Abstract
The N-acylhydrazone (NAH) moiety is considered a privileged structure, being present in many compounds with diverse pharmacological activities. Among the activities attributed to NAH derivatives anti-inflammatory and analgesic ones are recurrent. As part of a research program aiming at the design of new analgesic and anti-inflammatory lead-candidates, a series of cyclohexyl-N-acylhydrazones 10–26 were structurally designed from molecular modification on the prototype LASSBio-294, representing a new class of cycloalkyl analogues. Compounds 10–26 and their conformationally restricted analogue 9 were synthetized and evaluated as analgesic and anti-inflammatory agents in classical pharmacologic protocols. The cyclohexyl-N-acylhydrazones 10–26 and the cyclohexenyl analogue 9 showed great anti-inflammatory and/or analgesic activities, but compound 13 stood out as a new prototype to treat acute and chronic painful states due to its important analgesic activity in a neuropathic pain model.
Keywords: molecular simplification, N-acylhydrazone, anti-inflammatory, neuropathic pain, privileged structure, adenosine receptor
1. Introduction
Defined as a minimum common subunit present in the structure of several drug candidates, privileged structures are characterized by their ability to be recognized by plural and distinct receptors. This concept has been widely used in the literature for the design of kinase-protein and serine-protease inhibitors, and ligands of G-protein coupled receptors (GPCR) and ionic channels [1]. Recently, based on several works with bioactive N-acylhydrazone (NAH) derivatives [2,3,4,5,6,7], the privileged structure nature of this bioactive framework was proposed [5].
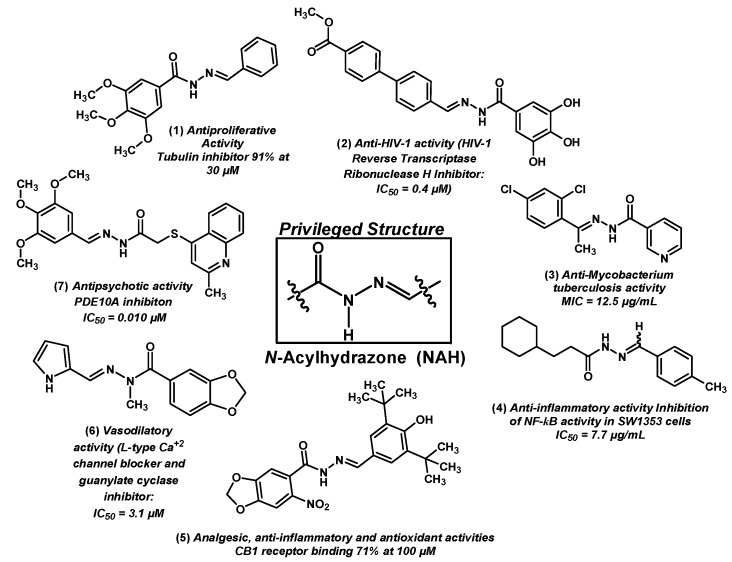
Compounds 1–7 containing the NAH subunit have been described to possess antitumor (compound 1) [6], antiviral (compound 2) [8], antibacterial (compound 3) [9], anti-inflammatory (compound 4) [4], analgesic (compound 5) [10,11], vasodilatory (compound 6) [12] or antipsychotic (compound 7) [13] activity (Figure 1).
Figure 1.
Examples of N-acylhydrazones with different mechanism of action and pharmacological activity.
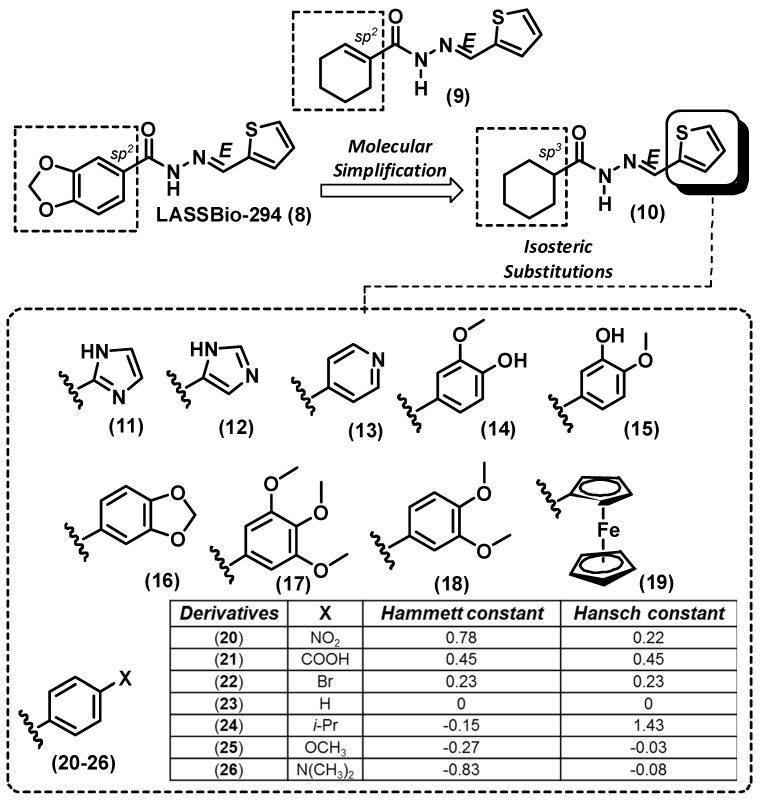
As part of a research program aiming at the design of new analgesic and anti-inflammatory lead-candidates, especially useful in the treatment of neuropathic pain, molecular changes in the prototype (E)-N'-((thiophen-2-yl)methylene)benzo[d][1,3]dioxole-5-carbohydrazide (LASSBio-294, 8) were performed in order to improve its analgesic and anti-inflammatory profile. LASSBio-294 has been described as a potent positive inotropic agent with modest vasodilatory [14], analgesic and anti-inflammatory activities [15]. Structural modification in the derivative 8 was planned applying the concept of molecular simplification [16,17], resulting in the replacement of the 1,3-benzodioxole system by a cyclohexane moiety (Figure 2).
Figure 2.
Design concept of novel cyclohexyl-N-acylhydrazones 10–26 from the prototype LASSBio-294 and its cyclohexenyl analogue 9.
Considering the changes in the hybridization of the carbon atom linked to the carbonyl function in the cyclohexyl-N-acylhydrazone derivatives 10–26, compared to the prototype LASSBio-294 (8), the cyclohexenyl analogue 9 was also designed. Aiming to construct a congeneric series, the 2-thienyl ring was replaced by several isosteric rings (compounds 10–19) and the resulting phenyl nucleus was subsequently substituted by different monovalent substituents [18] (compounds 20–26; Figure 2). The election of these substituents considered their electronic effects and their lipophilicity, in accordance with Hammett and Hansch parameters, as illustrated in Figure 2.
2. Results and Discussion
2.1. Chemistry
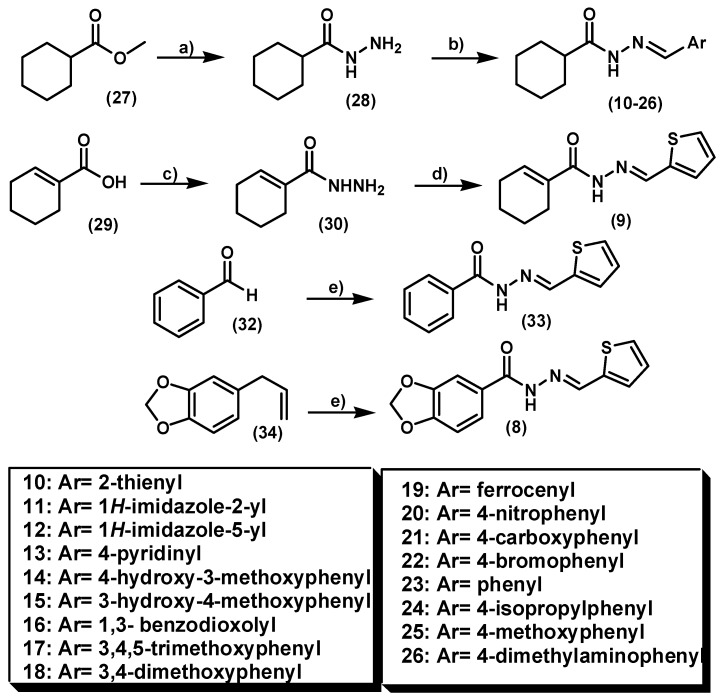
The cyclohexyl-N-acylhydrazones 10–26 and the cyclohexenyl analogue 9 were synthesized using classical methodology as depicted in Scheme 1. Compounds were obtained in good yields by exploring a linear synthesis in two and three steps, based on functional group interconversion and acid-catalyzed condensation of hydrazide intermediates 28 or 30 with aldehydes, previously selected in accordance with the design concept illustrated in Figure 1. LASSBio-294 (8) and LASSBio-322 (33) [19,20] were used in order to compare the biological results with the analogues 10–26 and 9; and to establish the contribution of 1,3-benzodioxole and phenyl subunits to the analgesic and anti-inflammatory activities.
Scheme 1.
Synthesis route to NAH compounds.
Reagents and conditions: (a) NH2NH2·H2O 64%, EtOH, reflux, 48 h, 79%; (b) aromatic aldehyde, EtOH, room temperature, 30–90 min, 46%–99%; (c) (1) SOCl2, DMF (cat.), room temperature, 4 h; (2) NH2NH2·H2O 64%, CH2Cl2, 0 °C, 2 h; (d) 2-thiophenecarboxaldehyde, EtOH, room temperature, 30 min, 51%; (e) as previously described [19,20].
