanced activity of P1 promoter in cervical cancer, ST6Gal I expression is induced by Ras oncogene in NIH3T3 cells via its P3 promoter [112] which suggests that the transcription of ST6Gal I is regulated by different mechanisms under different biological scenarios. In addition to transcription factors, the promoter activity of ST6Gal I is also regulated by the epigenetic modification [101]. ST6Gal I promoter methylation resulted in ST6gal I gene silencing in human bladder cancer. Beyond its promoter activity, ST6Gal1 expression could be regulated at a post-transcriptional level. Our recent report showed that ST6Gal I formed a complex with GOLPH3, an oncogene that functions in secretory trafficking at the Golgi. Knockdown of this gene in MDA-MB-231 breast cancer cells led to the down-regulation of both α2,3 sialylation and α2,6 sialylation, but had no effect on the transcription of sialyltransferases [73]. Further, alternative pathways could also regulate the α2,6 sialylation on the cell surface. For instance, although not a human cell line, CHO-Lec2 cells which are unable to transport CMP-sialic acid (substrate for sialyltranferases) into the Golgi vesicle, have a 70%–90% deficiency in sialic acids on the glycoproteins and gangliosides [118,119]. In addition, several sialidases in tumor cells are expressed abnormally and act at the cell surface [120,121,122,123,124]. A reduction of certain sialidases like Neu1, which removes sialic acid residues on oligosaccharides leads to higher levels of cell surface sialylation of human cancers [124]. Moreover, different sialyltransferases have different acceptor preferences. Recent structure studies of ST6Gal I and ST3GAL1 (which catalyzes 2,3 sialylation on O-glycans) clearly demonstrated the difference in their glycan acceptor binding domain [125,126]. Therefore, it is reasonable to hypothesize that the expression level as well as the distribution of α2,3 and α2,6 sialylation could be also modulated by the expression pattern of oligosaccharides that sialyltransferases catalyze. Consistent with this idea, it was shown that biantennary N-glycan could be easily sialylated by ST6Gal I but not α2,3 sialyltransferases and a higher degree of branching of the acceptors led to a decrease in the rate of sialylation [127,128]. Also, knockout of GnT-V, an enzyme catalyzes the α1,6 branched GlcNAc structure of N-glycan, led to increased α2,6 sialylation and decreased α2,3 sialylation [129]. Better understanding of the substrate specificity of ST6Gal I may contribute to the development of ST6Gal I-specific inhibitory drug.
Figure 2.
Schematic representation of the regulation of α2,6 sialylation expression. The expression level of surface α2,6 sialylation is increased in tumors by several mechanisms. (1) Most commonly, ST6Gal I transcription is regulated by some transcription factors and methylation modification [18,31,32,33,34,35,36,37,101,130]; (2) Some factors like GOLPH3, recently, has been shown to interact with ST6Gal I, thereby modulating the efficiency of the sialylation [73]. (3) In addition, the expression levels of sialic acid transporter could affect α2,6 sialylation expression by regulating the donor substrate reservoir of ST6Gal I [118,119]; (4) The distribution as well as the amount of α2,6 sialic acids on cell surface also depend on the expression pattern of oligosaccharide acceptors [127,128,129]; (5) Further, as observed in many types of tumors, down-regulation of sialidases is usually accompanied by the upregulation of α2,6 sialylation expression [120,121,122,123,124].
6. Conclusions and Future Directions
Given increasing evidence implicating ST6Gal I in multiple cancers, much effort has been undertaken to clarify the functions of this enzyme in cancer progression during the last decade. Furthermore, it is becoming clear that ST6Gal I plays important roles in regulating tumor metastatic behaviors including migration and enhanced cell survival. In contrast, there is a marked dearth of information on how tumor cells regulate the expression of ST6Gal I and α2,6 sialylation. Defining regulatory mechanisms and specific ST6Gal I substrates will be necessary for a complete understanding of the roles of ST6Gal I during cancer progression and may provide new insights for targeting aggressiveness and drug resistance of the cancer cell by manipulating the level of cell surface α2,6 sialylation. In addition, although ST6Gal I is mainly localized in the Golgi apparatus, this protein can also be proteolytically processed to a soluble form and secreted into the serum [51,131]. The serum ST6Gal I is involved in the generation of the cell-surface carbohydrate determinants and differentiation antigens HB-6, CD75, and CD76 [132]. Its enzymatic activity in serum is elevated during the hepatic inflammatory response, a cumulative homeostatic process executed in response to tissue injury, trauma, infection, or tumor burden [133]. Therefore, there is a critical need for elucidating the roles of soluble ST6Gal I in physiological and pathological processes.
