Abstract
α-Series gangliosides define a particular sub-class of glycosphingolipids containing sialic acid α2,6-linked to GalNAc residue that was isolated as a minor compound from the brain. The sialyltransferase ST6GalNAc V was cloned from mouse brain and showed α2,6-sialyltransferase activity almost exclusively for GM1b, to form GD1α and is considered as the main enzyme involved in the biosynthesis of α-series gangliosides. Recently, ST6GALNAC5 was identified as one of the genes over-expressed in breast cancer cell populations selected for their ability to produce brain metastasis. However, the capacity of human breast cancer cells to produce α-series gangliosides has never been clearly demonstrated. Here, we show by stable transfection and MS-MS analysis of total glycosphingolipids that ST6GALNAC5 expressing MDA-MB-231 breast cancer cells accumulate GD1α ganglioside (IV3Neu5Ac1, III6Neu5Ac1Gg4-Cer).
Keywords: ST6GalNAc V, breast cancer, MDA-MB-231, α-gangliosides, GD1α
1. Introduction
Gangliosides, the glycosphingolipids (GSL) carrying one or several sialic acid residues, are essentially located on the outer leaflet of the plasma membrane where they form lipid rafts with cholesterol and other sphingolipids. Gangliosides were demonstrated to be essential molecules in the modulation of signal transduction pathways by their interactions with signal transduction molecules including receptors tyrosine kinases. Gangliosides are therefore involved in cell adhesion, proliferation and recognition processes [1]. GSL from the ganglio-series are usually classified in four series (0-, a-, b- and c-series) according to the presence of 0 to 3 sialic acid residues linked to lactosylceramide [2]. Normal human tissues mainly express gangliosides from 0- and a-series whereas more ‘complex’ gangliosides from b- and c-series are mainly restricted to the nervous system [3]. The expression of complex gangliosides increases under several pathological conditions including neurodegenerative disorders [4], immune diseases [5] and cancers [6]. For example, GD3 and GD2 are over-expressed in neuroectoderm-derived tumors such as melanoma, neuroblastoma and triple-negative breast cancer, in which they mediate cell proliferation, migration, tumor growth and angiogenesis [6].
α-Series gangliosides define a particular sub-class of GSL containing Neu5Ac α2,6-linked to the GalNAc residue of the gangliopentaosyl backbone Neu5Acα2-3Galβ1-3GalNAcβ1-4Galβ1-4Glc (IV3Neu5Ac1Gg4). The typical α-series ganglioside GD1α (IV3Neu5Ac1,III6Neu5Ac1Gg4-Cer) was first isolated as a minor compound from rat ascites hepatoma AH 7974F cells [7] and from bovine brain [8], with an expression restricted to particular cell populations of the forebrain, the midbrain and the cerebellum [9]. Three members of the CMP-Neu5Ac: β-N-acetylgalactosaminide α2,6-sialyltransferase family (ST6GalNAc III, V and VI) were shown to catalyze in vitro the transfer of a sialic acid residue onto GM1b (IV3Neu5Ac1Gg4-Cer) to form GD1α[10]. However, according to its substrate specificity and expression pattern, ST6GalNAc V is generally considered as the main GD1α synthase. ST6GalNAc V cDNA was cloned from mouse brain [11,12] and st6galnac5 gene is specifically expressed in brain tissues, mostly in forebrain and cerebellum [12]. When expressed as a soluble recombinant protein, the mouse ST6GalNAc V showed α2,6-sialyltransferase activity almost exclusively for GM1b, while being inactive toward glycoproteins [11]. The recombinant mouse ST6GalNAc VI was also shown to convert in vitro GM1b, GD1a, and GT1b into α-series gangliosides GD1α, GT1aα, and GQ1bα, respectively [13]. However, this enzyme was lately demonstrated to be responsible for the synthesis of disialyl-Lea but not for α-series gangliosides in human colon tissues [14]. To our knowledge, the enzymatic activity of human ST6GalNAc V was never thoroughly investigated. However, it was shown that transfection of Human ST6GalNAc V into U373MG glioma cells produced the unusual α2,6-monosialoganglioside, GM2α (Neu5Acα2-6GalNAcβ1-4Galβ1-4Glc-Cer, III6Neu5Ac1Gg3-Cer) instead of GD1α[15].