Considering the possibility that N-acylhydrazones (-CONHN=CHR) may exist as syn/anti amide conformers and either as Z/E geometrical isomers (-HC=N-) [21], a careful analysis of the 1H-NMR spectra of the compounds was performed.
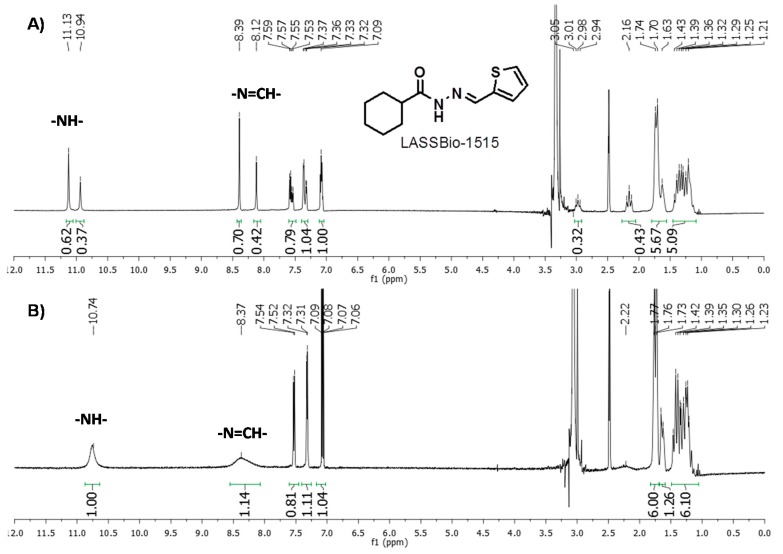
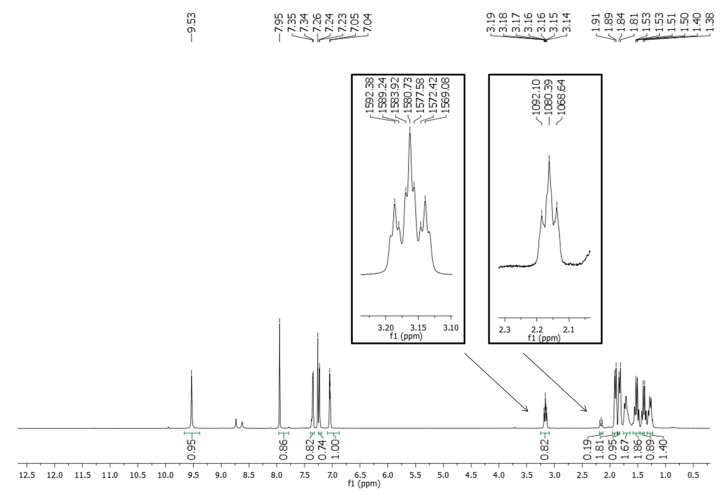
The 1H-NMR spectra of cyclohexyl-N-acylhydrazone derivative 10, recorded at room temperature using solvents with different polarity (i.e., DMSO-d6 and CDCl3), showed duplication of the singlet signals relative to amide and imine hydrogens (-CONHN=CH-) at 11 ppm in DMSO-d6. Curiously, the analysis of the same sample by reversed-phased HPLC, using different mobile phases, revealed only one peak at the chromatogram, indicating the presence of only one species. These data indicate that the mixture found in the 1H-NMR analysis does not correspond to possible geometric isomers (Z and E) of imine group (-N=CH-). In order to completely discard the possibility of diastereoisomers and to identify the possible presence of conformers of the amide bond, the 1H-NMR spectrum (300 MHz) of LASSBio-1515 (10) was recorded at 90 °C. This spectrum showed coalescence of the duplicated singlet signals seen in the 1H-NMR spectrum at room temperature (Figure 3). These results allowed us to discard the diastereoisomer possibility and reinforced the existence of conformers.
Figure 3.
1H-NMR (300 MHz) spectra of the NAH derivative LASSBio-1515 (10) in DMSOd6 at 25 °C (A) and 90 °C (B).
The possibility of chair conformers was also investigated by examining the JJ-coupling in the 500 MHz 1H-NMR of LASSBio-1515 (10). It was clear that both duplicated signals relative to the tertiary hydrogen had H-coupling constants of approximately 11 Hz (Figure 4). Data in the literature was found for axial-axial H-coupling [1Haxial-2Haxial or 1Haxial-6Haxial] of cyclohexyl derivatives, J1Ha/2Ha or J1-Ha/6Ha of around 8 to 14 Hz, while J1He/2Ha or J1-He/6Ha [1Hequatorial-2Haxial or 1Hequatorial-6Haxial] have J-couplings below 5 Hz [22]. Therefore, the result for LASSBio-1515 (10) indicated that the mixtures of conformers at 1H-NMR are amide rotamers and not chair conformers. This type of rotamers was previously reported for NAH compounds [21,23,24]. The same 1H-NMR spectra analysis performed with compounds 8, 9 and 33, revealed that the presence of rotamers seems to be dependent of the hybridization nature of carbon linked to the carbonyl-amide group. The rotamers were not seen for the cyclohexenyl derivative 9 or even for phenyl analogue 33 and LASSBio-294 (8).
Figure 4.
1H-NMR (500 MHz, CDCl3) spectrum of NAH derivative 10 with J-coupling of the tertiary hydrogen of the cyclohexyl functionalized system.
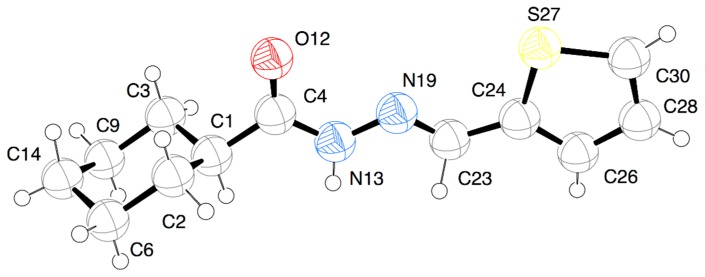
Aiming to determine unambiguously the relative configuration and conformation of the cyclohexyl-N-acylhydrazone derivative 10 X-ray powder diffraction was performed [25]. The results indicated that in the solid phase the compound is an E diastereoisomer (-N=CH-), having equatorial substitution of the cyclohexyl ring, with an antiperiplanar conformation of the hydrogen amide and oxygen atom of the carbonyl group (Figure 5).
Figure 5.
View of representative NAH derivative LASSBio-1515 (10) by X-ray powder diffraction Red = oxygen (O); blue = nitrogen (N); yellow = sulfur (S), black = carbon (C); and white = hydrogen (H).
2.2. Pharmacological Activities
The anti-inflammatory and analgesic activities of the cyclohexyl-N-acylhydrazones 10–26 and the cyclohexenyl analogue 9 were determined using classical murine models, exemplified by carrageenan-induced peritonitis, acetic acid-induced writhing and formalin-induced pain [26,27,28]. The results were compared with the standard drug indomethacin and with the prototype LASSBio-294 (8) and its simplified analogue LASSBio-382 (33). All compounds were tested by oral administration using a screening dose of 100 µmol/Kg.
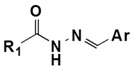
As illustrated in Table 1 the replacement of the 1,3-benzodiozole system (compound 8) by a cyclohexyl subunit (compound 10) resulted in the improvement of the anti-inflammatory and analgesic activities. The conformationally restricted cyclohexenyl analogue 9 was significantly less active than the cyclohexyl derivative 10 in the carrageenan-induced peritonitis model and showed similar antihipernociceptive profile in the acetic acid-induced writhing test (Table 1). The attempts to replace the 2-thienyl ring (in 10) by isosteric ones, like a 4-pyridinyl (compound 13), 1H-imidazole-2-yl (compound 11) and the 1H-imidazole-5-yl regioisomer (compound 12) resulted in loss of activity in these models for compounds 11 and 12, while derivative 13 showed a similar pharmacological profile as the lead 10 (Table 1) The isosteric replacement of the 2-thienyl unit in 10 by a phenyl group (compound 23) decreased the anti-inflammatory activity, although the analgesic profile in the writhing test was preserved (Table 1). The substitution of the phenyl ring of compound 23 by monovalent isosteric groups (e.g., 10–26) improves the anti-inflammatory and analgesic activities, with the regioisosmers 14 and 15 and the 4-dimethylamine (26) and 4-bromo (22) derivatives standing out. The ferrocenyl derivative 19, designed in accordance with literature reports showing its bioisosteric relationship with the phenyl group [29], showed an improvement in the analgesic profile (Table 1) in the acetic acid-induced writhing test and also in the 1st phase of the formalin model.
Table 1.