Acknowledgments
The authors apologize to colleagues and researchers who have made important contributions that could not incorporated in this review. This work was partly supported by a Grant-in-Aid for Scientific Research (15H04354 to JG), for Challenging Exploratory Research (15K14408 to JG) from the Japan Society for the Promotion of Science; and the Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author Contributions
Study concept and design (J.L., J.G.). Critical review of the manuscript (J.L., J.G.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kannagi R., Sakuma K., Cai B.-H., Yu S.-Y. Tumor-Associated Glycans and Their Functional Roles in the Multistep Process of Human Cancer Progression. In: Suzuki T., Ohtsubo K., Taniguchi N., editors. Sugar Chains. Springer; Tokyo, Japan: 2015. pp. 139–158. [Google Scholar]
- 2.Glavey S.V., Huynh D., Reagan M.R., Manier S., Moschetta M., Kawano Y., Roccaro A.M., Ghobrial I.M., Joshi L., O’Dwyer M.E. The cancer glycome: Carbohydrates as mediators of metastasis. Blood Rev. 2015 doi: 10.1016/j.blre.2015.1001.1003. [DOI] [PubMed] [Google Scholar]
- 3.Drake R.R., Jones E.E., Powers T.W., Nyalwidhe J.O. Altered Glycosylation in Prostate Cancer. In: Richard R.D., Lauren E.B., editors. Advances in Cancer Research. Volume 126. Academic Press; New York, NY, USA: 2015. pp. 345–382. [DOI] [PubMed] [Google Scholar]
- 4.Lemjabbar-Alaoui H., McKinney A., Yang Y.-W., Tran V.M., Phillips J.J. Glycosylation Alterations in Lung and Brain Cancer. In: Richard R.D., Lauren E.B., editors. Advances in Cancer Research. Volume 126. Academic Press; New York, NY, USA: 2015. pp. 305–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki N.M., Varki A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angata T., Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 7.Varki A., Schauer R. Sialic Acids. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor; New York, NY, USA: 2009. pp. 199–217. [Google Scholar]
- 8.Warren L. The Distribution of Sialic Acids in Nature. Comp. Biochem. Physiol. 1963;10:153–171. doi: 10.1016/0010-406X(63)90238-X. [DOI] [PubMed] [Google Scholar]
- 9.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou H.-H., Takematsu H., Diaz S., Iber J., Nickerson E., Wright K.L., Muchmore E.A., Nelson D.L., Warren S.T., Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz M.J., Swindall A.F., Bellis S.L. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bull C., den Brok M.H., Adema G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta. 2014;1846:238–246. doi: 10.1016/j.bbcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Varki A., Kannagi R., Toole B.P. Glycosylation Changes in Cancer. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor; New York, NY, USA: 2009. pp. 617–632. [PubMed] [Google Scholar]
- 15.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dall’Olio F., Malagolini N., Trinchera M., Chiricolo M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Julien S., Delannoy P. Sialic Acid and Cancer. In: Endo T., Seeberger P.H., Hart G.W., Wong C.-H., Taniguchi N., editors. Glycoscience: Biology and Medicine. Springer Japan; Tokyo, Japan: 2014. pp. 1–6. [Google Scholar]
- 18.Dall’Olio F., Chiricolo M. Sialyltransferases in cancer. Glycoconj. J. 2001;18:841–850. doi: 10.1023/A:1022288022969. [DOI] [PubMed] [Google Scholar]
- 19.Harduin-Lepers A., Krzewinski-Recchi M.A., Colomb F., Foulquier F., Groux-Degroote S., Delannoy P. Sialyltransferases functions in cancers. Front. Biosci. 2012;4:499–515. doi: 10.2741/E396. [DOI] [PubMed] [Google Scholar]
- 20.Burchell J., Poulsom R., Hanby A., Whitehouse C., Cooper L., Clausen H., Miles D., Taylor-Papadimitriou J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology. 1999;9:1307–1311. doi: 10.1093/glycob/9.12.1307. [DOI] [PubMed] [Google Scholar]
- 21.Videira P.A., Correia M., Malagolini N., Crespo H.J., Ligeiro D., Calais F.M., Trindade H., Dall’Olio F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer. 2009;9:357. doi: 10.1186/1471-2407-9-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo T., Ikehara Y., Togayachi A., Morozumi K., Watanabe M., Nakamura M., Nishihara S., Narimatsu H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Investig. 1998;78:797–811. [PubMed] [Google Scholar]