To date, little is known concerning the specific function of α-series gangliosides. It has been proposed that GD1α could play a role in Purkinje cell functions in the cerebellum [9] and that GD1α could serve as an adhesion molecule for high-metastatic murine lymphosarcoma cell line RAW117-H10 in the adhesion to hepatic sinusoidal endothelial cells [16]. ST6GALNAC5 gene was also shown playing a role in HeLa cell adhesion [17,18] and recently, ST6GALNAC5 was identified as one of the genes over-expressed in breast cancer cell populations selected for their ability to produce brain metastasis [19]. ShRNA inhibition of ST6GALNAC5 expression reduced the capacity of breast cancer cells to produce brain metastasis whereas the expression of ST6GALNAC5 in parental cell lines promoted brain metastasis formation [19]. Moreover, ST6GALNAC5 was demonstrated as the only gene specifically correlated with brain metastasis of breast cancer and up-regulated in human brain metastasis samples [20]. However, the capacity of Human breast cancer cells that express ST6GALNAC5 to produce α-series gangliosides has never been clearly demonstrated. Here, we show by MS analysis of total GSL that ST6GALNAC5 expressing MDA-MB-231 breast cancer cells accumulate GD1α ganglioside.
2. Results and Discussion
2.1. Quantitative Real-Time-PCR (qPCR) Analysis of ST6GalNAc V Expression in Transfected MDA-MB-231 Cells
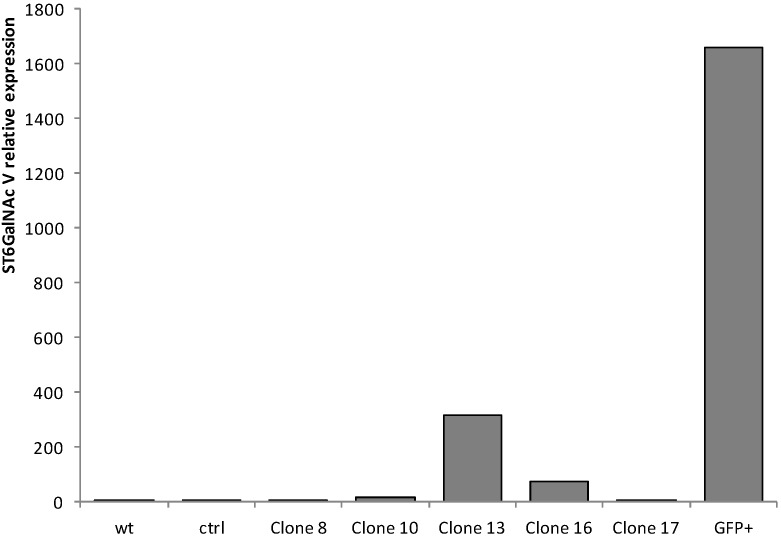
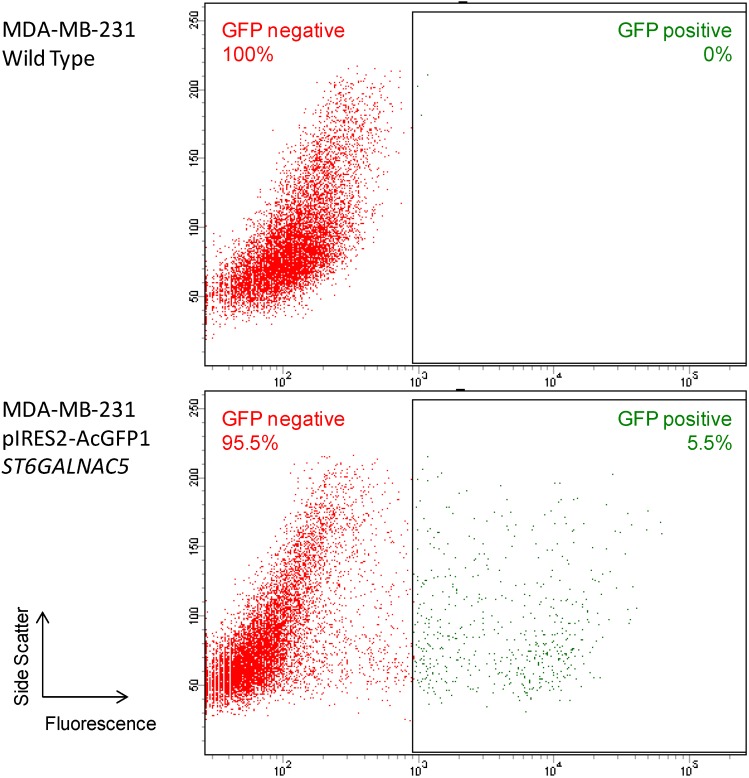
MDA-MB-231 cells were transfected with the pIRES2-AcGFP1 expression vector containing the full-length cDNA of human ST6GalNAc V or the empty vector as control. pIRES2-AcGFP1 is a bicistronic expression vector designed for the simultaneous expression of green fluorescent protein (GFP) and a protein of interest in mammalian cells. Transfected cells were cultured 14 days in the presence of 1 mg/mL G418. Eighteen individual G418-resistant colonies were isolated by limiting dilution and analyzed for the expression of ST6GalNAc V transcripts. No amplification was obtained for thirteen of the resistant clones (not shown) and of the 5 clones showing over-expression of ST6GalNAc V mRNA compared to control cells, the clone #13 displayed the highest level (317-fold) (Figure 1). In parallel, the polyclonal G418-resistant cell population was sorted for GFP expression and 5.5% of the cell population was selected (Figure 2).