Effect of N-acylhydrazones (10 or 30 µM in binding assay and 100 µmol/kg p.o. in the in vivo assays), dipyrone or indomethacin (100 µmol/kg, p.o in vivo assays).
| Biological Tests | |||||||
|---|---|---|---|---|---|---|---|
 |
Binding A2a | Carrageenan-induced peritonitis | Acetic acid-induced writhing test | Formalin-induced pain test | |||
| Compounds | (R1) | (Ar) | % of inhibition a | % of inhibition b | % of inhibition b | Phase I % of inhibition b | Phase II % of inhibition b |
| Dipyrone or Indomethacin | - | 65.1 ± 4.0 ** dipyrone | 82.5 ± 6.2 ** indomethacin | 23.5 ± 7.9 indomethacin | 57.4 ± 5.8 ** indomethacin | ||
|
(8) LASSBio-294 |
1,3-benzodioxolyl | 2-thienyl | (Ki = 8.2µM) | 55.3 ± 4.8 ** | 68.2 ± 13.6 ** | 37.4 ± 4.9 | 42.0 ± 4.7 ** |
|
(33) LASSBio-382 |
phenyl | 2-thienyl | 17% at 10 µM | 40.3 ± 2.3 ** | 57.2 ± 8.5 ** | 12.3 ± 6.1 | 23.2 ± 4.1 |
|
(9) LASSBio-1780 |
cyclohexenyl | 2-thienyl | 7% at 10 µM | 21.4 ± 5.4 ** | 77.3 ± 3.2 ** | - | - |
|
(10) LASSBio-1515 |
cyclohexyl | 2-thienyl | 13% at 30 µM | 79.2 ± 2.7 ** | 87.9 ± 3.4 ** | 32.9 ± 11.5 | 61.5 ± 2.9 ** |
|
(11) LASSBio-1600 |
cyclohexyl | 1H-imidazole-5-yl | 36% at 10 µM | 39.8 ± 4.9 ** | 20.9 ± 5.7 * | 5.2 ± 3.2 | 15.4 ± 10.6 |
|
(12) LASSBio-1602 |
cyclohexyl | 1H-imidazole-2-yl | 0% at 10 µM | 37.6 ± 1.9 ** | 69.2 ± 7.9 ** | 14.2 ± 5.8 | 5.7 ± 2.4 |
|
(13) LASSBio-1514 |
cyclohexyl | 4-pyridinyl | 0% at 10 µM | 81.9 ± 2.2 ** | 65.7 ± 9.5 ** | 36.9 ± 8.5 | 43.0 ± 5.7 ** |
|
(14) LASSBio-1688 |
cyclohexyl | 3-hydroxy-4-methoxyphenyl | 15% at 30 µM | 74.3 ± 4.4 ** | 90.7 ± 2.5 ** | 36.9 ± 8.5 | 38.3 ± 5.8 ** |
|
(15) LASSBio-1689 |
cyclohexyl | 4-hydroxy-3-methoxyphenyl | - | 68.2 ± 4.9 ** | 55.6 ± 5.3 ** | 31.5 ± 8.3 | 63.1 ± 7.7 ** |
|
(16) LASSBio-1691 |
cyclohexyl | 1,3- benzodioxolyl | 33% at 30 µM | 64.7 ± 3.0 ** | 56.0 ± 11.9 ** | 31.3 ± 6.7 | 25.6 ± 5.9 |
|
(17) LASSBio-1513 |
cyclohexyl | 3,4,5-trimethoxyphenyl | 10% at 30 µM | 76.3 ± 3.8 ** | 78.3 ± 8.4 ** | 26.9 ± 5.8 | 38.5 ± 6.8 ** |
|
(18) LASSBio-1509 |
cyclohexyl | 3,4-dimethoxyphenyl | 9% at 10 µM | 63.7± 3.2 ** | 67.3 ± 8.5 ** | 13.1 ± 7.8 | 75.97 ± 4.4 ** |
|
(19) LASSBio-1517 |
cyclohexyl | ferrocenyl | 5% at 30 µM | 46.9 ± 6.7 ** | 97.6 ± 0.9 ** | 47.3 ± 4.7 ** | 27.1 ± 12.0 |
|
(20) LASSBio-1603 |
cyclohexyl | 4-nitrophenyl | 0% at 10 µM | 42.8 ± 1.9 ** | 47.6 ± 7.0 ** | 31.3 ± 8.6 | 25.9 ± 6.9 |
|
(21) LASSBio-1516 |
cyclohexyl | 4-carboxylic-phenyl | 0%, at 10 µM | 58.6 ± 5.3 ** | 87.4 ± 6.0 ** | 17.5 ± 5.9 | 54.7 ± 6.9 ** |
|
(22) LASSBio-1511 |
cyclohexyl | 4-bromophenyl | 3% at 10 µM | 70.7 ± 3.1 ** | 62.2 ± 2.1 ** | 53.4 ± 11.1 ** | 77.0 ± 3.9 ** |
|
(23) LASSBio-1601 |
cyclohexyl | phenyl | 8% at 10 µM | 28.7 ± 4.4 ** | 57.3 ± 9.5 ** | 20.3± 8.4 | NA |
|
(24) LASSBio-1508 |
cyclohexyl | 4-isopropylphenyl | 12% at 10 µM | 78.6 ± 3.3 ** | 64.9 ± 3.3 ** | 13.4 ± 8.2 | 38.5 ± 6.8 * |
|
(25) LASSBio-1510 |
cyclohexyl | 4-methoxyphenyl | 0% at 10 µM | 67.4 ± 4.9 ** | 86.3 ± 4.0 ** | 30.1 ± 6.0 | 70.1 ± 5.3 ** |
|
(26) LASSBio-1512 |
cyclohexyl | 4-dimethylaminophenyl | 0% at 10 µM | 63.9 ± 4.0 ** | 68.3 ± 13.4 ** | 38.9 ± 13.1 * | 54.4 ± 4.9 ** |
a The % inhibition of specific binding displaced by 10 or 30µM competing compound. b The asterisks denote the significance levels in comparison with control groups (* p < 0.05, ** p < 0.01 at ANOVA). NA: not active.
In the formalin test, a neurogenic phase associated with the direct effect on sensory C fibers (1st phase) is followed by an inflammatory response related to analgesic mediators (2nd phase) [30]. With this in mind, compounds 19, 22 and 26, which were active in the neurogenic phase of the formalin test, were selected to be studied in the hot plate test [31]. These compounds were inactive in this model (data not shown), discarding the hypothesis of a central antinociceptive activity and reinforcing the peripheral analgesic profile of these bioactive N-acylhydrazones.
Considering recent works reporting the ability of the prototype 8 (LASSBio-294) to be a ligand of the adenosine receptor, subtype A2A [32], the cyclohexyl-N-acylhydrazones 10–26 and the cyclohexenyl analogue 9 were investigated using a specific binding assay for the A2A receptor (Table 1). As shown in Table 1, LASSBio-294 presented a Ki value of 8.2 µM. However, its simplified analogues 9, 10–26 and 33 were unable to bind to the A2A adenosine receptor, suggesting the pharmacophoric characteristic of 1,3-benzodiozole scaffold.
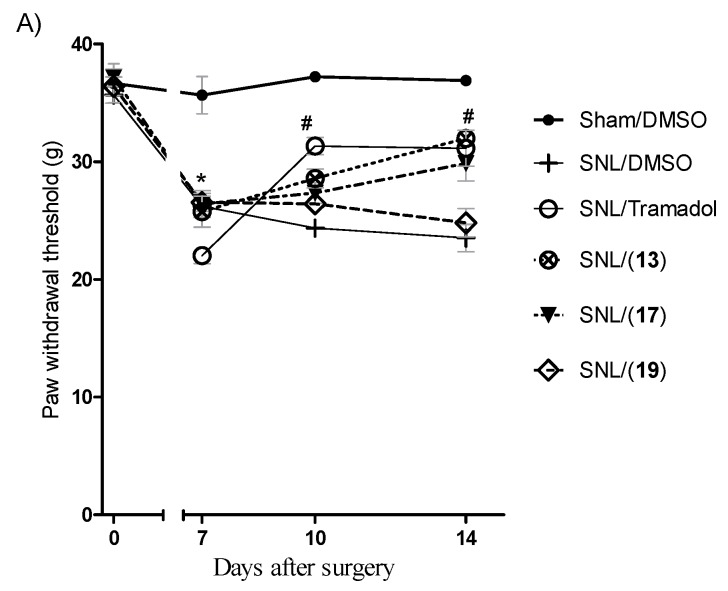
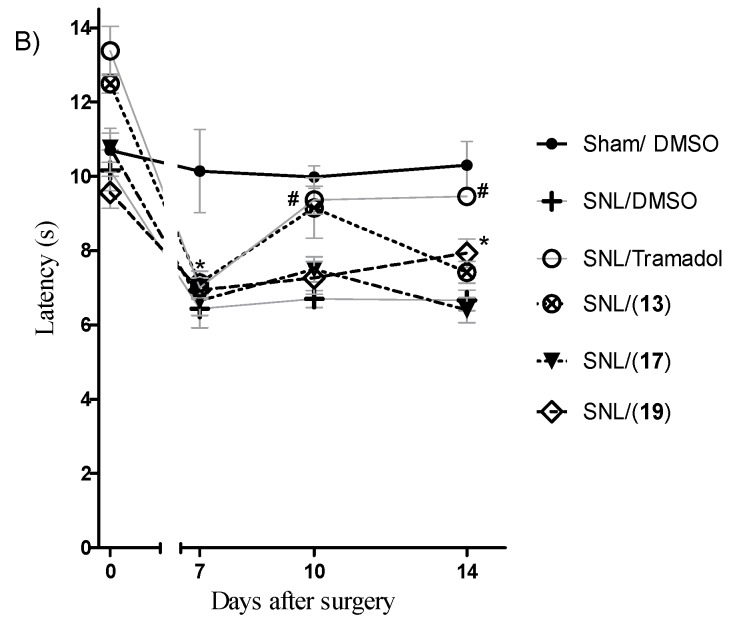
Bearing in mind that neuropathic pain represents a great challenge, and considering that its treatment needs more specific and effective drugs, the analgesic activity of the cyclohexyl-N-acylhydrazones in a murine neurophatic pain model was investigated. For this purpose, the spinal nerve ligation (SNL) model in rats [33] was selected, since involvement of inflammatory and analgesic components has been reported in this neuropathic pain model [34]. Compound 19 (LASSBio-1517), which presented a high analgesic activity in the acetic acid induced writhing test, 17 (LASSBio-1513) and 13 (LASSBio-1514), that showed comparable analgesic and anti-inflammatory activities (Table 1), were evaluated in the SNL model.