- 23.Hatano K., Miyamoto Y., Mori M., Nimura K., Nakai Y., Nonomura N., Kaneda Y. Androgen-regulated transcriptional control of sialyltransferases in prostate cancer cells. PLoS ONE. 2012;7:e31234. doi: 10.1371/journal.pone.0031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Garay M., Arteta B., Pages L., de Llorens R., de Bolos C., Vidal-Vanaclocha F., Peracaula R. alpha2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS ONE. 2010;5:e12524. doi: 10.1371/journal.pone.0012524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gretschel S., Haensch W., Schlag P.M., Kemmner W. Clinical relevance of sialyltransferases ST6GAL-I and ST3GAL-III in gastric cancer. Oncology. 2003;65:139–145. doi: 10.1159/000072339. [DOI] [PubMed] [Google Scholar]
- 26.Jin X.L., Zheng S.S., Wang B.S., Chen H.L. Correlation of glycosyltransferases mRNA expression in extrahepatic bile duct carcinoma with clinical pathological characteristics. Hepatobiliary Pancreat. Dis. Int. 2004;3:292–295. [PubMed] [Google Scholar]
- 27.Wang P.H., Li Y.F., Juang C.M., Lee Y.R., Chao H.T., Ng H.T., Tsai Y.C., Yuan C.C. Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol. Oncol. 2002;86:45–52. doi: 10.1006/gyno.2002.6714. [DOI] [PubMed] [Google Scholar]
- 28.Gomes C., Osorio H., Pinto M.T., Campos D., Oliveira M.J., Reis C.A. Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE. 2013;8:e66737. doi: 10.1371/journal.pone.0066737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito S., Yamashita S., Endoh M., Yamato T., Hoshi S., Ohyama C., Watanabe R., Ito A., Satoh M., Wada T., et al. Clinical significance of ST3Gal IV expression in human renal cell carcinoma. Oncol. Rep. 2002;9:1251–1255. [PubMed] [Google Scholar]
- 30.Mondal S., Chandra S., Mandal C. Elevated mRNA level of hST6Gal I and hST3Gal V positively correlates with the high risk of pediatric acute leukemia. Leuk. Res. 2010;34:463–470. doi: 10.1016/j.leukres.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Recchi M.A., Hebbar M., Hornez L., Harduin-Lepers A., Peyrat J.P., Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58:4066–4070. [PubMed] [Google Scholar]
- 32.Dall’Olio F., Malagolini N., di Stefano G., Minni F., Marrano D., Serafini-Cessi F. Increased CMP-NeuAc:Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity in human colorectal cancer tissues. Int. J. Cancer. 1989;44:434–439. doi: 10.1002/ijc.2910440309. [DOI] [PubMed] [Google Scholar]
- 33.Skacel P.O., Edwards A.J., Harrison C.T., Watkins W.M. Enzymic control of the expression of the X determinant (CD15) in human myeloid cells during maturation: The regulatory role of 6-sialytransferase. Blood. 1991;78:1452–1460. [PubMed] [Google Scholar]
- 34.Fukushima K., Hara-Kuge S., Seko A., Ikehara Y., Yamashita K. Elevation of alpha2-->6 sialyltransferase and alpha1-->2 fucosyltransferase activities in human choriocarcinoma. Cancer Res. 1998;58:4301–4306. [PubMed] [Google Scholar]
- 35.Wang P.H., Li Y.F., Juang C.M., Lee Y.R., Chao H.T., Tsai Y.C., Yuan C.C. Altered mRNA expression of sialyltransferase in squamous cell carcinomas of the cervix. Gynecol. Oncol. 2001;83:121–127. doi: 10.1006/gyno.2001.6358. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko Y., Yamamoto H., Kersey D.S., Colley K.J., Leestma J.E., Moskal J.R. The expression of Gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2,6-linked sialoglycoconjugates in human brain tumors. Acta Neuropathol. 1996;91:284–292. doi: 10.1007/s004010050427. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Li Y., Ma H., Dong W., Zhou H., Song X., Zhang J., Jia L. Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteomics. 2014;13:520–536. doi: 10.1074/mcp.M113.034025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Dall’Olio F., Trere D. Expression of alpha 2,6-sialylated sugar chains in normal and neoplastic colon tissues. Detection by digoxigenin-conjugated Sambucus nigra agglutinin. Eur. J. Histochem. 1993;37:257–265. [PubMed] [Google Scholar]
- 39.Sata T., Roth J., Zuber C., Stamm B., Heitz P.U. Expression of alpha 2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. A lectin-gold cytochemical study with Sambucus nigra and Maackia amurensis lectins. Am. J. Pathol. 1991;139:1435–1448. [PMC free article] [PubMed] [Google Scholar]
- 40.Dall’Olio F., Chiricolo M., D’Errico A., Gruppioni E., Altimari A., Fiorentino M., Grigioni W.F. Expression of beta-galactoside alpha2,6 sialyltransferase and of alpha2,6-sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology. 2004;14:39–49. doi: 10.1093/glycob/cwh002. [DOI] [PubMed] [Google Scholar]
- 41.Julien S., Adriaenssens E., Ottenberg K., Furlan A., Courtand G., Vercoutter-Edouart A.S., Hanisch F.G., Delannoy P., Le Bourhis X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology. 2006;16:54–64. doi: 10.1093/glycob/cwj033. [DOI] [PubMed] [Google Scholar]