Figure 1.
QPCR analysis of ST6GalNAc V expression in control and transfected MDA-MB-231 cells. Quantification of ST6GalNAc V expression was performed by the method described by Pfaffl [21] and normalized to HPRT. The expression of ST6GalNAc V was relative to wild-type (wt), which was regarded as 1. Ctrl, control cells transfected with the empty vector; GFP+, GFP-positive cell population.
Figure 2.
Cell sorting for GFP expression of the G418-resistant cell population. The G418-resistant cell population was sorted for GFP expression on an ARIA SORP flow cytometer.
The resulting GFP-positive polyclonal cell population was analyzed for the expression of ST6GalNAc V and shown a high level of ST6GalNAc V mRNA expression compared to control cells (1657-fold) (Figure 1).
2.2. Flow Cytometry Analysis of α2,6-Sialylation Using Sambucus Nigra Agglutinin (SNA)
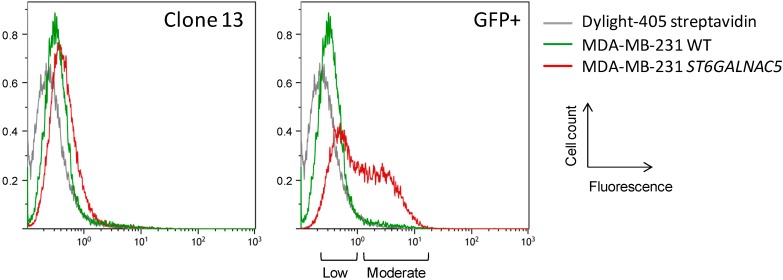
The α-2,6-sialylation of clone #13 and polyclonal GFP-positive cell population was analyzed by flow cytometry using SNA lectin that binds to Neu5Acα2,6-Gal/GalNAc sequence [22]. SNA binding to clone #13 was slightly increased compared to wild-type MDA-MB-231 (Figure 3). In parallel, SNA binding to the polyclonal GFP-positive cell population was stronger but heterogeneous, indicating the presence of at least two populations displaying ‘low’ and ‘moderate’ staining. According to the observed staining, the clone #13 may therefore derive from the low staining sub-population. To our knowledge, the affinity of SNA to Neu5Acα2-3Galβ1-3[Neu5Acα2-6]GalNAcβ- tetrasaccharide has never been clearly determined. Most (if not all) the glycan structures used to analyze SNA binding contain terminal α-2,6-linked sialic acid [23] and the affinity of the lectin could be reduced when sialic acid is α-2,6-linked to an internal GalNAc residue substituted by Neu5Acα2-3Galβ1-3 sequence. This could explain that only a slight increase of SNA binding was observed only for the GFP-positive cell population.
Figure 3.
Flow cytometry analysis of α-2,6-sialylation in ST6GalNAc V transfected MDA-MB-231 cells. Detection of α-2,6-sialylation was performed using biotin-labeled SNA and revealed with Dylight-405-conjugated streptavidin. WT, wild-type.