As demonstrated in Figure 6A, oral treatment for seven days with 100 µmol/kg of compounds 17 (LASSBio-1513) and 13 (LASSBio-1514) prevented the mechanical allodynia, while 19 (LASSBio-1517) was inactive. Derivative 13 (LASSBio-1514) was also able to increase the latency time in the thermal hyperalgesia component of the SNL model, revealing an important antihyperalgesic activity (Figure 6B). The better profile observed with acylhydrazones 13 and 17 on SNL model may be explained by their associative analgesic and anti-inflammatory activities (Table 1). Although the results presented in this study did not clarify the mechanism of action of these bioactive cyclohexyl-N-acylhydrazones, the results suggest that these compounds could be considered as new lead-candidates to treat acute and chronic painful states.
Figure 6.
Spinal nerve ligation (SNL) model in rats. (A) Treatment of mechanical allodynia for 7 days orally at a dose of 100 μmol·kg−1 (tramadol 33.3 μmol·kg−1), n = 6 animals. (B) Treatment of thermal hyperalgesia for 7 days orally at a dose of 100 μmol·kg−1 (tramadol 33.3 μmol·kg−1), n = 6 animals. * p < 0.05 versus day 0. # p < 0.05 versus day 7. One-way ANOVA, followed by the Newman–Keuls test. p values lower than 0.05 were considered significant.
3. Experimental Section
3.1. Chemistry
3.1.1. General Methods
NMR spectra were determined in deuterated chloroform or dimethyl sulfoxide containing ca. 1% tetramethylsilane as an internal standard, using a 200/50 MHz Bruker DPX-200, 250/62.5 MHz Bruker DPX-250, 400/100 MHz Varian 400-Mr, 300/75 MHz Varian Unity-300 and 500/125 MHz Varian VNMRSYS-500 spectrometer. The progress of all reactions was monitored by thin layer chromatography, which was performed on 2.0 cm × 6.0 cm aluminum sheets pre-coated with silica gel 60 (HF-254, Merck) to a thickness of 0.25 mm, or by infrared (IR) spectra obtained with Nicolet-550 Magna spectrophotometer by using potassium plates. The developed chromatograms were viewed under ultraviolet light at 254 nm. Merck silica gel (70–230 mesh) was used for column chromatography. Elemental analyses were carried out on a Thermo Scientific Flash EA 1112 Series CHN-Analyzer. Melting points were determined with a Quimis 340 apparatus and are uncorrected. All described products showed 1H and 13C NMR spectra according to the assigned structures. All organic solutions were dried over anhydrous sodium sulfate and all organic solvents were removed under reduced pressure in rotatory evaporator. HPLC for purity determinations were conducted using Shimadzu LC-20AD with a SHIM-PACK CLC-ODS analytical column (4.6 mm × 6250 mm) or Kromasil 100-5C18 (4.6 mm × 6250 mm) and a Shimadzu SPD-M20A detector at 254 nm wavelength. The solvent system for HPLC purity analyses was 70:30 acetonitrile:phosphate buffer solution at pH 7. The isocratic HPLC mode was used, and the flow rate was 1.0 mL/min.
3.1.2. General Procedure for the Preparation of the 3,4-Methylenedioxybenzoylacylhydrazone LASSBio-294 (8) and the Methylenedioxybenzoylacylhydrazone LASSBio-382 (33)
The methodology used in the synthesis of compounds 8 (LASSBio-294) and 33 (LASSBio-332) was previously reported by our research group [17,19,20] and was reproduced in this work.
3.1.3. Procedure for the Synthesis of Cyclohex-1-enecarbohydrazide Intermediate 30
A solution of cyclohex-1-enecarboxylic acid (29, 0.3 g, 2.38 mmol) and thionyl chloride (2 mL) in the presence of one drop of DMF was stirred at room temperature for 4 h until reaction completion. The solution was concentrated to dryness under reduced pressure to obtain a solid residue, which was solubilized in dichloromethane (10 mL) for the next step without further purification. To a reaction flask containing 80% hydrazine monohydrate (1.15 mL, 23.80 mmol) in dichloromethane (10 mL) was added dropwise the first solution at 0 °C for 2 h. At the end of the reaction, the solution was concentrated under reduced pressure. The solid 30 formed was filtered off and washed with n-hexane.
3.1.4. Procedure for the Synthesis of (E)-N'-(Thiophen-2-ylmethylene)cyclohex-1-enecarbohydrazide (LASSBio-1780, 9)
The cyclohex-1-enecarbohydrazide 30 previously obtained was immediately used without further purification or characterization. After solubilization in ethanol (21 mL) thiophene-2-carboxaldehyde (2.38 mmol) was added. The mixture was stirred at room temperature for 30 min, until TLC indicated the end of the reaction. The resulting suspension was poured onto ice and the resulting precipitate was filtered out and washed with n-hexane. Compound 9 (LASSBio-1780) was obtained as a yellow solid in 51% yield, m.p. 201–203 °C. 1H-NMR (400 MHz) DMSO-d6 (ppm): 11.17 (s, 1H, CONH), 8.55 (s, 1H, N=CH), 7.61 (d, 1H, J = 5 Hz), 7.38 (d, 1H, J = 5 Hz), 7.13 (dd, 1H, J = 5 Hz, J = 2 Hz), 6.57 (m, 1H), 2.21–1.17 (m, 4H, cyclohexenyl), 1.71–1.21 (m, 4H, cyclohexenyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 164.45 (C=O), 141.77 (1C), 139.36 (1C), 133.16 (1C), 132.62 (C1), 130.27 (1C), 128.46 (1C), 127.72 (1C), 23–25 (2C), 21–22 (2C). IR (KBr)νmax (cm−1): 3215 (NH); 2932 (CH3); 1660 (C=C); 1626 (C=O); 1596 (C=N). % purity = 96.93% by HPLC-C18 (acetonitrile/water 7:3) (Rt = 3.89 min).
3.1.5. Procedure for the Preparation of Cyclohexanecarbohydrazide (28)
Hydrazine hydrate (64%, 1.36 mL, 28.13 mmol) was added to a solution of methyl cyclohexanecarboxylate (27, 1.00 g, 7.03 mmol) in absolute ethanol (5 mL). The reaction mixture was kept under reflux for 48 h, whenupon infrared (IR) spectroscopy indicated the end of the reaction. Next, the ethanol was almost totally evaporated under reduced pressure. Ice was added to the residue and the resulting precipitate was filtered out affording the title compound in 79% yield, as white crystals, m.p. 154–156 °C. 1H-NMR (200 MHz, DMSO-d6, TMS) (ppm): 8.85 (s, 1H, CONH), 4.04 (s, 2H, NH2), 2.01 (m, 1H, cyclohexyl), 1.65 (m, 5H, cyclohexyl), 1.35 (m, 5H, cyclohexyl). 13C-NMR (50 MHz, DMSO-d6, TMS) (ppm): 174.92 (NC=O), 42.35 (1C, cyclohexyl), 29.19 (2C, cyclohexyl), 25.43 (1C, cyclohexyl), 25.33 (2C, cyclohexyl). IR (KBr) ν (cm−1): 3311, 3195 (NH); 2930 (CH); 1629 (C=O).
3.1.6. General Procedure for Preparation of Cyclohexylacylhydrazones 10–26
The corresponding aromatic or heteroaromatic aldehyde (2.11 mmol) was added to a solution of cyclohexanecarbohydrazide (28, 0.3 g; 2.11 mmol) in absolute ethanol (21 mL). The mixture was stirred for 2 h at room temperature. At the end of the reaction, the volume of ethanol was partially concentrated at reduced pressure and the resulting mixture was poured into cold water. The precipitate was filtered out, dried under vacuum and then the solid was washed with n-hexane and/or recrystallized from ethanol to give the desired cyclohexyl-N-acylhydrazones 10–26.
(E)-N'-(Thiophen-2-ylmethylene)cyclohexanecarbohydrazide (LASSBio-1515, 10): LASSBio-1515 (10) was obtained as orange crystals in 92% yield by condensation of cyclohexane-carbohydrazide (28) with thiophene-2-carboxylaldehyde, m.p. 209–211 °C. The data for this compound is in agreement with previous reports [35]. 1H-NMR (300 MHz) DMSO-d6 (ppm): 10.74 (s, 1H, CONH), 8.37 (s, 1H, N=CH), 7.55 (d, 1H, J = 5 Hz), 7.33 (d, 1H, J = 5 Hz), 7.09 (dd, 1H, J = 5 Hz, J = 1 Hz), 1,77 (m, 1H), 1.76 (m, 5H, cyclohexyl), 1.39 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.60 and 171.57 (NC=O), 141.18 (1C), 137.29 (HC=N), 130.30 (3C). IR (KBr) νmax (cm−1): 3185 (NH); 2926 (CH3); 1656 (C=O); 1590 (C=N). % purity = 96.55% by HPLC-C18 (acetonitrile/water 7:3) (Rt = 4.75 min).
(E)-N'-((1H-imidazol-2-yl)methylene)cyclohexanecarbohydrazide (LASSBio-1602, 11): LASSBio-1602 (11) was obtained as a white solid in 57% yield by condensation of cyclohexane-carbohydrazide (28) with 1H-imidazole-2-carbaldehyde, m.p. > 220 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 12.60 and 12.35 (s, NH, imidazole), 11.18 and 10.95 (s, 1H, CONH), 8.09 and 7.87 (s, 1H , HC=N), 7.21 (m, 2H), 2.86 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.75 and 172.54 (NC=O), 143.08 (HC=N), 134.46 (1C), 137.87 (2C), 43.26 (1C), 29.39 (2C), 25.75 (3C). IR (KBr) νmax (cm−1): 3188 (NH); 2925 (CH3); 1649 (C=O). Anal. Calcd. for C11H16N4O: C, 60.26; H, 6.90; N, 25.55. Found: C, 59.97; H, 6.95; N, 25.13.). % purity = 98.49% by HPLC-C18 (acetonitrile/water 6:4) (Rt = 2.98 min).