- 42.Marcos N.T., Pinho S., Grandela C., Cruz A., Samyn-Petit B., Harduin-Lepers A., Almeida R., Silva F., Morais V., Costa J., et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 43.Vázquez-Martín C., Cuevas E., Gil-Martín E., Fernández-Briera A. Correlation Analysis between Tumorous Associated Antigen Sialyl-Tn Expression and ST6GalNAc I Activity in Human Colon Adenocarcinoma. Oncology. 2004;67:159–165. doi: 10.1159/000081003. [DOI] [PubMed] [Google Scholar]
- 44.Pinho S., Marcos N.T., Ferreira B., Carvalho A.S., Oliveira M.J., Santos-Silva F., Harduin-Lepers A., Reis C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249:157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Schneider F., Kemmner W., Haensch W., Franke G., Gretschel S., Karsten U., Schlag P.M. Overexpression of sialyltransferase CMP-sialic acid: Galbeta1,3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001;61:4605–4611. [PubMed] [Google Scholar]
- 46.Bos P.D., Zhang X.H.F., Nadal C., Shu W.P., Gomis R.R., Nguyen D.X., Minn A.J., van de Vijver M.J., Gerald W.L., Foekens J.A., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchida A., Okajima T., Furukawa K., Ando T., Ishida H., Yoshida A., Nakamura Y., Kannagi R., Kiso M., Furukawa K. Synthesis of disialyl Lewis a (Le(a)) structure in colon cancer cell lines by a sialyltransferase, ST6GalNAc VI, responsible for the synthesis of alpha-series gangliosides. J. Biol. Chem. 2003;278:22787–22794. doi: 10.1074/jbc.M211034200. [DOI] [PubMed] [Google Scholar]
- 48.Miyazaki K., Ohmori K., Izawa M., Koike T., Kumamoto K., Furukawa K., Ando T., Kiso M., Yamaji T., Hashimoto Y., et al. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 49.Steenackers A., Vanbeselaere J., Cazet A., Bobowski M., Rombouts Y., Colomb F., Le Bourhis X., Guerardel Y., Delannoy P. Accumulation of unusual gangliosides G(Q3) and G(P3) in breast cancer cells expressing the G(D3) synthase. Molecules. 2012;17:9559–9572. doi: 10.3390/molecules17089559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S.J., Chung T.W., Jin U.H., Suh S.J., Lee Y.C., Kim C.H. Molecular mechanisms involved in transcriptional activation of the human Sia-alpha2,3-Gal-beta1,4-GlcNAc-R: Alpha2,8-sialyltransferase (hST8Sia III) gene induced by KCl in human glioblastoma cells. Biochem. Biophys. Res. Commun. 2006;344:1057–1064. doi: 10.1016/j.bbrc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein J., Lee E.U., McEntee K., Lai P.H., Paulson J.C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J. Biol. Chem. 1987;262:17735–17743. [PubMed] [Google Scholar]
- 52.Wen D.X., Svensson E.C., Paulson J.C. Tissue-specific alternative splicing of the beta-galactoside alpha 2,6-sialyltransferase gene. J. Biol. Chem. 1992;267:2512–2518. [PubMed] [Google Scholar]
- 53.Wang X., Vertino A., Eddy R.L., Byers M.G., Jani-Sait S.N., Shows T.B., Lau J.T. Chromosome mapping and organization of the human beta-galactoside alpha 2,6-sialyltransferase gene. Differential and cell-type specific usage of upstream exon sequences in B-lymphoblastoid cells. J. Biol. Chem. 1993;268:4355–4361. [PubMed] [Google Scholar]
- 54.Dall’Olio F., Chiricolo M., Lau J.T.Y. Differential expression of the hepatic transcript of β-galactoside α2,6-sialyltransferase in human colon cancer cell lines. Int. J. Cancer. 1999;81:243–247. doi: 10.1002/(SICI)1097-0215(19990412)81:2<243::AID-IJC13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 55.Grundmann U., Nerlich C., Rein T., Zettlmeissl G. Complete cDNA sequence encoding human beta-galactoside alpha-2,6-sialyltransferase. Nucleic Acids Res. 1990;18:667. doi: 10.1093/nar/18.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keppler O.T., Moldenhauer G., Oppenlander M., Schwartz-Albiez R., Berger E.G., Funderud S., Pawlita M. Human Golgi beta-galactoside alpha-2,6-sialyltransferase generates a group of sialylated B lymphocyte differentiation antigens. Eur. J. Immunol. 1992;22:2777–2781. doi: 10.1002/eji.1830221104. [DOI] [PubMed] [Google Scholar]
- 57.Aas-Eng D.A., Asheim H.C., Deggerdal A., Smeland E., Funderud S. Characterization of a promoter region supporting transcription of a novel human beta-galactoside alpha-2,6-sialyltransferase transcript in HepG2 cells. Biochim. Biophys. Acta. 1995;1261:166–169. doi: 10.1016/0167-4781(94)00250-7. [DOI] [PubMed] [Google Scholar]