2.3. MS Analysis of GSL in ST6GalNAc V Transfected MDA-MB-231 Cells
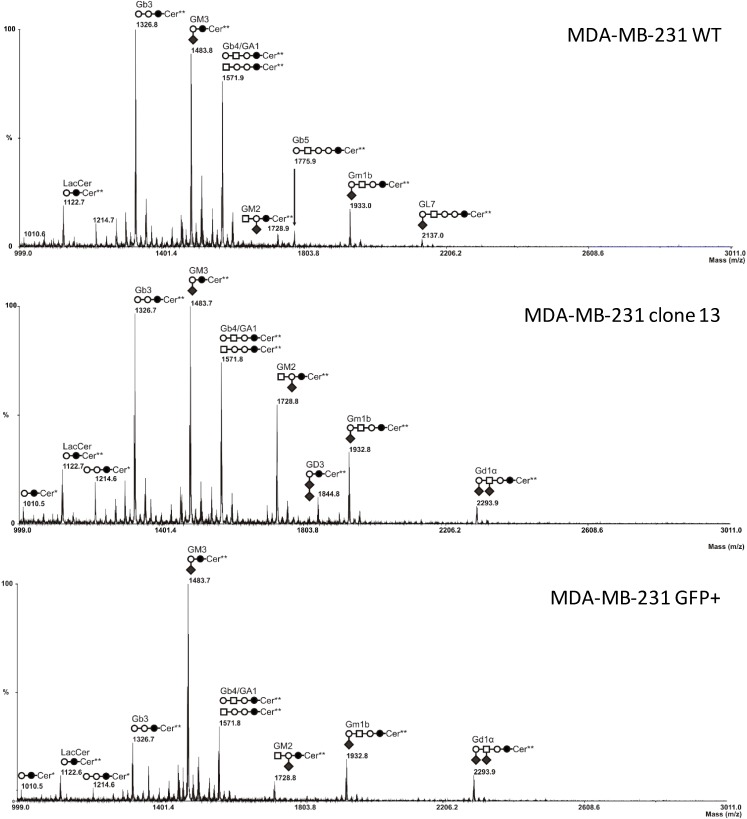
Total glycosphingolipids were extracted from control and ST6GalNAc V expressing cells, purified by reverse phase chromatography and permethylated prior to MS analysis. As previously shown [24], wild-type or empty vector-transfected MDA-MB-231 (not shown) cells expressed neutral globosides Gb3 and Gb4 and monosialylated gangliosides, mainly GM3 (Figure 4A). The precursor lactosylceramide was also detected, as well as a monosialoganglioside at m/z 1933, which was confirmed to correspond to GM1b by MALDI-TOF/TOF fragmentation analysis (data not shown). Two ceramide isoforms are commonly expressed in human tissues due to the substitution of the sphingosine moiety by palmitic acid C16:0 (Cer*) or lignoceric acid C24:0 (Cer**) (Figure 4).
Figure 4.
Comparison of MS profiles of permethylated glycosphingolipids purified from MDA-MB-231 wt, clone #13 and GFP+ ST6GalNAc V transfected cells. GSL are present as d18:1/C16:0 (Cer*) and d18:1/C24:0 (Cer**) isomers. ○, Gal; ●, Glc; □, GalNAc; ♦, Neu5Ac.
As shown in Figure 4B,C, the composition in GSL of clone #13 and polyclonal GFP-positive cell population was similar to control cells with the notable expression at an additional signal at m/z 2294.5 that was tentatively identified as an isomer of GD1 ganglioside (GD1a, GD1b, GD1c or GD1α) with 3 hexoses, one N-acetylhexosamine and 2 N-acetylneuraminic acid residues. Surprisingly, the presence of GD3 at m/z 1,844.8 was also noticed in clone #13. However GD3 was not detected in the polyclonal GFP+ cell population, despite its higher level of ST6GALNAC5 transcripts (Figure 2). This suggests that GD3 expression in clone #13 is probably an artefact due to the clone selection process rather than a consequence of ST6GALNAC5 expression.
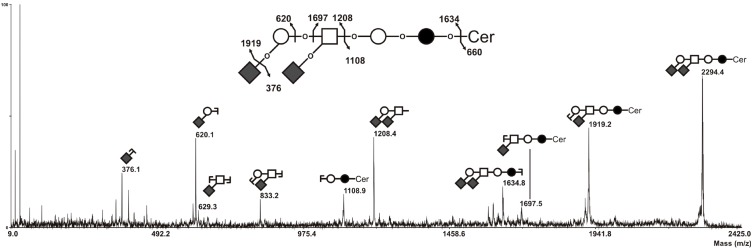
MALDI-TOF/TOF fragmentation analysis established that this signal corresponded to GD1α, as shown in Figure 5. Indeed, the [M+Na]+ B/Y-ions at m/z 1208/1108 attested the presence of a terminal HexNAc1Hex1Neu5Ac2 tetrasaccharide, excluding GD1a and GD1b isomers in which at least one N-acetylneuraminic acid residue is linked to the internal galactose residue and characterised by B/Y-ions at m/z 847/1,469 and m/z 486/1,830, respectively [25]. Then, the presence of [M+Na]+ secondary ion at m/z 629 testified the presence of an internal HexNAc1Neu5Ac1 disaccharide unit, characteristic from the α-series gangliosides. Finally, the absence of [M+Na]+ B-ions at m/z 759, which corresponds to a disialylated sequence finally excluded GD1c isomer. Altogether, these data clearly demonstrate that the additional signal at m/z 2,295 that appeared in the GSL composition of clone #13 and GFP-positive cell population corresponded exclusively to GD1α.