(E)-N'-((1H-Imidazol-5-yl)methylene)cyclohexanecarbohydrazide (LASSBio-1600, 12): LASSBio-1600 (12) was obtained as a white solid in 46% yield by condensation of cyclohexane-carbohydrazide (28) with 1H-imidazole-5-carbaldehyde, m.p. > 220 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 12.36 (NH, imidazole), 11.05 and 10.80 (s, 1H, CONH), 8.13 and 7.91 (s, 1H, HC=N), 7.69 (s, 1H), 7,42 (s, 1H), 3,13 and 2,19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl).13C-NMR (50 MHz) DMSO-d6 (ppm): 171.83 and 177.20 (NC=O), 137.51 (HC=N), 136.23 (1C), 132.14 (1C), 121.44 (1C), 43.34 (1C), 29.52 (2C), 25.82 (3C). IR (KBr) νmax (cm−1): 3188 (NH); 2925 (CH3); 1649 (C=O). Anal. Calcd. for C11H16N4O: C, 60.26; H, 6.90; N, 25.55. Found: C, 60.01; H, 7.23; N, 26.00.
(E)-N'-(Pyridin-4-ylmethylene)cyclohexanecarbohydrazide (LASSBio-1514, 13): LASSBio-1514 (13) was obtained in 79% yield as yellow crystals by condensation of cyclohexane-carbohydrazide (28) with 4-pyridinecarboxaldehyde, m.p. 151–153 °C. The compound’s data was in agreement with previous reports [35]. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.55 and 11.46 (s, 1H, CONH), 8.63 (m, 2H), 8.34 and 8.17(s, 1H, N=CH), 7.59 (m, 2H), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.28 and 172.08 (NC=O), 150.22 (2C), 143.46 and 139.82 (HC=N), 141.66 (1C), 120.84 (2C), 43.40 (1C), 28.89 (2C), 25.31 (3C). IR (KBr) νmax (cm−1): 3478 (NH); 2934 (CH3); 1664 (C=O); 1599 (C=N); 1397 (Py). Anal. Calcd. for C13H17N3O.H2O: C, 62.63; H, 7.68; N, 16.85. Found: C, 62.22; H, 7.61; N, 16,69.
(E)-N'-(4-Hydroxy-3-methoxybenzylidene)cyclohexanecarbohydrazide (LASSBio-1689, 14): LASSBio-1689 (14) was obtained in 90% yield as white crystals by condensation of cyclohexane-carbohydrazide (28) with 4-hydroxy-3-methoxybenzaldehyde, m.p. 198–200 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.43 and 11.10 (s, 1H, CONH), 9.47 (s, 1H, OH), 8.00 (s, 1H, N=CH), 7.24 (d, 1H, J = 8 Hz), 7.04 (d, 1H, J = 8 Hz), 6.82 (d, 1H, J = 8 Hz), 3.80 (s, 3H, CH3), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl).13C-NMR (50 MHz) DMSO-d6 (ppm): 176.66 and 171.44 (NC=O), 148.76 (1C), 148.02 (1C), 146.50 and 142.53 (N=CH), 125.90 (1C), 121.81 (1C), 115.63 (1C), 109.39 (1C), 55.58 (1C), 42.89 (1C), 29.09 (2C), 25.73 (3C). IR (KBr) νmax (cm−1): 3528–2850 (OH); 3528 (NH); 2930 (CH3); 1641 (C=O); 1281, 1204 (OCH3). % purity = 99.87% by HPLC-C18 (acetonitrile/water 7:3) (Rt = 3.48 min).
(E)-N'-(3-Hydroxy-4-methoxybenzylidene)cyclohexanecarbohydrazide (LASSBio-1688, 15): LASSBio-1688 (15) was obtained in 92% yield as white crystals by condensation of cyclohexane-carbohydrazide (28) with 3-hydroxy-4-methoxybenzaldehyde, m.p. 192–194 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.51 and 11.11 (s, 1H, CONH), 9.29 (s, 1H, OH), 8.00 (s, 1H, N=CH), 7.19 (d, 1H, J = 8 Hz), 6.99 (d, 2H, J = 8 Hz), 3.80 (s, 3H, CH3), 3.10 and 2.19(m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.71 and 171.48 (NC=O), 149.61 (1C), 146.83 (N=CH), 142.59 (1C), 127.33 (1C), 120.03 (1C), 112.30 (1C), 111.90 (1C), 55.62 (1C), 43.91 (1C), 29.08 (2C), 25.41 (3C). IR (KBr) νmax (cm−1): 3234–2850 (OH); 3234 (NH); 2930 (CH3); 1656 (C=O) 1259, 1204 (OCH3). % purity = 98.51% by HPLC-C18 (acetonitrile/water 6:4) (Rt = 3.97 min).
(E)-N'-(Benzo[d][1,3]dioxol-5-ylmethylene)cyclohexanecarbohydrazide (LASSBio-1691, 16): LASSBio-1691 (16) was obtained in 98% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with benzo[d][1,3]dioxole-5-carbaldehyde, m.p. 206–208 °C. The compound’s data was in agreement with previous reports [36]. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.18 and 10.98 (s, 1H, CONH), 8.08 and 7.84 (s, 1H, N=CH), 7.22 (s, 1H), 7.09 (d, 1H, J = 8 Hz), 6.94 (d, 1H, J = 8 Hz), 6.11 (s, 2H, CH2), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.31 and 172.07 (NC=O), 149.41 (1C), 148.47 (1C), 146.28 and 142.54 (N=CH), 129.42 (1C), 123.53 (1C), 108.94 (1C), 105.58 (1C), 102.01 (1C, O-CH2-O), 43.40 (1C), 29.56 (2C), 25,83 (3C). IR (KBr) νmax (cm−1): 3228 (NH); 2942 (CH3); 1659 (C=O) 1247 (OCH3). % purity = 95.33% by HPLC-C18 (acetonitrile/water 7:3) (Rt = 4.68 min).
(E)-N'-(3,4,5-Trimethoxybenzylidene)cyclohexanecarbohydrazide (LASSBio-1513, 17): LASSBio-1513 (17) was obtained in 99% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with 3,4,5-trimethoxybenzaldehyde, m.p. 215–217 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.27 and 11.11 (s, 1H, CONH), 8.65 and 8.10 (s, 1H, N=CH), 7.22 (s, 2H), 3.81 (s, 9H), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.42 and 172.21 (NC=O), 153.69 (2C), 146.49 and 142.24 (HC=N), 139.60 (1C), 130.60 (1C), 106.21 (2C), 60.71 (1C), 56.49 (2C), 43.40 (1C), 29.56 (2C), 25.73 (3C). IR (KBr) νmax (cm−1): 3230 (NH); 2925 (CH3); 1660 (C=O); 1236 (O-CH3). Anal. Calcd. for C17H24N2O4: C, 63.73; H, 7.55; N, 8.74. Found: C, 63.54; H, 7.42; N, 8.51.
(E)-N'-(3,4-Dimethoxybenzylidene)cyclohexanecarbohydrazide (LASSBio-1509, 18): LASSBio-1509 (18) was obtained in 94% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with 3,4-dimethoxybenzaldehyde, m.p. 193–195 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.15 and 10.98 (s, 1H, CONH), 8.09 and 7.89 (s, 1H, N=CH), 7.27 (d, 1H, J = 2 Hz), 7.14 (dd, 1H, J = 8 Hz, J = 2 Hz), 6.99 (d, 1H, J = 8 Hz), 3.82 (s, 6H, OCH3), 3.10 na 2.19 (m,1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.24 and 172.00 (NC=O), 161.08 and 152.10 (1C), 150.90 (1C), 146.47 and 142.82 (HC=N), 127.79 (1C), 122.04 (1C), 112.14 (1C), 108.83 (1C), 55.98 (2C), 43.40 (1C), 29.57 and 28.92 (2C), 25.97 (3C). IR (KBr) νmax (cm−1): 3223 (NH); 2926 (CH3); 1659 (C=O); 1603 (C=N); 1266, 1210(C-O). Anal. Calcd. for C16H22N2O3: C, 66.18; H, 7.64; N, 9.65. Found: C: 66.17; H: 7.48; N: 9.40.
(E)-N'-Ferrocenylcyclohexanecarbohydrazide (LASSBio-1517, 19): LASSBio-1517 (19) was obtained in 99% yield as a brown solid by condensation of cyclohexane-carbohydrazide (28) with ferrocenecarboxaldehyde, m.p. > 220 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.01 and 10.72 (s, 1H, CONH), 7.98 and 7.83 (s, 1H, N=CH), 4.57 (s, 2H), 4.42 (s, 2H), 4.19 (s, 5H), 2.86 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.09 (NC=O), 142.67 (HC=N), 79.42 (1C), 69.83 (2C), 68.79 (5C), 67.28 (2C), 43.40 (1C), 29.51 (2C), 25.89 (3C). IR (KBr) νmax (cm−1): 3189 (NH); 2929 (CH3); 1652 (C=O). Anal. Calcd. for C18H22FeN2O: C, 63.92; H, 6.56; N, 8.28. Found: C, 63.54; H, 6.37; N 8.28.