- 58.Dall’Olio F., Chiricolo M., Ceccarelli C., Minni F., Marrano D., Santini D. Beta-galactoside alpha2,6 sialyltransferase in human colon cancer: Contribution of multiple transcripts to regulation of enzyme activity and reactivity with Sambucus nigra agglutinin. Int. J. Cancer. 2000;88:58–65. doi: 10.1002/1097-0215(20001001)88:1<58::AID-IJC9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 59.Shibuya N., Goldstein I.J., Broekaert W.F., Nsimba-Lubaki M., Peeters B., Peumans W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J. Biol. Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 60.Yamashita K., Umetsu K., Suzuki T., Ohkura T. Purification and characterization of a Neu5Ac alpha 2-->6Gal beta 1-->4GlcNAc and HSO3(-)-->6Gal beta 1-->GlcNAc specific lectin in tuberous roots of Trichosanthes japonica. Biochemistry. 1992;31:11647–11650. doi: 10.1021/bi00161a052. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita K., Fukushima K., Sakiyama T., Murata F., Kuroki M., Matsuoka Y. Expression of Sia alpha 2-->6Gal beta 1-->4GlcNAc residues on sugar chains of glycoproteins including carcinoembryonic antigens in human colon adenocarcinoma: Applications of Trichosanthes japonica agglutinin I for early diagnosis. Cancer Res. 1995;55:1675–1679. [PubMed] [Google Scholar]
- 62.Korekane H., Matsumoto A., Ota F., Hasegawa T., Misonou Y., Shida K., Miyamoto Y., Taniguchi N. Involvement of ST6Gal I in the biosynthesis of a unique human colon cancer biomarker candidate, alpha2,6-sialylated blood group type 2H (ST2H) antigen. J. Biochem. 2010;148:359–370. doi: 10.1093/jb/mvq077. [DOI] [PubMed] [Google Scholar]
- 63.Vierbuchen M.J., Fruechtnicht W., Brackrock S., Krause K.T., Zienkiewicz T.J. Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features. Cancer. 1995;76:727–735. doi: 10.1002/1097-0142(19950901)76:5<727::AID-CNCR2820760504>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 64.Hedlund M., Ng E., Varki A., Varki N.M. alpha 2–6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. 2008;68:388–394. doi: 10.1158/0008-5472.CAN-07-1340. [DOI] [PubMed] [Google Scholar]
- 65.Chiricolo M., Malagolini N., Bonfiglioli S., Dall’Olio F. Phenotypic changes induced by expression of beta-galactoside alpha2,6 sialyltransferase I in the human colon cancer cell line SW948. Glycobiology. 2006;16:146–154. doi: 10.1093/glycob/cwj045. [DOI] [PubMed] [Google Scholar]
- 66.Park J.J., Yi J.Y., Jin Y.B., Lee Y.J., Lee J.S., Lee Y.S., Ko Y.G., Lee M. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem. Pharmacol. 2012;83:849–857. doi: 10.1016/j.bcp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto H., Oviedo A., Sweeley C., Saito T., Moskal J.R. Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Res. 2001;61:6822–6829. [PubMed] [Google Scholar]
- 68.Zhang Z., Sun J., Hao L., Liu C., Ma H., Jia L. Modification of glycosylation mediates the invasive properties of murine hepatocarcinoma cell lines to lymph nodes. PLoS ONE. 2013;8:e65218. doi: 10.1371/journal.pone.0065218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Lin S., Kemmner W., Grigull S., Schlag P.M. Cell surface alpha 2,6 sialylation affects adhesion of breast carcinoma cells. Exp. Cell Res. 2002;276:101–110. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y., Srivatana U., Ullah A., Gagneja H., Berenson C.S., Lance P. Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochim. Biophys. Acta. 2001;1536:148–160. doi: 10.1016/S0925-4439(01)00044-8. [DOI] [PubMed] [Google Scholar]
- 71.Christie D.R., Shaikh F.M., Lucas J.A., 4th, Lucas J.A., 3rd, Bellis S.L. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J. Ovarian Res. 2008;1 doi: 10.1186/1757-2215-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bresalier R.S., Rockwell R.W., Dahiya R., Duh Q.Y., Kim Y.S. Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res. 1990;50:1299–1307. [PubMed] [Google Scholar]
- 73.Isaji T., Im S., Gu W., Wang Y., Hang Q., Lu J., Fukuda T., Hashii N., Takakura D., Kawasaki N., et al. An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 2014;289:20694–20705. doi: 10.1074/jbc.M113.542688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seales E.C., Jurado G.A., Brunson B.A., Wakefield J.K., Frost A.R., Bellis S.L. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 75.Shaikh F.M., Seales E.C., Clem W.C., Hennessy K.M., Zhuo Y., Bellis S.L. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp. Cell Res. 2008;314:2941–2950. doi: 10.1016/j.yexcr.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danguy A., Camby I., Kiss R. Galectins and cancer. Biochim. Biophys. Acta. 2002;1572:285–293. doi: 10.1016/S0304-4165(02)00315-X. [DOI] [PubMed] [Google Scholar]
- 77.Nakahara S., Raz A. Biological modulation by lectins and their ligands in tumor progression and metastasis. Anticancer Agents Med. Chem. 2008;8:22–36. doi: 10.2174/187152008783330833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu F.T., Rabinovich G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 79.He J.L., Baum L.G. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006;417:247–256. doi: 10.1016/S0076-6879(06)17017-2. [DOI] [PubMed] [Google Scholar]