Figure 5.
MS/MS sequencing of permethylated GD1 at m/z 2295 with ceramide moieties d18:1/C24:0 (Cer**). All fragments are observed as [M+Na]+ adducts. Fragment ions were annotated according to nomenclature of Domon and Costello [26]. The nature of monosaccharides was deduced from known biosynthesis of gangliosides. ○, Gal; ●, Glc; □, GalNAc; ♦, Neu5Ac.
3. Experimental Section
3.1. Cell Culture and Transfection
The breast cancer cell line MDA-MB-231 was obtained from the American Type Cell Culture Collection (Manassas, VA, USA) Cell culture reagents were purchased from Lonza (Levallois-Perret, France). Cells were routinely grown in monolayer and maintained at 37 °C in an atmosphere of 5% CO2, in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 units/mL penicillin-streptomycin. The full-length human ST6GalNAc V cDNA [11] was amplified by PCR from the Mammalian Gene Collection (MGC) clone 3356535 using sense 5'-gtagctagctcgagatgaagaccctgatgcgccatgg-3' and antisense 5'-atatatagatctgaattctcagtgtctcggtgtctgatgc-3' primers containing EcoRI and XhoI restriction sites, respectively (underlined) and inserted into the EcoRI and XhoI sites of the bicistronic pIRES2-AcGFP1 expression vector designed for the simultaneous expression in mammalian cells of green fluorescent protein (GFP) and the protein of interest (Clontech, Mountain View, CA, USA). The resulting plasmid was purified using NucleoSpin purification kit (Macherey-Nagel, Hoerdt, France) according to manufacturer’s instructions and fully sequenced. Transfection was performed by lipofection using Lipofectamine® 2000 (Invitrogen, Carlsbad, CA, USA). After transfection, cells were maintained for 48 h in DMEM at 37 °C in an atmosphere of 5% CO2 and then cultured in the presence of 1 mg/mL G418 (Invitrogen). After 14 days in the selective medium, individual G418-resistant colonies were isolated by limit dilution. Alternatively, the G418-resistant cell population was sorted for GFP expression on an ARIA SORP flow cytometer (BD Biosciences, Le Pont de Claix, France). Control cells (empty vector transfected) and ST6GalNAc V positive cells were cultured in the presence of 1 mg/mL G418 (Invitrogen).
3.2. QPCR Analysis of ST6GalNAc V
Total RNA was extracted using the Nucleospin RNA II kit (Macherey Nagel), quantified using DS-11 spectrophotometer (Denovix, Wilmington, DE, USA) and the purity of the preparation was checked by ratio of the absorbance at 260 and 280 nm. The cDNA was synthesized with 2 µg of RNA using the Maxima first strand cDNA Synthesis kit (Thermo Fisher Scientific, Langenselbold, Germany). PCR primers for Hypoxanthine PhosphoRibosylTransferase (HPRT) were previously described [27]. Primers for ST6GalNAc V (sense: 5'-ggatcccaatcacccttcag-3', antisense: 5'-tagcaagtgattctggtttcca-3') were designed using Primer 3 software. QPCR reactions (25 µL) were performed using Maxima SYBR Green Fluorescein qPCR MasterMix (Thermo Fisher Scientific), with 2 µL of cDNA solution and 300 nM final concentration of each primer in a Mx3005p qPCR System (Stratagene, La Jolla, CA, USA). PCR conditions were: 95 °C for 30 s, 51 °C for 45 s, 72 °C for 30 s (40 cycles). Assays were performed in triplicate and ST6GalNAc V transcript expression level was normalized to HPRT using the method described by Pfaffl [21]. Serial dilutions of the appropriate positive control cDNA sample were used to create standard curves for relative quantification and negative control reactions were performed by replacing cDNA templates by sterile water.