(E)-N'-(4-Nitrobenzylidene)cyclohexanecarbohydrazide (LASSBio-1603, 20): LASSBio-1603 (20) was obtained in 83% yield as white crystals by condensation of cyclohexane-carbohydrazide (28) with 4-nitrobenzaldehyde, m.p. 205–206 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.59 and 11.17 (s, 1H, CONH), 8.29 (d, 2H, J = 8 Hz), 8.07 (s, 1H, N=CH), 7.94 (d, 2H, J = 8 Hz), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.82 and 172.61 (NC=O), 148.33 (1C), 143.99 (N=CH), 141.03 (1C), 128.33 (2C), 124.63 (2C), 43.42 (1C), 29.47 (2C), 25.89 (3C). IR (KBr) νmax (cm−1): 3186 (NH); 2930 (CH3); 1668 (C=O) 1520, 1336 (NO2). Anal. Calcd. for C14H17N3O3: C, 61.08; H, 6.22; N, 15.28. Found: C, 60.81; H, 6.10; N. 14.97.
(E)-4-((2-(Cyclohexanecarbonyl)hydrazono)methyl)benzoic acid (LASSBio-1516, 21): LASSBio-1516 (21) was obtained in 83% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with 4-formylbenzoic acid, m.p. > 220 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.37 and 11.17 (s, 1H, CONH), 8.22 and 8.03 (s, 1H, N=CH), 7.97 (d, 2H, J = 8 Hz), 7.76 (d, 2H, J = 8 Hz), 3.39 (s, OH), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C- NMR (50 MHz) DMSO-d6 (ppm): 177.65 and 172.42 (NC=O), 167.45 (COOH), 145.24 and 141.81 (HC=N), 139.09 (1C), 132.03 (1C), 130.34 (2C), 127.43 (2C), 43.40 (1C), 29.51 (2C), 25.89 (3C). IR (KBr) νmax (cm−1): 3211–2855 (OH); 3211 (NH); 2925 (CH3); 1669 (C=O). Anal. Calcd. for C15H18N2O3: C, 65.68; H, 6.61; N, 10.21. Found: C, 65.46; H, 6.55; N, 9.88.
(E)-N'-(4-Bromobenzylidene)cyclohexanecarbohydrazide (LASSBio-1511, 22): LASSBio-1511 (22) was obtained in 98% yield as an orange solid by condensation of cyclohexane-carbohydrazide (28) with 4-bromobenzaldehyde, m.p. 215–216 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.36 and 11.14 (s, 1H, CONH), 8.15 and 7.94 (s, 1H, N=CH), 7.62 (m, 4H,), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.51 and 172.29 (NC=O), 145.19 and 141.69 (HC=N), 134.31 (1C), 132.29 (2C), 129.29 (2C), 123.52 (1C), 43.40 (1C), 29.51 (2C), 25.89 (2C), 18.92 (1C). IR (KBr) νmax (cm−1): 3188 (NH); 2925 (CH3); 1662 (C=O); 1601(C=N); 1138 (Ar-Br). Anal. Calcd. for C14H17BrN2O: C, 54.58; H, 5.54; N, 9.06. Found: C, 54.39; H, 5.50; N 8.92.
(E)-N'-Benzylidenecyclohexanecarbohydrazide (LASSBio-1601, 23): LASSBio-1601 (23) was obtained in 99% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with benzaldehyde, m.p. 165 °C. The compound’s data was in agreement with previous reports [36]. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.28 and 11.08 (s, 1H, CONH), 8.18 and 7.98 (s, 1H, HC=N), 7.66 (m, 2H), 7.43 (m, 3H), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.92 and 171.68 (NC=O), 145.90 and 142.28 (HC=N), 135.72 (1C), 129.79 (1C), 128.84 (2C), 126.89 (2C), 43.88 (1C), 29.01 (2C), 25.66 (3C). IR (KBr) νmax (cm−1): 3177 (NH); 2927 (CH3); 1663 (C=O); 1610 (C=N); 792 and 684 (Aryl). Anal. Calcd. for C14H18N2O: C, 63.92; H, 6.56; N, 8.28. Found: C, 63.44; H, 6.37; N, 8.28.
(E)-N'-(4-Isopropylbenzylidene)cyclohexanecarbohydrazide (LASSBio-1508, 24): LASSBio-1508 (24) was obtained in 98% yield as a yellow solid by condensation of cyclohexane-carbohydrazide (28) with 4-isopropylbenzaldehyde, m.p. 155–156 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.22 and 11.03 (s, 1H, CONH), 8.17 and 7.94 (s, 1H, N=CH), 7.56 (d, 2H, J = 8 Hz), 7.29 (d, 2H, J = 8 Hz), 3.10 and 2.19 (m, 1H), 2.90 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl), 1.20 (s, 6H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.35 and 172.10 (NC=O), 150.90 and 150.71 (1C), 146.47 and 142.82 (HC=N), 132.70 (1C), 127.52 and 127.33 (2C), 127.25 and 127.25 (2C), 43.42 (1C), 33.88 (1C), 29.57 and 28.92 (2C), 25.88 (3C), 24.20 (2C). IR (KBr) νmax (cm−1): 3231 (NH); 2930 (CH3); 1662 (C=O); 1601 (C=N). Anal. Calcd. for C17H24N2O:C, 74.96; H, 8.88; N, 10.30. Found: C, 74.82; H, 8.80; N, 10.14.
(E)-N'-(4-Methoxybenzylidene)cyclohexanecarbohydrazide (LASSBio-1510, 25): LASSBio-1510 (25) was obtained in 99% yield as a white solid by condensation of cyclohexane-carbohydrazide (28) with 4-methoxybenzaldehyde, m.p. 161–162 °C. The compound’s data was in agreement with previous reports [36]. 1H-NMR (200 MHz) DMSO-d6 (ppm): 11.15 and 10.96 (s, 1H, CONH), 8.11 and 7.92 (s, 1H, N=CH), 7.59 (d, 2H, J = 8 Hz), 7.02 (d, 2H, J = 8 Hz), 3.68 (s, 3H, CH3), 3.10 and 2.19 (m,1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.60 and 171.35 (NC=O), 160.56 (1C), 145.71 and 142.06 (HC=N), 129.90 (1C), 128.37 (2C), 114.27 (2C), 55.19 (1C), 42.80 (1C), 28.97 (2C), 25,27 (3C). IR (KBr) νmax (cm−1): 3184 (NH); 2929 (CH3); 1654 (C=O); 1608 (C=N), 1252, 1202 (C-O). Anal. Calcd. for C15H20N2O2: C, 69.20; H, 7.74; N, 10.76. Found: C, 69.23; H, 7.54; N, 10.46.
(E)-N'-(4-(Dimethylamino)benzylidene)cyclohexanecarbohydrazide (LASSBio-1512, 26): LASSBio-1512 (26) was obtained in 89% yield as an orange solid by condensation of cyclohexane-carbohydrazide (28) with 4-dimethylaminobenzaldehyde, m.p. 191 °C–192 °C. 1H-NMR (200 MHz) DMSO-d6 (ppm): 1H RMN (200 MHz) DMSO-d6 δ (ppm): 11.06 and 11.64 (s,1H, CONH), 7.95 and 7.82 (s, 1H, N=CH), 7.49 (d, 2H, J = 8 Hz), 6.69 (d, 2H, J = 8 Hz), 2.91(s, 6H, CH3), 3.10 and 2.19 (m, 1H), 1.72 (m, 5H, cyclohexyl), 1.32 (m, 5H, cyclohexyl).13C-NMR (50 MHz) DMSO-d6 (ppm): 176.46 and 171.24 (NC=O), 159.84 (1C), 146.81 and 143.16 (HC=N), 129.53 (2C), 121.78 (1C), 112.41 (2C), 43.40 (1C), 40.00 (2C), 29.51 (2C), 25.89 (3C). IR (KBr) νmax (cm−1): 3222 (NH); 2926 (CH3); 1658 (C=O); 1607 (C=N); 1362 (Ar-N(CH3)2). Anal. Calcd. for C16H23N3O: C, 70.30; H, 8.48; N, 15.37. Found: C, 70.44; H, 8.35; N, 15.70.
3.2. X-ray Crystallography
In this work, X-ray powder diffraction data and a simulated annealing algorithm implemented in the DASH software program [37] were used to determine the crystal structure of LASSBio-1515, on the basis of previous procedures [38,39]. Thus, a Rietveld refinement of the final crystal structure was conducted using the Topas Academic v. 5 software [40]. Data pertaining to the X-ray crystallographic determination of LASSBio-1515 have been deposited in the Cambridge Crystallographic Data Centre with CCDC ID: 929463. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3.3. Binding Assay
A binding assay was performed between NAH compounds (10 μM or 30 μM) and A2A receptors of rats [41]. [3H]CGS21680 (10 nM) was used as A2A agonist radioligand and 30 µM NECA was used to estimate the nonspecific binding. The data were expressed as percent inhibition of control specific binding using the equation: % inhibition =100 − [(measured specific binding/control specific binding) ×100].
3.4. Antinociceptive and Anti-Inflammatory Pharmacological Evaluation
3.4.1. Animals
Swiss mice weighing 20–30 g (from the BIOCEN-UFAL) were housed in group cages and maintained on a 12 h light/12 h dark cycle. Animals had free access to food and water at all times. Experiments were carried out according to a protocol approved by the Animal Welfare Committee of Federal University of Alagoas (UFAL) (Number: 026681/2009-23), and in according with the ethical guidelines for investigation of experimental pain in conscious animals.