- 80.Ochieng J., Furtak V., Lukyanov P. Extracellular functions of galectin-3. Glycoconj. J. 2002;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 81.Elola M.T., Wolfenstein-Todel C., Troncoso M.F., Vasta G.R., Rabinovich G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell. Mol. Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuo Y., Bellis S.L. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 2011;286:5935–5941. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W.E., et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002;1572:232–254. doi: 10.1016/S0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 84.Zhuo Y., Chammas R., Bellis S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swindall A.F., Bellis S.L. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 2011;286:22982–22990. doi: 10.1074/jbc.M110.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Z., Swindall A.F., Kesterson R.A., Schoeb T.R., Bullard D.C., Bellis S.L. ST6Gal-I regulates macrophage apoptosis via alpha2–6 sialylation of the TNFR1 death receptor. J. Biol. Chem. 2011;286:39654–39662. doi: 10.1074/jbc.M111.276063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moller P., Koretz K., Leithauser F., Bruderlein S., Henne C., Quentmeier A., Krammer P.H. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int. J. Cancer. 1994;57:371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 88.Lebel M., Bertrand R., Mes-Masson A.M. Decreased Fas antigen receptor expression in testicular tumor cell lines derived from polyomavirus large T-antigen transgenic mice. Oncogene. 1996;12:1127–1135. [PubMed] [Google Scholar]
- 89.Strand S., Hofmann W.J., Hug H., Muller M., Otto G., Strand D., Mariani S.M., Stremmel W., Krammer P.H., Galle P.R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—A mechanism of immune evasion? Nat. Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 90.Landowski T.H., GleasonGuzman M.C., Dalton W.S. Selection for drug resistance results in resistance to Fas-mediated apoptosis. Blood. 1997;89:1854–1861. [PubMed] [Google Scholar]
- 91.Natoli G., Ianni A., Costanzo A., Depetrillo G., Ilari I., Chirillo P., Balsano C., Levrero M. Resistance to Fas-Mediated Apoptosis in Human Hepatoma-Cells. Oncogene. 1995;11:1157–1164. [PubMed] [Google Scholar]
- 92.O’Connell J., Bennett M.W., O’Sullivan G.C., Roche D., Kelly J., Collins J.K., Shanahan F. Fas ligand expression in primary colon adenocarcinomas: Evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J. Pathol. 1998;186:240–246. doi: 10.1002/(SICI)1096-9896(199811)186:3<240::AID-PATH173>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 93.Keppler O.T., Peter M.E., Hinderlich S., Moldenhauer G., Stehling P., Schmitz I., Schwartz-Albiez R., Reutter W., Pawlita M. Differential sialylation of cell surface glycoconjugates in a human B lymphoma cell line regulates susceptibility for CD95 (APO-1/Fas)-mediated apoptosis and for infection by a lymphotropic virus. Glycobiology. 1999;9:557–569. doi: 10.1093/glycob/9.6.557. [DOI] [PubMed] [Google Scholar]
- 94.Peter M.E., Hellbardt S., Schwartz-Albiez R., Westendorp M.O., Walczak H., Moldenhauer G., Grell M., Krammer P.H. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated apoptotic cell death. Cell Death Differ. 1995;2:163–171. [PubMed] [Google Scholar]
- 95.Suzuki O., Nozawa Y., Abe M. Sialic acids linked to glycoconjugates of Fas regulate the caspase-9-dependent and mitochondria-mediated pathway of Fas-induced apoptosis in Jurkat T cell lymphoma. Int. J. Oncol. 2003;23:769–774. [PubMed] [Google Scholar]
- 96.Galea G.L., Price J.S., Lanyon L.E. Emerging role of estrogen receptor-alpha in bone formation and bone sparing. Bonekey Rep. 2013;2 doi: 10.1038/bonekey.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma H., Zhou H., Song X., Shi S., Zhang J., Jia L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene. 2015;34:726–740. doi: 10.1038/onc.2014.7. [DOI] [PubMed] [Google Scholar]
- 98.Swindall A.F., Londono-Joshi A.I., Schultz M.J., Fineberg N., Buchsbaum D.J., Bellis S.L. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013;73:2368–2378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu J., Isaji T., Im S., Fukuda T., Hashii N., Takakura D., Kawasaki N., Gu J. beta-Galactoside alpha2,6-sialyltranferase 1 promotes transforming growth factor-beta-mediated epithelial-mesenchymal transition. J. Biol. Chem. 2014;289:34627–34641. doi: 10.1074/jbc.M114.593392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dawson G., Moskal J.R., Dawson S.A. Transfection of 2,6 and 2,3-sialyltransferase genes and GlcNAc-transferase genes into human glioma cell line U-373 MG affects glycoconjugate expression and enhances cell death. J. Neurochem. 2004;89:1436–1444. doi: 10.1111/j.1471-4159.2004.02435.x. [DOI] [PubMed] [Google Scholar]