3.3. Flow Cytometry Analysis
Cells (3 × 105) were washed in cold PBS and detached by: 5 mM ethylenediaminetetraacetic acid (EDTA). Cells were incubated at 4 °C during 1 h with 10 µg/mL Biotin-labeled SNA (Vector Laboratories, Burlingame, CA, USA) diluted in phosphate buffered saline (PBS) containing 1% bovine serum albumin (PBS-BSA) (Sigma-Aldrich, St. Louis, MO, USA). After washing with PBS-BSA, cells were incubated 30 min on ice with Dylight-405-conjugated streptavidin (Jackson Immunoresearch, West Grove, PA, USA) and analyzed by flow cytometry (Cyan ADP Analyzer, Beckman Coulter, Lille, France).
3.4. Extraction and Preparation of Glycolipids
Twenty dishes (10 cm diameter) of cultured cells were washed twice with ice-cold PBS and cells were sonicated on ice in 200 µL of water. The resulting material was dried under vacuum and sequentially extracted by CHCl3/CH3OH (2:1, v/v), CHCl3/CH3OH (1:1, v/v) and CHCl3/CH3OH/H2O (1:2:0.8, v/v/v). Supernatants were pooled, dried and subjected to a mild saponification in 0.1 M NaOH in CHCl3/CH3OH (1:1) at 37 °C for 2 h and then evaporated to dryness [28]. Samples were reconstituted in CH3OH/H2O (1:1, v/v) and applied to a reverse phase C18 cartridge (Waters, Milford, MA, USA) equilibrated in the same solvent. After washing with CH3OH/H2O (1:1, v/v), GSL were eluted by CH3OH, CHCl3/CH3OH (1:1, v/v) and CHCl3/CH3OH (2:1, v/v).
3.5. Mass Spectrometry Analysis of GSL
Prior to mass spectrometry analysis, GSL were permethylated according to Ciucanu and Kerek [29]. Briefly, compounds were incubated 2 h in a suspension of 200 mg/mL NaOH in dry DMSO (400 µL) and CH3I (200 µL). The methylated derivatives were extracted in CHCl3 and washed several times with water. The reagents were evaporated and the sample was dissolved in CHCl3 in the appropriate dilution. MALDI-MS and MS/MS analyses of permethylated GSL were performed on 4800 Proteomics Analyzer (Applied Biosystems, Framingham, MA, USA) mass spectrometer, operated in the positive reflectron mode. For MS acquisition, 5 µL of diluted permethylated samples in CHCl3 were mixed with 5 µL of 2,5-dihydroxybenzoic acid matrix solution (10 mg/mL dissolved in CHCl3/CH3OH (1:1, v/v)). The mixtures (2 µL) were then spotted on the target plate and air dried. MS survey data comprises a total of 50 sub-spectra of 1500 laser shots. Peaks observed in the MS spectra were selected for further MS/MS. CID MS/MS data comprises a total of 100 sub-spectra of 3000 laser shots. Two or more spectra can be combined post-acquisition with mass tolerance set at 0.1 Da to improve S/N ratio. The potential difference between the source acceleration voltage and the collision cell was set to 1 kV and argon was used as collision gas.
4. Conclusions
The α-series gangliosides define a rare subclass of GSL essentially restricted to some area of mammalian brain. α-gangliosides biological function, however, remains mostly unknown. Based on the substrate specificity of soluble recombinant enzymes, the α2,6-sialytransferase ST6GalNAc V is considered as the main GD1α synthase. Strikingly, ST6GalNAc V expression is also restricted to the brain. The identification of ST6GALNAC5 as one of the genes involved in breast cancer brain metastasis [19] raised the question of the capacity of breast cancer cells to synthesize α-series gangliosides. Here, we show for the first time that the expression of human ST6GalNAc V cDNA in human cancer cells (MDA-MB-231) results in the accumulation of GD1α. However, the question of the role of α-series gangliosides in breast cancer brain metastasis remains open. To our knowledge, no recognition protein was identified to date to specifically bind α-series gangliosides [30]. ST6GALNAC5 gene was previously identified playing a role in controlling the degree of cell adhesion in Hela cells. It was shown that higher ST6GALNAC5 transcription correlated with a lower degree of adhesion, siRNA inhibition of ST6GALNAC5 transcription being followed by enhanced adhesion [18]. Furthermore, the expression of ST6GALNAC5, presumably increasing the expression of α-series gangliosides in breast cancer cells could promote their capacity to form brain metastasis. Although the authors showed ST6GALNAC5 over-expression increased transmigration through a brain-like endothelial barrier, the in vitro model using HUVEC endothelial cells may be questionable. Further investigation are required in order to delineate the molecular mechanism that allows the recognition of α-series gangliosides and the role of these glycolipids in the brain metastasis cascade.