3.4.2. Reagents
Acetic acid (Merck, Rio de Janeiro, Brasil), arabic gum (Sigma-Aldrich, Rio de Janeiro, RJ, Brasil), morphine sulphate (Dimorf-Cristalia-BR, São Paulo, Brasil), dipyrone (Sigma-Aldrich) and indomethacin (Sigma Aldrich) were obtained from commercial sources. A solution of formalin 2.5% was prepared with formaldehyde (Merck) in saline (NaCl 0.9%).
3.4.3. Acetic Acid-Induced Writhing Test
This test was performed as described [27]. Acetic acid (0.6%, v/v) was administered i.p. in a volume of 0.1 mL/10 g. The number of writhes, a response consisting of contraction of an abdominal wall, pelvic rotation followed by hind limb extension, was counted during continuous observation for 20 min beginning from 5 min after the acetic acid injection. Dipyrone and compounds (all 100 µmol/kg, oral administration) were administered 60 min before the acetic acid injection. Antinociceptive activity was expressed as inhibition percent of the usual number of writhing observed in control animals.
3.4.4. Formalin-Induced Nociception
The procedure used was essentially the same as that described previously [26]. Animals received 20 mL of 2.5% formalin solution (0.92% formaldehyde in saline) in the ventral surface of the right hind paw. Animals were observed from 0 to 5 min (neurogenic phase) and from 15 to 30 min (inflammatory phase) and the time that they spent licking the injected paw was recorded and considered as indicative of nociception. Animals received indomethacin or compounds (100 µmol/kg, oral administration) 40 min beforehand. Control animals received vehicle (Arabic gum).
3.4.5. Hot-Plate Test
Mice were treated according to the method described by Kuraishi et al. [42]. Animals (n = 6) were placed on a hot-plate set at 55 ± 1 °C. Reaction time was recorded when the animals licked their fore and hind-paws and jumped at 30, 60, 90 and 120 min after oral administration of 100 µmol/kg of compounds or reference drug (morphine, 15 µmol/kg. i.p.). Baseline was considered as the mean of reaction time obtained at 30 and 60 min before administration of derivatives or morphine and was defined as normal reaction of animal to the temperature.
3.4.6. Carrageenan-Induced Peritonitis
Peritoneal inflammation was induced according to the method described by Ferrandiz and Alcaraz [28]. A solution of carrageenan 1% (Sigma-Aldrich) was prepared in saline (NaCl 0.9%) and injected into the peritoneal cavity of mice (250 µL/animal). Four h after injection of carrageenan, the animals were killed by cervical dislocation and the peritoneal cavity was washed with 3 mL of cold Hank’s. Compounds and indomethacin were administered at the dose of 100 µmol/kg (p.o.), 30 min before carrageenan injection. Control group received 10 mL/kg of vehicle (Arabic gum, p.o.). The number of cells was quantified by optical microscope, using 100 × lens.
3.4.7. Statistical Analysis
Data obtained from animal experiments are represented by mean ± standard error of the mean (Mean ± S.E.M.). Statistical differences between the treated and the control groups were evaluated by ANOVA in the Prisma® tutorial. Values were considered significant if * p < 0.05 and ** p < 0.01.
3.5. Thermal Sensitization and Mechanical Allodynia Induced by Spinal Nerve Ligation
3.5.1. Animals
Male Wistar rats (Rattus norvegicus) weighing 180 to 220 g were used at the start of surgery. The animals were maintained in a temperature (25 °C) and humidity (50%–60%) controlled room on a 12 h light: 12 h dark cycle. The Institutional Animal Care and Use Committee at Universidade Federal do Rio de Janeiro (UFRJ) approved the procedures in this study. Animals were acclimated to the laboratory for at least 30 min before experimental initiation. The study was approved by the Animal Care and Use Committee at Universidade Federal do Rio de Janeiro (UFRJ; Rio de Janeiro, Brazil)
3.5.2. Surgery
Peripheral neuropathy was induced in rats by spinal nerve ligation [33]. Briefly, rats were anesthetized with ketamine (100 mg·kg−1 i.p.) and xylazine (5 mg·kg−1 i.p.), the skin was sterilized with 0.5% chlorhexidine. A small incision to the skin overlying L5-S1 was made followed by retraction of the paravertebral musculature from the vertebral transverse processes. The L6 transverse process was partially removed, exposing the L4 and L5 spinal nerve. The L5 spinal nerve was tightly ligated using 6-0 silk suture. For sham animals (animals false operated), the same process was carried out, but the spinal nerve was not ligated.
3.5.3. Behavioral Tests
The withdrawal latency was assessed by applying a radiant heat source to the hind paw of the animals [43]. The light beam was interrupted when the animal lifted the hind paw, allowing measurement the time of paw remained in contact with the heat source. Withdrawal latency was determined using a Dynamic Plantar Anesthesiometer (model 37450, UgoBasile SRL, Varese, Italy). The latency to evoke paw-withdrawal was determined with a cut-off value of 30 s. Control latency was determined using the average response of three measurements. Withdrawal threshold to pressure applied in the hind paw, expressed in grams, was measured using a digital analgesic meter (model EFF301, Insight, Sao Paulo, Brazil) [44]. The stimulation of the paw was repeated five times. Withdrawal threshold was determined with a cut-off value of 120 g to avoid potential tissue injury in the absence of response.
3.5.4. Experimental Design
Forty two Wistar rats were randomly divided into sham and spinal nerve ligation (SNL) groups, which were subdivided into six groups: SNL/DMSO, SHAM/DMSO, SNL/(13), SNL/(17), SNL/(19) and SNL/tramadol. At seven days post-surgery, SNL animals were treated by oral administration with compounds during seven or fourteen days. Thermal hyperalgesia and mechanical allodynia were measured at the 10th and 14th day after surgery. In other group, thermal hyperalgesia and mechanical allodynia were measured at the 10th, 14th, 17th and 21th days after surgery.
3.5.5. Statistical Analysis
Values are expressed as means ± standard error of means (SEM) and one-way ANOVA followed by the Newman–Keuls test for multiple comparisons, using GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA). p values of <0.05 were considered significant.
4. Conclusions
A series of cyclohexyl-N-acylhydrazones were designed as simplified analogues of the prototype LASSBio-294, synthesized and evaluated as analgesic and anti-inflammatory agents. The configuration and conformation of the privileged structure N-acylhydrazone (NAH) were determined from compound 10. Employing X-ray powder diffraction, the configuration E (N=CH) and the antiperiplanar conformation of the amide subunit (-CONH-) of the NAH framework were unequivocally determined. These compounds 10–26 and their conformational restricted analogue 9 showed great anti-inflammatory and/or analgesic activity, with compound 13 (LASSBio-1514) standing out due to its important analgesic activity in a neuropathic pain model.
Acknowledgments
The authors would like to thank INCT-INOFAR (BR, 573.564/2008-6 and E-26/170.020/2008), FAPERJ (BR), FAPEAL (BR), CNPq (BR) and CAPES (BR) for fellowship and financial support.
Author Contributions
T.F.S performed the synthesis and structure characterization; W.B.J. and M.S.A-M. performed the analgesic and antiinflamatory evaluation; F.N.C; F.F.F. and R.C.R.B. performed X-ray experiments; C.E.S.M; R.T.S. and G.Z-S. performed the neuropathic pain experiment; F.N. performed the binding assay; L.M.L and E.J.B. designed the research. All the authors contributed in some way to the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 8–26 are available from the authors.