- 101.Antony P., Rose M., Heidenreich A., Knuchel R., Gaisa N.T., Dahl E. Epigenetic inactivation of ST6GAL1 in human bladder cancer. BMC Cancer. 2014;14:901. doi: 10.1186/1471-2407-14-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Croci D.O., Cerliani J.P., Dalotto-Moreno T., Mendez-Huergo S.P., Mascanfroni I.D., Dergan-Dylon S., Toscano M.A., Caramelo J.J., Garcia-Vallejo J.J., Ouyang J., et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 103.Kimura N., Ohmori K., Miyazaki K., Izawa M., Matsuzaki Y., Yasuda Y., Takematsu H., Kozutsumi Y., Moriyama A., Kannagi R. Human B-lymphocytes express alpha2–6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. J. Biol. Chem. 2007;282:32200–32207. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- 104.Woodard-Grice A.V., McBrayer A.C., Wakefield J.K., Zhuo Y., Bellis S.L. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J. Biol. Chem. 2008;283:26364–26373. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amano M., Galvan M., He J., Baum L.G. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J. Biol. Chem. 2003;278:7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y.C., Yen H.Y., Chen C.Y., Chen C.H., Cheng P.F., Juan Y.H., Chen C.H., Khoo K.H., Yu C.J., Yang P.C., et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc. Natl. Acad. Sci. USA. 2011;108:11332–11337. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kitazume S., Imamaki R., Ogawa K., Komi Y., Futakawa S., Kojima S., Hashimoto Y., Marth J.D., Paulson J.C., Taniguchi N. Alpha2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J. Biol. Chem. 2010;285:6515–6521. doi: 10.1074/jbc.M109.073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cha S.K., Ortega B., Kurosu H., Rosenblatt K.P., Kuro O.M., Huang C.L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Delannoy P., Pelczar H., Vandamme V., Verbert A. Sialyltransferase activity in FR3T3 cells transformed withras oncogene: Decreased CMP-Neu5Ac:Galβ1–3GalNAc α-2,3-sialyltransferase. Glycoconj. J. 1993;10:91–98. doi: 10.1007/BF00731192. [DOI] [PubMed] [Google Scholar]
- 110.Easton E.W., Bolscher J.G., van den Eijnden D.H. Enzymatic amplification involving glycosyltransferases forms the basis for the increased size of asparagine-linked glycans at the surface of NIH 3T3 cells expressing the N-ras proto-oncogene. J. Biol. Chem. 1991;266:21674–21680. [PubMed] [Google Scholar]
- 111.Le Marer N., Laudet V., Svensson E.C., Cazlaris H., van Hille B., Lagrou C., Stehelin D., Montreuil J., Verbert A., Delannoy P. The c-Ha-ras oncogene induces increased expression of beta-galactoside alpha-2, 6-sialyltransferase in rat fibroblast (FR3T3) cells. Glycobiology. 1992;2:49–56. doi: 10.1093/glycob/2.1.49. [DOI] [PubMed] [Google Scholar]
- 112.Dalziel M., Dall’Olio F., Mungul A., Piller V., Piller F. Ras oncogene induces β-galactoside α2,6-sialyltransferase (ST6Gal I) via a RalGEF-mediated signal to its housekeeping promoter. Eur. J. Biochem. 2004;271:3623–3634. doi: 10.1111/j.1432-1033.2004.04284.x. [DOI] [PubMed] [Google Scholar]
- 113.Sakakura C., Hasegawa K., Miyagawa K., Nakashima S., Yoshikawa T., Kin S., Nakase Y., Yazumi S., Yamagishi H., Okanoue T., et al. Possible involvement of RUNX3 silencing in the peritoneal metastases of gastric cancers. Clin. Cancer Res. 2005;11:6479–6488. doi: 10.1158/1078-0432.CCR-05-0729. [DOI] [PubMed] [Google Scholar]
- 114.Yu S., Fan J., Liu L., Zhang L., Wang S., Zhang J. Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett. 2013;587:782–787. doi: 10.1016/j.febslet.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., O’Hanlon T.P., Young R.F., Lau J.T. Rat beta-galactoside alpha 2,6-sialyltransferase genomic organization: Alternate promoters direct the synthesis of liver and kidney transcripts. Glycobiology. 1990;1:25–31. doi: 10.1093/glycob/1.1.25. [DOI] [PubMed] [Google Scholar]
- 116.Lopez-Morales D., Velazquez-Marquez N., Valenzuela O., Santos-Lopez G., Reyes-Leyva J., Vallejo-Ruiz V. Enhanced sialyltransferases transcription in cervical intraepithelial neoplasia. Investig. Clin. 2009;50:45–53. [PubMed] [Google Scholar]
- 117.Milflores-Flores L., Millan-Perez L., Santos-Lopez G., Reyes-Leyva J., Vallejo-Ruiz V. Characterization of P1 promoter activity of the beta-galactoside alpha2,6-sialyltransferase I gene (siat 1) in cervical and hepatic cancer cell lines. J. Biosci. 2012;37:259–267. doi: 10.1007/s12038-012-9194-6. [DOI] [PubMed] [Google Scholar]