Acknowledgments
This work was supported by the University of Sciences and Technologies of Lille, the CNRS and the comité du Pas-de-Calais de La Ligue contre le Cancer.
Abbreviations
- BAS
Bovine Serum Albumin
- Cer
ceramide
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
Fetal Bovine Serum
- GFP
green fluorescent protein
- GSL
glycosphingolipid
- HPRT
Hypoxanthine PhosphoRibosylTransferase
- LacCer
Lactosylceramide
- MALDI-TOF
matrix assisted laser desorption-ionization time-of-flight
- MS
Mass Spectrometry
- PBS
Phosphate Buffered Saline
- PCR
Polymerase Chain Reaction
- qPCR
Quantitative real-time PCR
- SNA
Sambucus nigra agglutinin
- WT
Wild-Type
Author Contributions
S.V., J.V., C.P.D., A.D. performed the experiments; Y.G. analyzed the data and participated to the redaction; C.M. participated to the redaction; P.D. and S.J. conceived and designed the experiments, analyzed the data and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Julien S., Bobowski M., Steenackers A., Le Bourhis X., Delannoy P. How Do Gangliosides Regulate RTKs Signaling? Cells. 2013;2:751–767. doi: 10.3390/cells2040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svennerholm L. Ganglioside designation. Adv. Exp. Med. Biol. 1980;125:11–19. doi: 10.1007/978-1-4684-7844-0_2. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T., Wada R., Sasaki T., Deng C., Bierfreund U., Sandhoff K., Proia R.L. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA. 1999;96:9142–9147. doi: 10.1073/pnas.96.16.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariga T., McDonald M.P., Yu R.K. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—A review. J. Lipid Res. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahrizaila N., Yuki N. Guillain-Barré syndrome animal model: The first proof of molecular mimicry in human autoimmune disorder. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/829129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobowski M., Cazet A., Steenackers A., Delannoy P. Role of Complex Gangliosides in Cancer Progression. Carbohydr. Chem. 2012;37:1–20. [Google Scholar]
- 7.Taki T., Hirabayashi Y., Ishikawa H., Ando S., Kon K., Tanaka Y., Matsumoto M. A ganglioside of rat ascites hepatoma AH 7974F cells. Occurrence of a novel disialoganglioside (GD1 alpha) with a unique N-acetylneuraminosyl (alpha 2–6)-N-acetylgalactosamine structure. J. Biol. Chem. 1986;261:3075–3078. [PubMed] [Google Scholar]
- 8.Hirabayashi Y., Hyogo A., Nakao T., Tsuchiya K., Suzuki Y., Matsumoto M., Kon K., Ando S. Isolation and characterization of extremely minor gangliosides; GM1b and GD1 alpha; in adult bovine brains as developmentally regulated antigens. J. Biol. Chem. 1990;265:8144–8151. [PubMed] [Google Scholar]
- 9.Furuya S., Irie F., Hashikawa T., Nakazawa K., Kozakai A., Hasegawa A., Sudo K., Hirabayashi Y. Ganglioside GD1 alpha in cerebellar Purkinje cells. Its specific absence in mouse mutants with Purkinje cell abnormality and altered immunoreactivity in response to conjunctive stimuli causing long-term desensitization. J. Biol. Chem. 1994;269:32418–32425. [PubMed] [Google Scholar]
- 10.Harduin-Lepers A. Vertebrate Sialyltransferases. In: Martínez-Duncker J.T., editor. Sialobiology: Structure, Biosynthesis and Function. Sialic Acid Glycoconjugates in Health and Disease. Bentham Science Publishers; Sharjah, United Arab Emirates: 2013. pp. 139–187. [Google Scholar]
- 11.Okajima T., Fukumoto S., Ito H., Kiso M., Hirabayashi Y., Urano T., Furukawa K. Molecular cloning of brain-specific GD1alpha synthase (ST6GalNAc V) containing CAG/Glutamine repeats. J. Biol. Chem. 1999;274:30557–30562. doi: 10.1074/jbc.274.43.30557. [DOI] [PubMed] [Google Scholar]
- 12.Ikehara Y., Shimizu N., Kono M., Nishihara S., Nakanishi H., Kitamura T., Narimatsu H., Tsuji S., Tatematsu M. A novel glycosyltransferase with a polyglutamine repeat; a new candidate for GD1alpha synthase (ST6GalNAc V) FEBS Lett. 1999;463:92–96. doi: 10.1016/S0014-5793(99)01605-1. [DOI] [PubMed] [Google Scholar]