References
- 1.Evans B.E., Rittle K.E., Bock M.G., DiPardo R.M., Freidinger R.M., Whitter W.L., Lundell G.F., Veber D.F., Anderson P.S., Chang R.S., et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 2.Asif M., Husain A. Analgesic, anti-Inflammatory, and antiplatelet profile of hydrazones containing synthetic molecules. J. Appl. Chem. 2013;2013 doi: 10.1155/2013/247203. [DOI] [Google Scholar]
- 3.Rollas S., Küçükgüzel S. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplancikli Z.A., Altintop M.D., Ozdemir A., Turan-Zitouni G., Khan S.I., Tabanca N. Synthesis and biological evaluation of some hydrazone derivatives as anti-inflammatory agents. Lett. Drug Des. Discov. 2012;9:310–315. doi: 10.2174/157018012799129828. [DOI] [Google Scholar]
- 5.Duarte C.D., Barreiro E.J., Fraga C.A. Privileged structures: A useful concept for the rational design of new lead drug candidates. Mini Rev. Med. Chem. 2007;7:1108–1119. doi: 10.2174/138955707782331722. [DOI] [PubMed] [Google Scholar]
- 6.Do Amaral D.N., Cavalcanti B.C., Bezerra D.P., Ferreira P.M.P., Castro R.P., Sabino J.R., Machado C.M.L., Chammas R., Pessoa C., Sant’Anna C.M.R., et al. Docking, synthesis and antiproliferative activity of N-acylhydrazone derivatives designed as combretastatin A4 analogues. PLoS One. 2014;9:e85380. doi: 10.1371/journal.pone.0085380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraga C.A., Barreiro E.J. Medicinal chemistry of N-acylhydrazones: New lead-compounds of analgesic, antiinflammatory and antithrombotic drugs. Curr. Med. Chem. 2006;13:167–198. doi: 10.2174/092986706775197881. [DOI] [PubMed] [Google Scholar]
- 8.Gong Q., Menon L., Ilina T., Miller L.G., Ahn J., Parniak M.A., Ishima R. Interaction of HIV-1 reverse transcriptase ribonuclease H with an acylhydrazone inhibitor. Chem. Biol. Drug Des. 2011;77:39–47. doi: 10.1111/j.1747-0285.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narang R., Narasimhan B., Sharma S., Sriram D., Yogeeswari P., Clercq E.D., Pannecouque C., Balzarini J. Nicotinic acid benzylidene/phenyl-ethylidene hydrazides: synthesis, antimicrobial evaluation and QSAR studies. Lett. Drug Des. Discov. 2011;8:733–749. doi: 10.2174/157018011796575999. [DOI] [Google Scholar]
- 10.Tributino J.L., Duarte C.D., Correa R.S., Doriguetto A.C., Ellena J., Romeiro N.C., Castro N.G., Miranda A.L., Barreiro E.J., Fraga C.A. Novel 6-methanesulfonamide-3,4-methylenedioxyphenyl-N-acylhydrazones: Orally effective anti-inflammatory drug candidates. Bioorg. Med. Chem. 2009;17:1125–1131. doi: 10.1016/j.bmc.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Tributino J.L., Santos M.L., Mesquita C.M., Lima C.K., Silva L.L., Maia R.C., Duarte C.D., Barreiro E.J., Fraga C.A., Castro N.G., et al. LASSBio-881: An N-acylhydrazone transient receptor potential vanilloid subfamily type 1 antagonist orally effective against the hypernociception induced by capsaicin or partial sciatic ligation. Br. J. Pharmacol. 2010;159:1716–1723. doi: 10.1111/j.1476-5381.2010.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kummerle A.E., Raimundo J.M., Leal C.M., da Silva G.S., Balliano T.L., Pereira M.A., de Simone C.A., Sudo R.T., Zapata-Sudo G., Fraga C.A., et al. Studies towards the identification of putative bioactive conformation of potent vasodilator arylidene N-acylhydrazone derivatives. Eur. J. Med. Chem. 2009;44:4004–4009. doi: 10.1016/j.ejmech.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 13.Gage J.L., Onrust R., Johnston D., Osnowski A., Macdonald W., Mitchell L., Urogdi L., Rohde A., Harbol K., Gragerov S., et al. N-Acylhydrazones as inhibitors of PDE10A. Bioorg. Med. Chem. Lett. 2011;21:4155–4159. doi: 10.1016/j.bmcl.2011.05.100. [DOI] [PubMed] [Google Scholar]
- 14.Silva A.G., Zapata-Sudo G., Kummerle A.E., Fraga C.A., Barreiro E.J., Sudo R.T. Synthesis and vasodilatory activity of new N-acylhydrazone derivatives, designed as LASSBio-294 analogues. Bioorg. Med. Chem. 2005;13:3431–3437. doi: 10.1016/j.bmc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Barreiro E.J. Estratégia de simplificação molecular no planejamento racional de fármacos: A descoberta de novo agente cardioativo. Quim. Nova. 2002;25:1172–1180. doi: 10.1590/S0100-40422002000700018. [DOI] [Google Scholar]
- 16.Barreiro E.J., Fraga C.A.M., Miranda A.L.P., Rodrigues C.R. A química medicinal de N-acilidrazonas: Novos compostos-protótipos de fármacos analgésicos, antiinflamatórios e anti-trombóticos. Quim. Nova. 2002;25:129–148. doi: 10.1590/S0100-40422002000100022. [DOI] [Google Scholar]
- 17.Gonzalez-Serratos H., Chang R., Pereira E.F., Castro N.G., Aracava Y., Melo P.A., Lima P.C., Fraga C.A., Barreiro E.J., Albuquerque E.X. A novel thienylhydrazone, (2-thienylidene)3,4-methylenedioxybenzoylhydrazine, increases inotropism and decreases fatigue of skeletal muscle. J. Pharmacol. Exp. Ther. 2001;299:558–566. [PubMed] [Google Scholar]
- 18.Lima L.M., Barreiro E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005;12:23–49. doi: 10.2174/0929867053363540. [DOI] [PubMed] [Google Scholar]
- 19.Albuquerque E.X., Barreiro E.J., Sudo T.R. Thienylhydrazon with Digitalis-Like Properties (Positive Inotropic Effects) CA2384525, C. Patent. 2007 Mar 6;
- 20.Lima P.C., Lima L.M., da Silva K.C., Leda P.H., de Miranda A.L., Fraga C.A., Barreiro E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000;35:187–203. doi: 10.1016/S0223-5234(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 21.Palla G.P.G., Domiano P., Vignali C., Turner W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron. 1986;42:3649–3654. doi: 10.1016/S0040-4020(01)87332-4. [DOI] [Google Scholar]
- 22.Basso E.A., Oliveira P.R., Caetano J., Schuquel T.A. Semiempirical and ab initio Calculations versus Dynamic NMR on Conformational Analysis of Cyclohexyl-N,N-dimethylcarbamate. J. Braz. Chem. Soc. 2001;12:215–222. doi: 10.1590/S0103-50532001000200015. [DOI] [Google Scholar]
- 23.Palla G., Predieri C., Predieri G., Vignali C. Conformational study on N-acylhydrazones of aromatic aldehydes by NMR spectroscopy. Gazz. Chim. Ital. 1982;112:339–341. [Google Scholar]
- 24.Lopes A.B., Miguez E., Kummerle A.E., Rumjanek V.M., Fraga C.A., Barreiro E.J. Characterization of amide bond conformers for a novel heterocyclic template of N-acylhydrazone derivatives. Molecules. 2013;18:11683–11704. doi: 10.3390/molecules181011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa F.N., Braz D., Ferreira F.F., da Silva T.F., Barreiro E.J., Lima L.M., Colaço M.V., Kuplich L., Barroso R.C. Synchrotron X-ray powder diffraction data of LASSBio-1515: A new N-acylhydrazone derivative compound. Radiat. Phys. Chem. 2014;95:292–294. doi: 10.1016/j.radphyschem.2013.02.014. [DOI] [Google Scholar]
- 26.Hunskaar S., Fasmer O.B., Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods. 1985;14:69–76. doi: 10.1016/0165-0270(85)90116-5. [DOI] [PubMed] [Google Scholar]
- 27.Collier H.O., Dinneen L.C., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrandiz M.L., Alcaraz M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 1991;32:283–288. doi: 10.1007/BF01980887. [DOI] [PubMed] [Google Scholar]
- 29.Top S., Vessieres A., Leclercq G., Quivy J., Tang J., Vaissermann J., Huche M., Jaouen G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chemistry (Easton) 2003;9:5223–5236. doi: 10.1002/chem.200305024. [DOI] [PubMed] [Google Scholar]
- 30.Hunskaar S., Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 31.Woolfe G., Mcdonald A.L. The evaluation of the analgesic action of pethidine hydrochloride [Demerol] J. Pharmacol. Exp. Ther. 1944;80:300–307. [Google Scholar]
- 32.Leal C.M., Pereira S.L., Kummerle A.E., Leal D.M., Tesch R., de Sant’Anna C.M., Fraga C.A., Barreiro E.J., Sudo R.T., Zapata-Sudo G. Antihypertensive profile of 2-thienyl-3,4-methylenedioxybenzoylhydrazone is mediated by activation of the A2A adenosine receptor. Eur. J. Med. Chem. 2012;55:49–57. doi: 10.1016/j.ejmech.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.H., Chung J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang Z.Y., Wen Y.R., Zhang D.R., Borsello T., Bonny C., Strichartz G.R., Decosterd I., Ji R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rector D.L., Conder G.A., Folz S.D. Anthelmintic acylhydrazones, method of use and compositions. WO 1987006127, A1. Patent. 1987 Apr 3;
- 36.Olsen S., Enkemeyer E.-M. Notiz über Hexahydrobenzhydrazid. Chem. Ber. 1948;81:359–361. doi: 10.1002/cber.19480810415. [DOI] [Google Scholar]
- 37.David W.I.F., Shankland K., van de Streek J., Pidcock E., Motherwell W.D.S. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006;39:910–915. doi: 10.1107/S0021889806042117. [DOI] [Google Scholar]
- 38.Costa F.N., Ferreira F.F., da Silva T.F., Barreiro E.J., Lima L.M., Braz D., Barroso R.C. Structure Re-determination of LASSBio-294-a cardioactive compound of the N-acylhydrazone class—Using X-ray powder diffraction data. Powder Diffr. 2013;28:S491–S509. doi: 10.1017/S0885715613000808. [DOI] [Google Scholar]
- 39.Ferreira F.F., Antonio S.G., Rosa P.C., Paiva-Santos Cde O. Crystal structure determination of mebendazole form A using high-resolution synchrotron x-ray powder diffraction data. J. Pharm. Sci. 2010;99:1734–1744. doi: 10.1002/jps.21902. [DOI] [PubMed] [Google Scholar]
- 40.Coelho A.A., Evans J., Evans I., Kern A., Parsons S. The TOPAS symboliccomputation system. Powder Diffr. 2011;26:S22–S25. doi: 10.1154/1.3661087. [DOI] [Google Scholar]
- 41.Luthin D.R., Linden J. Comparison of A4 and A2a binding sites in striatum and COS cells transfected with adenosine A2a receptors. J. Pharmacol. Exp. Ther. 1995;272:511–518. [PubMed] [Google Scholar]
- 42.Kuraishi Y., Harada Y., Aratani S., Satoh M., Takagi H. Separate involvement of the spinal noradrenergic and serotonergic systems in morphine analgesia: The differences in mechanical and thermal algesic tests. Brain Res. 1983;273:245–252. doi: 10.1016/0006-8993(83)90849-1. [DOI] [PubMed] [Google Scholar]
- 43.Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 44.Vivancos G.G., Verri W.A., Jr., Cunha T.M., Schivo I.R., Parada C.A., Cunha F.Q., Ferreira S.H. An electronic pressure-meter nociception paw test for rats. Braz. J. Med. Biol. Res. 2004;37:391–399. doi: 10.1590/S0100-879X2004000300017. [DOI] [PubMed] [Google Scholar]