- 118.Stanley P. Altered Glycolipids of Cho Cells Resistant to Wheat-Germ Agglutinin. Abstr. Pap. Am. Chem. Soc. 1979:70. [Google Scholar]
- 119.Stanley P. Membrane Mutants of Animal-Cells: Rapid Identification of Those with a Primary Defect in Glycosylation. Mol. Cell. Biol. 1985;5:923–929. doi: 10.1128/mcb.5.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyagi T., Sagawa J., Kuroki T., Matsuya Y., Tsuiki S. Tumor-promoting phorbol ester induces alterations of sialidase and sialyltransferase activities of JB6 cells. Jpn. J. Cancer Res. 1990;81:1286–1292. doi: 10.1111/j.1349-7006.1990.tb02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sawada M., Moriya S., Saito S., Shineha R., Satomi S., Yamori T., Tsuruo T., Kannagi R., Miyagi T. Reduced sialidase expression in highly metastatic variants of mouse colon adenocarcinoma 26 and retardation of their metastatic ability by sialidase overexpression. Int. J. Cancer. 2002;97:180–185. doi: 10.1002/ijc.1598. [DOI] [PubMed] [Google Scholar]
- 122.Tokuyama S., Moriya S., Taniguchi S., Yasui A., Miyazaki J., Orikasa S., Miyagi T. Suppression of pulmonary metastasis in murine B16 melanoma cells by transfection of a sialidase cDNA. Int. J. Cancer. 1997;73:410–415. doi: 10.1002/(SICI)1097-0215(19971104)73:3<410::AID-IJC16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 123.Miyagi T. Aberrant expression of sialidase and cancer progression. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008;84:407–418. doi: 10.2183/pjab.84.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uemura T., Shiozaki K., Yamaguchi K., Miyazaki S., Satomi S., Kato K., Sakuraba H., Miyagi T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–1229. doi: 10.1038/onc.2008.471. [DOI] [PubMed] [Google Scholar]
- 125.Meng L., Forouhar F., Gao Z., Ramiah A., Moniz H., Thieker D., Seetharaman J., Milaninia S., Su M., Veillon L., et al. Enzymatic basis for N-glycan sialylation: Structure of rat ST6GAL1 reveals conserved and unique features for glycan sialylation. Glycobiology. 2013;23:1373–1374. doi: 10.1074/jbc.M113.519041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rao F.V., Rich J.R., Rakic B., Buddai S., Schwartz M.F., Johnson K., Bowe C., Wakarchuk W.W., DeFrees S., Withers S.G., et al. Structural insight into mammalian sialyltransferases. Nat. Struct. Mol. Biol. 2009;16:1186–1188. doi: 10.1038/nsmb.1685. [DOI] [PubMed] [Google Scholar]
- 127.Joziasse D.H., Schiphorst W.E.C.M., Vandeneijnden D.H., Vankuik J.A., Vanhalbeek H., Vliegenthart J.F.G. Branch Specificity of Bovine Colostrum Cmp-Sialic Acid: N-Acetyllactosaminide Alpha-2,6-Sialyltransferase. Interaction with Biantennary Oligosaccharides and Glycopeptides of N-Glycosylproteins. J. Biol. Chem. 1985;260:714–719. [PubMed] [Google Scholar]
- 128.Joziasse D.H., Schiphorst W.E.C.M., Vandeneijnden D.H., Vankuik J.A., Vanhalbeek H., Vliegenthart J.F.G. Branch Specificity of Bovine Colostrum Cmp-Sialic Acid: Gal-Beta-1,4glcnac-R Alpha-2,6-Sialyltransferase. Sialylation of Biantennary, Triantennary, and Tetraantennary Oligosaccharides and Glycopeptides of the N-Acetyllactosamine Type. J. Biol. Chem. 1987;262:2025–2033. [PubMed] [Google Scholar]
- 129.Guo H.B., Nairn A., Harris K., Randolph M., Alvarez-Manilla G., Moremen K., Pierce M. Loss of expression of N-acetylglucosaminyltransferase Va results in altered gene expression of glycosyltransferases and galectins. FEBS Lett. 2008;582:527–535. doi: 10.1016/j.febslet.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang P.H., Lee W.L., Lee Y.R., Juang C.M., Chen Y.J., Chao H.T., Tsai Y.C., Yuan C.C. Enhanced expression of alpha 2,6-sialyltransferase ST6Gal I in cervical squamous cell carcinoma. Gynecol. Oncol. 2003;89:395–401. doi: 10.1016/S0090-8258(03)00127-6. [DOI] [PubMed] [Google Scholar]
- 131.Sugimoto I., Futakawa S., Oka R., Ogawa K., Marth J.D., Miyoshi E., Taniguchi N., Hashimoto Y., Kitazume S. Beta-galactoside alpha2,6-sialyltransferase I cleavage by BACE1 enhances the sialylation of soluble glycoproteins. A novel regulatory mechanism for alpha2,6-sialylation. J. Biol. Chem. 2007;282:34896–34903. doi: 10.1074/jbc.M704766200. [DOI] [PubMed] [Google Scholar]
- 132.Bast B.J.E.G., Zhou L.J., Freeman G.J., Colley K.J., Ernst T.J., Munro J.M., Tedder T.F. The Hb-6, Cdw75, and Cd76 Differentiation Antigens Are Unique Cell-Surface Carbohydrate Determinants Generated by the Beta-Galactoside Alpha-2,6-Sialyltransferase. J. Cell Biol. 1992;116:423–435. doi: 10.1083/jcb.116.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baumann H., Gauldie J. The Acute-Phase Response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]