- 13.Okajima T., Chen H.H., Ito H., Kiso M., Tai T., Furukawa K., Urano T., Furukawa K. Molecular cloning and expression of mouse GD1alpha/GT1aalpha/GQ1balpha synthase (ST6GalNAc VI) gene. J. Biol. Chem. 2000;275:6717–6723. doi: 10.1074/jbc.275.10.6717. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchida A., Okajima T., Furukawa K., Ando T., Ishida H., Yoshida A., Nakamura Y., Kannagi R., Kiso M., Furukawa K. Synthesis of disialyl Lewis a (Le(a)) structure in colon cancer cell lines by a sialyltransferase; ST6GalNAc VI; responsible for the synthesis of alpha-series gangliosides. J. Biol. Chem. 2003;278:22787–22794. doi: 10.1074/jbc.M211034200. [DOI] [PubMed] [Google Scholar]
- 15.Kroes R.A., He H., Emmett M.R., Nilsson C.L., Leach F.E., 3rd, Amster I.J., Marshall A.G., Moskal J.R. Overexpression of ST6GalNAcV, a ganglioside-specific alpha2,6-sialyltransferase, inhibits glioma growth in vivo. Proc. Natl. Acad. Sci. USA. 2010;107:12646–12651. doi: 10.1073/pnas.0909862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taki T., Ishikawa D., Ogura M., Nakajima M., Handa S. Ganglioside GD1alpha functions in the adhesion of metastatic tumor cells to endothelial cells of the target tissue. Cancer Res. 1997;57:1882–1888. [PubMed] [Google Scholar]
- 17.Chu C., Lugovtsev V., Golding H., Betenbaugh M., Shiloach J. Conversion of MDCK cell line to suspension culture by transfecting with human siat7e gene and its application for influenza virus production. Proc. Natl. Acad. Sci. USA. 2009;106:14802–14807. doi: 10.1073/pnas.0905912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaluria P., Betenbaugh M., Konstantopoulos K., Frank B., Shiloach J. Application of microarrays to identify and characterize genes involved in attachment dependence in HeLa cells. Metab. Eng. 2007;9:241–251. doi: 10.1016/j.ymben.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos P.D., Zhang X.H., Nadal C., Shu W., Gomis R.R., Nguyen D.X., Minn A.J., van de Vijver M.J., Gerald W.L., Foekens J.A., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorusso G., Rüegg C. New insights into the mechanisms of organ-specific breast cancer metastasis. Semin. Cancer Biol. 2012;22:226–233. doi: 10.1016/j.semcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibuya N., Goldstein I.J., Broekaert W.F., Nsimba-Lubaki M., Peeters B., Peumans W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha2–6)Gal/GalNAc sequence. J. Biol. Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 23.Smith D.F., Song X., Cummings R.D. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 24.Cazet A., Bobowski M., Rombouts Y., Lefebvre J., Steenackers A., Popa I., Guérardel Y., Le Bourhis X., Tulasne D., Delannoy P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology. 2012;22:806–816. doi: 10.1093/glycob/cws049. [DOI] [PubMed] [Google Scholar]
- 25.Steenackers A., Cazet A., Bobowski M., Rombouts Y., Lefebvre J., Guérardel Y., Tulasne D., Le Bourhis X., Delannoy P. Expression of GD3 synthase modifies ganglioside profile and increases migration of MCF-7 breast cancer cells. Comptes Rendus Chim. 2012;15:3–14. doi: 10.1016/j.crci.2011.05.004. [DOI] [Google Scholar]
- 26.Domon B., Costello C.E. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988;27:1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X., Ding L., Sandford A.J. Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol. Biol. 2005;6 doi: 10.1186/1471-2199-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnaar R.L. Isolation of glycosphingolipids. Methods Enzymol. 1994;230:348–370. doi: 10.1016/0076-6879(94)30024-0. [DOI] [PubMed] [Google Scholar]
- 29.Ciucanu I., Kerek F. Rapid and simultaneous methylation of fatty and hydroxy fatty acids for gas-liquid chromatographic analysis. J. Chromatogr. A. 1984;284:179–185. doi: 10.1016/S0021-9673(01)87813-4. [DOI] [Google Scholar]
- 30.Krengel U., Bousquet P.A. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]